Risk Assessment and Area-Wide Crop Rotation to Keep Western Corn Rootworm below Damage Thresholds and Avoid Insecticide Use in European Maize Production
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
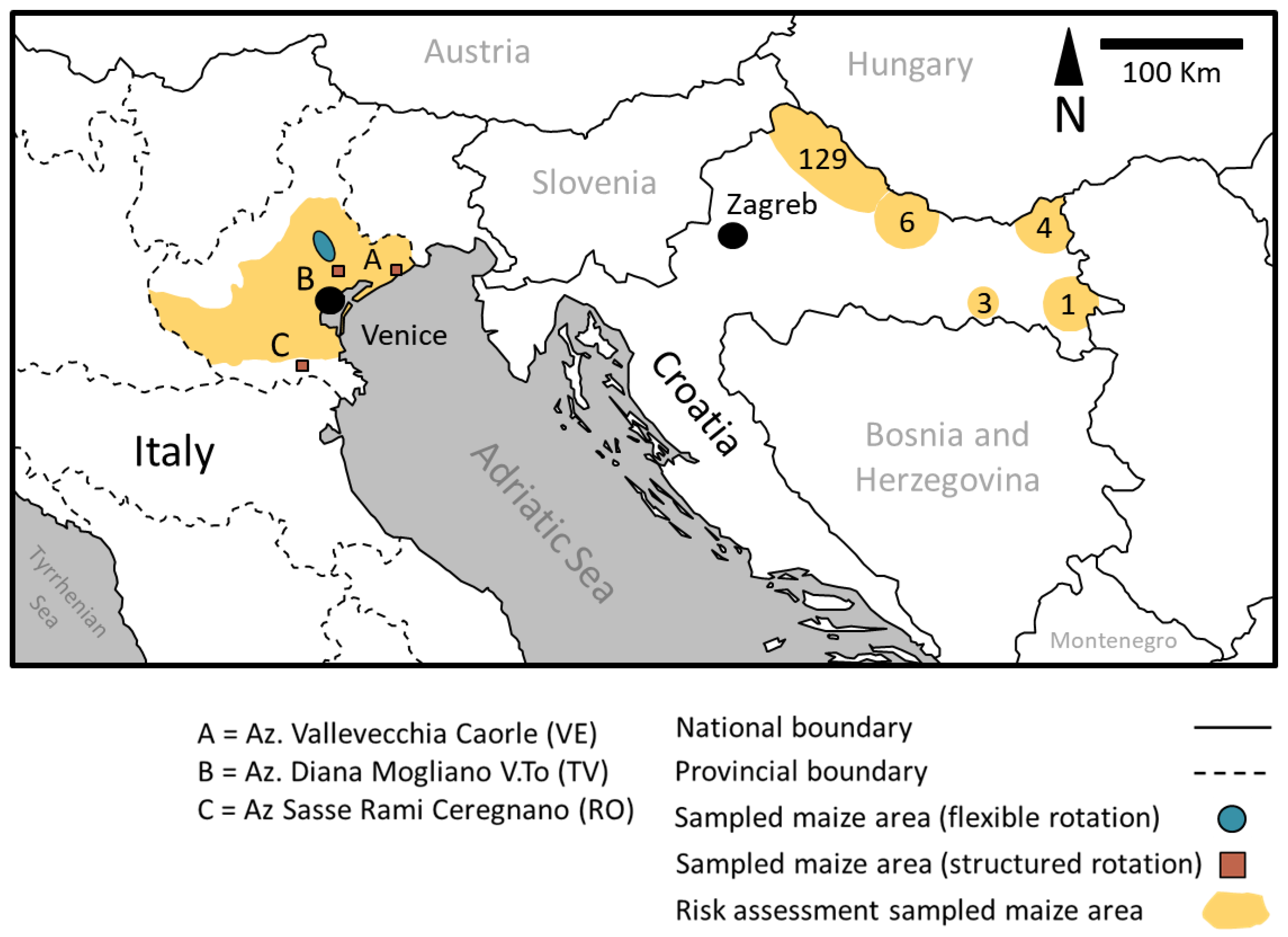
- A WCR risk assessment survey that included damaged fields in both Italian and Croatian maize cultivation areas (Figure 1) and that considered a large number of potential risk factors;
- A WCR area-wide management survey in northern Italy where some maize cultivation areas under different rotation approaches (structural or flexible rotation) and pest control practices were monitored to assess the effects of these practices on WCR populations and on the consequent risks of damage.
2.1. WCR Risk Factors Study in Italy and Croatia
2.1.1. Soil Properties
2.1.2. Agronomic Practices
Rotation Regimes
Sowing Date
Hybrids
2.1.3. Insecticide Treatments
2.1.4. Damage Assessment
2.1.5. WCR Population Level
2.2. Crop Rotation Study in Italy
2.2.1. Structural Crop Rotation
2.2.2. Flexible Crop Rotation
- Intensive rotation (agronomic control with intensive rotation) (IR): Maize generally rotated every two years (maize area 66% per year), with no prolonged maize cultivation; monitoring of pests with traps; no use or reduced use of chemical treatments;
- Chemical approach (chemical control) (CA): Presence of continuous maize plots over many years with adult pest treatments or seed treatments at seeding with insecticides on most or all of the cultivated area.
