Simple Summary
Melanaphis sorghi has been a perennial economically important pest to U.S. sorghum since 2013. Previous research has shown its recent infestation on sorghum has been spreading as a super-clone, a highly abundant clone that is distributed over a large geographic area and persists over time, in the U.S. To continuously monitor the genotypes present in the U.S. and to determine the genotype present in Brazil on sorghum, Melanaphis spp. were collected in 2019 and 2020. Genotyping of aphid samples with microsatellite markers revealed that the super-clone predominated in the U.S. in 2019 and 2020 and Brazil in 2020. Thus, the M. sorghi super-clone remains in the U.S. on sorghum, Johnsongrass, and giant miscanthus and is present in Brazil on sorghum.
Abstract
Melanaphis sorghi (Hemiptera: Aphididae), are an economically important pest to sorghum in the Americas. Previous studies have found that a super-clone that belongs to multilocus lineage (MLL)-F predominated in the U.S. from 2013 to 2018 and uses multiple hosts besides sorghum. In contrast, previous studies found that aphids in South America belong to MLL-C, but these studies only examined aphids collected from sugarcane. In this study we sought to determine if the superclone persisted in the U.S. in 2019–2020 and to determine the MLL of aphids found on sorghum in the largest country in South America, Brazil. Melanaphis spp. samples (121) were collected from the U.S. in 2019–2020 and Brazil in 2020 and were genotyped with 8–9 Melanaphis spp. microsatellite markers. Genotyping results showed that all samples from the U.S. in 2019 and Brazil in 2020 had alleles identical to the predominant superclone. Of the 52 samples collected in the U.S. in 2020, 50 samples were identical to the predominant super-clone (multilocus lineage-F; M. sorghi), while two samples from Texas differed from the super-clone by a single allele. The results demonstrated that the super-clone remains in the U.S. on sorghum, Johnsongrass, and giant miscanthus and is also present on sorghum within Brazil.
1. Introduction
Melanaphis sorghi have been an economically important pest to U.S. sorghum (Sorghum bicolor) since their discovery on grain sorghum near Beaumont, TX in the late summer of 2013 [1]. This invasive parthenogenic aphid pest has spread rapidly, and within two years, it has infested sorghum crops in 17 U.S. states, which accounted for 98% of the total sorghum production in the U.S. [2]. This recent invasive aphid species has been found to feed on all types of sorghum, Johnsongrass (Sorghum halepense), sudangrass (Sorghum verticilliflorum), energycane (Saccharum spp.), Columbus grass (Sorghum almum; a hybrid between sorghum and Johnsongrass), and giant miscanthus (Miscanthus x giganteus) [1,3,4,5]. Interestingly, this new M. sorghi invader reproduces poorly on sugarcane [5].
M. sorghi damages sorghum by feeding on the sap from phloem tissue of the leaves, stems, and panicles causing a loss of plant nutrients and sugars [6,7]. Aphid populations can reach tremendous numbers (exceeding 10,000 aphids per plant) on sorghum and aphid feeding can result in leaf chlorosis, leaf necrosis, stunted growth, delayed or prevention of panicle emergence, and plant death [2,7,8,9,10]. Additionally, M. sorghi also causes a reduction in photosynthetic efficiency due to its secretion of honeydew that causes a sooty mold to buildup on the sorghum leaves [7]. This sticky honeydew also causes problems at harvest as combines become clogged and grain may be expelled from the combine discharge [1]. Yield decline on susceptible grain sorghum hybrids ranged from 50–100% in infested fields [6,10].
Prior to the U.S. invasion in 2013, Melanaphis spp. diversity was examined worldwide, and aphids were collected on sugarcane and Johnsongrass in 2007 from Louisiana and Hawaii [11]. These aphids from the U.S. were classified as multilocus lineage (MLL)—D. Melanaphis spp. diversity in the U.S. has been examined from 2013–2018. Harris-Shultz et al. [12] found that M. sorghi collected on sorghum in 2015 from 17 locations across seven states and one U.S. territory were predominantly one “superclone”. Nibouche et al. [13] collected Melanaphis spp. samples from 2013–2017 from primarily North America and the Caribbean and found that this new invasive aphid pest on sorghum had not been identified previously in their worldwide study and classified this “superclone” as belonging to MLL-F. In the U.S. since 2013, the MLL-F “superclone” (M. sorghi) has been found feeding on sorghum, sugarcane, Johnsongrass, giant miscanthus, and Columbus grass whereas aphids classified as MLL-D have been found feeding only on sugarcane [13,14]. Recently, MLL-C, and D aphids have been classified as M. sacchari, MLL-A and F aphids have been reclassified as M. sorghi, and MLL B and E aphids have not been assigned a species designation although SSR and EF1-α sequence data suggest both belongs to M. sorghi [15]. Although the Entomological Society of America has not adopted this change, the common names have also been altered and aphids that are classified as M. sacchari have a common name as sugarcane aphids and aphids classified as M. sorghi are now called sorghum aphids [15].
