1. Introduction
Studies of sex allocation have been the most intriguing and productive areas in evolutionary biology [
1]. There has been theoretical and empirical research supporting sex allocation. The theory predicts how individuals should divide their investment of resources between male and female offspring in response to environmental conditions, and there are an enormous number of supporting empirical studies [
1,
2,
3,
4,
5]. The theory of sex allocation states that, under certain circumstances, the marginal fitness benefit of allocating resources to male or female reproduction differs when selecting for biased sex allocation [
6,
7].
The biased sex ratio in progeny is the most obvious way to adjust sex allocation [
8]. This aspect of sex allocation has been widely studied in a variety of both invertebrate and vertebrate organisms [
9,
10]. According to several authors, parental investment in eggs can affect offspring fitness [
11,
12]. Offspring that benefit from inheriting more resources from their parents have greater fitness, and one of the most widely used predictors of such investment is egg size. However, the adjustment of egg size depending on the sex of the embryo has been less investigated. These sex-biased parental strategies have been demonstrated in birds [
13,
14,
15,
16] and lizards [
17]. Haplodiploidy is a sex-determination system that enables females to control the sex of their offspring through fertilization control. That is, females are diploid and develop from fertilized eggs while males are haploid and develop from unfertilized eggs [
1]; evidence is the rearing of only male progeny from eggs laid by virgin females. With arrhenotokous haplodiploid sex determination, females control the sex of their offspring by regulating the access of sperm to the egg. Following insemination, sperm is stored in a spermatheca. Evidence for the ability of females to control the sex ratio in thrips by selective fertilization was provided by Priesner [
18], who described the thrips spermatheca and associated musculature. Males can also influence sex allocation in different ways [
19]. First, the ability of males to fertilize eggs is variable, with some males producing sperm that is unable to successfully fertilize eggs. Second, the incompatibility of the paternal and maternal genomes leads to the embryonic death of female offspring. Third, males can attempt to increase daughter production because males can only pass genes to their daughters [
20]. In haplodiploid arthropods, egg-size-mediated sex allocation studies have been reported for the two-spotted spider mite
Tetranychus urticae Koch (Prostigmata: Tetranychidae) [
21] and Kelly’s citrus thrips
Pezothrips kellyanus (Bagnall) (Thysanoptera: Thripidae) [
22]. In these two species, egg size determines not only the larval size, juvenile survival, and adult size but also the probability of fertilization, with female (fertilized) eggs being larger than male (unfertilized) eggs. Since there are no further reports available for other haplodiploid arthropods, we intended to study this mechanism in the two arrhenotokous lineages of onion thrips.
Onion thrips,
Thrips tabaci Lindeman (Thysanoptera: Thripidae), has received considerable attention due to its cryptic life habitat and mode of reproduction. The pest status of onion thrips can be attributed to its polyphagous nature, high reproductive capacity, short generation time, transmission of plant viruses, and development of resistance to insecticides [
23,
24]. It has been one of the most intensively studied thrips species, though some of the biological aspects of this species remain unknown [
25]. DNA sequences of the mitochondrial COI gene have confirmed that
T. tabaci is a species complex, and it has been divided into three lineages: L1, L2 (leek-associated), and T (tobacco-associated) [
26]. L1 leek-associated and T tobacco-associated lineages have arrhenotokous reproduction [
27,
28], while the L2 leek-associated lineage has thelytokous reproduction [
29]. Despite the great potential of thrips for the study of sex allocation, data regarding sex allocation for this order is rather sparse.
T. tabaci has an arrhenotokous haplodiploid sex-determination system that is common in the order Thysanoptera [
30]. Virgin arrhenotokous females produce only haploid male eggs, while mated arrhenotokous females produce diploid female and haploid male eggs. Different investigations into
T. tabaci reproductive modes have been carried out so far. According to Nault et al. [
31], neither high temperatures nor
Wolbachia bacteria have any effect on sex ratios in either thelytokous or male-producing populations.
The aim of our study was to investigate the influence of egg size on sex allocation in the two arrhenotokous lineages of the T. tabaci cryptic species complex. Prior to this, we tested the effect of mating and egg size on hatching probability. Furthermore, we investigated the effect of maternal mating status on the size of male eggs produced by virgin and mated mothers, as well as the effect of maternal age on egg size regardless of the offspring sex.