2.2.3. WCR Population Density and Damage Estimation
WCR Density
Root Damage
2.3. Climatic Conditions
2.4. Statistical Analysis
2.4.1. WCR Risk Factors Study in Italy and Croatia
2.4.2. Crop Rotation Study in Italy
3. Results
3.1. WCR Risk Factors Study in Italy and Croatia
Multifactorial Model
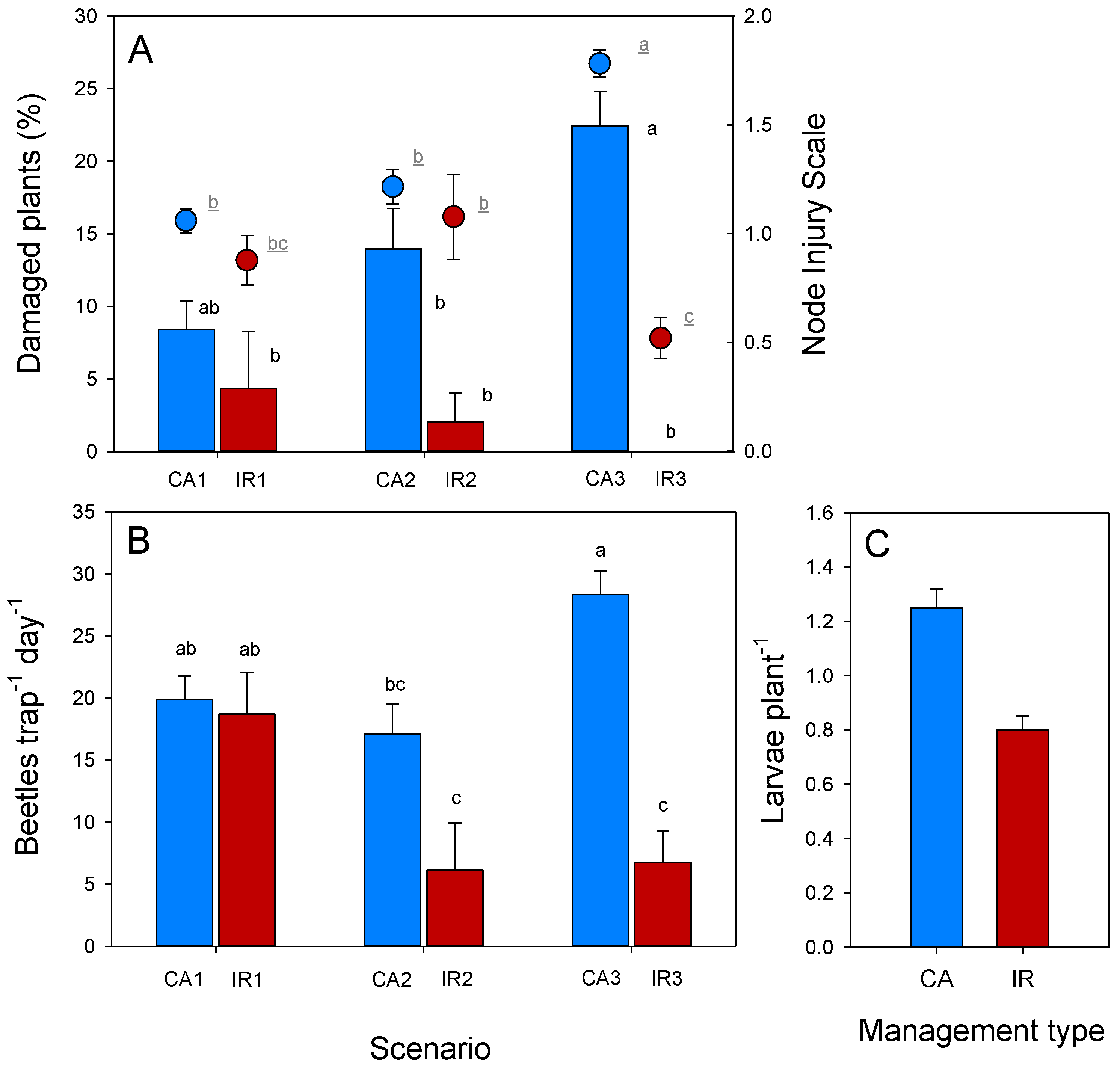
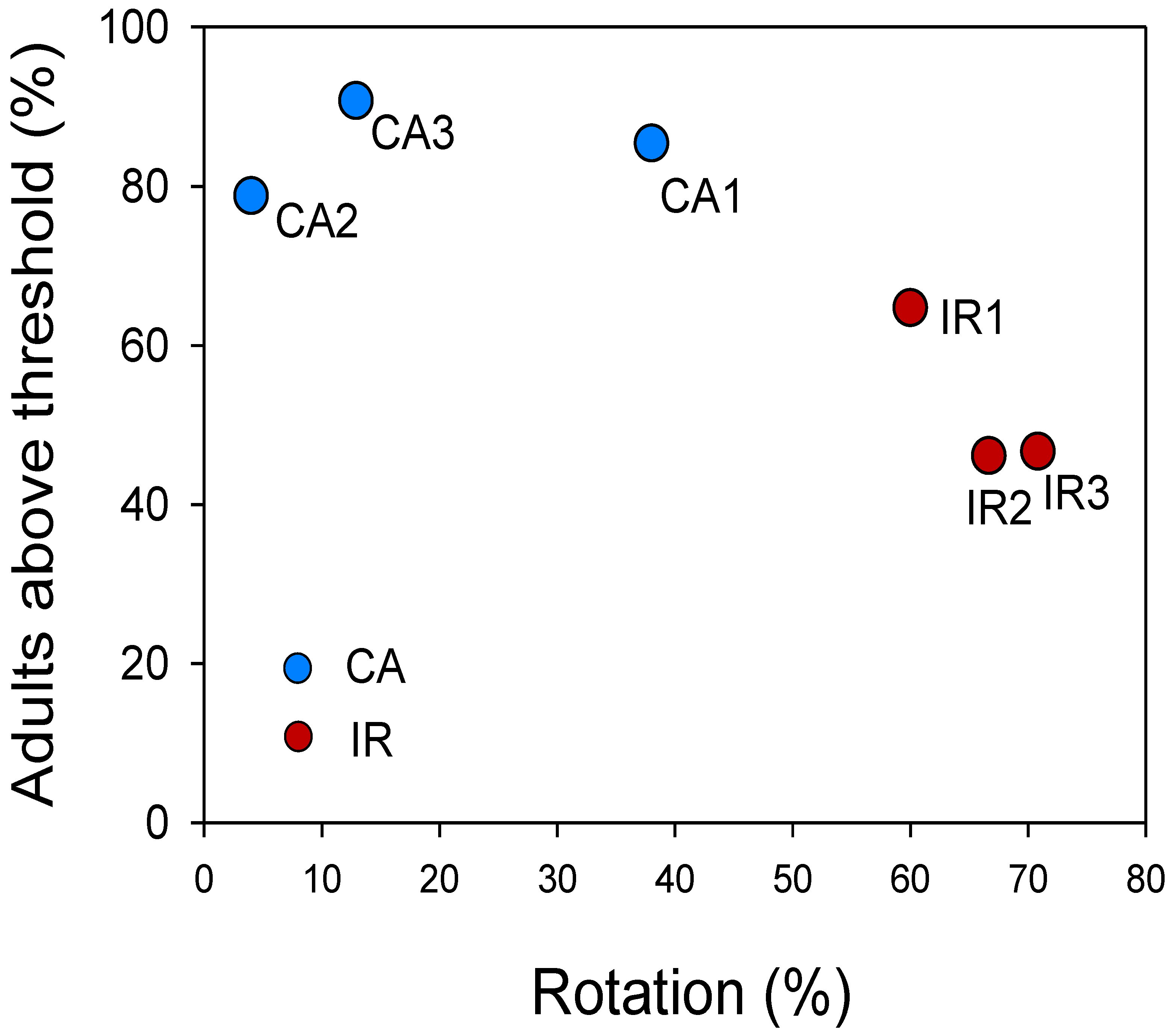
3.2. Crop Rotation Study in Italy
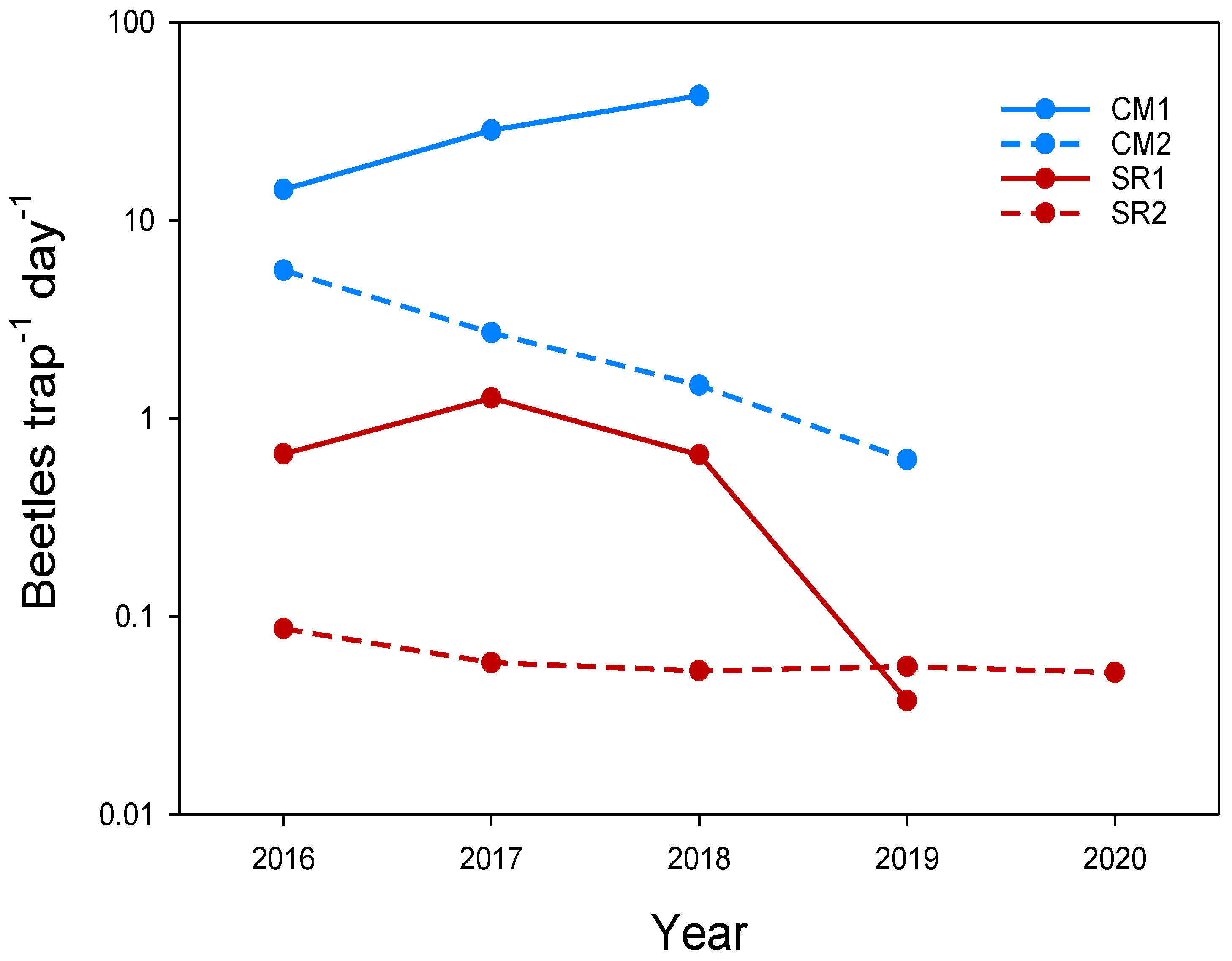
3.2.1. Structural Crop Rotation
3.2.2. Flexible Crop Rotation
4. Discussion
4.1. WCR Risk Factors Study in Italy and Croatia
4.2. Crop Rotation Study in Italy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Metcalf, R.L. Foreword. In Methods for the Study of Pest Diabrotica; Krysan, J.L., Miller, T.A., Eds.; Springer: New York, NY, USA, 1986; pp. vii–xvi. [Google Scholar]
- Bažok, R.; Lemić, D.; Chiarini, F.; Furlan, L. Western corn rootworm (Diabrotica virgifera virgifera LeConte) in Europe: Current status and sustainable pest management. Insects 2021, 12, 195. [Google Scholar] [CrossRef] [PubMed]
- EUR-Lex. Directive 2009/128/EC of the European Parliament and of the Council of 21 October 2009 Establishing a Framework for Community Action to Achieve the Sustainable Use of Pesticides. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32009L0128 (accessed on 28 December 2021).
- Darlington, M.; Reinders, J.D.; Sethi, A.; Lu, A.L.; Ramaseshadri, P.; Fischer, J.R.; Boeckman, C.J.; Petrick, J.S.; Roper, J.M.; Narva, K.E.; et al. RNAi for western corn rootworm management: Lessons learned, challenges, and future directions. Insects 2022, 13, 57. [Google Scholar] [CrossRef] [PubMed]
- EUR-Lex. 2006/564/EC: Commission Decision of 11 August 2006 Amending Decision 2003/766/EC on Emergency Measures to Prevent the Spread within the Community of Diabrotica virgifera Le Conte (Notified under Document Number C (2006) 3582). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32006D0564 (accessed on 28 December 2021).