Diversity of Melanaphis spp. in South America has been previously examined. Samples (131) from Brazil, Ecuador, and Columbia collected from 2008–2009 on sugarcane all belonged to MLL-C [11]. In 2016, four Melanaphis spp. samples were collected from Peru on sugarcane and were also found to be MLL-C [13]. In a personal communication, three samples collected from Brazil in 2020 were reported to be M. sorghi but the host plant (sorghum or sugarcane) from which it was collected or the MLL it was assigned was not reported [15]. Diversity of Melanaphis spp. on sorghum in South America warrants further investigation.
Seed companies have responded to the M. sorghi outbreak by providing sorghum producers with hybrids that express antibiosis or tolerance (and in some cases both) [16] to the MLL-F “superclone” [17]. To date, Melanaphis spp. worldwide have been classified into six MLL [13] and with global trade and prevailing jet streams it is possible to have a new MLL enter the U.S. Changes in the predominant clonal genotype in the U.S. is also possible especially with the use of insecticides listed within the same Insecticide Resistance Action Committee (IRAC) group to aide in the selection of resistant genotypes [18]. The sorghum industry could be severely impacted if a new Melanaphis spp. biotype or pesticide resistant genotype spreads throughout the sorghum growing region. The sorghum industry suffered when new economically important greenbug biotypes overcame host resistance from the 1970s–1990s [as reviewed in 18]. In this study we examined Melanaphis spp. diversity in the U.S. from 2019 to 2020 to confirm if the “superclone” was still present as the dominant genotype. We also examined Melanaphis spp. diversity in Brazil on sorghum to determine the MLL that was present on sorghum in 2020.
2. Materials and Methods
Aphid samples were collected from sorghum, Johnsongrass, and giant miscanthus in 2019 and 2020 (Supplemental Table S1). For the samples from the U.S., for large fields, 2–3 samples were collected per field where a sample consists of 4–5 infested leaves. Samples were mailed overnight to Tifton and a pooled sample and a clonal sample was created from each mailed sample. The pooled sample consisted of aphids filled to the 0.5 mL mark in a 2 mL tube. For the clonal sample, a single aphid was moved to a benzimidazole agar plate [19,20] containing either cut sorghum or Johnsongrass leaves as the food source. Aphids were reared on the agar plates until approximately 0.1 mL of aphids were obtained. The aphids were then moved into a 2 mL tube and were immediately frozen in a −80 °C freezer. Additionally, some of the Melanaphis spp. samples were shipped frozen on dry ice. For these samples, the microcentrifuge tubes were placed in the −80 °C freezer until DNA extraction.
For the samples from Brazil, aphids were shipped in 90% alcohol. The day prior to DNA extraction, the alcohol was removed and 350 µL of Lysis buffer A (Thermo Fisher Scientific, Waltham, MA, USA) was added to each tube. Samples were placed overnight at 4 °C and the following morning a DNA extraction was performed.
For each sample, four Zn-plated BBs (Daisy Outdoor Products, Rogers, AR, USA) was added into each 2 mL tube and the tubes were placed in liquid nitrogen. Samples were ground using a vortexer by repeatedly taking the samples out of the liquid nitrogen, grinding for less than 10 s, and then placing the tube back into the liquid nitrogen. Samples were ground into a fine powder. DNA was extracted using a GeneJET Plant Genomic DNA Purification kit (Thermo Fisher Scientific) following the manufacturer’s recommendations, except aphids were used instead of plant tissue and one elution was performed. The purified DNA was quantified using a NanoDrop 2000c (Thermo Fisher Scientific), and DNA quality was determined by visualization of the DNA on a 1% agarose gel.
DNA fragments containing Simple Sequence Repeats (SSR) were amplified from each Melanaphis spp. sample by performing 10 µL PCR reactions. The Melanaphis spp. SSR markers used have been previously published [21]. For the 2019 and 2020 U.S. aphid samples, the SSR markers CIR-Ms-B09, CIR-Ms-D02, CIR-Ms-E01, CIR-Ms-G08, CIR-Ms-G403, CIR-Ms-C08, CIR-Ms-G01, CIR-Ms-E03, and CIR-Ms-G02 were amplified. The 2020 aphid samples from Brazil were amplified with the same SSR markers except CIR-Ms-B09. Each reaction contained 2 µL of 5× Colorless GoTaq Flexi buffer (Promega, Madison, WI, USA), 1 µL of 25 mM MgCl2, 0.8 µL of 2.5 mM dNTP mix, 1.8 µL of 1 μM M13 primer (M13-TGTAAAACGACGGCCAGT) 5′ labeled with FAM or HEX, 0.5 µL of 1 μM forward primer with a 5′ M13 tag, 2 µL of 1 μM reverse primer, 0.04 μL of GoTaq Flexi DNA polymerase (Promega), 0.86 µL of water, and 1 μL of sample DNA diluted to 2.5 ng-µL The template arrays (three in total: 2019 USA, 2020 USA, 2020 Brazil) contained all aphid samples as well as the controls Armstrong 1, Armstrong 48, Bellflower1, Brewer4, and at least two no template controls (water only). The thermocycler conditions were an initial denaturation at 94 °C for 3 min, 39 cycles of 94 °C for 30 s, 50 °C for 1 min, 72 °C for 1 min and 10 s, and a final elongation step at 72 °C for 10 min. PCR amplicons were then diluted with 20 µL of molecular biology grade water. To load the PCR fragments onto the SeqStudio Genetic Analyzer (Thermo Fisher Scientific, Waltham, MA, USA), each sample contained 8.5 µL of Hi-Di formamide (Thermo Fisher) and 0.5 µL of GeneScan 500 ROX dye size standard, and 1 µL of diluted PCR sample. Samples were denatured on a thermocycler using 94 °C for 5 min, then loaded onto the SeqStudio, and run using the default program ”fragment analysis”. The fragment data were analyzed using GeneMapper software version 6 (Thermo Fisher Scientific).
