Dissecting the Isoform-Specific Roles of FTZ-F1 in the Larval–Larval and Larval–Pupal Ecdyses in Henosepilachna vigintioctopunctata
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods and Materials
2.1. Insect
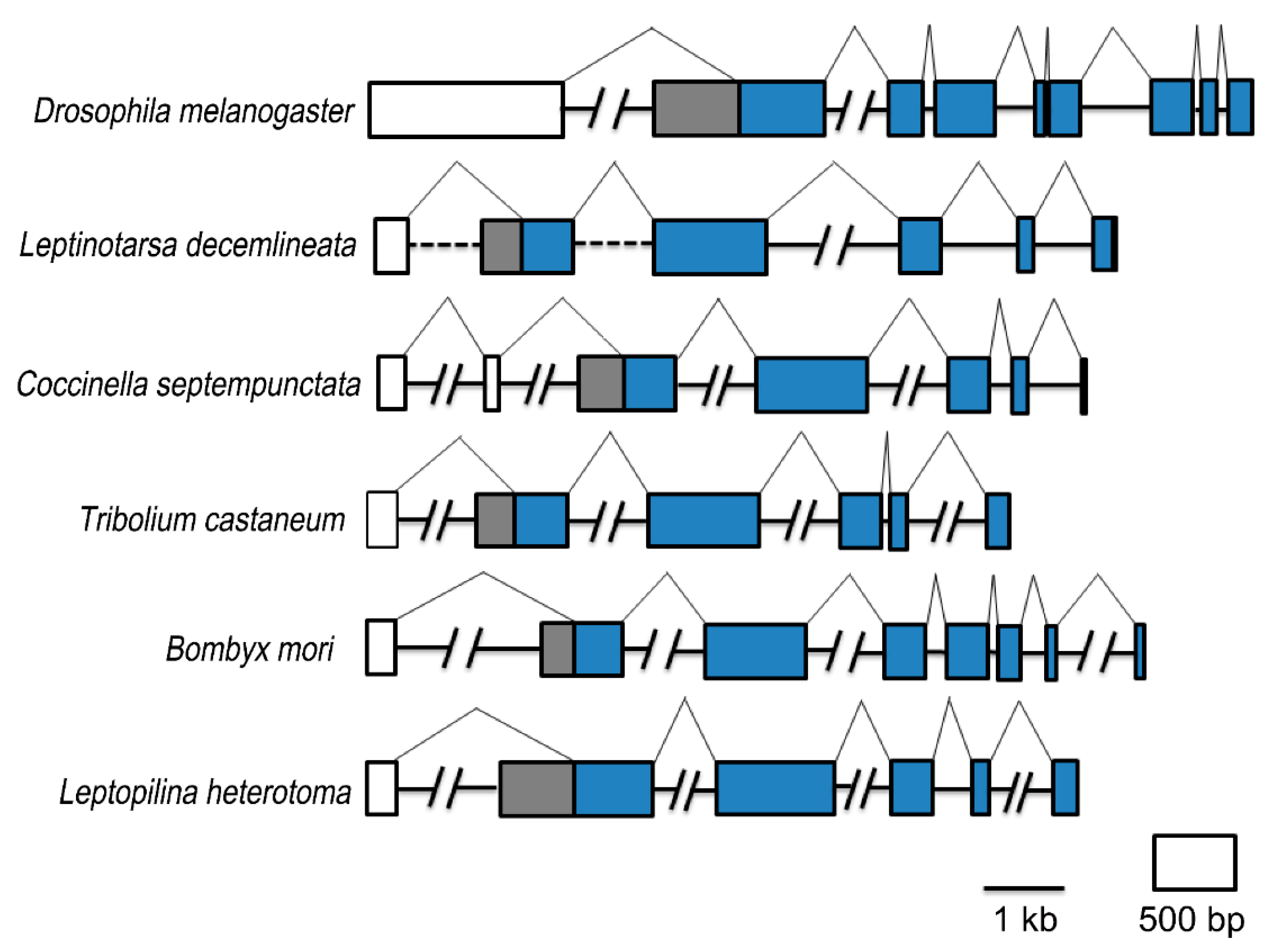
2.2. Gene Structure Comparison
2.3. Molecular Cloning and Bioinformatic Analysis
2.4. Synthesis of dsRNA Molecules
2.5. Introduction of dsRNA
2.6. Real-Time Quantitative PCR (qRT-PCR)
2.7. Data Analysis
3. Results
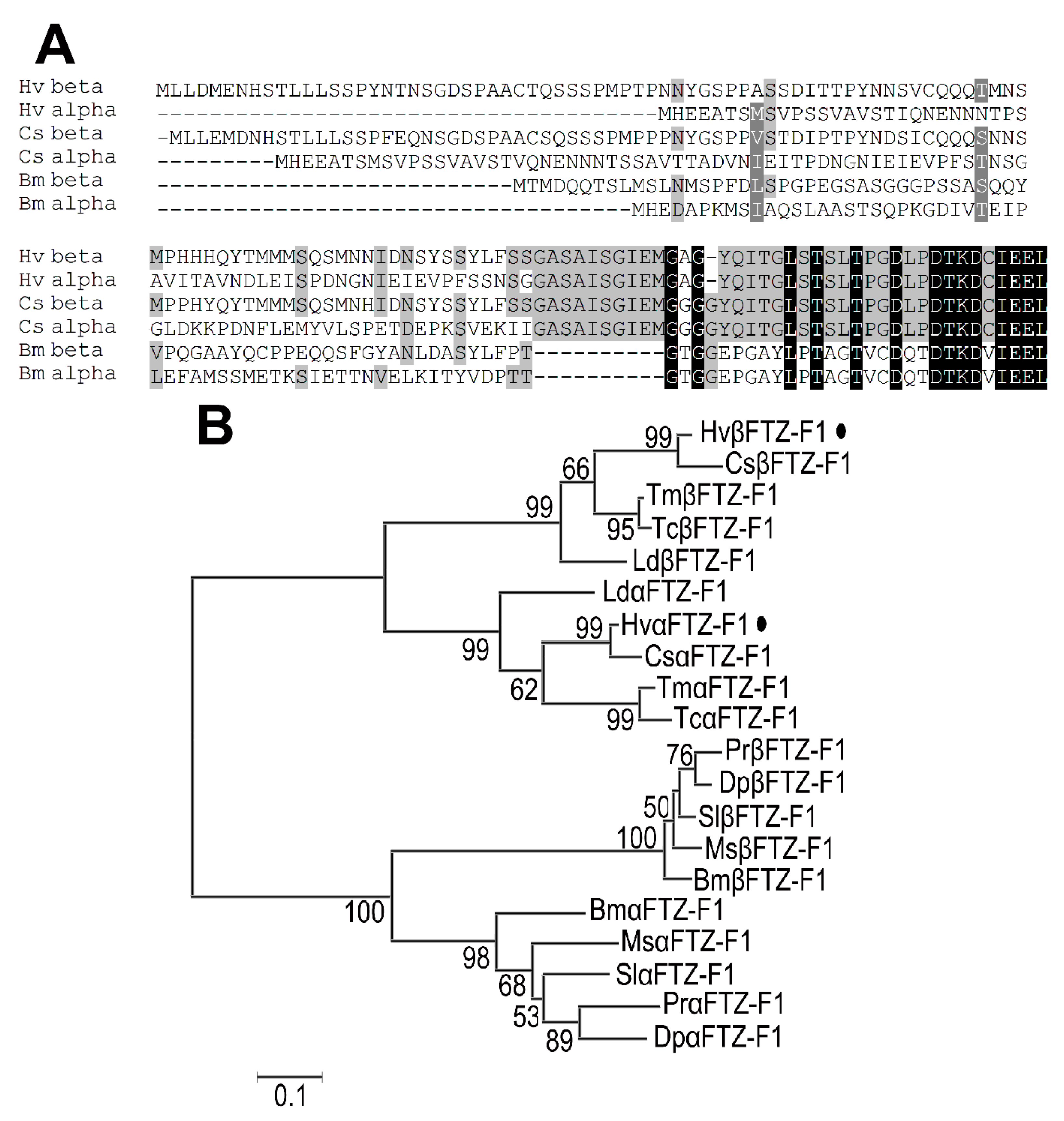
3.1. FTZ-F1 Isoforms Are Widely Distributed among Insects
3.2. Identification of FTZ-F1 Isoforms in H. vigintioctopunctata
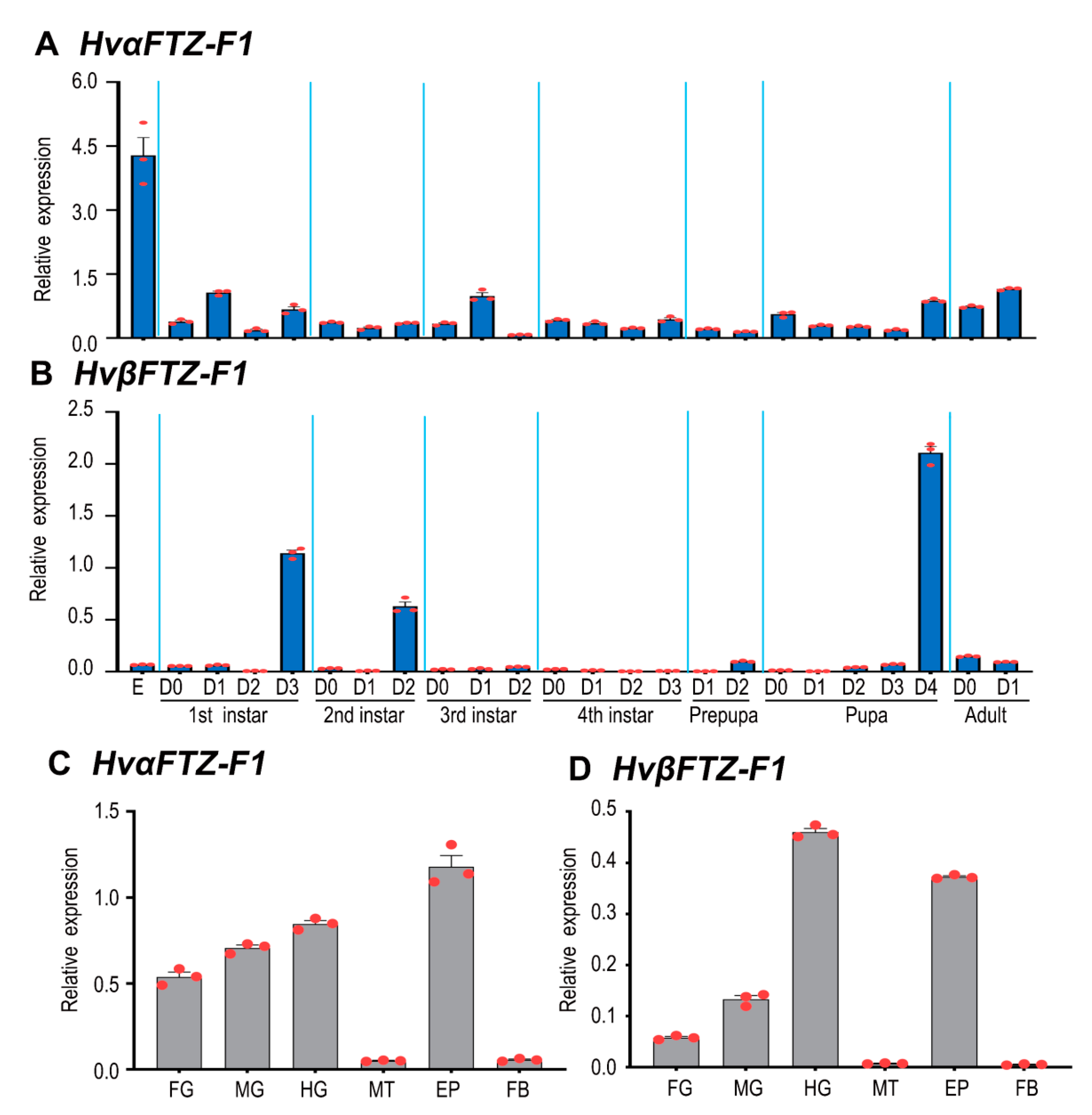
3.3. Expression Profiles of HvFTZ-F1 Isoforms
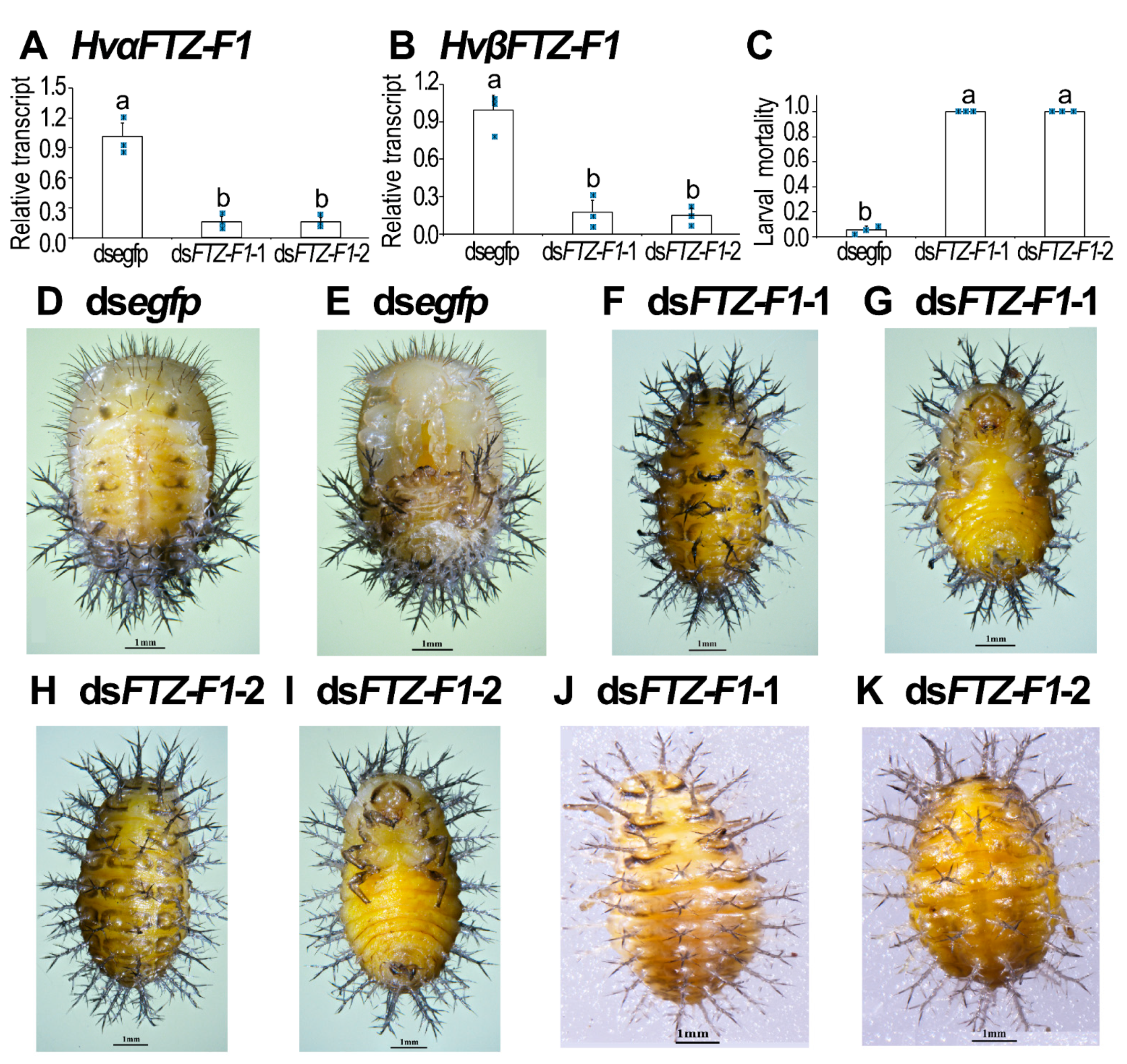
3.4. RNAi of Both HvFTZ-F1 Isoforms in Fourth-Instar Larvae
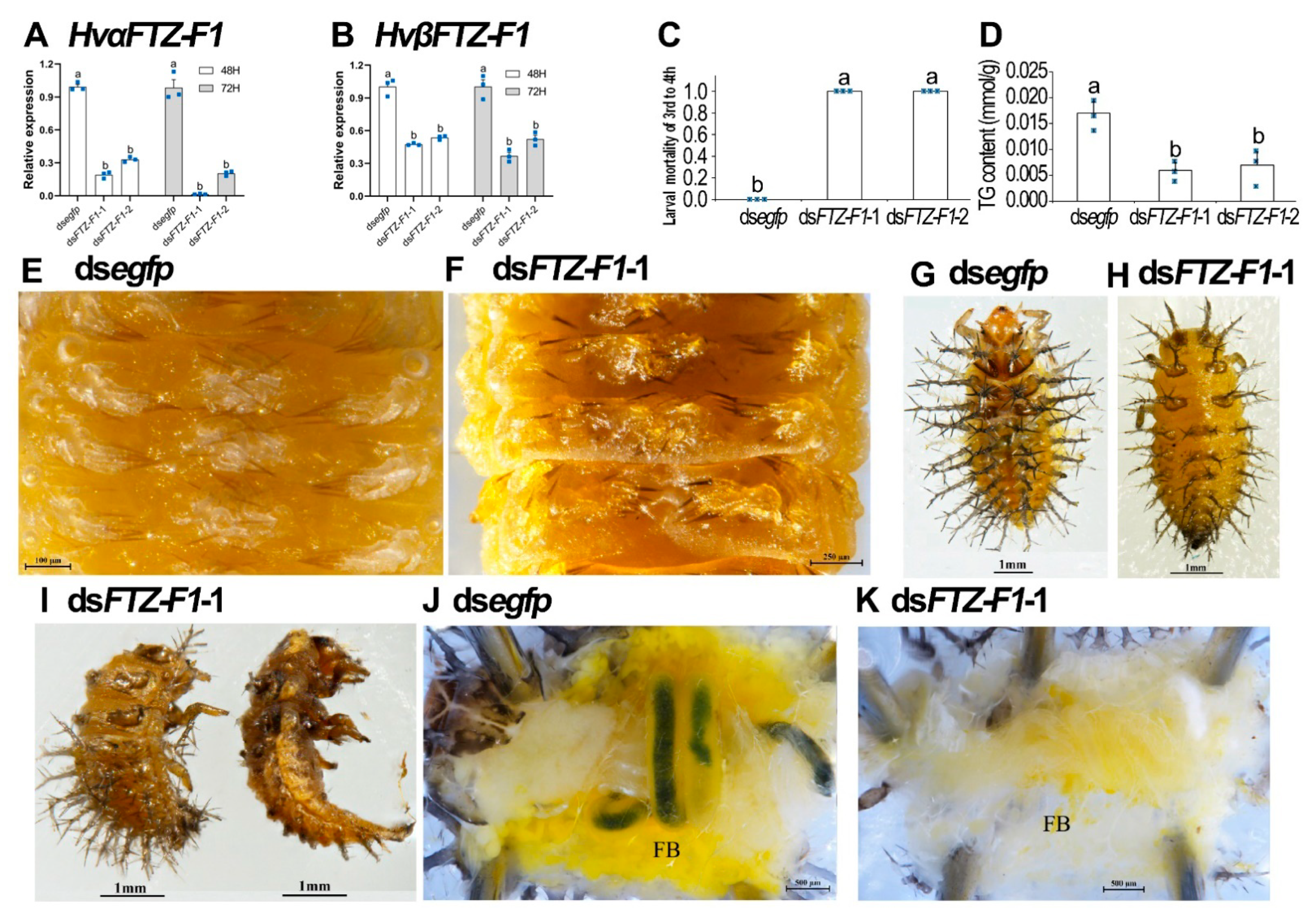
3.5. Knockdown of Both HvFTZ-F1 Isoforms in Third-Instar Larvae
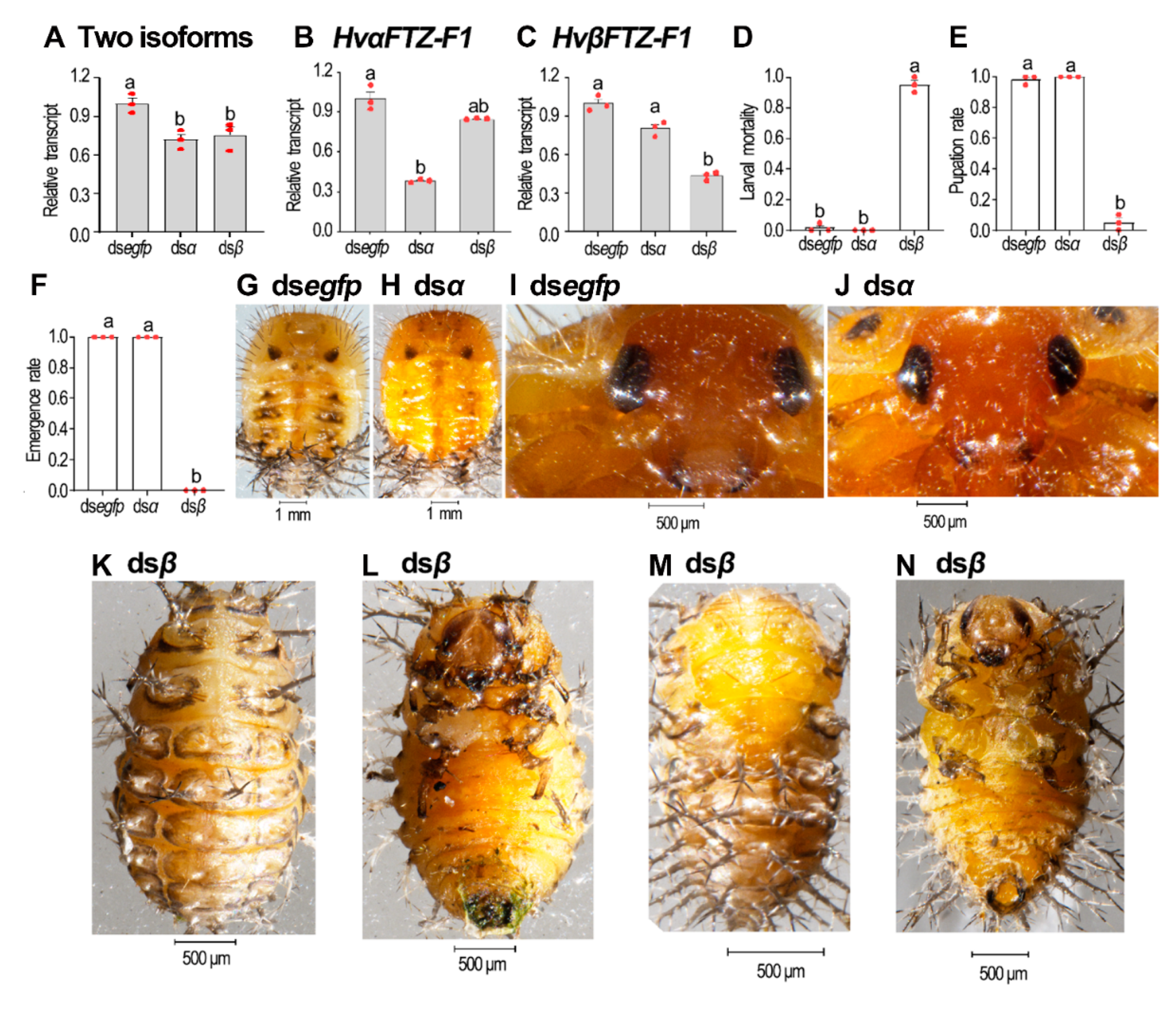
3.6. Isoform-Specific RNAi of HvFTZ-F1 in Fourth-Instar Larvae
3.7. Isoform-Specific Depletion of HvFTZ-F1 in Third-Instar Larvae
4. Discussion
4.1. HvβFTZ-F1 Is Necessary and Sufficient for Larval–Pupal Transition
4.2. Both HvαFTZ-F1 and HvβFTZ-F1 Are Critical but Mutually Interchangeable for Larval–Larval Ecdysis
4.3. HvαFTZ-F1 Is Involved in Embryonic Development in H. vigintioctopunctata
