Toxicity, Sublethal and Low Dose Effects of Imidacloprid and Deltamethrin on the Aphidophagous Predator Ceratomegilla undecimnotata (Coleoptera: Coccinellidae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insecticide Properties and Treatments
2.2. Insects and Plant Material
2.3. Lethal Toxicity
2.4. Life History Study
2.5. Data Analysis
3. Results
3.1. Toxicity of Imidacloprid and Deltamethrin on Fourth Instar Larvae of C. undecimnotata
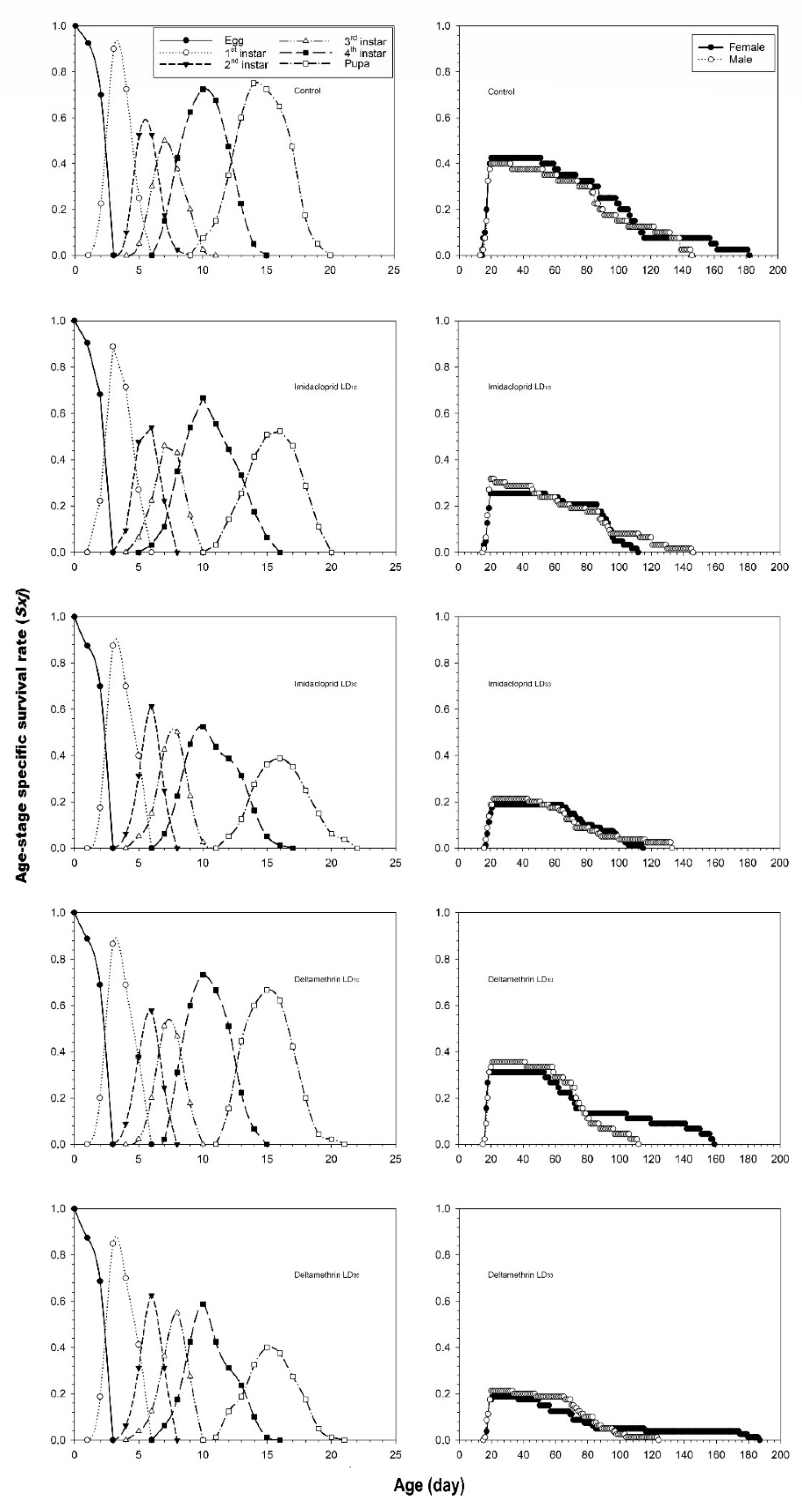
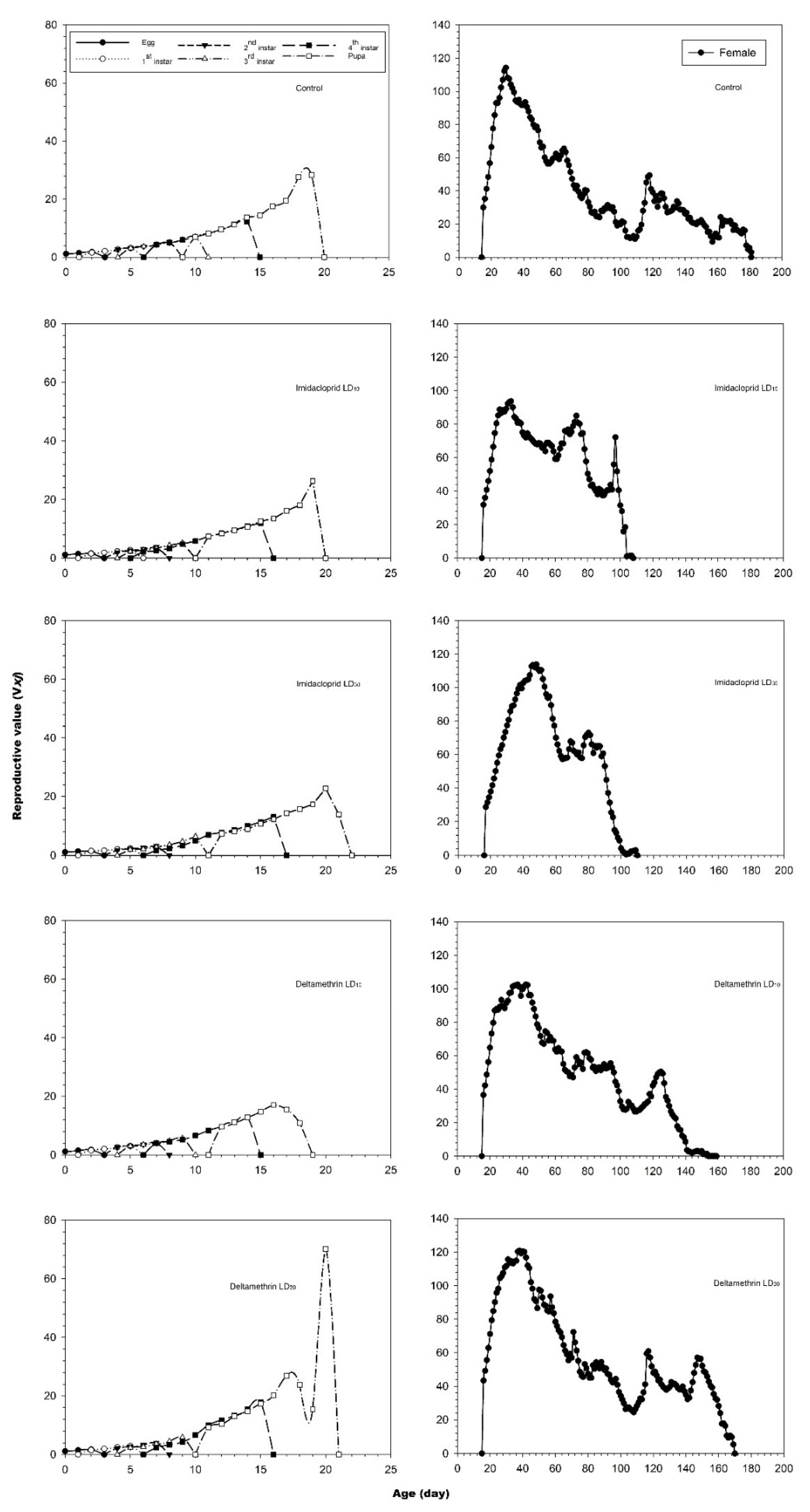
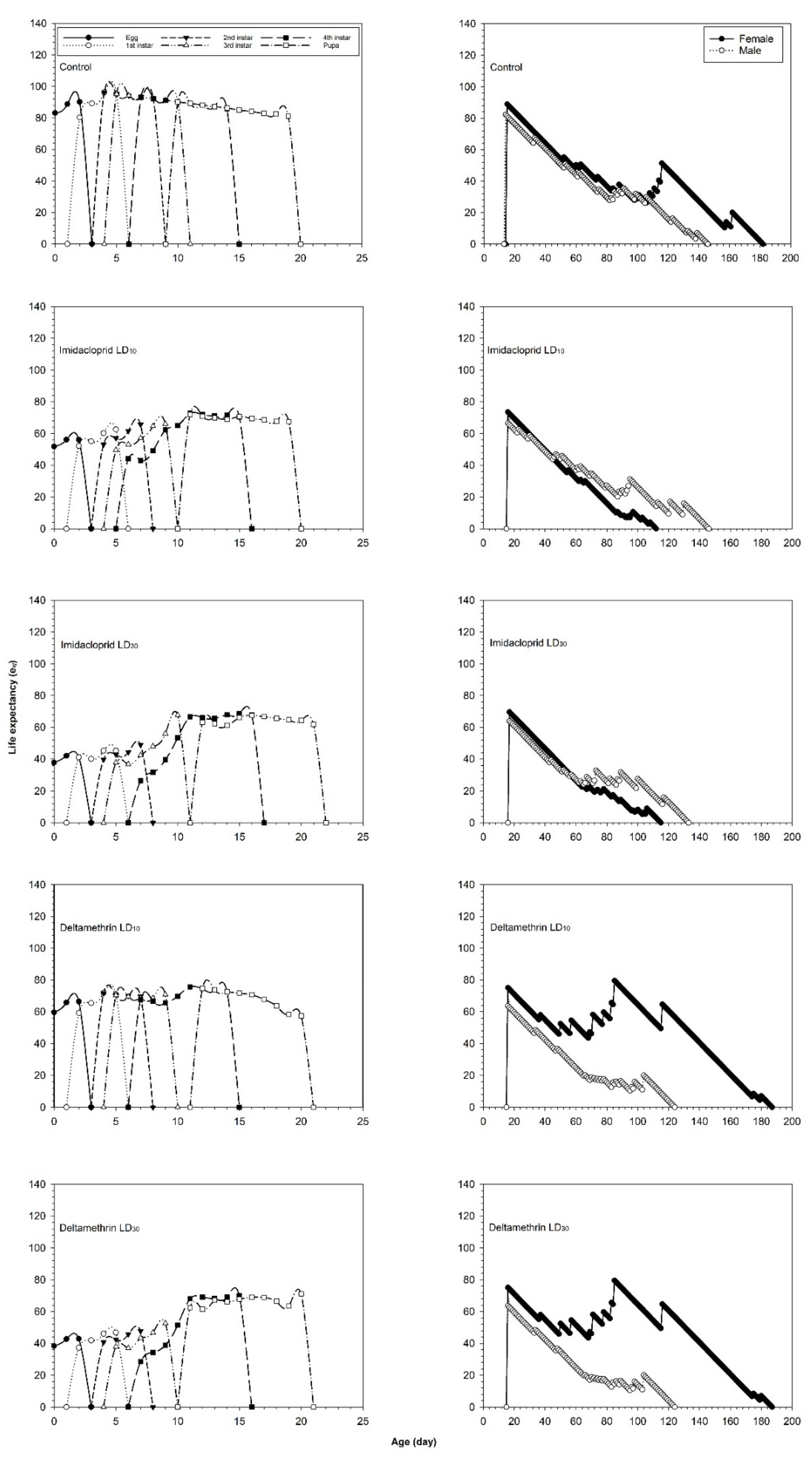
3.2. Effects of Imidacloprid and Deltamethrin on the Developmental Duration Time, Longevity and Fecundity of C. undecimnotata
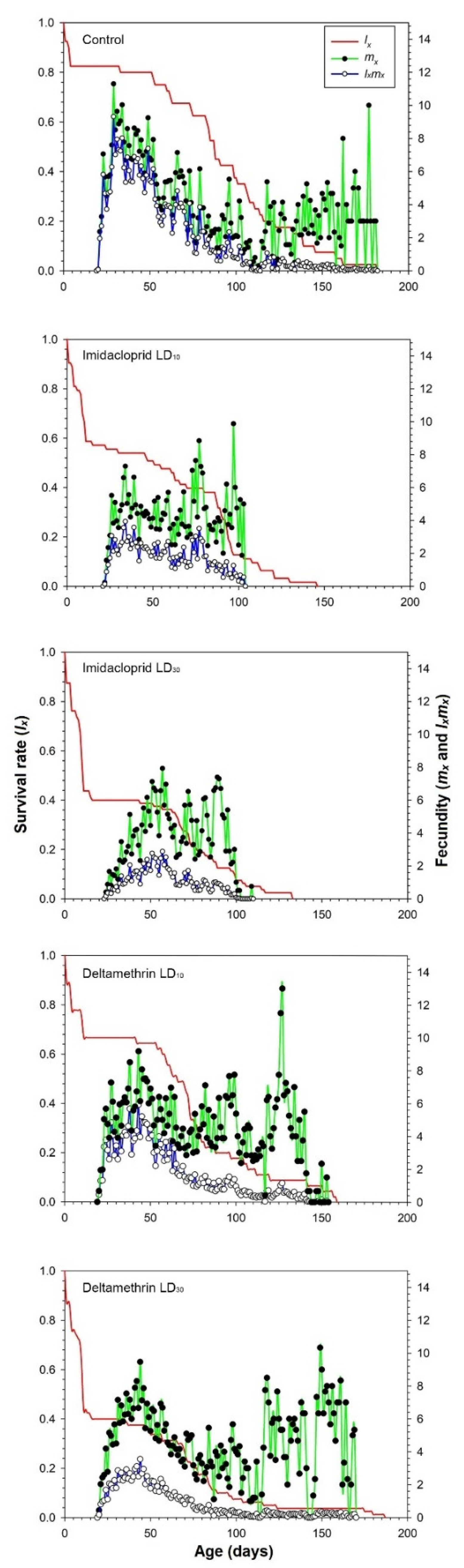
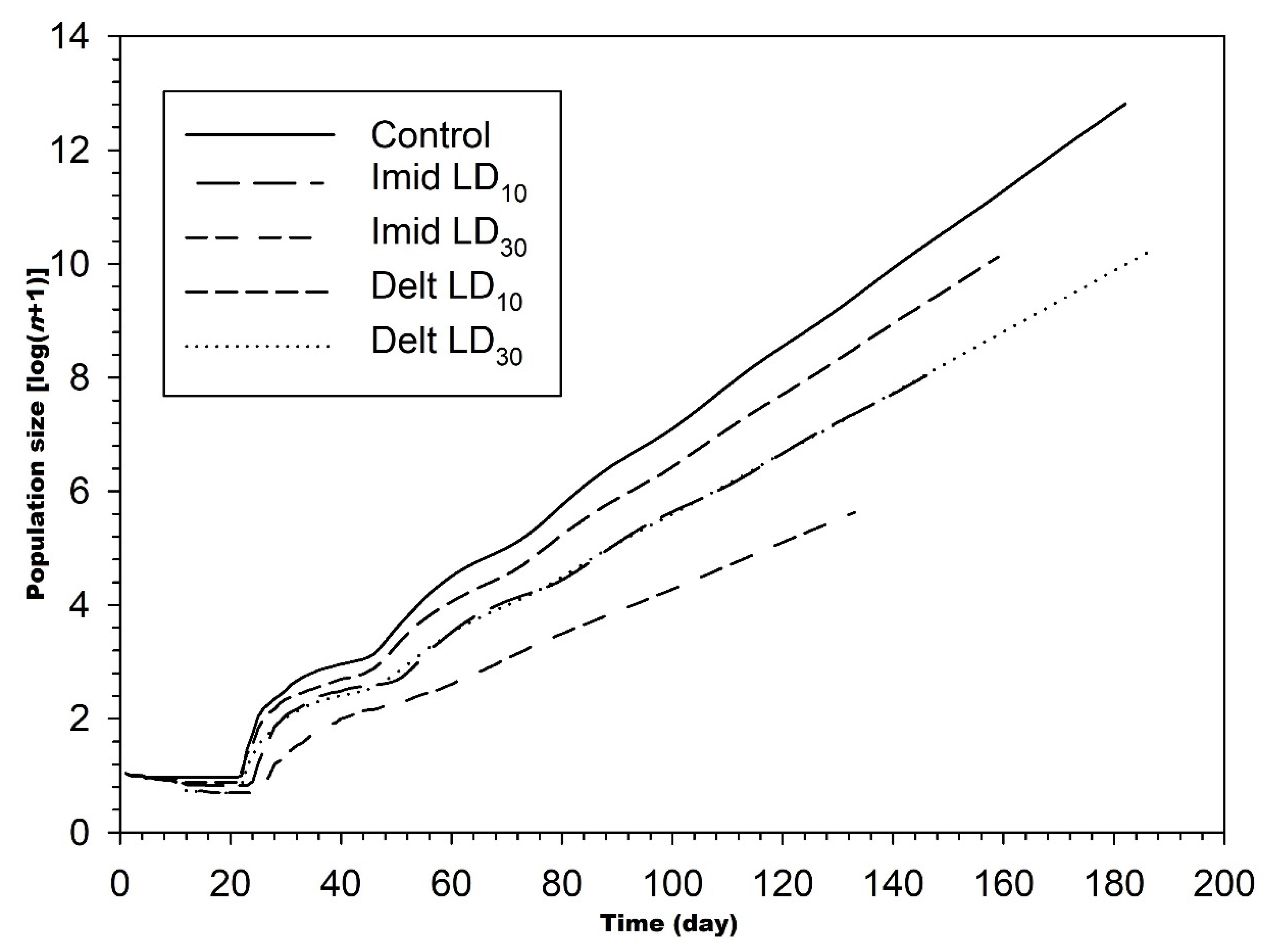
3.3. Influence of Imidacloprid and Deltamethrin on the Population Parameters of C. undecimnotata
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hodek, I.; Evans, E.W. Food Relationships. In Ecology and Behaviour of the Ladybird Beetles (Coccinellidae); Hodek, I.A., van Emden, H.F., Honěk, A., Eds.; Wiley-Blackwell: Oxford, UK, 2012; pp. 141–274. [Google Scholar]
- Karagounis, C.; Kourdoumbalos, A.K.; Margaritopoulos, J.T.; Nanos, G.D.; Tsitsipis, J.A. Organic farming-compatible insecticides against the aphid Myzus persicae (Sulzer) in peach orchards. J. Appl. Entomol. 2006, 130, 150–154. [Google Scholar] [CrossRef]