Fragments were coded for the program NTSYSpc [22] where a ”0” is the absence of a band, ”1” is the presence of a band, and “9” is a missing fragment due to a failed reaction. For each sized fragment, this coding was used for each sample. Genetic similarity between each pair of samples was calculated using the SIMQUAL module using the DICE coefficient of similarity [23]. An unweighted pair-group method using arithmetic averages (UPGMA) dendrogram was created from the similarity matrix by using the SAHN module in NTSYSpc.
3. Results
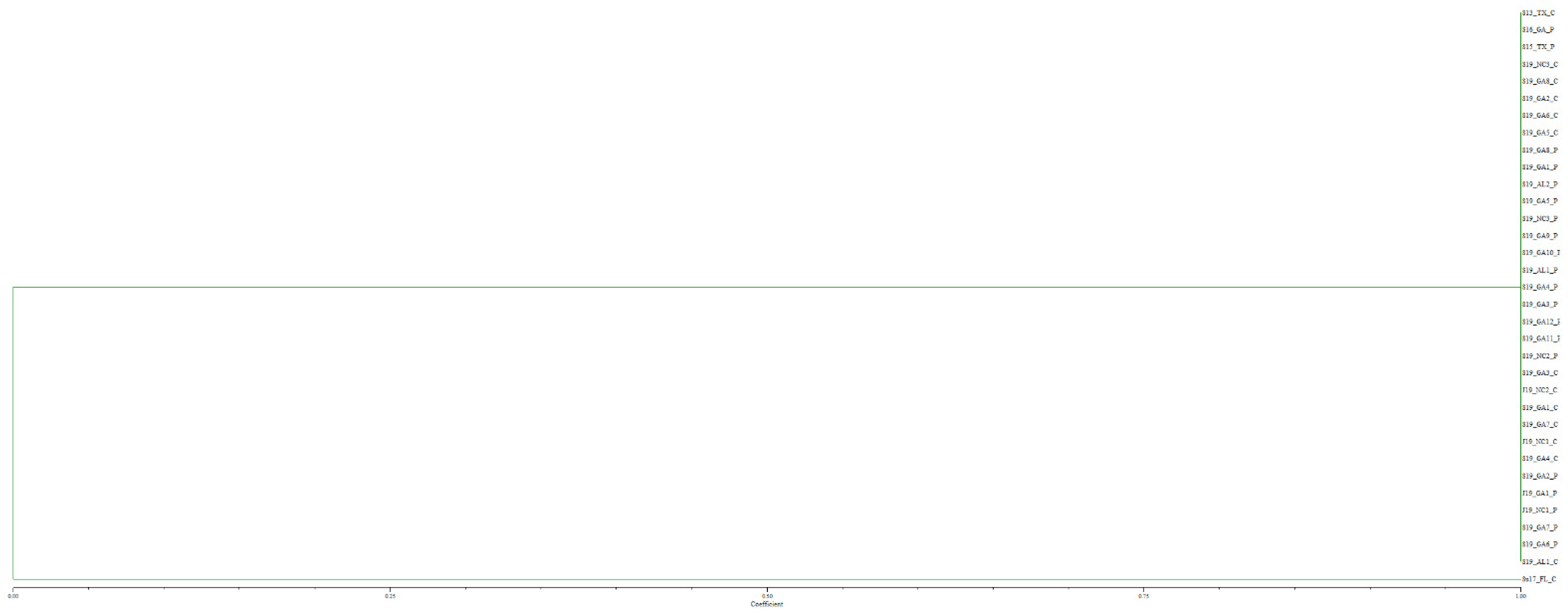
Genotyping of the 2019 U.S. Melanaphis spp. samples collected on sorghum and Johnsongrass from seven cities encompassing three states with nine SSR markers, generated 26 alleles (Supplemental Table S2). This analysis revealed that all 2019 samples (N = 30) had alleles that were identical to S13_TX_C, the sample used to represent the predominant genotype of MLL-F (Figure 1). The sample Su17_FL_C, the sample used to represent the MLL-D lineage grouped by itself.
Figure 1.
Unweighted pair group method with arithmetic mean dendrogram of 2019 Melanaphis spp. samples collected on sorghum and Johnsongrass from seven United States cities and genotyped using nine simple sequence repeat markers. All the Melanaphis samples collected in the United States in 2019 were found to be MLL-F (Melanaphis sorghi).