2. Materials and Methods
The L1 and T arrhenotokous onion thrips colonies were established in 2014 at the Department of Entomology, Institute of Plant Protection. The lineages of
T. tabaci were identified with molecular identification [
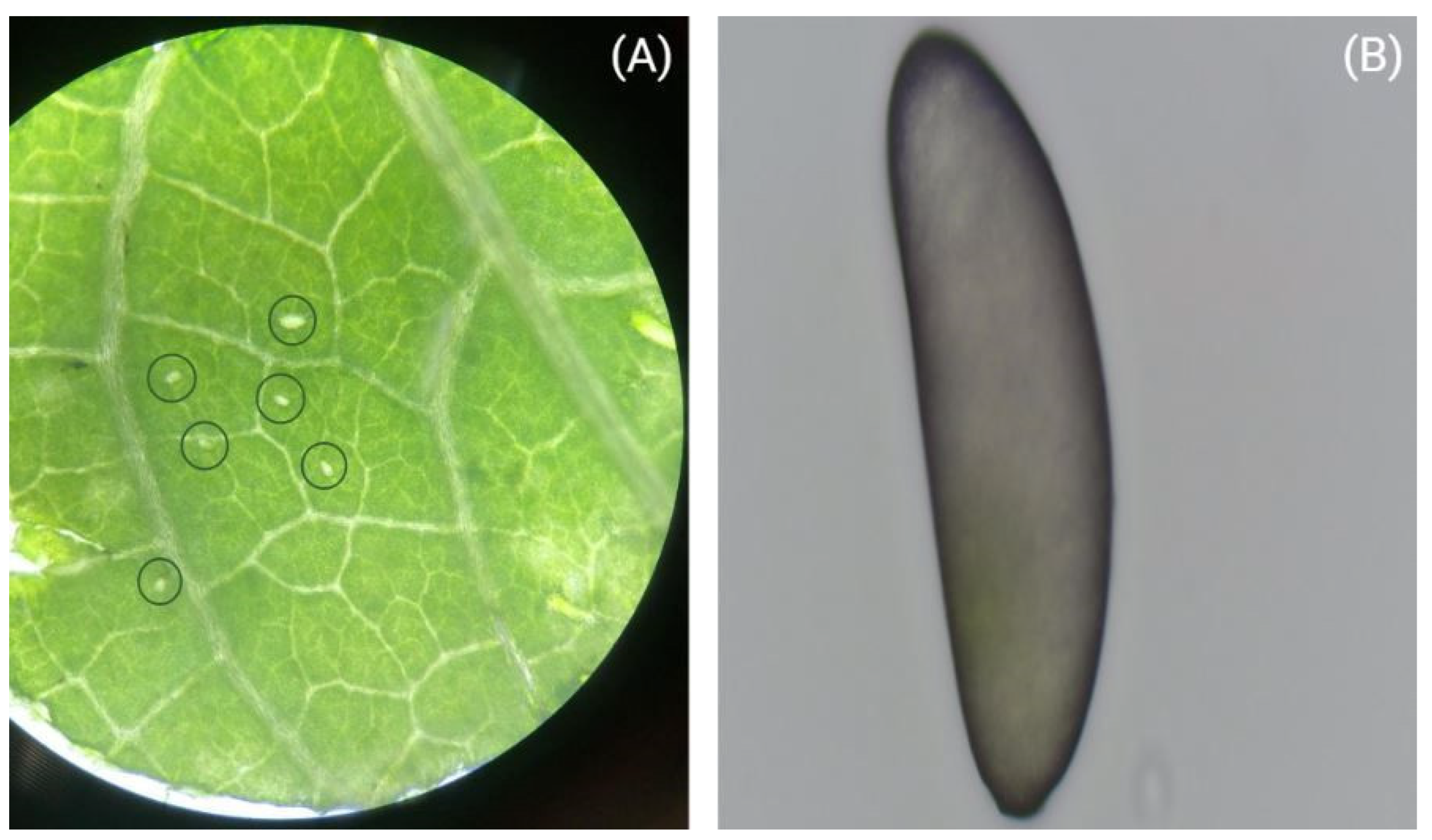
28]. As the eggs of terebrantian thrips are laid into the leaf tissue (
Figure 1A), bean leaves (
Phaseolus vulgaris L.) were used for all treatments to facilitate the careful excavation of eggs (
Figure 1B), since bean leaves are soft and provide the opportunity to handle the eggs easily. All experiments were performed in a growth chamber (Peltier-cooled incubator IPP260plus, Memmert GmbH + Co.KG, Schwabach, Germany) under controlled conditions at 23 °C with a light and dark photoperiod regime of L16: D8 h.
2.1. Egg Collection from Virgin and Mated Females of L1 and T Lineages
In order to measure the size of eggs produced by virgin and mated females of the L1 and T lineages of T. tabaci, pupae were collected from the stock colonies and separately isolated in order to ensure the virginity of the females. Pupae were observed every twelve hours in order to record the time of adult emergence. In total, 77 pupae of the L1 lineage and 69 pupae of the T lineages were collected and individually reared in a 2 mL Eppendorf tube on bean (P. vulgaris) leaf discs less than 1 cm in diameter until they reached the adult stage. From 77 pupae collected from the L1 colony, 42 females and 15 males emerged. In the case of the T lineage, 45 females and 21 male adults emerged. Right after adult emergence, 16 females from the L1 lineage and 19 females from the T lineage were paired with the emerging males, one female and one male in each Eppendorf tube containing a bean leaf disk. Females and males were kept together for 48 h in order to ensure mating. Then, males were removed and preserved in 96% ethanol for DNA-based identification. Meanwhile, the remaining females, of which there were 26 for each lineage, were kept as virgins. All the virgin and mated females were transferred to new bean leaf disks for oviposition throughout their lifespan. The bean leaf disks were changed every twelve hours until the given female died.
