A Preliminary Survey of Olive Grove in Biskra (Southeast Algeria) Reveals a High Diversity of Thrips and New Records
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Thrips Community Composition and Thrips Attacks on Olive Trees
2.3. Mounting and Identification
2.4. Analyzing Abundance and Population Fluctuation of Thrips on Olive Trees
2.5. Data Analysis
3. Results and Discussion
3.1. Thrips Community Composition and Thrips Attacks on Olive Trees
3.2. Analyzing Abundance of Thrips on Olive Trees
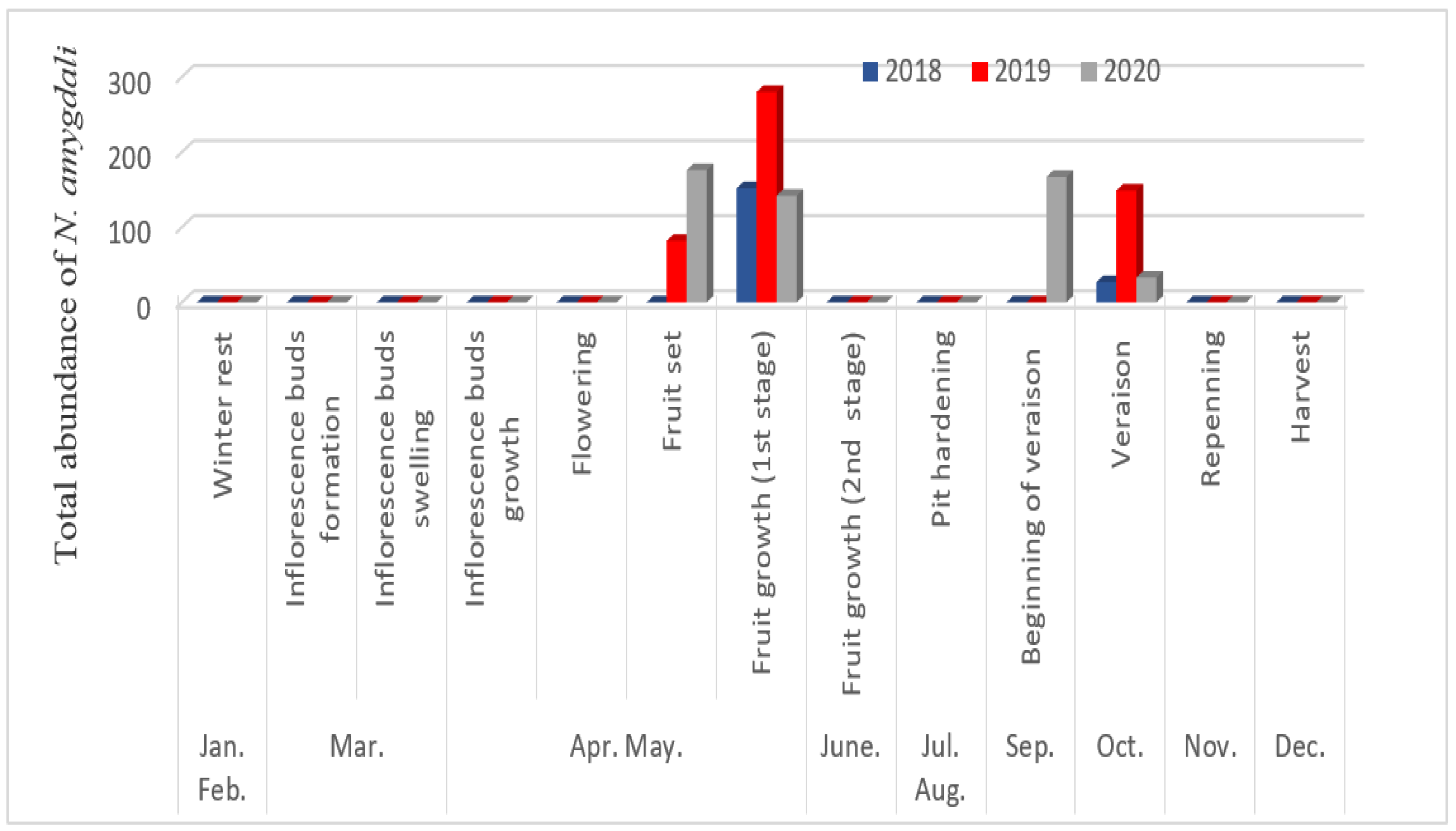
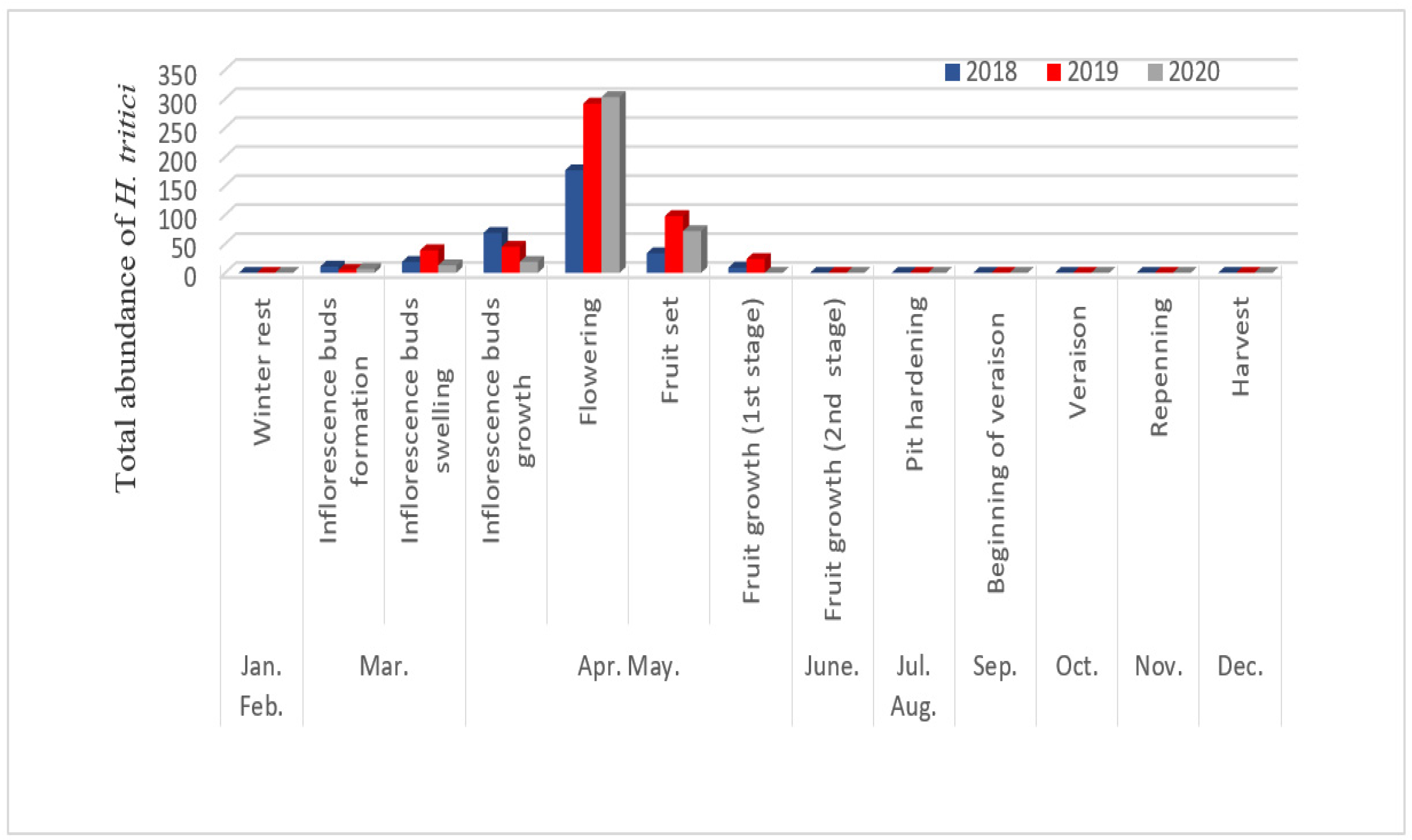
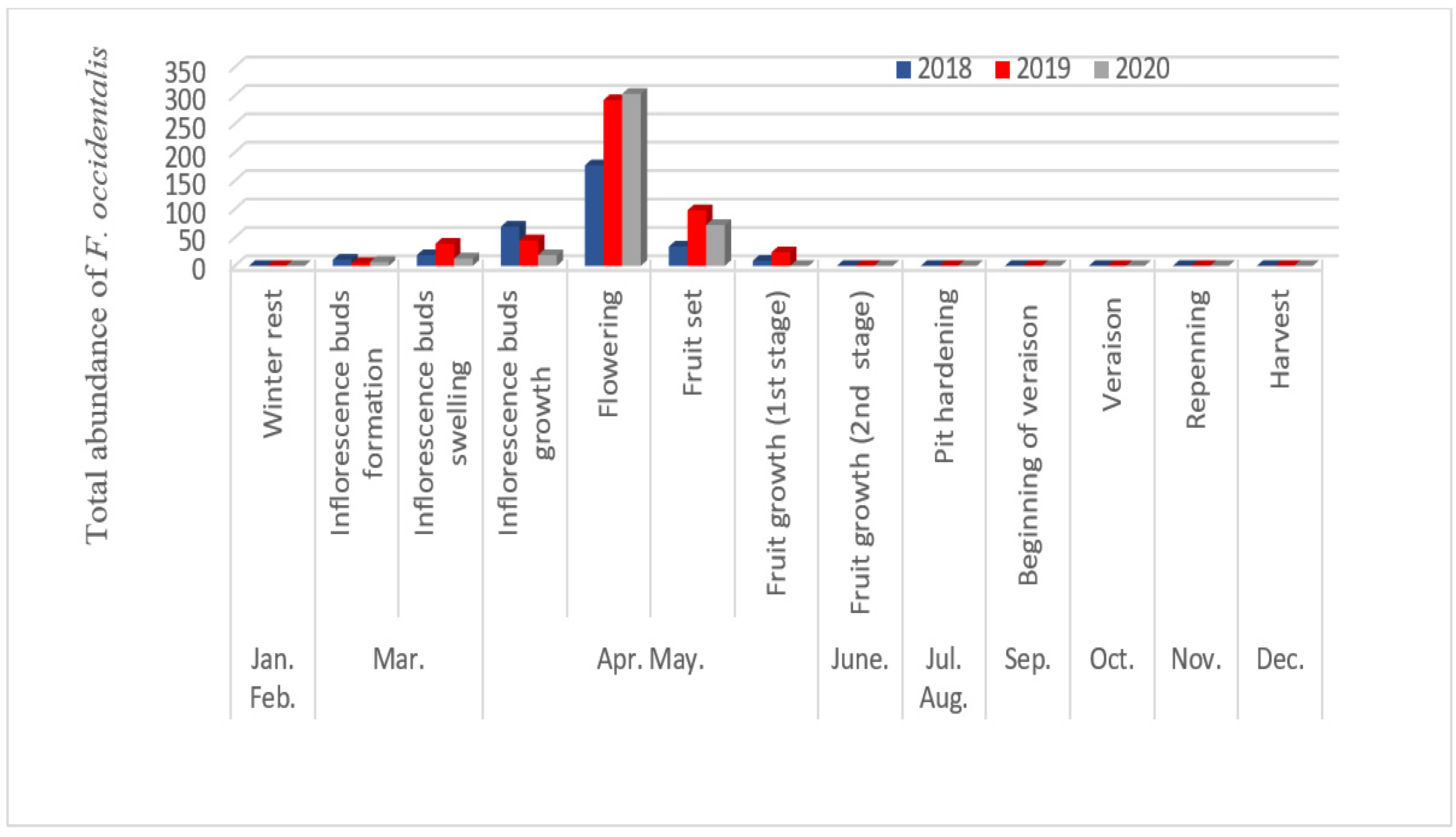
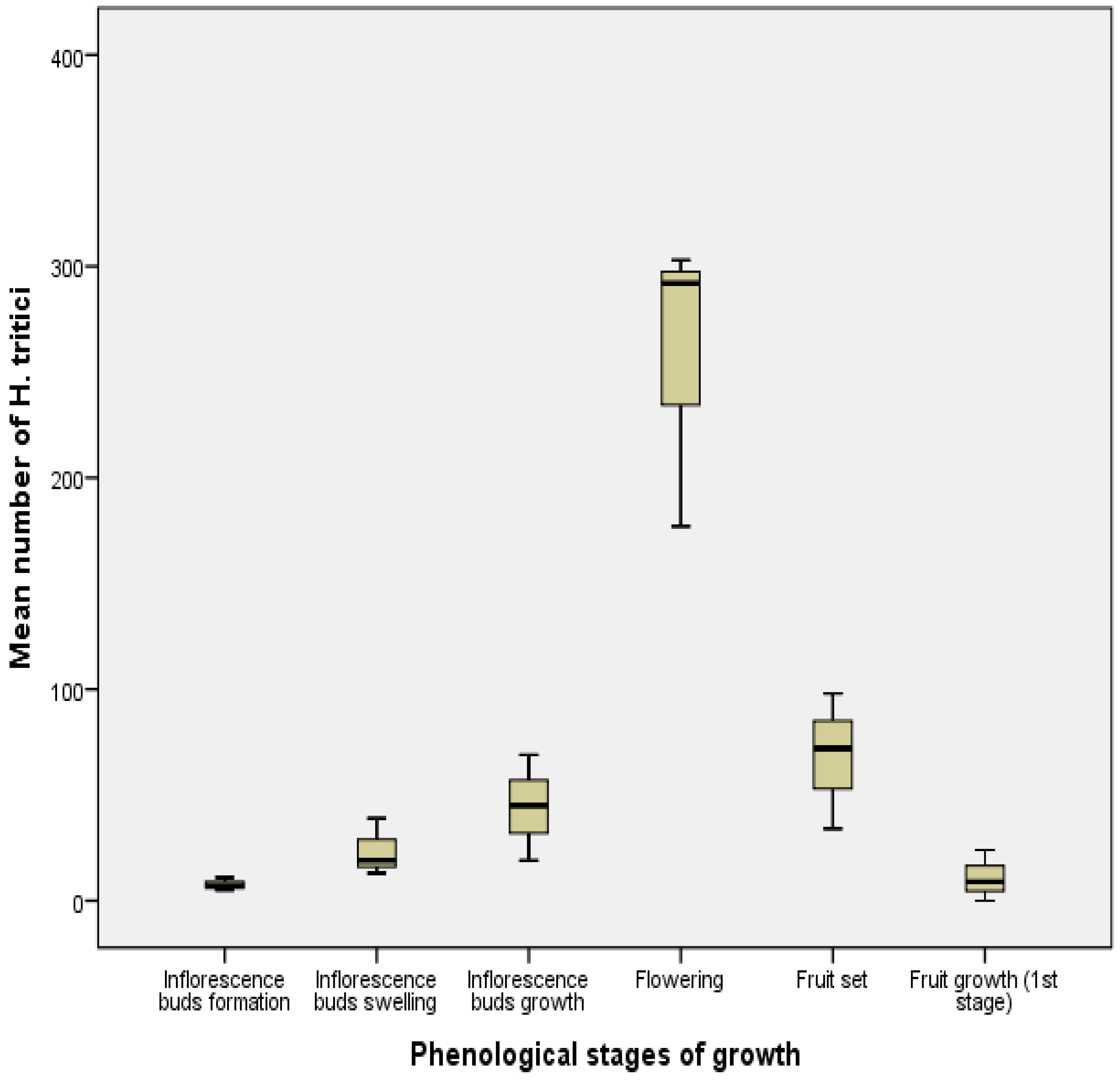
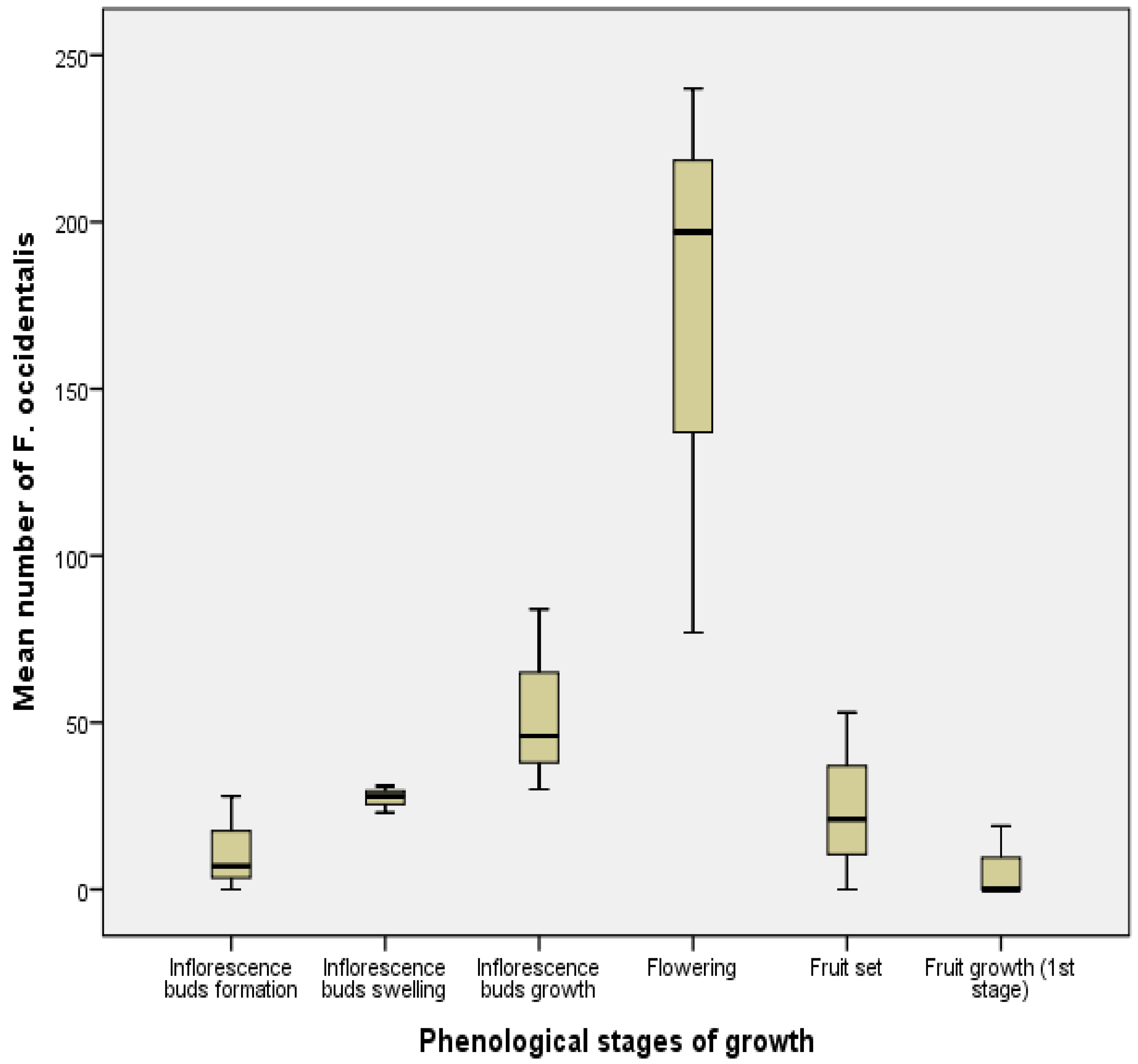
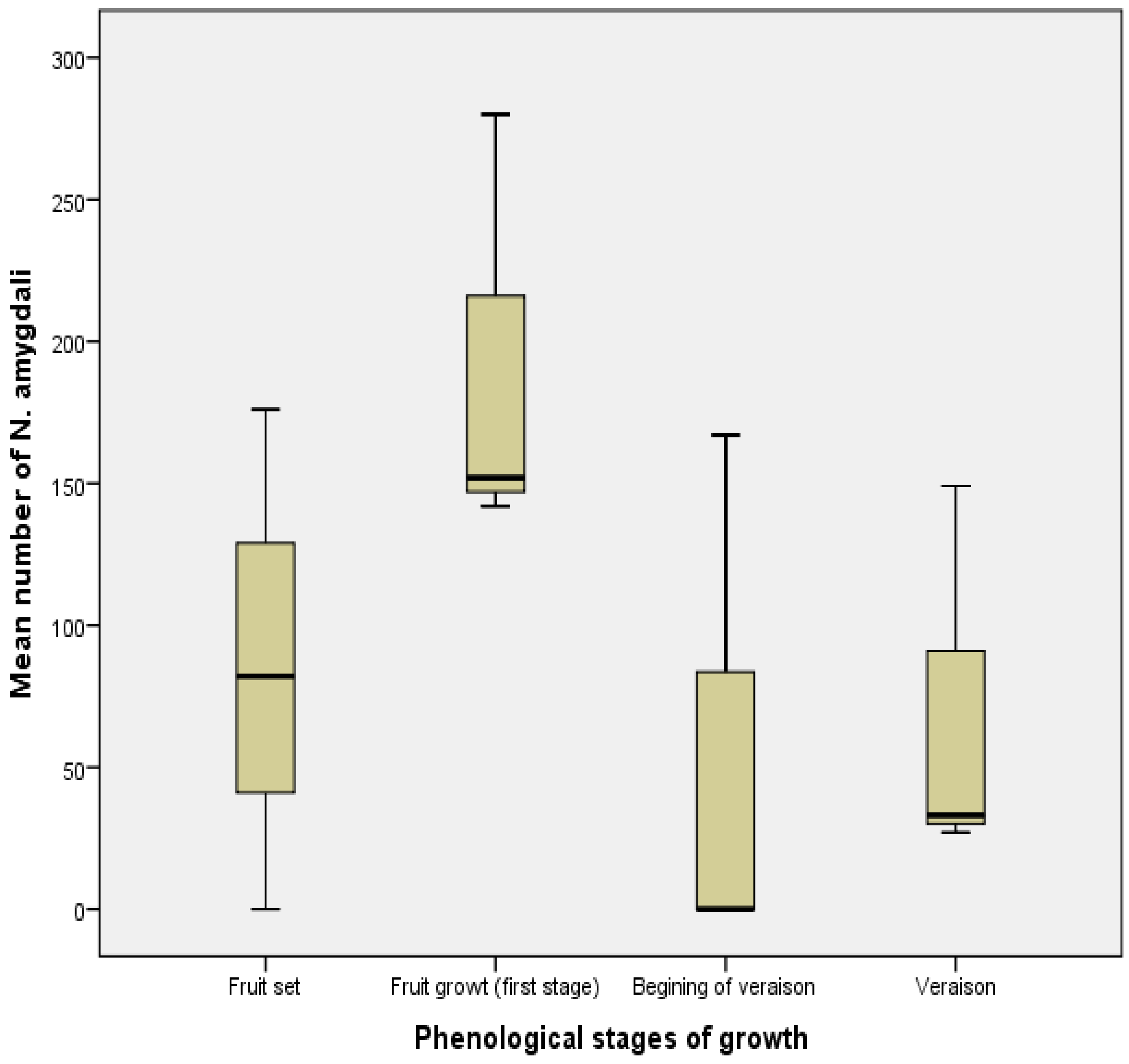
3.3. Fluctuations in Numbers According to Phenological Stages
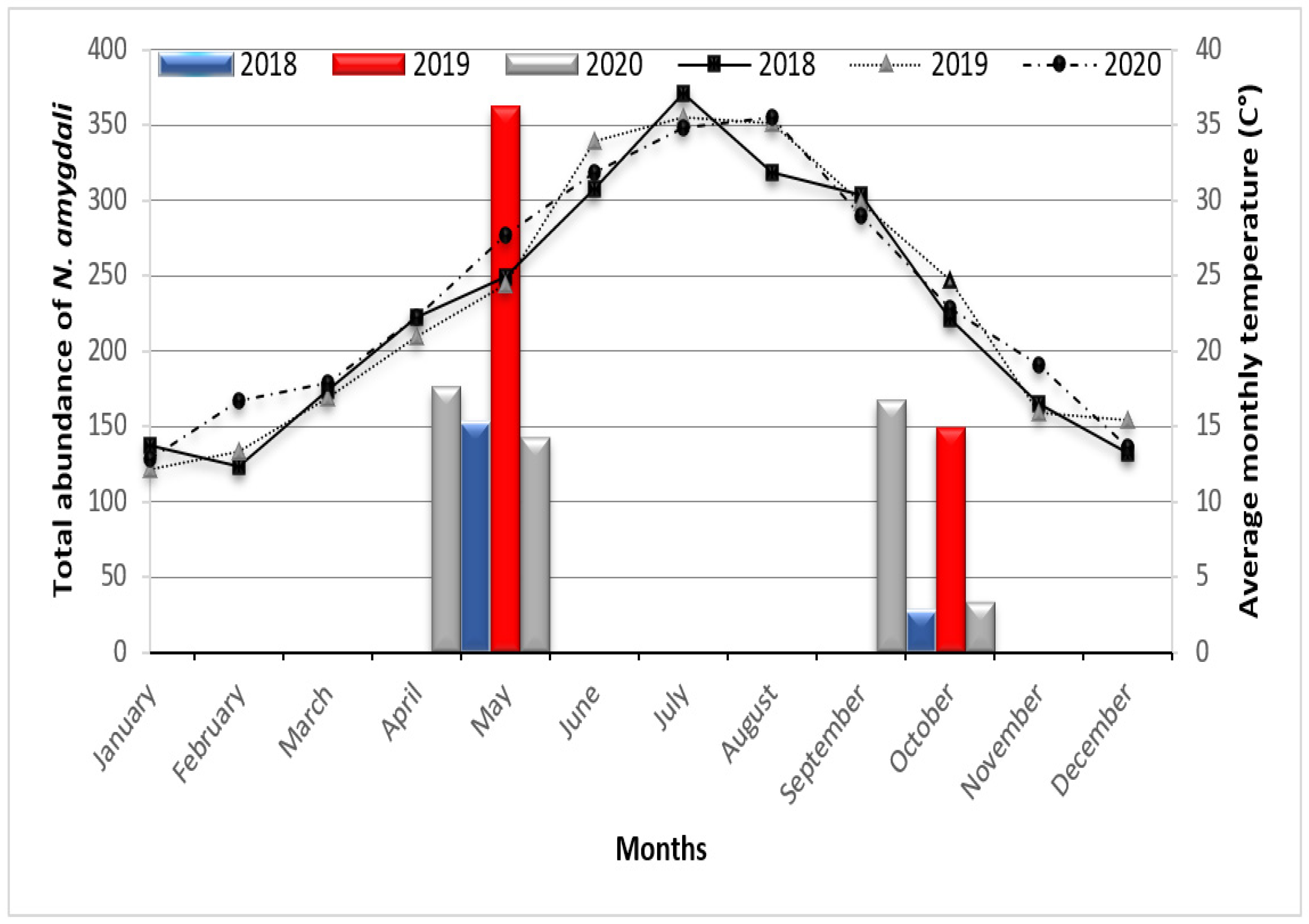
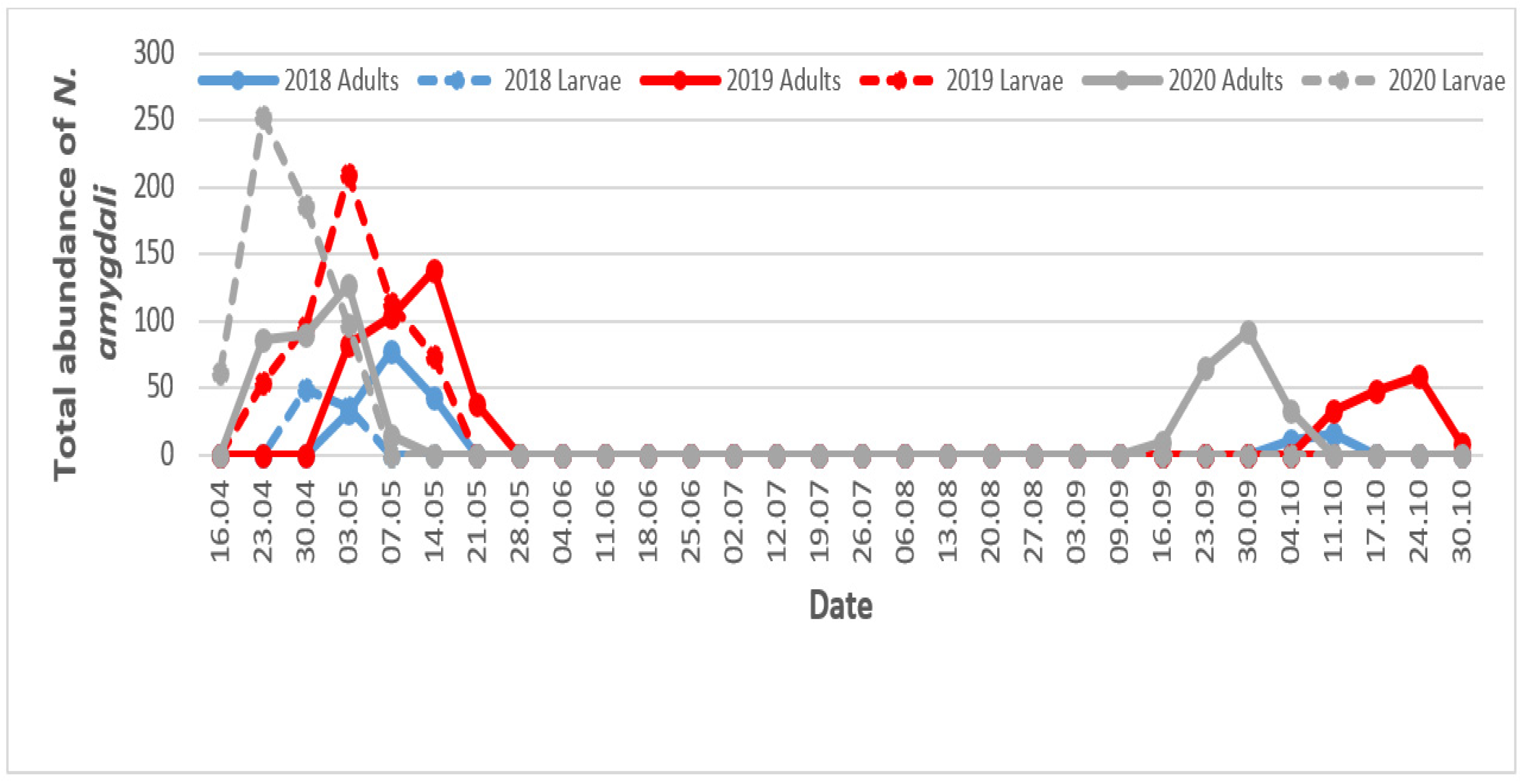
3.4. Temporal Fluctuation of Neohydatothrips Amygdali Based on Environmental Conditions
3.5. Sex Ratio of Neohydatothrips amygdali
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ministry of Agriculture and Rural Development of Algeria. Available online: https://www.aps.dz/economie/129572-la-production-agricole-disponible-en-quantites-suffisantes (accessed on 20 August 2021).
- Direction of Agriculture Services of Biskra Region. Available online: https://www.aps.dz/regions/83476-biskra-une-production-record-de-177-000-quintaux-d-olive-realisee (accessed on 13 April 2020).
- EIP-AGRI. Focus Group Pests and Diseases of the Olive Tree; Final Report; Eip-Agri: Brusells, Belgium, 2020. [Google Scholar]