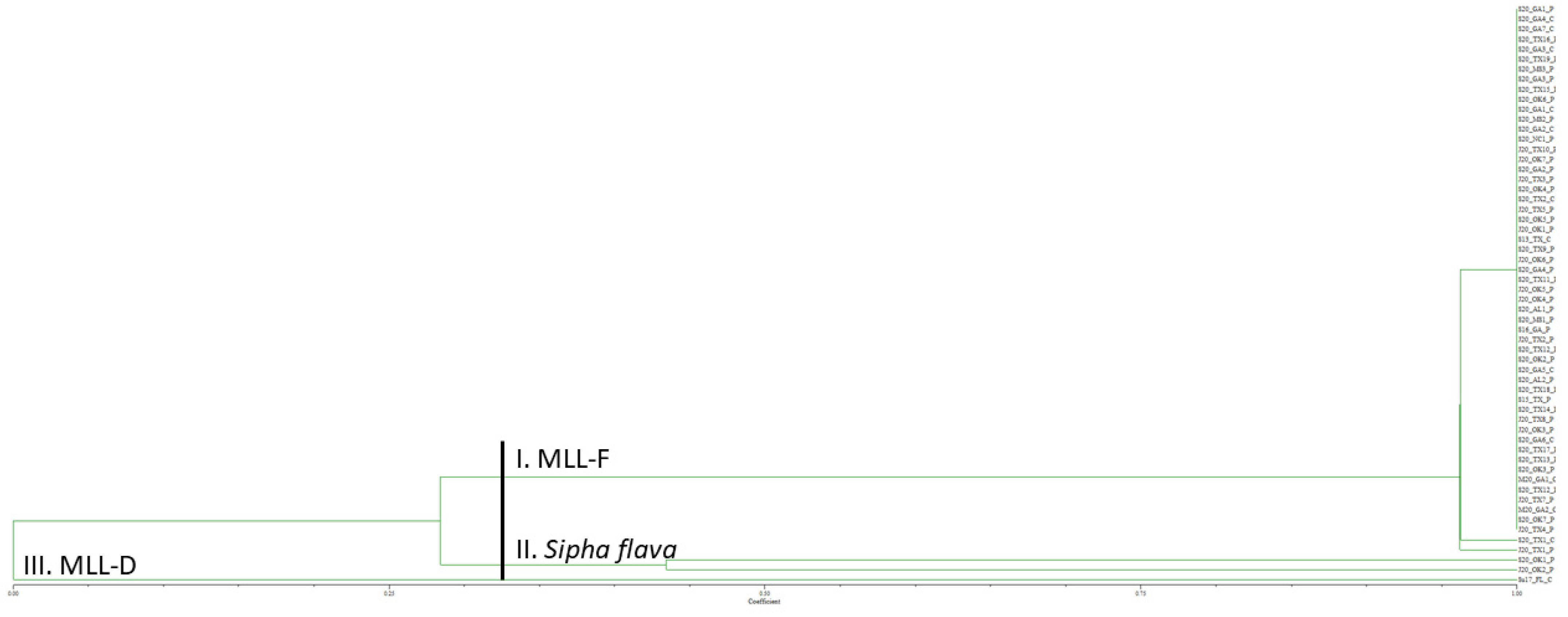
Genotyping of the 2020 U.S. Melanaphis spp. samples collected on giant miscanthus, Johnsongrass, and sorghum from 13 cities encompassing six states with nine SSR markers generated 41 alleles (Supplemental Table S2). Of the 54 U.S. aphid samples collected in 2020, 52 were Melanaphis spp. samples. All 52 of the Melanaphis samples grouped with S13_TX_C, the MLL-F control (Figure 2). Within this group, 50 of the 2020 U.S. samples had identical alleles to S13_TX_C and two samples from Texas differed from S13_TX_C by one allele. The sample representing MLL-D, Su17_FL_C, formed a group by itself. Two samples from Oklahoma, S20_OK1_P and J20_OK2_P grouped together, had markedly different allele sizes than the other samples, and are yellow sugarcane aphid, Sipha flava, samples (our outgroup). Furthermore, an error was observed for samples S20_OK1_P and J20_OK1_P as samples appeared to have been switched as S20_OK1_P groups with the yellow sugarcane aphid sample and J20_OK1_P groups with the M. sorghi samples.
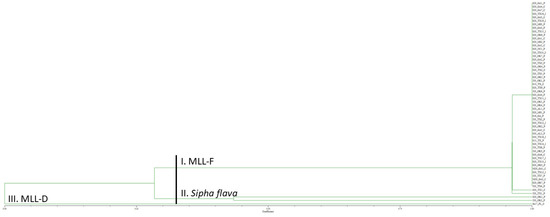
Figure 2.
Unweighted pair group method with arithmetic mean dendrogram of 2020 Melanaphis spp. samples collected on giant miscanthus, sorghum, and Johnsongrass from 13 United States cities and genotyped using nine simple sequence repeat markers. Sipha flava was used as an outgroup. All the Melanaphis samples collected in the United States in 2020 were found to be MLL-F (Melanaphis sorghi). MLL, multilocus lineage.