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Mello, T.R.P.; Aleixo, A.C.; Pinheiro, D.G.; Nunes, F.; Cristino, A.; Bitondi, M.M.G.; Barchuk, A.R.; Simões, Z.L.P. Hormonal control and target genes of ftz-f1 expression in the honeybee Apis mellifera: A positive loop linking juvenile hormone, and vitellogenin. Insect Mol. Biol. 2018, 28, 145–159. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.-P.; Fu, K.-Y.; Lü, F.-G.; Meng, Q.-W.; Guo, W.-C.; Li, G.-Q. Involvement of FTZ-F1 in the regulation of pupation in Leptinotarsa decemlineata (Say). Insect Biochem. Mol. Biol. 2014, 55, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Ueda, H.; Sonoda, S.; Brown, J.L.; Scott, M.P.; Wu, C. A sequence-specific DNA-binding protein that activates fushi tarazu segmentation gene expression. Genes Dev. 1990, 4, 624–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lala, D.S.; Parker, K.L.; Rice, D.A. Steroidogenic factor I, a key regulator of steroidogenic enzyme expression, is the mouse homolog of fushi tarazu-factor I. Mol. Endocrinol. 1992, 6, 1249–1258. [Google Scholar] [CrossRef]
- Honda, S.; Morohashi, K.; Nomura, M.; Takeya, H.; Kitajima, M.; Omura, T. Ad4BP regulating steroidogenic P-450 gene is a member of steroid hormone receptor superfamily. J. Biol. Chem. 1993, 268, 7494–7502. [Google Scholar] [CrossRef]
- Gissendanner, C.R.; E Sluder, A. nhr-25, the Caenorhabditis elegans ortholog of ftz-f1, is required for epidermal and somatic gonad development. Dev. Biol. 2000, 221, 259–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, A.; Palli, S.R. Identification and characterization of nuclear receptors from the red flour beetle, Tribolium castaneum. Insect Biochem. Mol. Biol. 2008, 38, 430–439. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Ma, L.; Liu, X.; Peng, Y.; Liang, G.; Xiao, H. Dissecting the roles of FTZ-F1 in larval molting and pupation, and the sublethal effects of methoxyfenozide on Helicoverpa armigera. Pest Manag. Sci. 2021, 77, 1328–1338. [Google Scholar] [CrossRef]
- Žitňan, D.; Kim, Y.J.; Žitňanová, I.; Roller, L.; Adams, M.E. Complex steroid-peptide-receptor cascade controls insect ecdysis. Gen. Comp. Endocrinol. 2007, 153, 88–96. [Google Scholar] [CrossRef] [Green Version]
- Bialecki, M.; Shilton, A.; Fichtenberg, C.; Segraves, W.A.; Thummel, C.S. Loss of the ecdysteroid-inducible E75A orphan nuclear receptor uncouples molting from metamorphosis in Drosophila. Dev. Cell 2002, 3, 209–220. [Google Scholar] [CrossRef] [Green Version]
- Bernardo, T.; Dubrovsky, E.B. The Drosophila juvenile hormone receptor candidates methoprene-tolerant (MET) and germ cell-expressed (GCE) utilize a conserved LIXXL motif to bind the FTZ-F1 nuclear receptor. J. Biol. Chem. 2012, 287, 7821–7833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavorgna, G.; Karim, F.D.; Thummel, C.S.; Wu, C. Potential role for a FTZ-F1 steroid receptor superfamily member in the control of Drosophila metamorphosis. Proc. Natl. Acad. Sci. USA 1993, 90, 3004–3008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sultan, A.R.S.; Oish, Y.; Ueda, H. Function of the nuclear receptor FTZ-F1 during the pupal stage in Drosophila melanogaster. Dev. Growth Differ. 