- Kavallieratos, N.G.; Athanassiou, C.G.; Tomanović, Ž.; Papadopoulos, G.D.; Vayias, B.J. Seasonal abundance and effect of predators (Coleoptera, Coccinellidae) and parasitoids (Hymenoptera: Braconidae, Aphidiinae) on Myzus persicae (Hemiptera, Aphidoidea) densities on tobacco: A two-year study from Central Greece. Biologia 2004, 59, 613–619. [Google Scholar]
- Skouras, P.J.; Margaritopoulos, J.T.; Zarpas, K.D.; Tsitsipis, J.A. Development, growth, feeding and reproduction of Ceratomegilla undecimnotata, Hippodamia variegata and Coccinella septempunctata fed on the tobacco aphid, Myzus persicae nicotianae. Phytoparasitica 2015, 43, 159–169. [Google Scholar] [CrossRef]
- Katsoyannos, P.; Kontodimas, D.C.; & Stathas, G.J. (1997) Phenology of Hippodamia undecimnotata (Col.: Coccinellidae) in Greece. Entomophaga 1997, 42, 283–293. [Google Scholar] [CrossRef]
- Kontodimas, D.C.; Milonas, P.G.; Stathas, G.J.; Papanikolaou, N.E.; Skourti, A.; Matsinos, Y.G. Life table parameters of the aphid predators Coccinella septempunctata, Ceratomegilla undecimnotata and Propylea quatuordecimpunctata (Coleoptera: Coccinellidae). Eur. J. Entomol. 2008, 105, 427–430. [Google Scholar] [CrossRef] [Green Version]