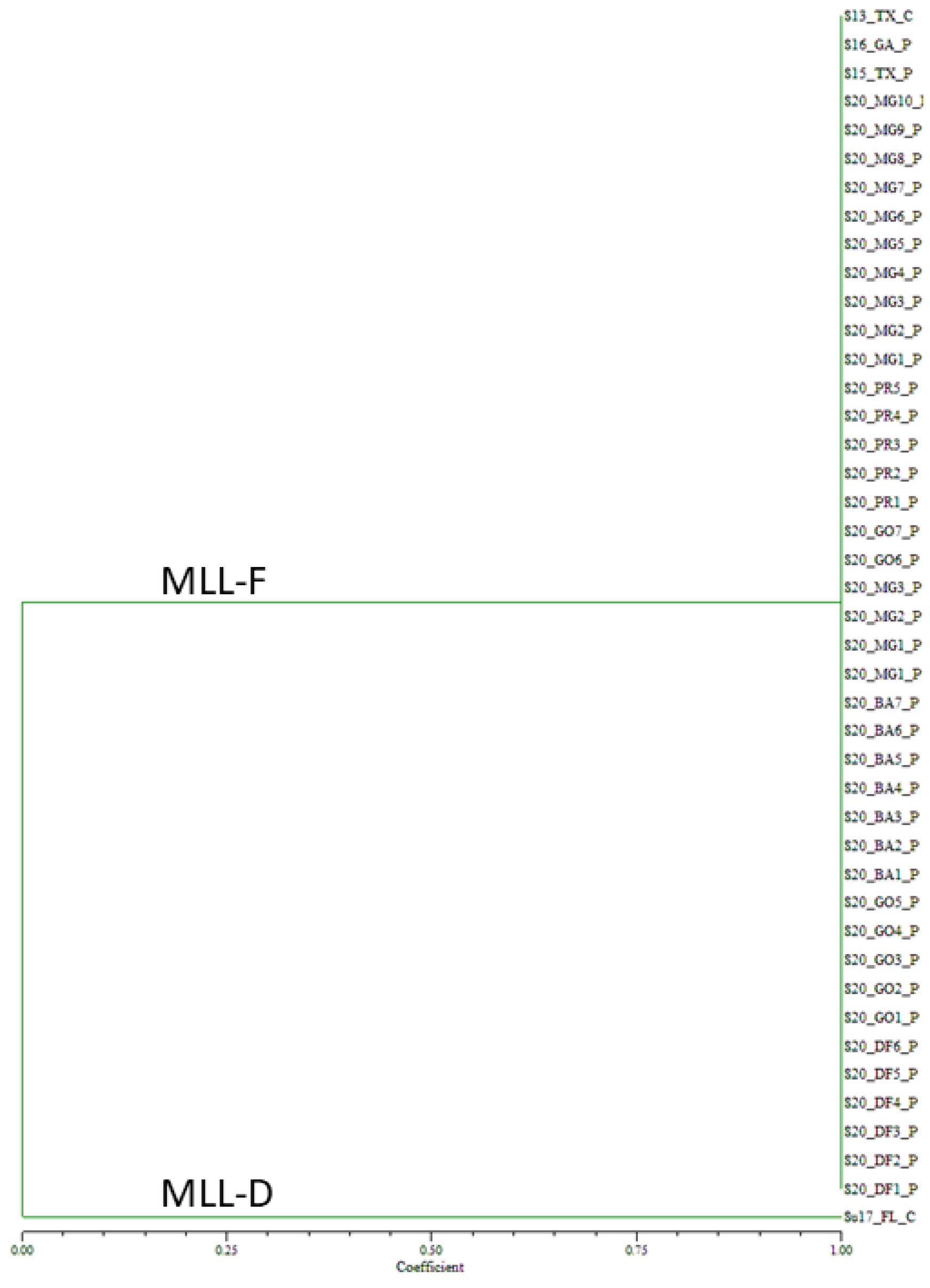
Genotyping of the 2020 Brazilian samples collected on sorghum from ten cities encompassing four states and one federal district, and the controls with eight SSR markers generated 23 alleles (Supplemental Table S2). For the 39 Brazilian samples collected from 2020, all the samples showed identical alleles to the MLL-F control, S13_TX_C (Figure 3). In contrast, the MLL-D control sample, Su17_FL_C formed its own group.
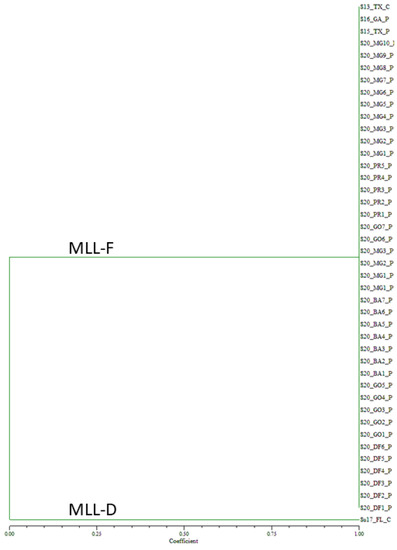
Figure 3.