- EUR-Lex. 2006/565/EC: Commission Recommendation of 11 August 2006 on Containment Programmes to Limit the Further Spread of Diabrotica virgifera Le Conte in Community Areas Where Its Presence Is Confirmed. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32006H0565 (accessed on 28 December 2021).
- Bažok, R.; Sivčev, I.; Kos, T.; Igrc-Barčić, J.; Kiss, J.; Janković, S. Pherocon AM trapping and the “Whole plant count” method—A comparison of two sampling techniques to estimate the WCR adult densities in Central Europe. Cereal Res. Commun. 2011, 39, 298–305. [Google Scholar] [CrossRef]
- Kos, T.; Bažok, R.; Gunjača, J.; Igrc Barčić, J. Western corn rootworm adult captures as a tool for the larval damage prediction in continuous maize. J. Appl. Entomol. 2014, 138, 173–182. [Google Scholar] [CrossRef]
- Baca, F. New member of the harmful entomofauna of Yugoslavia, Diabrotica virgifera virgifera LeConte (Coleoptera, Chrysomelidae). Zast. Bilja 1994, 45, 125–131. [Google Scholar]
- Igrc Barčić, J.; Bažok, R.; Maceljski, M. Research on the western corn rootworm (Diabrotica virgifera virgifera LeConte, Coleoptera: Chrysomelidae) in Croatia (1994–2003). Entomol. Croat. 2003, 7, 63–83. [Google Scholar]
- Szalai, M.; Komáromi, J.P.; Bažok, R.; Igrc Barčić, J.; Kiss, J.; Toepfer, S. The growth rate of Diabrotica virgifera virgifera populations in Europe. J. Pest Sci. 2010, 84, 133–142. [Google Scholar] [CrossRef]
- Miller, N.; Estoup, A.; Toepfer, S.; Bourguet, D.; Lapchin, L.; Derridj, S. Multiple transatlantic introductions of the western corn rootworm. Science 2005, 310, 992. [Google Scholar] [CrossRef] [Green Version]
- Ciosi, M.; Miller, N.J.; Kim, K.S.; Giordano, R.; Estoup, A.; Guillemaud, T. Invasion of Europe by the western corn rootworm, Diabrotica virgifera virgifera: Multiple transatlantic introductions with various reductions of genetic diversity. Mol. Ecol. 2008, 17, 3614–3627. [Google Scholar] [CrossRef] [Green Version]