- Bejarano-Alcázar, J.D.; Rodríguez-Jurado, J.M.; Durán-Álvaro, M.; Ruiz-Torres, M.; Herrera-Mármol. Unidad Didáctica 5. Control de enfermedades y plagas en producción integrada del olivar. In Producción Integrada de Olivar; Instituto de Investigación y Formación Agraria y Pesquera: Junta de Andalucía, Spain, 2011; pp. 55–90. [Google Scholar]
- ThripsWiki. Providing Information on the World’s Thrips. Available online: http://thrips.info/wiki/Main_Page (accessed on 30 August 2021).
- Canale, A.; Conti, B.; Petacchi, R.; Rizzi, I. Thysanoptera collected in an olive-growing area of the northern Tuscany (Italy). Entomol. Probl. 2003, 33, 105–110. [Google Scholar]
- Rei, F.T.; Mateus, C.; Torres, L. Thrips in Oleae europaea L.: Organic versus Conventional Production. Acta Hortic. 2011, 924, 151–156. [Google Scholar] [CrossRef]
- Trdan, S.; Andjus, L.; Raspudic, E.; Kac, M. Distribution of Aeolothrips intermedius Bagnall (Thysanoptera: Aeolothripidae) and its potential prey Thysanoptera species on different cultivated host plants. J. Pest. Sci 2005, 78, 217–226. [Google Scholar] [CrossRef]
- Trdan, S.; Vidrih, M.; Vierbergen, G. First record of Aeolothrips gloriosus Bagnall (Thysanoptera: Aeolothripidae) in Slovenia. Arch. Biol. Sci. 2012, 64, 767–770. [Google Scholar] [CrossRef]
- Agamy, E.A.; El-Husseini, M.M.; El-Sebaey, I.I.; Maaly, E. The Egyptian Thripid Species in Olive Groves at Ismaialia, Egypt. Egypt Acad. J. Biolog. Sci. A Entomol. 2017, 10, 1–17. [Google Scholar] [CrossRef]
- Marullo, R.; Vono, G. Forti attacchi di Liothrips oleae su olivo in Calabria. L’informatore Agrar. 2017, 36, 51–55. [Google Scholar]
- Mound, L.A. Biodiversity of Thysanoptera. In Insect Biodiversity: Science and Society; Foottit, R.G., Adler, P.H., Eds.; Wiley-Blackwell: Chichester, UK, 2018; Volume II, pp. 483–499. [Google Scholar] [CrossRef]