- Bass, C.; Denholm, I.; Williamson, M.S.; Nauen, R. The global status of insect resistance to neonicotinoid insecticides. Pestic. Biochem. Physiol. 2015, 121, 78–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomizawa, M.; Yamamoto, I. Structure-activity relationships of nicotinoids and imidacloprid analogs. J. Pestic. Sci. 1993, 18, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Field, L.M.; Emyr Davies, T.G.; O’Reilly, A.O.; Williamson, M.S.; Wallace, B.A. Voltage-gated sodium channels as targets for pyrethroid insecticides. Eur. Biophys. J. 2017, 46, 675–679. [Google Scholar] [CrossRef] [Green Version]
- Jactel, H.; Verheggen, F.; Thiéry, D.; Escobar-Gutiérrez, A.J.; Gachet, E.; Desneux, N. Alternatives to neonicotinoids. Environ. Int. 2019, 129, 423–429. [Google Scholar] [CrossRef]
- Desneux, N.; Decourtye, A.; Delpuech, J.M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef]
- Guedes, R.N.C.; Smagghe, G.; Stark, J.D.; Desneux, N. Pesticide-induced stress in Arthropod Pests for Optimized Integrated Pest Management Programs. Annu. Rev. Entomol. 2016, 61, 43–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricupero, M.; Desneux, N.; Zappalà, L.; Biondi, A. Target and non-target impact of systemic insecticides on a polyphagous aphid pest and its parasitoid. Chemosphere 2020, 247, 125728. [Google Scholar] [CrossRef] [PubMed]
- Skouras, P.J.; Brokaki, M.; Stathas, G.J.; Demopoulos, V.; Louloudakis, G.; Margaritopoulos, J.T. Lethal and sub-lethal effects of imidacloprid on the aphidophagous coccinellid Hippodamia variegate. Chemosphere 2019, 229, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Ullah, F.; Gul, H.; Desneux, N.; Gao, X.; Song, D. Imidacloprid-induced hormesis effects on demographic traits of the melon aphid, Aphis gossypii. Entomol. Gen. 2019, 39, 325–337. [Google Scholar] [CrossRef]
- Ullah, F.; Gul, H.; Desneux, N.; Qu, Y.; Xiao, X.; Khattak, A.M.; Gao, X.; Song, D. Acetamiprid-induced hormetic effects and vitellogenin gene (Vg) expression in the melon aphid, Aphis gossypii. Entomol. Gen. 2019, 39, 259–270. [Google Scholar] [CrossRef]
- Ullah, F.; Gul, H.; Desneux, N.; Tariq, K.; Ali, A.; Gao, X.; Song, D. Clothianidin-induced sublethal effects and expression changes of vitellogenin and ecdysone receptors genes in the melon aphid, Aphis gossypii. Entomol. Gen. 2019, 39, 137–149. [Google Scholar] [CrossRef]
- Voudouris, C.C.; Kati, A.; Sachinoglou, E.; Williamson, M.; Skouras, P.J.; Dimotsiou, O.; Georgiou, S.; Fenton, B.; Skavdis, G.; Margaritopoulos, J.T. Insecticide resistance status of Myzus persicae in Greece: Long-term surveys and new diagnostics for resistance mechanisms. Pest Manag. Sci. 2016, 72, 671–683. [Google Scholar] [CrossRef]
- Voudouris, C.C.; Williamson, M.S.; Skouras, P.J.; Kati, A.N.; Sahinoglou, A.J.; Margaritopoulos, J.T. Evolution of imidacloprid resistance in Myzus persicae in Greece and susceptibility data for spirotetramat. Pest Manag. Sci. 2017, 73, 1804–1812. [Google Scholar] [CrossRef]