- Ciosi, M.; Miller, N.J.; Toepfer, S.; Estoup, A.; Guillemaud, T. Stratified dispersal and increasing genetic variation during the invasion of Central Europe by the western corn rootworm, Diabrotica virgifera virgifera. Evol. Appl. 2011, 4, 54–70. [Google Scholar] [CrossRef]
- Lemic, D.; Mikac, K.M.; Ivkosic, S.A.; Bažok, R. The temporal and spatial invasion genetics of the western corn rootworm (Coleoptera: Chrysomelidae) in Southern Europe. PLoS ONE 2015, 10, e0138796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levine, E.; Spencer, J.L.; Isard, S.A.; Onstad, D.W.; Gray, M.E. Adaptation of the western corn rootworm to crop rotation: Evolution of a new strain in response to a cultural management practice. Am. Entomol. 2002, 48, 94–107. [Google Scholar] [CrossRef] [Green Version]
- De Luigi, V.; Furlan, L.; Palmieri, S.; Vettorazzo, M.; Zanini, G.; Edwards, C.R.; Burgio, G. Results of WCR monitoring plans and evaluation of an eradication programme using GIS and Indicator Kriging. J. Appl. Entomol. 2011, 135, 38–46. [Google Scholar] [CrossRef]
- Bermond, G.; Ciosi, M.; Lombaert, E.; Blin, A.; Boriani, M.; Furlan, L.; Toepfer, S.; Guillemaud, T. Secondary contact and admixture between independently invading populations of the western corn rootworm, Diabrotica virgifera virgifera in Europe. PLoS ONE 2012, 7, e50129. [Google Scholar] [CrossRef] [Green Version]
- Bermond, G.; Cavigliasso, F.; Mallez, S.; Spencer, J.; Guillemaud, T. No clear effect of admixture between two European invading outbreaks of Diabrotica virgifera virgifera in Natura. PLoS ONE 2014, 9, e106139. [Google Scholar] [CrossRef] [Green Version]
- ARPAV. Carta dei Suoli del Veneto. Available online: https://www.arpa.veneto.it/arpavinforma/pubblicazioni/carta-dei-suoli-del-veneto (accessed on 15 November 2021).
- USDA. Soil Survey Manual (SSM). Available online: https://www.nrcs.usda.gov/wps/portal/nrcs/detail/soils/scientists/?cid=nrcs142p2_054262 (accessed on 15 November 2021).