- Lewis, T. Thrips as Crop Pests; CAB International: New York, NY, USA, 1997; p. 740. [Google Scholar]
- Mound, L.A.; Kibby, G. Thysanoptera: An Identification Guide; CABI International: Wallingford, UK, 1998; p. 70. [Google Scholar]
- Mound, L.A. Thysanoptera—Diversity and Interactions. Annu. Rev. Entomol. 2004, 50, 247–269. [Google Scholar] [CrossRef]
- Mound, L.A. The Thysanoptera vector species of tospoviruses. Acta Hortic. 1996, 431, 298–309. [Google Scholar] [CrossRef]
- Lewis, T. Thrips, Their Biology, Ecology and Economic Importance; Academic Press: London, UK; New York, NY, USA, 1973; p. 349. [Google Scholar]
- Palmer, J.M.; Mound, L.A. Thysanoptera as predators. In Armoured Scale Insects; Elsevier: Amsterdam, The Netherlands, 1990; pp. 67–76. [Google Scholar]
- Beattie, A.J. The Evolutionary Ecology of Ant–Plant Mutualisms; Cambridge University Press: Cambridge, UK, 1985. [Google Scholar]
- Hoddle, M.S.; Oevering, P.; Phillips, P.A.; Faber, B.A. Evaluation of augmentative releases of Franklinothrips orizabensis for control of Scirtothrips perseae in California avocado orchards. Biol. Control 2004, 30, 456–465. [Google Scholar] [CrossRef]
- Mirab-balou, M.; Chen, X.X. A new method for preparing and mounting thrips for microscopic examination. J. Environ. Entomol. 2010, 32, 115–121. [Google Scholar]
- Goldarazena, A.; Mound, L. The Biodiversity of Thysanoptera at the Great Smoky Mountains National Park (U.S.A.), An Introduction. Boletín Soc. Entomológica Aragonesa 2006, 38, 291–299. [Google Scholar]
- Zur-Strassen, R. Die terebranten Thysanopteren Europas und des Mittelmeer-Gebietes. Die Tierwelt Dtschl. 2003, 74, 1–277. [Google Scholar]