2014, 56, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Ishak, N.S.M.; Kato, Y.; Matsuura, T.; Watanabe, H. Sequence conservation and sexually dimorphic expression of the Ftz-F1 gene in the crustacean Daphnia magna. PLoS ONE 2016, 11, e0154636. [Google Scholar] [CrossRef] [Green Version]
- Brunet, J.; Eichner, C.; Male, R. The FTZ-F1 gene encodes two functionally distinct nuclear receptor isoforms in the ectoparasitic copepod salmon louse (Lepeophtheirus salmonis). PLoS ONE 2021, 16, e0251575. [Google Scholar] [CrossRef]
- Lu, Z.; Li, M.; Fang, Y.; Qu, J.; Ye, W.; Dai, M.; Bian, D.; Mao, T.; Li, F.; Sun, H.; et al. The mechanism of damage to the posterior silk gland by trace amounts of acetamiprid in the silkworm, Bombyx mori. Pestic. Biochem. Physiol. 2020, 170, 104676. [Google Scholar] [CrossRef]
- Heffer, A.; Grubbs, N.; Mahaffey, J.; Pick, L. The evolving role of the orphan nuclear receptor ftz-f1, a pair-rule segmentation gene. Evol. Dev. 2013, 15, 406–417. [Google Scholar] [CrossRef]
- Cruz, J.; Nieva, C.; Mané-Padrós, D.; Martín, D.; Bellés, X. Nuclear receptor BgFTZ-F1 regulates molting and the timing of ecdysteroid production during nymphal development in the hemimetabolous insect Blattella germanica. Dev. Dynam. 2010, 237, 3179–3191. [Google Scholar] [CrossRef]
- Lin, T.; Zhana, C.; Lin, L.; Feng, Q.; Zheng, S. Cloning and expression of the nuclear transcription factor SlβFTZ-F1 cDNA from Spodoptera litura. Insect Sci. 2011, 18, 635–644. [Google Scholar]