- Wang, S.; Qi, Y.; Desneux, N.; Shi, X.; Biondi, A.; Gao, X. Sublethal and transgenerational effects of short-term and chronic exposures to the neonicotinoid nitenpyram on the cotton aphid Aphis gossypii. J. Pest Sci. 2017, 90, 389–396. [Google Scholar] [CrossRef]
- Youn, Y.N.; Seo, M.J.; Shin, J.G.; Jang, C.; Yu, Y.M. Toxicity of greenhouse pesticides to multicolored Asian lady beetles, Harmonia axyridis (Coleoptera: Coccinellidae). Biol. Control 2003, 28, 164–170. [Google Scholar] [CrossRef]
- Calvo-Agudo, M.; González-Cabrera, J.; Picó, Y.; Calatayud-Vernich, P.; Urbaneja, A.; Dicke, M.; Tena, A. Neonicotinoids in excretion product of phloem-feeding insects kill beneficial insects. Proc. Natl. Acad. Sci. USA 2019, 116, 16817–16822. [Google Scholar] [CrossRef] [Green Version]
- Douglas, M.R.; Tooker, J.F. Large-scale deployment of seed treatments has driven rapid increase in use of neonicotinoid insecticides and preemptive pest management in U.S. field crops. Environ. Sci. Technol. 2015, 49, 5088–5097. [Google Scholar] [CrossRef]
- Desneux, N.; Ramirez-Romero, R.; Kaiser, L. Multistep bioassay to predict recolonization potential of emerging parasitoids after a pesticide treatment. Environ. Toxicol. Chem. 2006, 25, 2675–2682. [Google Scholar] [CrossRef] [Green Version]
- Torres, J.B.; Bueno, A.D.F. Conservation biological control using selective insecticides—A valuable tool for IPM. Biol. Control 2018, 126, 53–64. [Google Scholar] [CrossRef]