- PEDOLOGIJA.COM. Interaktivna Pedološka Karta RH na Podlozi Google Earth. Available online: http://pedologija.com.hr/Zem_resursi.html (accessed on 27 December 2021).
- Igrc Barcic, J.; Bazok, R.; Edwards, C.R.; Kos, T. Western corn rootworm adult movement and possible egg laying in fields bordering maize. J. Appl. Entomol. 2007, 131, 400–405. [Google Scholar] [CrossRef]
- Hill, T.M.; Peters, D.C. A method for evaluating post planting insecticide treatments for control western corn rootworm larva. J. Econ. Entomol. 1971, 64, 764–765. [Google Scholar] [CrossRef]
- Oleson, J.D.; Park, Y.; Nowatzki, T.; Tollefson, J.J. Node—Injury scale to evaluate root injury by corn rootworms (Coleoptera: Chrysomelidae). J. Econ. Entomol. 2005, 98, 1–8. [Google Scholar] [CrossRef]
- ISRIC. FAO-UNESCO. Soil Map of the World. Available online: https://www.isric.org/sites/default/files/ISRIC_TechPap20.pdf (accessed on 15 November 2021).
- Furlan, L.; Cossalter, S.; Chiarini, F.; Signori, A.; Bincoletto, S.; Faraon, F.; Codato, F. Strategie di difesa integrata dalla diabrotica del mais. L’Inf. Agrar. 2018, 10, 74–77. [Google Scholar]
- Blandino, M.; Ferracini, C.; Rigamonti, I.; Testa, G.; Saladini, M.A.; Jucker, C.; Agosti, M.; Alma, A.; Reyneri, A. Control of western corn rootworm damage by application of soil insecticides at different maize planting times. Crop Prot. 2017, 93, 19–27. [Google Scholar] [CrossRef]
- Davis, P.M.; Brenes, N.; Allee, L.L. Temperature dependent models to predict regional differences in corn rootworm (Coleoptera Chrysomelidae) phenology. Environ. Entomol. 1996, 25, 767–775. [Google Scholar] [CrossRef]
- ARPAV. Dati Meteorologici Ultimi Anni. Available online: www.arpa.veneto.it/bollettini/storico/Mappa_2021_TEMP.htm (accessed on 27 October 2021).
- Croatian Meteorological and Hydrological Service. Climate Monitoring. Available online: https://meteo.hr/klima_e.php?section=klima_pracenje¶m=srednja_temperatura (accessed on 27 October 2021).