- Velarde, R.A.; Robinson, G.E.; Fahrbach, S.E. Nuclear receptors of the honey bee: Annotation and expression in the adult brain. Insect Mol. Biol. 2006, 15, 583–595. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Kapitskaya, M.Z.; Zhu, J.; Miura, K.; Segraves, W.; Raikhel, A.S. Conserved molecular mechanism for the stage specificity of the mosquito vitellogenic response to ecdysone. Dev. Biol. 2000, 224, 96–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, K.H.; Daubnerová, I.; Park, Y.; Zitnan, D.; Adams, M.E. Secretory competence in a gateway endocrine cell conferred by the nuclear receptor βFTZ-F1 enables stage-specific ecdysone responses throughout development in Drosophila. Dev. Biol. 2014, 385, 253–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.; Li, W.; Su, K.; Yussa, M.; Han, W.; Perrimon, N.; Pick, L. The nuclear hormone receptor Ftz-F1 is a cofactor for the Drosophila homeodomain protein Ftz. Nature 1997, 385, 552–555. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.A.; Murata, T.; Hirose, S.; Lavorgna, G.; Suzuki, E.; Ueda, H. Temporally restricted expression of transcription factor βFTZ-F1: Significance for embryogenesis, molting and metamorphosis in Drosophila melanogaster. Development 2000, 127, 5083–5092. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-L.; Wang, F.; Guo, J.; Deng, X.-Y.; Chen, J.-Y.; Lin, L.-B. Characterization of ladybird Henosepilachna vigintioctopunctata transcriptomes across various life stages. Sci. Data 2018, 5, 180093. [Google Scholar] [CrossRef] [PubMed]