Unweighted pair group method with arithmetic mean dendrogram of 2020 Melanaphis spp. Samples collected on sorghum from ten Brazilian cities. Samples were genotyped using eight simple sequence repeat markers. All the samples collected in Brazil in 2020 were found to be MLL-F (Melanaphis sorghi). MLL, multilocus lineage.
4. Discussion
Genotyping of the 2019 and 2020 U.S. aphid samples on sorghum, Johnsongrass, and giant miscanthus and the 2020 Brazilian aphid samples on sorghum revealed that the MLL-F superclone is still predominant in the U.S. and is present in all 10 cities of Brazil examined (five states). MLL-F aphids have spread to all sorghum growing areas and persisted in the U.S. on sorghum and other hosts since 2013. This is the first identification of the MLL-F superclone in Brazil. An outbreak started in the state of Goias in 2020 and heavy aphid infestations were observed on sorghum (personal communication, Geraldo Afonso Carvalho). Besides the continental U.S. and Brazil, MLL-F aphids have also been detected in Mexico, Haiti, and Puerto Rico [11].
For the 2020 U.S. Melanaphis samples, two of the 52 samples (S20_TX1_C and J20_TX1_P) differed by one allele from the predominant genotype. This may be due to mutation or genotyping error. Both samples were from Texas, one collected from Johnsongrass next to a plot of sorghum and the other was collected from sorghum. Along the Mexico/US border, two crops of grain sorghum can be grown in the same year and Johnsongrass, a perennial grass species that is a pervasive weed in the U.S. that is also a very good host to M. sorghi, is present year-round. Furthermore, in Texas and Louisiana, MLL-D and MLL-F aphids are present as sugarcane and sorghum are grown in these areas but only asexual reproduction has been reported in the U.S. [2,24,25]. Southern regions (latitude ≤ 31° N) provide environments where M. sorghi can survive year-round. Many more generations of M. sorghi can be produced in southern environments than in more northern regions that experience harsher winters that kill the aphids. These southern locations may provide an ideal environment for the accumulation of mutations in M. sorghi. For example, Harrington [26] stated, in theory, a single asexual female aphid in just one growing season can produce 7.6 × 1028 offspring and aphid asexual lineages have been found to be mutating, even within a few generations [27,28,29]. Most of these mutations would not cause a change in phenotype but with time (more generations produced) and selection pressure, some would impact phenotype [29].
In agroecosystems, there is a trend for invasive asexual aphid lineages to have a few genotypes or super-clones as compared with species with cyclical parthenogenesis which have many unique genotypes [30]. The main variables allowing these aphids to thrive in these areas are obligate parthenogenesis, host availability, and phenotypic plasticity. The MLL-F M. sorghi super-clone in the U.S. and Brazil follows this trend as it does reproduce by obligate parthenogenesis, feeds on multiple species of which one, Johnsongrass, is a perennial invasive weed found from 55° N to 45° S in latitude and thrives in a wide range of environments.
Melanaphis sorghi remains a critically important pest for all types (i.e., grain, forage, biomass, and sweet) of sorghum production. Despite natural enemies including Coccinellidae, Syrphidae, Chrysopidae, Hemerobiidae, Anthocoridae, and Hymenopterans being recruited to the aphid-infested sorghum, they cannot decrease aphid populations below economic threshold [2,31,32]. Genotyping of Melanaphis spp. from the U.S. in 2019 and 2020, and from Brazil in 2020 revealed that the MLL-F super-clone persists in the U.S. and is present throughout Brazil. The utilization of multiple sources of resistant sorghum and use of insecticides with differing modes of action may be beneficial to prevent the development of new biotypes and insecticide resistant genotypes of M. sorghi.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/insects13050416/s1. Table S1: Melanaphis spp. and Sipha flava collection information for samples collected in 2019 from the United States, 2020 from the United States and Brazil, and samples used as the controls. Table S2: Allele sizes of 2019 and 2020 Melanaphis sorghi samples collected in the United States or Brazil.
Author Contributions
Conceptualization, K.H.-S. and J.S.A.; formal analysis, K.H.-S.; funding acquisition, K.H.-S. and X.N.; investigation, K.H.-S.; project administration, K.H.-S. and X.N.; resources, J.S.A., G.C.J. and J.P.S.; writing—original draft, K.H.-S. and J.S.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the USDA-ARS Areawide Pest Management Program, grant number USDA-ARS/: 6048-21220-018-00D and the USDA-ARS.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Raw fragment data can be found in Supplemental Table S2 and analyzed data can be found in the figures in this paper.