- Vierbergen, G.; Kucharczyk, H.; Kirk, W.D.J. A key to the second instar larvae of the Thripidae of the Western Palaearctic region (Thysanoptera). Tijdschr. voor Èntomol. 2010, 153, 99–160. [Google Scholar] [CrossRef]
- McCloskey, W.B.; Baker, P.B.; Sherman, W. Survey of Cotton Weeds and Weed Control Practices in Arizona Upland Cotton Fields. In Cotton: A College of Agriculture Report for 1998; Silvertooth, J., Ed.; University of Arizona: Tucson, AZ, USA; p. 7. Available online: https://repository.arizona.edu/handle/10150/210369 (accessed on 25 August 2021).
- Kucharczyk, H.; Bereś, P.K.; Dąbrowski, Z.T. The species composition and seasonal dynamics of thrips (Thysanoptera) populations on maize (Zea mays L.) in southeastern Poland. J. Plant Prot. Res. 2011, 51, 210–216. [Google Scholar] [CrossRef]
- IBM Corp. Released (2013): IBM SPSS Statistics for Windows, Version 22.0; IBM Corp.: Armonk, NY, USA, 2013. [Google Scholar]
- Vono, G.; Bonsignore, C.P.; Gullo, G.; Marullo, R. Olive production threatened by a resurgent pest Liothrips oleae (Costa, 1857). (Thysanoptera: Phlaeothripidae) in Southern Italy. Insects 2020, 11, 887. [Google Scholar] [CrossRef]
- Childers, C.C.; Achor, D.S. Feeding and Oviposition Injury to Flowers and Developing Floral Buds of ‘Navel’ Orange by Frankliniella bispinosa (Thysanoptera: Thripidae) in Florida. Ann. Entomol. Soc. Am. 1991, 84, 272–282. [Google Scholar] [CrossRef]
- Gerin, C.; Hance, T.; Van Impe, G. Impact of flowers on the demography of western flower thrips Frankliniella occidentalis (Thysan., Thripidae). J. Appl. Ent. 1999, 123, 569–574. [Google Scholar] [CrossRef]