- Ze, L.J.; Xu, P.; Kang, W.N.; Wu, J.J.; Jin, L.; Anjum, A.A.; Li, G.Q. Disruption of ommochrome biosynthesis affects eye coloration, phototaxis and climbing in Henosepilachna vigintioctopunctata. Amino Acids 2021, 53, 1091–1104. [Google Scholar] [CrossRef]
- Xu, P.; Ze, L.J.; Kang, W.N.; Wu, J.J.; Jin, L.; Anjum, A.A.; Li, G.Q. Functional divergence of white genes in Henosepilachna vigintioctopunctata revealed by RNA interference. Insect Mol. Biol. 2020, 29, 466–476. [Google Scholar]
- Lü, J.; Chen, S.; Guo, M.; Ye, C.; Qiu, B.; Wu, J.; Yang, C.; Pan, H. Selection and validation of reference genes for RT-qPCR analysis of the ladybird beetle Henosepilachna vigintioctomaculata. Front. Physiol. 2018, 9, 1614. [Google Scholar] [CrossRef] [Green Version]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [Green Version]
- Ueda, H.; Sun, G.C.; Murata, T.; Hirose, S. A novel DNA-binding motif abuts the zinc finger domain of insect nuclear hormone receptor FTZ-F1 and mouse embryonal long terminal repeat-binding protein. Mol. Cell. Biol. 1992, 12, 5667–5672. [Google Scholar] [CrossRef]
- Laudet, V.; Hänni, C.; Coll, J.; Catzeflis, F.; Stéhelin, D. Evolution of the nuclear receptor gene superfamily. EMBO J. 1992, 11, 1003–1013. [Google Scholar] [CrossRef] [PubMed]
- Savkur, R.S.; Burris, T.P. The coactivator LXXLL nuclear receptor recognition motif. J. Pept. Res. 2004, 63, 207–212. [Google Scholar] [CrossRef] [PubMed]

| Species | αFTZ-F1 | βFTZ-F1 |
|---|---|---|
| Coleoptera | ||
| Tribolium castaneum | XP_008191375.1 | XP_008191374.1 |
| Tribolium madens | XP_044256208.1 | XP_044256191.1 |
| Leptinotarsa decemlineata | AJF93909.1 | AJF93908.1 |