- Furlan, L.; Contiero, B.; Chiarini, F.; Colauzzi, M.; Sartori, E.; Benvegnù, I.; Fracasso, F.; Giandon, P. Risk assessment of maize damage by wireworms (Coleoptera: Elateridae) as the first step in implementing IPM and in reducing the environmental impact of soil insecticides. Environ. Sci. Pollut. Res. 2017, 24, 236–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szalai, M.; Kiss, J.; Kover, S.; Toepfer, S. Simulating crop rotation strategies with a spatiotemporal lattice model to improve legislation for the management of the maize pest Diabrotica virgifera virgifera. Agric. Syst. 2014, 124, 39–50. [Google Scholar] [CrossRef]
- Stamm, D.E.; Mayo, Z.B.; Campbell, J.B.; Witkowski, J.F.; Andersen, L.W.; Kozub, R. Western corn rootworm (Coleoptera: Chrysomelidae) beetle counts as a means of making larval control recommendations in Nebraska. J. Econ. Entomol. 1985, 78, 794–798. [Google Scholar] [CrossRef]
- Modic, S.; Žigon, P.; Koromanič, A.; Trdan, S.; Razinger, J. Evaluation of the field efficacy of Heterorhabditis bacteriophora Poinar (Rhabditida: Heterorhabditidae) and synthetic insecticides for the control of western corn rootworm larvae. Insects 2020, 11, 202. [Google Scholar] [CrossRef] [Green Version]
- Souza, D.; Peterson, J.A.; Wright, R.J.; Meinke, L.J. Field efficacy of soil insecticides on pyrethroid-resistant western corn rootworms (Diabrotica virgifera virgifera LeConte). Pest Manag. Sci. 2019, 76, 827–833. [Google Scholar] [CrossRef] [Green Version]
- Furlan, L.; Canzi, S.; Di Bernardo, A.; Edwards, C.R. The ineffectiveness of insecticide seed coatings and planting-time soil insecticides as Diabrotica virgifera virgifera LeConte population-suppressors. J. Appl. Entomol. 2006, 130, 485–490. [Google Scholar] [CrossRef]
- Szalai, M. Modelling the Population Dynamics of Western Corn Rootworm (Diabrotica virgifera virgifera LeConte) on Landscape Level. Ph.D. Thesis, Szent István University, Godollo, Hungary, 2012. [Google Scholar]
- Furlan, L.; Faraglia, B.; Michelatti, G.; Vettorazzo, M.; Palmieri, S.; Minuzzo, R.; Martini, G.; Mingardo, A.; Donantoni, L.; Perissinotto, G.; et al. Diffusione nazionale della diabrotica nel 2005. L’Inf. Agrar. 2006, 7, 52–56. [Google Scholar]
- Furlan, L.; Capellari, C.; Porrini, C.; Radeghieri, P.; Ferrari, R.; Pozzati, M.; Davanzo, M.; Canzi, S.; Saladini, M.A.; Alma, A.; et al. Difesa integrata del mais: Come effettuarla nelle prime fasi. L’Inf. Agrar. 2011, 7, 15–19. [Google Scholar]
- Spencer, J.L.; Levine, E. Resistance to Crop Rotation. In Insect Resistance Management: Biology, Economics and Prediction; Onstadt, D.W., Ed.; Elsevier: New York, NY, USA, 2008; pp. 153–184. [Google Scholar] [CrossRef]
- Kos, T. Damage Forecast and Risk Assessment for Western Corn Rootworm (Diabrotica virgifera virgifera LeConte). Ph.D. Thesis, University of Zagreb Faculty of Agriculture, Zagreb, Croatia, 2010. [Google Scholar]
- Veneto Agricoltura. Data Processing from ISTAT. Available online: https://www.istat.it/it/dati-analisi-e-prodotti/banche-dati; (accessed on 10 January 2022).
- Knolhoff, L.M.; Onstad, D.W.; Spencer, J.L.; Levine, E. Behavioral differences between rotation-resistant and wild-type Diabrotica virgifera virgifera (Coleoptera: Chrysomelidae). Environ. Entomol. 2006, 35, 1049–1057. [Google Scholar] [CrossRef] [Green Version]
- Mikac, K.M.; Douglas, J.; Spencer, J.L. Wing shape and size of the western corn rootworm (Coleoptera: Chrysomelidae) is related to sex and resistance to soybean-maize crop rotation. J. Econ. Entomol. 2013, 106, 1517–1524. [Google Scholar] [CrossRef] [PubMed]
- Kadoić Balaško, M.; Mikac, K.M.; Benítez, H.A.; Bažok, R.; Lemic, D. Genetic and morphological approach for western corn rootworm resistance management. Agriculture 2021, 11, 585. [Google Scholar] [CrossRef]
- Lemic, D.; Mikac, K.M.; Bažok, R. Historical and contemporary population genetics of the invasive western corn rootworm (Coleoptera: Chrysomelidae) in Croatia. Environ. Entomol. 2013, 42, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.L.; Mabry, T.R.; Levine, E.; Isard, S.A. Soybean foliage consumption reduces adult western corn rootworm (Diabrotica virgifera virgifera)(Coleoptera: Chrysomelidae) survival and stimulates flight. J. Econ. Entomol. 2021, 114, 2390–2399. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Sappington, T.W. Population genetics strategies to characterize long-distance dispersal of insects. J. Asia-Pac. Entomol. 2013, 16, 87–97. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.M.; Sun, J.T.; Xue, X.F.; Li, J.B.; Hong, X.Y. Invasion genetics of the western flower thrips in China: Evidence for genetic bottleneck, hybridization and bridgehead effect. PLoS ONE 2012, 7, e34567. [Google Scholar] [CrossRef] [Green Version]
- Luikart, G.; Allendorf, F.; Cornuet, J.M.; Sherwin, W. Distortion of allele frequency distributions provides a test for recent population bottlenecks. J. Hered. 1998, 89, 238–247. [Google Scholar] [CrossRef]
- Agrifondo Mutualistico Veneto e Friuli. Available online: https://www.condifesatvb.it/campagna-mutualistica (accessed on 10 January 2022).