- Hazir, M.; Ulusoy, R. Population Fluctuation of Thrips Species (Thysanoptera: Thripidae) in Nectarine Orchards and Damage Levels in East Mediterranean Region of Turkey. J. Entomol. Res. Soc. 2012, 14, 41–52. [Google Scholar]
- Hanafy, A.R.I. Population Fluctuation, Host Preference, Damage and Control of Western Flower Thrips, Frankliniella occidentalis (Pergande) on Some Vegetable Crops in Egypt. Egypt. Acad. J. Biol. Sci. 2015, 8, 117–128. [Google Scholar]
- Bournier, A. Les Thrips. Biologie-Importance Agronomique; Inra: Paris, France, 1983; 128p. [Google Scholar]
- Goldarazena, A.; Gattesco, F.; Atencio, R.; Korytowski, C. An updated checklist of the Thysanoptera of Panama with comments on host associations. Check List 2012, 8, 1232–1247. [Google Scholar] [CrossRef][Green Version]
- Loomans, A.J.M.; van Lenteren, J.C. Biological Control of Thrips Pests: A Review on Thrips Parasitoids. Wageningen Agricultural University: Wageningen, The Netherlands, 1995; 237p. [Google Scholar]
- Beres, P.K.; Kucharczyk, H.; Kucharczyk, M. Thrips abundance on sweet corn in southeastern Poland and the impact of weather conditions on their population dynamics. Bull. Insectol. 2013, 66, 143–152. [Google Scholar]
- Hurej, M.; Kucharczyk, H.; Twardowski, J.P.; Kozak, M. Thrips (Thysanoptera) associated with narrow-leafed lupin (Lupinus angustifolius L., 1753) intercropped with spring triticale (X Triticosecale Wittm. ex A. Camus, 1927). Rom. Agric. Res. 2014, 31, 337–345. [Google Scholar]