Eggs that were laid into a bean leaf disk were carefully excavated with a needle under a stereo microscope (Alpha, NSZ-606, Novel optics, Ningbo Yongxin, Ningbo, China). Subsequently, eggs were placed on a microscopic slide under a calibrated compound light microscope (LEICA DM LB, Leica Microsystem GmBH, Wetzlar, Germany) with an ocular graticule. Two dimensions (width and length) of an egg were measured under 600× magnification. After the measurement, the eggs were carefully placed back into the tube with the help of the needle, each egg being individually placed on a leaf disk to facilitate hatching and consequent juvenile development. Eggs were characterized by the mother ID, mother age, time of oviposition, size, hatching date, and (later on) sex when it became detectable (first stage larvae of mated females were slide-mounted and sexed according to the procedure of Vierbergen et al. [
32]).
2.2. Egg Volume
The formula below for calculating the volume of eggs was taken from the work of Church et al. [
33], which we think is more tailored for the shape of
T. tabaci eggs than the formula for the prolate ellipsoid shape used by Katlav et al. [
22]. Using the formula, the volume was calculated from two measured dimensions, namely length and width, assuming that thickness and width are equal:
2.3. Effect of Mating and Egg Size on the Hatching Probability of the Eggs
To test whether egg size is a predictor of energy content in the L1 and T arrhenotokous lineages, we assessed the effect of mating and egg size on the hatching probability of the eggs in the progeny of virgin and mated mothers. Egg hatchability was recorded as hatched and unhatched eggs for all the eggs that were laid during the entire lifespan of the mothers. We compared the egg hatchability rates between virgin and mated mothers of the L1 and T lineages using the chi-squared test. Using the one-way random block design MANOVA, we compared the size of eggs (width, length, and volume) laid by virgin and mated mothers of the L1 and T lineages according to their hatchability (hatched and did not hatch), with the factor of ‘hatchability’ and mother ID as a block.
2.4. Comparison of Male and Female Egg Size Produced by Mated Mothers of the L1 and T Lineages
To quantify maternal investment in eggs, we compared the size of eggs in male and female offspring produced by mated mothers during their entire lifespan. In two studied species,
T. urticae and P.
kellyanus, egg size and gender were found to be correlated with the egg mass produced by young arrhenotokous females. Corresponding to these approaches, we compared the size of male and female eggs in different age groups of mothers: 3–5-day-old mothers (just like in
T. urticae [
21]), 7–10-day-old mothers (similar to
P. kellyanus [
22]), and above-10-day-old mothers. The analysis of the egg size (width, length, and volume) of male and female offspring produced by mated mothers of the L1 and T lineages was performed using one-way multivariate analysis of variance (MANOVA) with the factor of ‘gender’. We carried out the same analysis for all age groups.