Acknowledgments
The authors would like to thank Alana Jacobson, Somashekhar Punnuri, David Buntin, Anders Huseth, Greg Wilson, Michael Brewer, Ada Szczepaniec, James Glover, Brent Bean, and Hongliang Wang for collection of aphids. The authors would also like to thank Michael Purvis and Tyler Bailey for technical support and Kathy Marchant for administrative support.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Armstrong, J.S.; Rooney, W.L.; Peterson, G.C.; Villenueva, R.T.; Brewer, M.J.; Sekula-Ortiz, D. Sugarcane aphid (Hemiptera: Aphididae): Host range and sorghum resistance including cross-resistance from greenbug sources. J. Econ. Entomol. 2015, 108, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Bowling, R.D.; Brewer, M.J.; Kerns, D.L.; Gordy, J.; Seiter, N.; Elliott, N.E.; Buntin, G.D.; Way, M.O.; Royer, T.A.; Biles, S.; et al. Sugarcane aphid (Hemiptera: Aphididae): A new pest on sorghum in North America. J. Integr. Pest Manag. 2016, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, J.S.; Mbulwe, L.; Sekula-Ortiz, D.; Villanueva, R.T.; Rooney, W.L. Resistance to Melanaphis sacchari (Hemiptera: Aphididae) in forage and grain sorghums. J. Econ. Entomol. 2017, 110, 259–265. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Armstrong, J.S.; Harris-Shultz, K.R.; Ni, X.; Wang, H.; Knoll, J.E.; Anderson, W.F. Utilizing biodemographic indices to identify perennial bioenergy grasses as sugarcane aphid (Hemiptera: Aphididae) host plants. Trends Entomol. 2019, 15, 1–14. [Google Scholar]
- Paudyal, S.; Armstrong, J.S.; Harris-Shultz, K.R.; Wang, H.; Giles, K.L.; Rott, P.C.; Payton, M.E. Evidence of host plant specialization among the U.S. sugarcane aphid (Hemiptera: Aphididae) genotypes. Trends Entomol. 2019, 15, 47–58. [Google Scholar]
- Brewer, M.J.; Gordy, J.W.; Kerns, D.L.; Woolley, J.B.; Rooney, W.L.; Bowling, R.D. Sugarcane aphid population growth, plant injury, and natural enemies on selected grain sorghum hybrids in Texas and Louisiana. J. Econ. Entomol. 2017, 110, 2109–2118. [Google Scholar] [CrossRef]
- Singh, B.U.; Padmaja, P.G.; Seetharama, N. Biology and management of the sugarcane aphid, Melanaphis sacchari (Zehntner) (Homoptera: Aphididae), in sorghum: A review. Crop Prot. 2004, 23, 739–755. [Google Scholar] [CrossRef]
- Brewer, M.J.; Bowling, R.; Michaud, J.P.; Jacobson, A.L. 2016, Sugarcane Aphid: A new Pest in North America. ENTO-056. Texas A&M AgriLife Extension Service, College Station, TX. Available online: https://agrilifecdn.tamu.edu/sca/files/2017/03/Sugarcane-Aphid-a-New-Sorghum-Pest-in-North-America.pdf (accessed on 18 April 2022).
- Peterson, G.C.; Armstrong, J.S.; Pendleton, B.B.; Stelter, M.; Brewer, M.J. Registration of RTx3410 through RTx3428 sorghum germplasm resistant to sugarcane aphid [Melanaphis sacchari (Zehntner)]. J. Plant Regist. 2018, 12, 391–398. [Google Scholar] [CrossRef]
- Villanueva, R.T.; Brewer, M.; Way, M.O.; Biles, S.; Sekula-Ortiz, D.; Bynum, E.; Swart, J.; Crumley, C.; Knutson, A.; Porter, P.; et al. Sugarcane Aphid: A New Pest of Sorghum. Texas A&M Agrilife Extension, Ento-035. 2014. Available online: http://denton.agrilife.org/files/2013/08/ENTO-035-The-Sugarcane-Aphid-2014.pdf (accessed on 25 April 2022).
- Nibouche, S.; Fartek, B.; Mississipi, S.; Delatte, H.; Reynaud, B.; Costet, L. Low genetic diversity in Melanaphis sacchari aphid populations at the worldwide scale. PLoS ONE 2014, 9, e106067. [Google Scholar] [CrossRef]
- Harris-Shultz, K.; Ni, X.; Wadl, P.A.; Wang, X.; Wang, H.; Huang, F.; Flanders, K.; Seiter, N.; Kerns, D.; Meagher, R.; et al. Microsatellite markers reveal a predominant sugarcane aphid (Homoptera: Aphididae) clone is found on sorghum in seven states and one territory of the USA. Crop Sci. 2017, 57, 2064–2072. [Google Scholar] [CrossRef]
- Nibouche, S.; Costet, L.; Holt, J.R.; Jacobson, A.; Pekarcik, A.; Sadeyen, J.; Armstrong, J.S.; Peterson, G.C.; McLaren, N.; Medina, R.F. Invasion of sorghum in the Americas by a new sugarcane aphid (Melanaphis sacchari) superclone. PLoS ONE 2018, 13, e0196124. [Google Scholar] [CrossRef] [PubMed]
- Harris-Shultz, K.; Ni, X. A sugarcane aphid (Hemiptera: Aphididae) ‘super-clone’ remains on U.S. sorghum and Johnsongrass and feeds on Giant Miscanthus. J. Entomol. Sci. 2021, 56, 43–52. [Google Scholar] [CrossRef]
- Nibouche, S.; Costet, L.; Medina, R.F.; Holt, J.R.; Sadeyen, J.; Zoogones, A.-S.; Brown, P.; Blackman, R.L. Morphometric and molecular discrimination of the sugarcane aphid, Melanaphis sacchari, (Zehntner, 1897) and the sorghum aphid Melanaphis sorghi (Theobald, 1904). PLoS ONE 2021, 16, e0241881. [Google Scholar] [CrossRef] [PubMed]
- Paudyal, S.; Armstrong, J.S.; Giles, K.L.; Payton, M.E.; Opit, G.P.; Limaje, A. Categories of resistance to sugarcane aphid (Hemiptera: Aphididae) among sorghum genotypes. J. Econ. Entomol. 2019, 112, 1932–1940. [Google Scholar] [CrossRef]
- MyFields. Pest Profile. 2021. Available online: https://www.myfields.info/pests/sugarcane-aphid (accessed on 18 April 2022).