- Minaei, K. The genus Neohydatothrips (Thysanoptera: Thripidae) in Iran with one new species and first record of a micropterous form. Zootaxa 2016, 4189, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Rasool, I.; Soliman, A.M.; Zaki Alattal, Y.; Al Dhafer, H.M. The Thripidae subfamily Sericothripinae (Thysanoptera) from Saudi Arabia with a new species of Hydatothrips Karny. Zootaxa 2021, 4908, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Reitz, S.R. Biology and Ecology of the Western Flower Thrips (Thysanoptera: Thripidae): The Making of a Pest. Fla. Entomol. 2009, 92, 7–13. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Zhang, Y.J.; Xu, B.Y.; Zhu, G.R.; Wu, Q.J. Effects of temperature on development, reproduction and population growth of the western flower thrips, Frankliniella occidentalis (Thysanoptera: Thripidae). Acta Entomol. Sin. 2012, 55, 1168–1177. [Google Scholar]
- Vasiliu-Oromulu, L. The dynamics of the sex ratio index of thrips populations in mountainous meadows. In Thrips and Tospoviruses, Proceedings of the 7th International Symposium on Thysanoptera, 2002, Reggio Calabria, Italy, 2–7 July 2001; Marullo, R., Mound, L.A., Eds.; Australian National Insect Collection: Canberra, Australia, 2002; pp. 315–324. [Google Scholar]
- Normark, B.B.; Kirkendall, L.R. Parthenogenesis in insects and mites. In Encyclopedia of Insects, 2nd ed.; Resh, V.H., Carde’, R.T., Eds.; Academic Press: Amsterdam, The Netherlands, 2009; pp. 753–757. [Google Scholar]
- Moritz, G. Structure, growth and development. In Thrips as Crop Pests; Lewis, T., Ed.; CAB International: New York, NY, USA, 1997; pp. 15–63. [Google Scholar]
- Risler, H.; Kempter, E. Die Haploidie der Männchen und die Polyploidie in einigen Geweben von Haplothrips (Thysanoptera). Chromosoma 1961, 12, 351–361. [Google Scholar] [CrossRef]
- Stannard, L.J. The thrips, or Thysanoptera, of Illinois. Bull. Ill. Nat. Hist. Surv. 1968, 2, 215–552. [Google Scholar] [CrossRef]