- Biondi, A.; Desneux, N.; Siscaro, G.; Zappalà, L. (2012) Using organic-certified rather than synthetic pesticides may not be safer for biological control agents: Selectivity and side effects of 14 pesticides on the predator Orius laevigatus. Chemosphere 2012, 87, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Cabral, S.; Soares, A.O.; Garcia, P. Voracity of Coccinella undecimpunctata: Effects of insecticides when foraging in a prey/plant system. J. Pest Sci. 2011, 84, 373–379. [Google Scholar] [CrossRef] [Green Version]
- Desneux, N.; Denoyelle, R.; Kaiser, L. A multi-step bioassay to assess the effect of the deltamethrin on the parasitic wasp Aphidius ervi. Chemosphere 2006, 65, 1697–1706. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Zhao, J.; Guo, X.; Chen, H.; Qu, M.; Zhai, W.; Desneux, N.; Biondi, A.; Zhang, F.; Wang, S. Sublethal effects of imidacloprid on the predatory seven-spot ladybird beetle Coccinella septempunctata. Ecotoxicology 2016, 25, 1782–1793. [Google Scholar] [CrossRef] [PubMed]
- Santos, K.F.A.; Zanuzo Zanardi, O.; de Morais, M.R.; Jacob, C.R.O.; de Oliveira, M.B.; Yamamoto, P.T. The impact of six insecticides commonly used in control of agricultural pests on the generalist predator Hippodamia convergens (Coleoptera: Coccinellidae). Chemosphere 2017, 186, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Skouras, P.J.; Stathas, G.J.; Demopoulos, V.; Louloudakis, G.; Margaritopoulos, J.T. The effect of five insecticides on the predators Coccinella septempunctata and Hippodamia variegate. Phytoparasitica 2019, 47, 197–205. [Google Scholar] [CrossRef]
- Taravati, S.; Mannion, C.; McKenzie, C.; Osborne, L. Lethal and Sublethal Effects of Selected Systemic and Contact Insecticides on Nephaspis oculata (Coleoptera: Coccinellidae), in a Tri-Trophic System. J. Econ. Entomol. 2019, 112, 543–548. [Google Scholar] [CrossRef]
- He, Y.; Zhao, J.; Zheng, Y.; Desneux, N.; Wu, K. Lethal effect of imidacloprid on the coccinellid predator Serangium japonicum and sublethal effects on predator voracity and on functional response to the whitefly Bemisia tabaci. Ecotoxicology 2012, 21, 1291–1300. [Google Scholar] [CrossRef]
- Dai, C.; Ricupero, M.; Puglisi, R.; Lu, Y.; Desneux, N.; Biondi, A.; Zappalà, A. Can contamination by major systemic insecticides affect the voracity of the harlequin ladybird? Chemosphere 2020, 256, 126986. [Google Scholar] [CrossRef]
- Skouras, P.J.; Stathas, G.J.; Voudouris, C.C.; Darras, A.I.; Tsitsipis, J.A.; Margaritopoulos, J.T. Effect of synthetic insecticides on the larvae of Coccinella septempunctata from Greek populations. Phytoparasitica 2017, 45, 165–173. [Google Scholar] [CrossRef]