- Harris-Shultz, K.; Armstrong, S.; Jacobson, A. Invasive cereal aphids of North America: Biotypes, genetic variation, management, and lessons learned. Trends Entomol. 2019, 15, 99–122. [Google Scholar]
- Harris-Shultz, K.; Ni, X.; Wang, H.; Knoll, J.E.; Anderson, W.F. Use of benzimidazole agar plates to assess fall armyworm (Lepidoptera: Noctuidae) feeding on excised maize and sorghum leaves. Florida Entomol. 2015, 98, 394–397. [Google Scholar] [CrossRef]
- Nowierski, R.M.; Zeng, Z.; Scharen, A. Age-specific life table modeling of the Russian wheat aphid (Homoptera: Aphididae) on barley grown in benzimidazole agar. Environ. Entomol. 1995, 24, 1284–1290. [Google Scholar] [CrossRef]
- Molecular Ecology Resources Primer Development Consortium; Andris, M.; Aradottir, G.I.; Arnau, G.; Audzijonyte, A.; Bess, E.C.; Bonadonna, F.; Bourdel, G.; Bried, J.; Bugbee, G.; et al. Permanent genetic resources added to Molecular Ecology Resources Database 1 June 2010–31 July 2010. Mol. Ecol. Resour. 2010, 10, 1106–1108. [Google Scholar] [CrossRef]
- Rohlf, F.J. NTSYSpc: Numerical Taxonomy System; Version 2.20; Exeter Publ.: Setauket, NY, USA, 2008. [Google Scholar]
- Nei, M.; Li, W.H. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc. Natl. Acad. Sci. USA 1979, 76, 5269–5273. Available online: http://www.jstor.org/stable/70430 (accessed on 18 April 2022). [CrossRef]
- Hall, D.G. The sugarcane aphid, Melanaphis sacchari, in Florida sugarcane. J. Am. Soc. Sugar Cane Technol. 1987, 7, 26–29. [Google Scholar]
- White, W.H.; Reagan, T.E.; Hall, D.G. Melanaphis sacchari (Homoptera: Aphididae), a sugarcane pest new to Louisiana. Fla. Entomol. 2001, 84, 435–436. [Google Scholar] [CrossRef]
- Harrington, R. Aphid layer (letter). Antenna Bull. R. Entomol. Soc. 1994, 18, 50. [Google Scholar]
- Lushai, G.; De Barro, P.J.; David, O.; Sherratt, T.N.; Maclean, N. Genetic variation within a parthenogenetic lineage. Insect Mol. Biol. 1998, 7, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Forneck, A.; Walker, M.A.; Blaich, R. Ecological and genetic aspects of grape phylloxera (Daktulosphaira vitifoliae Fitch) performance on rootstock hosts. Bull. Entomol. Res. 2001, 91, 445–451. [Google Scholar] [CrossRef]
- Loxdale, H.D.; Lushai, G. Rapid changes in clonal lines: The death of a ‘sacred cow’. Biol. J. Linn. Soc. Lond. 2003, 79, 3–16. [Google Scholar] [CrossRef]
- Figueroa, C.C.; Fuentes-Contreras, E.; Molina-Montenegro, M.A.; Ramirez, C.C. Biological and genetic features of introduced aphid populations in agroecosystems. Curr. Opin. Insect Sci. 2018, 26, 63–68. [Google Scholar] [CrossRef]
- Colares, F.; Michaud, J.P.; Bain, C.L.; Torres, J.B. Recruitment of aphidophagous arthropods to sorghum plants infested with Melanaphis sacchari and Schizaphis graminum (Hemiptera: Aphididae). Biol. Control 2015, 90, 16–24. [Google Scholar] [CrossRef]
- Szczepaniec, A. Interactive effects of crop variety, insecticide seed treatment, and planting date on population dynamics of sugarcane aphid (Melanaphis sacchari) and their predators in late-colonized sorghum. Crop Prot. 2018, 109, 72–79. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).