- Stouthamer, R.; Breeuwer, R.F.; Luck, R.F.; Werren, J.H. Molecular identification of microorganisms associated with parthenogenesis. Nature 1993, 631, 66–68. [Google Scholar] [CrossRef]
- Arakaki, N.; Miyoshi, T.; Noda, H. Wolbachia-mediated parthenogenesis in the predatory thrips Franklinothrips vespiformis (Thysanoptera: Insecta). Proc. R. Soc. Lond. 2015, 268, 1011–1016. [Google Scholar] [CrossRef]
- Kumm, S.; Moritz, G. First Detection of Wolbachia in Arrhenotokous Populations of Thrips Species (Thysanoptera: Thripidae and Phlaeothripidae) and Its Role in Reproduction. Environ. Entomol. 2008, 37, 1422–1428. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.T.; Spooner-Hart, R.N.; Riegler, M. Polyploidy versus endosymbionts in obligately thelytokous thrips. BMC Evol. Biol. 2015, 15, 23. [Google Scholar] [CrossRef] [PubMed]

| Suborder | Family | Species |
|---|---|---|
| Terebrantia | * Aeolothrips collaris Priesner, 1919 | |
| Aeolothripidae | Aeolothrips intermedius Bagnall, 1934 | |
| *° Franklinothrips megalops (Trybom, 1912) | ||
| Melanthripidae | ° Melanthrips fuscus Sulzer, 1776 | |
| Frankliniella occidentalis (Pergande, 1895) | ||
| Thripidae | *° Neohydatothrips amygdali Minaei, 2016 | |
| Thrips tabaci Lindeman, 1889 | ||
| Tubulifera | Phlaeothripidae | * Haplothrips andresi Priesner, 1931 |
| ° Haplothrips tritici (Kurdjumov, 1912) |
| Species | 2018 | 2019 | 2020 | Total of Three Years | ||||
|---|---|---|---|---|---|---|---|---|
| Value | RA% | Value | RA% | Value | RA% | Value | RA% | |
| (●) Aeolothrips collaris | 10 | 1.04% | 8 | 0.50% | 37 | 2.09% | 55 | 1.27% |
| (●) Aeolothrips intermedius | 4 | 0.42% | 1 | 0.06% | 20 | 1.13% | 25 | 0.58% |