2.5. Testing the Model of Egg Size and Sex Allocation
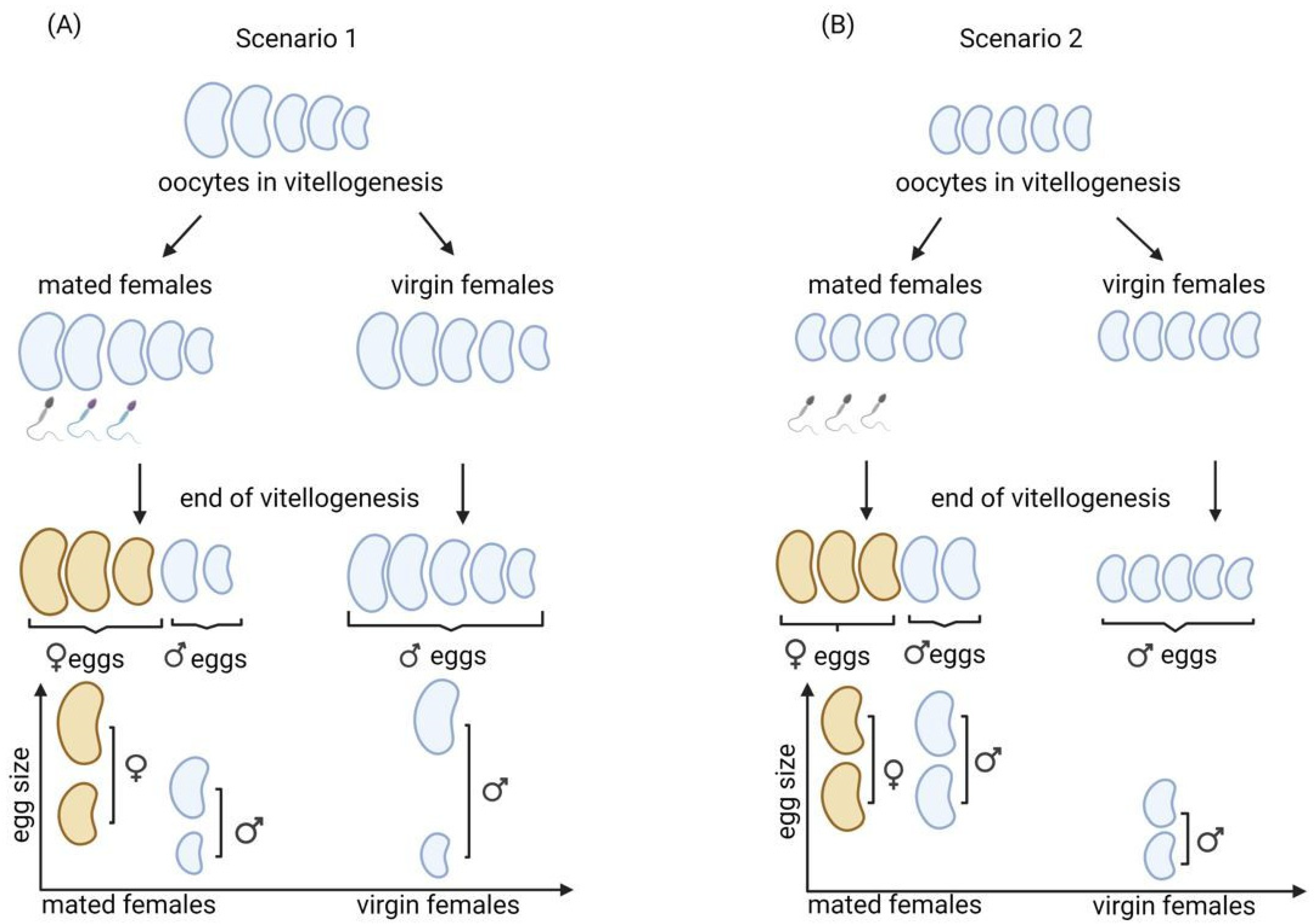
We tested whether an asymmetric allocation by mothers in the male and female eggs could occur before fertilization and whether egg size determines the probability of an egg being fertilized (a female fertilized egg being larger than a male unfertilized egg) (
Figure 2A, scenario 1). Under this scenario, the range of egg size should be similar between virgin and mated mothers. Moreover, any egg size sex-specific differences would result from the selective fertilization of larger eggs, leading to the larger egg size of males produced by virgin mothers than by mated mothers.
Second, we tested whether mating affects the female resource allocation strategy if eggs draw more resources once they are fertilized, which is also possible because eggs in the majority of insects, including thrips, are fertilized just before the end of vitellogenesis [
34] (
Figure 2B, scenario 2). Under this scenario, the size of the eggs is expected to be larger among eggs from mated mothers than among virgins, and mating should increase the egg size in general, leading to an equal egg size in males and females produced by mated mothers and, therefore, a smaller egg size in males produced by virgin mothers.