| Onthophagus taurus | XP_022917389.1 | XP_022917387.1 |
| Coccinella septempunctata | XP_044746177.1 | XP_044746169.1 |
| Dendroctonus ponderosae | XP_019760699.1 | XP_019760698.1 |
| Anoplophora glabripennis | XP_018574370.1 | - |
| Rhynchophorus ferrugineus | - | ATU47257.1 |
| Dermestes maculatus | - | ATU89126.1 |
| Diabrotica virgifera virgifera | XP_028132013.1 | - |
| Lepidoptera | ||
| Bombyx mori | XP_021205999.1 | XP_021205997.1 |
| Spodoptera litura | XP_022832322.1 | XP_022832321.1 |
| Manduca sexta | XP_030023965.1 | XP_030023964.1 |
| Pieris rapae | XP_022123667.1 | XP_022123650.1 |
| Papilio xuthus | XP_013162170.1 | XP_013162169.1 |
| Danaus plexippus plexippus | XP_032527344.1 | XP_032527343.1 |
| Operophtera brumata | KOB72997.1 | - |
| Grapholitha molesta | ALG36655.1 | - |
| Spodoptera exigua | AMP42756.1 | - |
| Diptera | ||
| Drosophila melanogaster | AAA28542.1 | AAA28915.1 |
| Bactrocera oleae | XP_036216375.1 | XP_014088530.1 |
| Bactrocera tryoni | XP_039966955.1 | XP_039966958.1 |
| Aedes aegypti | XP_021697813.1 | XP_021697818.1 |
| Aedes albopictus | XP_029734936.1 | XP_029734942.1 |
| Bactrocera dorsalis | XP_011200628.1 | XP_029405286.1 |
| Rhagoletis pomonella | XP_036326029.1 | - |
| Bactrocera dorsalis | - | XP_011200628.1 |
| Culex pipiens pallens | - | XP_039436203.1 |
| Culex quinquefasciatus | - | XP_038118974.1 |
| Hymenoptera | ||
| Leptopilina heterotoma | XP_043462940.1 | XP_043462939.1 |
| Chelonus insularis | XP_034947592.1 | XP_034947593.1 |