- Furlan, L.; Pozzebon, A.; Duso, C.; Simon-Delso, N.; Sànchez-Bayo, F.; Marchand, P.A.; Codato, F.; van Lexmond, M.B.; Bonmatin, J.M. An update of the worldwide integrated assessment (WIA) on systemic insecticides. Part 3: Alternatives to systemic insecticides. Environ. Sci. Pollut. Res. 2018, 28, 11798–11820. [Google Scholar] [CrossRef] [Green Version]
| Variables | Explanation | Type | Classification 1 | Number of Records | Surveyed Maize-Cultivated Land (ha) | |
|---|---|---|---|---|---|---|
| Croatia | Italy | |||||
| Year | Year of data collection | Ordinal | 2003–2005 | 6 | 0 | 10 |
| 2006–2010 | 60 | 5 | 62 | |||
| 2011–2015 | 77 | 449 | 790 | |||
| 2016–2018 | 0 | 148 | 393 | |||
| Crop damage | Damage index: percentage of total plants damaged (gooseneck and lodged plants %) | Quantitative | <5% | 98 | 345 | 864 |
| ≥5% | 45 | 257 | 391 | |||
| WCR beetle population level PhAM Y-1 | Beetle population level (from pheromone trapping) in the previous year | Qualitative | Low | 23 | 65 | 137 |
| Medium | 72 | 83 | 204 | |||
| High | 28 | 110 | 241 | |||
| Very high | 20 | 152 | 368 | |||
| NA * | 0 | 192 | 305 | |||
| Soil properties | Texture | Qualitative | F * | 0 | 223 | 436 |
| FL ** | 134 | 21 | 216 | |||
| FA ***, FLA **** | 1 | 207 | 269 | |||
| FS ***** | 0 | 49 | 117 | |||
| NA | 8 | 102 | 217 | |||
| Agronomic practices | Rotation type (see Section 2.1.3) | Qualitative | A (100% maize) | 21 | 377 | 768 |
| B (70–80% maize) | 116 | 183 | 416 | |||
| C (30–40% maize) | 6 | 42 | 71 | |||
| Sowing date | Qualitative | Early (March) | 1 | 226 | 414 | |
| Ordinary (April–mid May) | 139 | 240 | 619 | |||
| Late (second half of May) | 3 | 100 | 143 | |||
| Very late (June) | 0 | 36 | 79 | |||
| Hybrid | Qualitative | Dekalb | 3 | 69 | 140 | |
| KWS | 0 | 37 | 54 | |||
| Pioneer | 50 | 311 | 595 | |||
| BC Institute | 84 | 0 | 81 | |||
| Others | 6 | 67 | 125 | |||
| NA | 0 | 118 | 260 | |||
| Treatments | Insecticide treatments against adults in previous year | Qualitative | No | 143 | 444 | 1022 |
| Yes | 0 | 147 | 177 | |||
| NA | 0 | 11 | 56 | |||
| Soil insecticide application | Qualitative | No | 103 | 123 | 354 | |
| In furrow micro-granular insecticide | 0 | 306 | 510 | |||
| Insecticide coating | 40 | 128 | 329 | |||
| Both (granular and coating) | 5 | 27 | 43 | |||
| NA | 0 | 13 | 19 | |||
| Insecticide (active ingredient) | Qualitative | No | 0 | 39 | 354 | |
| Tefluthrin in furrow | 0 | 22 | 346 | |||
| Teflutrin as seed treatment | 0 | 72 | 131 | |||
| Lambda-cyhalothrin | 0 | 41 | ||||
| Others | 0 | 35 | ||||
| Country (Number of Fields) | Product | Active Ingredients | Dose | Type |
|---|---|---|---|---|
| Croatia (14) | Cruiser® | Thiametoxam | 0.63 mg/seed | coating |
| Croatia (3) | Gaucho® | Imidacloprid | 1.2 mg/seed | coating |
| Croatia (3) | Macho® | |||
| Croatia (18) | Poncho® | Chlothianidin | 1 mg/seed | coating |
| Croatia (1) | Mesurol FS500® | Methiocarb 50% | 1.8 L/100 kg of seed | coating |
| Croatia (6) | Force 1.5G® | Tefluthrin 1.5% | 10–12 kg/ha | granules applied in-furrow |
| Italy (52) | Force ST® | Tefluthrin | 0.5 mg/seed | coating granules applied in-furrow |
| Italy (91) | Force 0.5 G® | Tefluthrin | 10–12 kg/ha | |
| Italy (17) | Poncho® | Clothianidin | 0.5 mg/seed | Coating granules applied in-furrow coating |
| Italy (77) | Santana® | Clothiadinin 0.7% | 11 kg/ha | |
| Italy (6) | Gaucho® | Imidacloprid | 1.2 mg/seed |
| Scenario | Townland | Surface (ha) | No Maize | Maize | Number of Plots | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st Year | 2nd Year | 3rd Year | 4th Year | 5th Year | 6th Year | 2nd–6th Year | |||||
| IR1 | Paese/Trevignano | 26.8 | 30.1 | 37.7 | 10.6 | 6.5 | 1.5 | 1.9 | 11.6 | 32.1 | 17 |
| CA1 | Montebelluna/Trevignano | 30.6 | 19.8 | 18.3 | 1.6 | 7.2 | 17.9 | 1.8 | 33.2 | 61.9 | 54 |
| IR2 | Paese | 27.3 | 57.9 | 16.0 | 6.9 | 1.9 | 0.0 | 3.5 | 13.7 | 26.0 | 13 |
| CA2 | Paese | 17.0 | 0.0 | 13.4 | 0.0 | 6.3 | 6.8 | 5.6 | 67.9 | 86.6 | 33 |