To discriminate between these two scenarios, we compared the mean and the distribution of the egg size produced by virgin and mated mothers. The analysis of egg size (width, length, and volume) produced by virgin and mated mothers of the L1 and T lineages was performed for the entire lifespan of the mothers and age groups using one-way MANOVA, where the factor was ‘mating status’.
2.6. Comparison of Male Egg Size Produced by Virgin and Mated Mothers of the L1 and T Lineages
Using only male eggs, we compared the egg size (width, length, and volume) of males produced by virgin and mated mothers in their whole lifespan and in the above-mentioned age groups. For this analysis, a one-way multivariate analysis of variance (MANOVA) was run with the factor of ‘mating status’.
2.7. Effect of Maternal Age on Egg Size
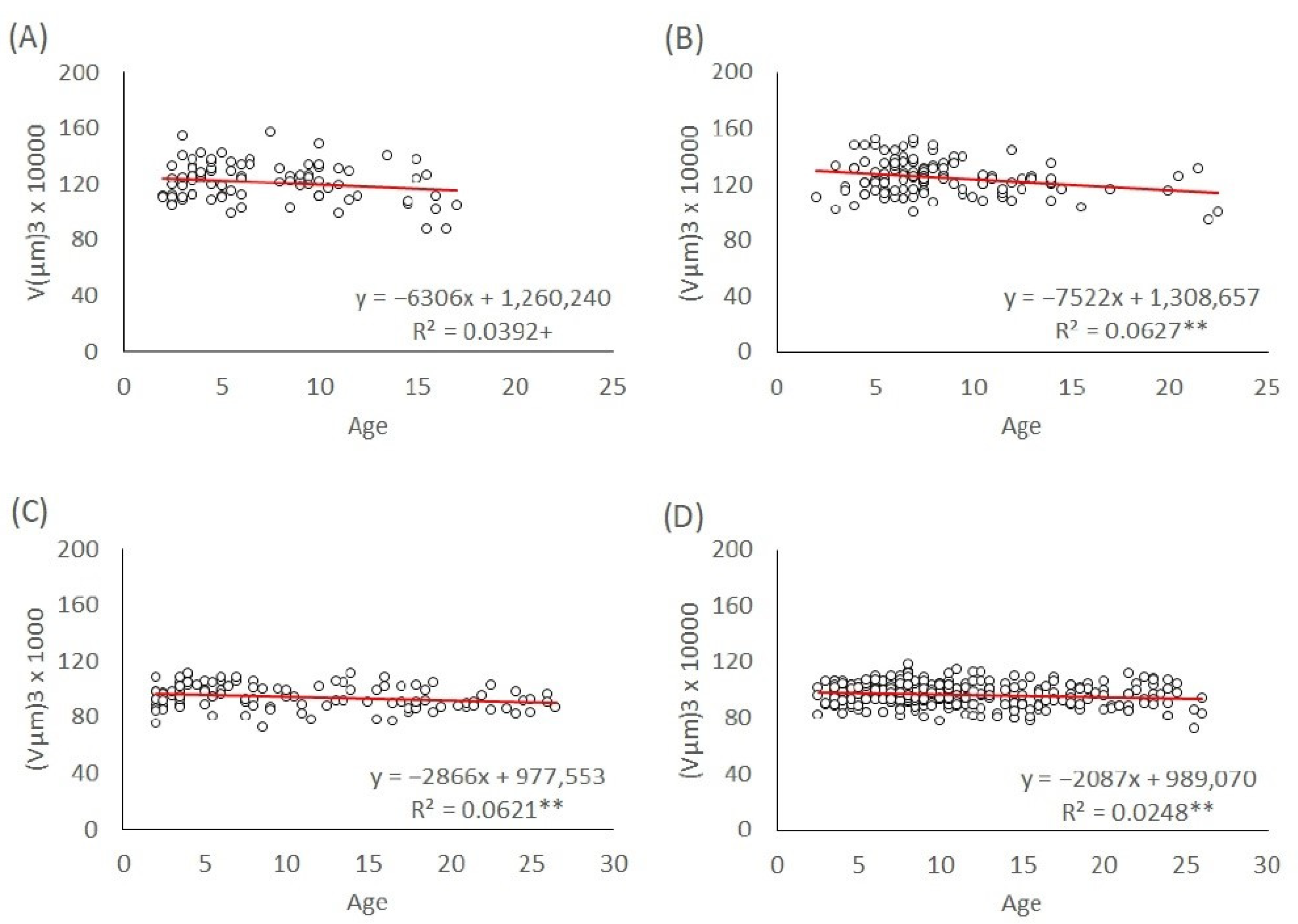
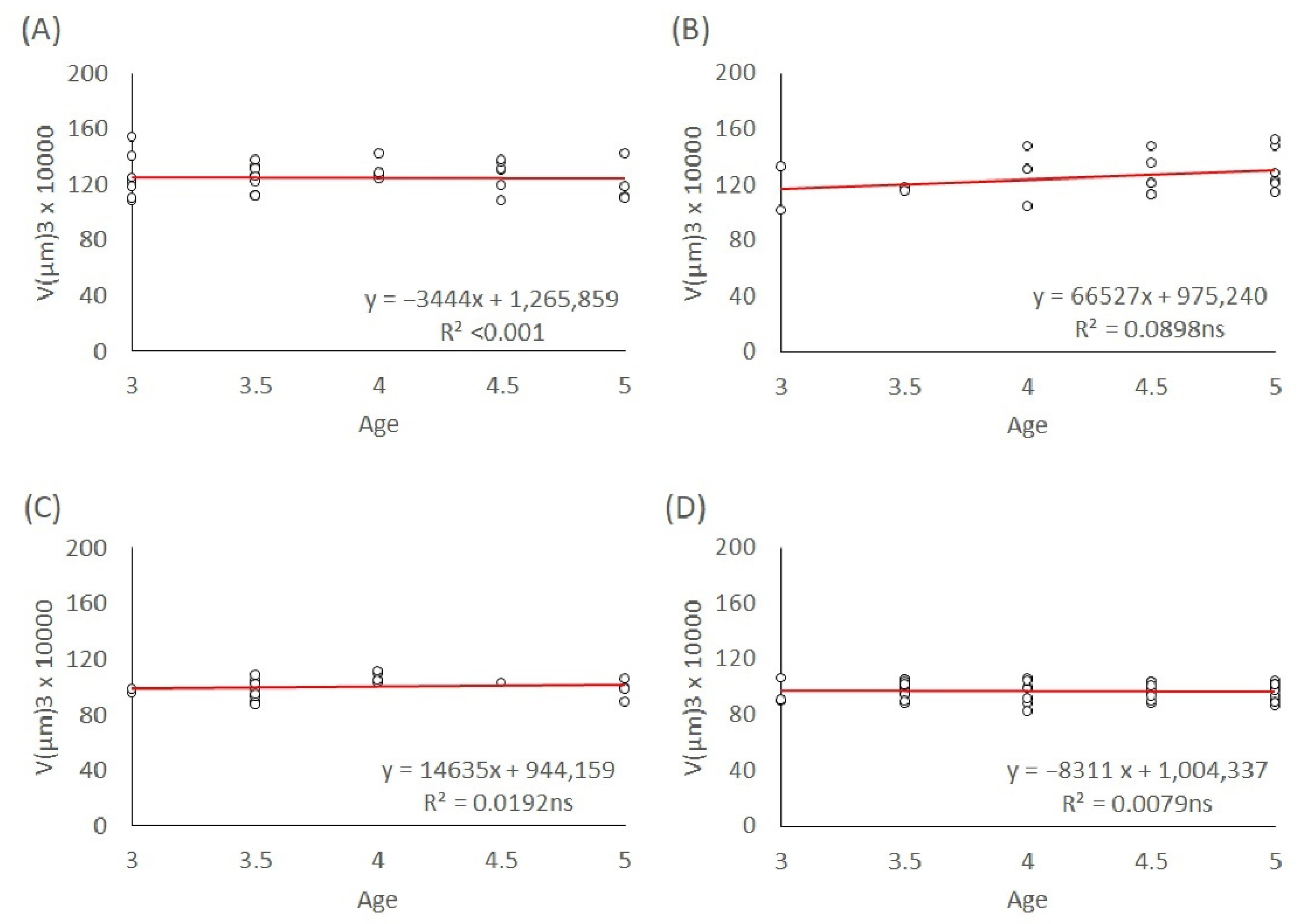
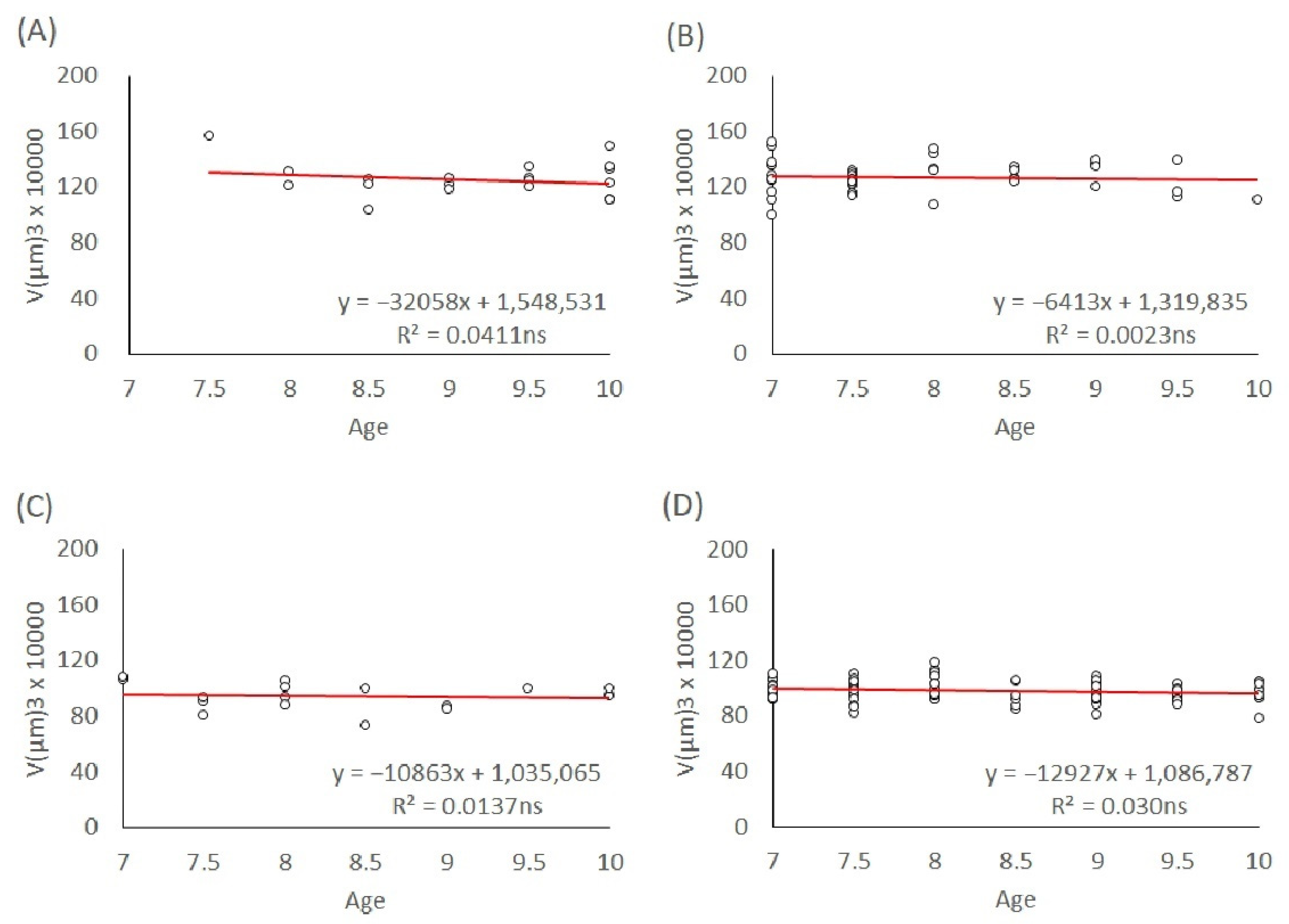
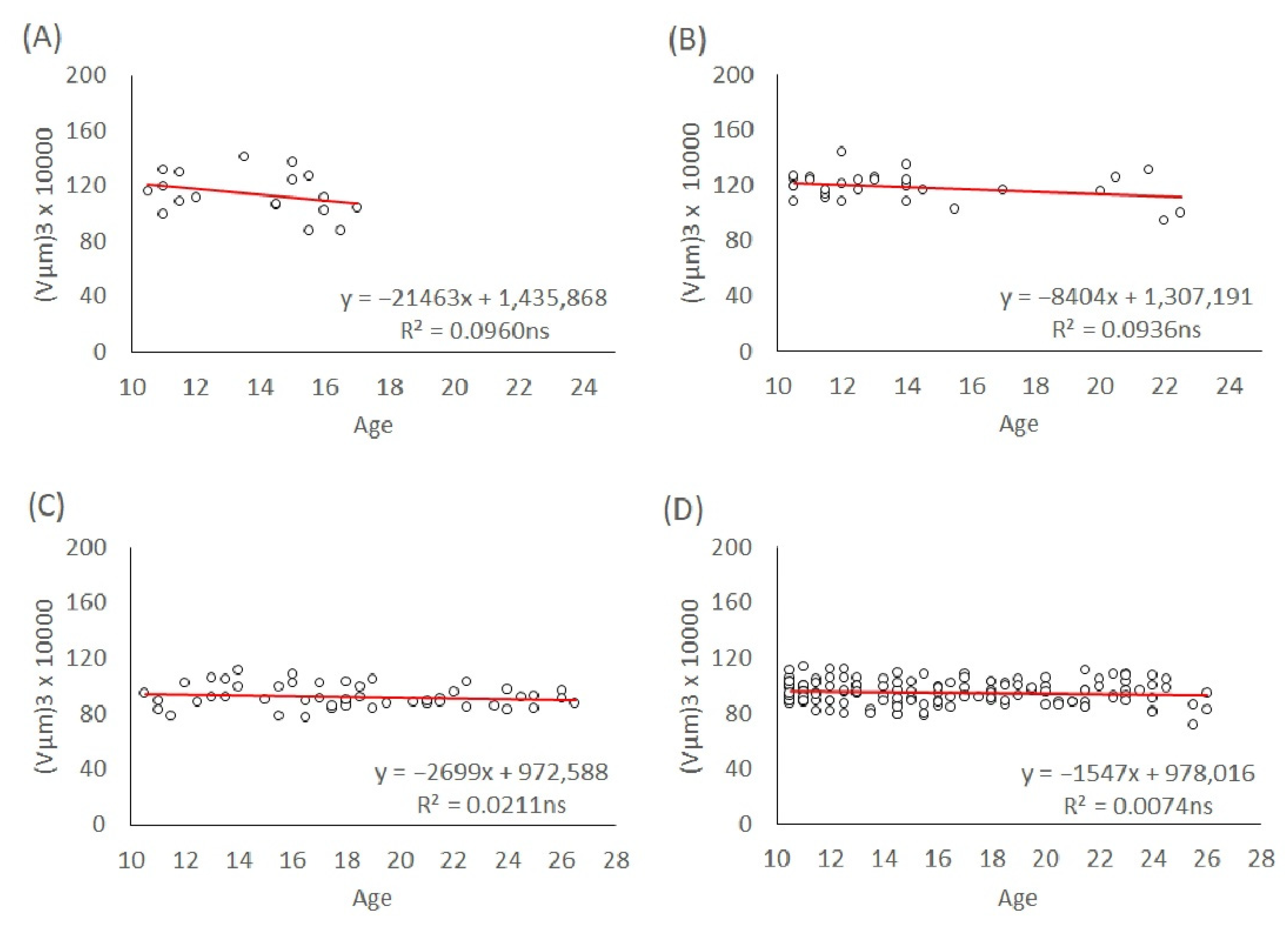
To investigate the effect of maternal age on egg size independently of the offspring sex, we used eggs produced by mated mothers of the L1 and T lineages. The age of the ovipositing mothers was recorded for each egg they laid until the mothers died. We modelled the egg volumes depending on the maternal age for their entire lifespan and age groups: 3–5 days, 7–10 days, and above 10 days. The maternal age effect on egg size in both genders of the L1 and T lineages was analyzed with linear regression models. The slopes of the trends were calculated and tested to see if they were significant.
2.8. Statistical Analysis
The statistical analyses were performed using IBM SPSS 25 [
35]. The normality of the residuals was accepted by the absolute values of their skewness and kurtosis, as they were all below 1. Homogeneity of variance was tested with Levene’s test (
p > 0.05). In the case of a significant overall MANOVA result, one-way between-subject effects were tested for all three variables (width, length, and volume) with Bonferroni’s adjustment.
4. Discussion
The relationship between egg size and hatchability has already been studied for mites [
21], but never in thrips. The fact that larger eggs are generally more likely to hatch and produce fast-developing juveniles with higher survivorship than smaller eggs in the spider mite
T. urticae indicates that egg size might be a predictor of energy content in some cases [
21], and our results confirm this: hatched eggs were significantly larger than the eggs that did not hatch in the L1 lineage laid by mated mothers and in the T lineage of
T. tabaci for all eggs, independently of mating status. Mating also affected the hatching probability of the eggs in the L1 lineage, with more eggs hatching from virgins than from mated mothers, and mating did not affect the hatching probability of the eggs in the T lineage. In the bruchid beetle
Callosobruchus maculatus F., mating (mated once or multiple times) had no detectable effect on the hatching probability of the eggs [
36]. To our knowledge, no other study has addressed the effect of mating status on the hatching probability of the eggs.