| (◊) Franklinothrips megalops | 4 | 0.42% | 17 | 1.07% | 113 | 6.37% | 134 | 3.10% |
| (*) Melanthrips fuscus | 6 | 0.63% | 4 | 0.25% | 3 | 0.17% | 13 | 0.30% |
| (*) Frankliniella occidentalis | 216 | 22.57% | 380 | 23.93% | 414 | 23.35% | 1010 | 23.39% |
| (*) Neohydatothrips amygdali | 179 | 18.70% | 511 | 32.18% | 518 | 29.22% | 1208 | 27.98% |
| (*) Thrips tabaci | 29 | 3.03% | 20 | 1.26% | 0 | 0.00% | 49 | 1.13% |
| (◊) Haplothrips andresi | 189 | 19.75% | 144 | 9.07% | 254 | 14.33% | 587 | 13.59% |
| (*) Haplothrips tritici | 320 | 33.44% | 503 | 31.68% | 414 | 23.35% | 1237 | 28.65% |
| Total | 957 | 100.00% | 1588 | 100.00% | 1773 | 100.00% | 4318 | 100.00% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Halimi, C.W.; Laamari, M.; Goldarazena, A. A Preliminary Survey of Olive Grove in Biskra (Southeast Algeria) Reveals a High Diversity of Thrips and New Records. Insects 2022, 13, 397. https://doi.org/10.3390/insects13050397
Halimi CW, Laamari M, Goldarazena A. A Preliminary Survey of Olive Grove in Biskra (Southeast Algeria) Reveals a High Diversity of Thrips and New Records. Insects. 2022; 13(5):397. https://doi.org/10.3390/insects13050397
Chicago/Turabian StyleHalimi, Chahrazed Warda, Malik Laamari, and Arturo Goldarazena. 2022. "A Preliminary Survey of Olive Grove in Biskra (Southeast Algeria) Reveals a High Diversity of Thrips and New Records" Insects 13, no. 5: 397. https://doi.org/10.3390/insects13050397
APA StyleHalimi, C. W., Laamari, M., & Goldarazena, A. (2022). A Preliminary Survey of Olive Grove in Biskra (Southeast Algeria) Reveals a High Diversity of Thrips and New Records. Insects, 13(5), 397. https://doi.org/10.3390/insects13050397