| IR3 | Quinto TV | 15.2 | 37.9 | 34.6 | 23.3 | 0.0 | 4.2 | 0.0 | 0.0 | 27.5 | 30 |
| CA3 | Treviso/Quinto/Paese | 23.2 | 9.0 | 4.5 | 14.7 | 8.6 | 0.0 | 0.0 | 60.8 | 84.1 | 54 |
| Variables | Level | LS Means: % Cases for Total Damage ≥5% | Comparisons | p | RR 95%CI |
|---|---|---|---|---|---|
| WCR beetle population level PhAM Y-1 | Low | 8 | reference level | ||
| Medium | 23 | M vs. L | 0.006 | 2.92 (1.36–6.28) | |
| High | 42 | H vs. L | <0.001 | 5.28 (2.53–11.04) | |
| Very high | 63 | VH vs. L | <0.001 | 7.97 (3.88–16.36) | |
| Soil texture | FS * | 26 | reference level | ||
| F ** | 38 | F vs. FS | 0.994 | ||
| FA *** | 37 | FA vs. FS | 0.990 | ||
| FL **** + FLA ***** | 32 | FL + FLA vs. FS | 0.990 | ||
| Rotation type | A (100% maize) | 23 | C vs. A | 0.038 | 0.57 (0.33–0.97) |
| B (70–80% maize) | 40 | C vs. B | 0.017 | 0.52 (0.31–0.89) | |
| C (30–40% maize) | 44 | reference level | |||
| Sowing date | Early (March) | 41 | early vs. ord. | 0.039 | 1.21 (1.01–1.44) |
| Ordinary (April–mid May) | 50 | reference level | |||
| Late (second half of May) + Very late (June) | 25 | very late + late vs. Ordinary | 0.002 | 0.61 (0.45–0.84) | |
| Hybrid variety (producer) | Dekalb | 22 | De Kalb vs. KWS | 0.142 | |
| KWS | 36 | reference level | |||
| Pioneer | 40 | Pioneer vs. KWS | 0.053 | ||
| BC Institute | 32 | BC Institute vs. KWS | 0.259 | ||
| Others | 55 | Other vs. KWS | 0.005 | 2.53 (1.32–4.84) | |
| Insecticide treatment against adults in previous year | No | 40 | reference level | ||
| Yes | 43 | yes vs. no | 0.527 | ||
| Soil insecticide application | No | 39 | reference level | ||
| In furrow micro-granular insecticide | 57 | seed vs. No | <0.001 | 1.45 (1.18–1.79) | |
| Insecticide coating | 33 | yes vs. No | 0.109 | ||
| Both (granular and coating) | 31 | yes + seed vs. no | 0.400 | ||
| Insecticide (active ingredient) | No | 39 | reference level | ||
| Tefluthrin in furrow | 22 | teflutrin in furrow vs. No | 0.006 | 0.56 (0.37–0.85) | |
| Teflutrin as seed treatment | 72 | tefluthrin seed treat. vs. No | <0.001 | 1.82 (1.44–2.30) | |
| Lambda-cyhalothrin | 41 | lambda-cyhalothrin vs. No | 0.739 | ||
| Other | 35 | other vs. No | 0.325 |
| Rotation | Fields | Previous Crop | Ha | Adult Population Density (Number of Beetles/Trap Per Day) 1 |
|---|---|---|---|---|
| C | 8 | Alfalfa (4) Barley (4) | 10.04 | Very high * |
| C | 1 | Alfalfa | 0.14 | High ** |
| C | 1 | Sorghum | 1.6 | NA *** |
| B | 16 | Soybean (2), Winter Wheat (10) Barley (3) Pumpkin (1) | 47.46 | Very high |
| B | 6 | Soybean (4), Winter Wheat (2) | 5.77 | High |
| B | 8 | Soybean (4), Winter Wheat (2) Barley (2) | 5.98 | NA |
| Total | 40 | 70.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furlan, L.; Chiarini, F.; Contiero, B.; Benvegnù, I.; Horgan, F.G.; Kos, T.; Lemić, D.; Bažok, R. Risk Assessment and Area-Wide Crop Rotation to Keep Western Corn Rootworm below Damage Thresholds and Avoid Insecticide Use in European Maize Production. Insects 2022, 13, 415. https://doi.org/10.3390/insects13050415
Furlan L, Chiarini F, Contiero B, Benvegnù I, Horgan FG, Kos T, Lemić D, Bažok R. Risk Assessment and Area-Wide Crop Rotation to Keep Western Corn Rootworm below Damage Thresholds and Avoid Insecticide Use in European Maize Production. Insects. 2022; 13(5):415. https://doi.org/10.3390/insects13050415
Chicago/Turabian StyleFurlan, Lorenzo, Francesca Chiarini, Barbara Contiero, Isadora Benvegnù, Finbarr G. Horgan, Tomislav Kos, Darija Lemić, and Renata Bažok. 2022. "Risk Assessment and Area-Wide Crop Rotation to Keep Western Corn Rootworm below Damage Thresholds and Avoid Insecticide Use in European Maize Production" Insects 13, no. 5: 415. https://doi.org/10.3390/insects13050415
APA StyleFurlan, L., Chiarini, F., Contiero, B., Benvegnù, I., Horgan, F. G., Kos, T., Lemić, D., & Bažok, R. (2022). Risk Assessment and Area-Wide Crop Rotation to Keep Western Corn Rootworm below Damage Thresholds and Avoid Insecticide Use in European Maize Production. Insects, 13(5), 415. https://doi.org/10.3390/insects13050415