Our key finding is that sex allocation in haplodiploid
T. tabaci is not mediated via egg size like in the two-spotted spider mite
T. urticae [
21] and Kelly’s citrus thrips
P. kellyanus [
22]. In both species, egg size and gender were found to be related to the egg mass that was produced by young arrhenotokous females, 3–5-day-old mothers in
T. urticae and 7–10-day-old mothers in
P. kellyanus. The size of male and female eggs produced by mated mothers of the L1 lineage was not significantly different for the whole lifespan and at any age group, indicating that gender and egg size are independent of each other in this lineage regardless of maternal age. In the T lineage, however, gender only seemed to be dependent on egg size in the maternal age group of 7–10 days, but gender was independent of egg size in the progeny of younger and older mothers. Our results demonstrate that studying the relationship between egg size and gender in a narrow age group of mothers might lead to misconclusions unless there are different mechanisms working in mated females depending on their age.
By comparing the size of eggs produced by virgin and mated mothers, we showed that mated females laid significantly larger eggs throughout their lifespan and in all other age groups in the L1 lineage. The mean value of the egg size distribution of mated mothers in the L1 lineage was larger than that of virgins throughout their lifespan. This may suggest that there is no egg size that determines fertilization in this lineage—rather, it is the fertilization that may influence egg size. Eggs produced by mated mothers might receive more resources than those produced by virgins, and mating just increases egg size in general, thus leading to a larger size of male eggs produced by mated mothers than by virgins (
Figure 2B, scenario 2). Contrary to the L1 lineage, in the T lineage, an entirely new scenario is needed to assess the obtained results; eggs laid throughout the lifespan of virgin mothers were significantly larger than those of mated mothers.
Our findings may indicate that these two lineages of
T. tabaci have different resource allocation strategies in response to maternal mating status. In
P. kellyanus [
22], mating increases the early-life reproductive investment of females; therefore, mated females produce larger eggs than virgin females, just like our L1 lineage. However, the male offspring of mated mothers had smaller eggs than those of virgins [
22]. In
Tetranychus ludeni Zacher, virgin mothers laid significantly larger eggs than mated mothers, indicating a strategic resource allocation in response to mating status, with more resources being allocated to their male offspring when the mothers do not have the chance to produce female offspring [
37]. Females may receive nutritional benefits during mating. Because males may provide nutrients to females with their ejaculate, mating is an activity of females that manipulates her nutritional status and therefore influences the size of her eggs. These nuptial gifts serve as paternal investment by increasing reproductive fitness (e.g., increasing egg size; Gwynne [
38]). For the bruchid beetle
C. maculatus F., multiple mating of a female has been proven to increase the size of eggs laid by her [
36]. In haplodiploids, males can only pass their genes to female offspring, whereas the genes of females are passed on to both males and females. The effort of males to influence female production results in sexual conflict between males and females over the sex ratio of the progeny. During mating, males may transfer some seminal proteins that increase the sperm release from the spermatheca, which facilitates the fertilization rate [
39]. A higher fertilization rate could also be achieved by increasing egg size after mating [
40]. Moreover, if the selection of females has optimized egg size, females will use male nutrients to increase egg numbers. In contrast, male selection would favor increasing the size of the eggs he fertilizes rather than increasing the number of eggs [
41]. This change in the egg size could be manipulated by shifting adaptations between early and late reproduction or between size and the number of offspring [
42]. Further studies into sexual conflicts and sex allocation patterns need to be carried out in order to better understand the observed phenomenon in this cryptic species complex.
Maternal age influences the egg size in a wide group of insect species, including Coleoptera [
36], Lepidoptera [
43], Orthoptera [
44], and Diptera [
45]. According to studies of various authors, some data support the reduction of egg size over time with respect to maternal age [
36,
46]. Some other authors have observed an increase in egg size with increasing maternal age [
47]. Some others have emphasized that there are no significant differences in egg size with respect to maternal age [
48,
49]. The most common explanation for this phenomenon is the resource depletion hypothesis, which suggests that it may be a physiological limitation of the females to produce offspring of the same size [
36,
50]. The observed decrease in egg size with increasing maternal age has been proven in two related species of bruchid beetles,
C. maculatus F. and
Callosobruchus chinensis Fab. (Coleoptera) [
36,
46]. It was found that females with diminished resources for egg production laid smaller eggs with increasing maternal age, and those who were allowed to access food laid larger eggs, even at later maternal ages. Alternatively, Begon and Parker [
50] proposed that the decrease in egg size with increasing maternal age may be adaptive when the female clutch size is constrained. However, our experiment was not designed to test such a hypothesis.
In T. tabaci, maternal age had an overall effect on the egg size of both males and females in the L1 and T lineages throughout their lifespan. Egg size in both genders and lineages decreased with increasing maternal age. Because the maternal diet was not manipulated in this study, our results are not consistent with the resource depletion hypothesis. However, in the very young maternal age groups (3–5-day-old mothers, 7–10-day-old mothers, and older than 10 days), male and female egg size in both L1 and T lineages stayed at a constant level, without significant changes in egg size.