| Diachasma alloeum | XP_015121710.1 | XP_015121711.1 |
| Cephus cinctus | XP_015598229.1 | XP_015598228.1 |
| Athalia rosae | XP_012261847.1 | - |
| Cotesia glomerata | KAH0535355.1 | - |
| Apis mellifera | XP_006557455.1 | - |
| Apis cerana | XP_016904299.1 | - |
| Aphidius gifuensis | XP_044014300.1 | - |
| Colletes gigas | - | XP_043249000.1 |
| Frieseomelitta varia | XP_043521209.1 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.-J.; Cheng, M.-D.; Ze, L.-J.; Shen, C.-H.; Jin, L.; Li, G.-Q. Dissecting the Isoform-Specific Roles of FTZ-F1 in the Larval–Larval and Larval–Pupal Ecdyses in Henosepilachna vigintioctopunctata. Insects 2022, 13, 228. https://doi.org/10.3390/insects13030228
Wu J-J, Cheng M-D, Ze L-J, Shen C-H, Jin L, Li G-Q. Dissecting the Isoform-Specific Roles of FTZ-F1 in the Larval–Larval and Larval–Pupal Ecdyses in Henosepilachna vigintioctopunctata. Insects. 2022; 13(3):228. https://doi.org/10.3390/insects13030228
Chicago/Turabian StyleWu, Jian-Jian, Min-Di Cheng, Long-Ji Ze, Chen-Hui Shen, Lin Jin, and Guo-Qing Li. 2022. "Dissecting the Isoform-Specific Roles of FTZ-F1 in the Larval–Larval and Larval–Pupal Ecdyses in Henosepilachna vigintioctopunctata" Insects 13, no. 3: 228. https://doi.org/10.3390/insects13030228
APA StyleWu, J.-J., Cheng, M.-D., Ze, L.-J., Shen, C.-H., Jin, L., & Li, G.-Q. (2022). Dissecting the Isoform-Specific Roles of FTZ-F1 in the Larval–Larval and Larval–Pupal Ecdyses in Henosepilachna vigintioctopunctata. Insects, 13(3), 228. https://doi.org/10.3390/insects13030228