- Chi, H.; You, M.; Atlıhan, R.; Smith, C.; Kavousi, A.; Özgökçe, M.S.; Güncan, A.; Tuan, S.J.; Fu, J.W.; Xu, Y.Y.; et al. Age-stage, two-sex life table: An introduction to theory, data analysis, and application. Entomol. Gen. 2020, 40, 102–123. [Google Scholar] [CrossRef]
- Wei, M.F.; Chi, H.; Guo, Y.F.; Li, X.W.; Zhao, L.L.; Ma, R.Y. Demography of Cacopsylla chinensis (Hemiptera: Psyllidae) reared on four cultivars of Pyrus bretschneideri and P. communis pears with estimations of confidence intervals of specific life table statistics. J. Econ. Entomol. 2020, 113, 2343–2353. [Google Scholar] [CrossRef]
- Nawaz, M.; Cai, W.; Jing, Z.; Zhou, X.; Mabubu, J.I.; Hua, H. Toxicity and sublethal effects of chlorantraniliprole on the development and fecundity of a non-specific predator, the multicolored Asian lady beetle, Harmonia axyridis (Pallas). Chemosphere 2017, 178, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Blackman, R.L. Variation in the photoperiodic response within natural populations of Myzus persicae (Sulz.). Bull. Entomol. Res. 1971, 60, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Chi, H. Life-Table Analysis Incorporating Both Sexes and Variable Development Rates Among Individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Chi, H.; Liu, H. (1985) Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sin. 1985, 24, 225–240. [Google Scholar]
- Chi, H. TWOSEX-MSChart: A Computer Program for the Age-Stage, Two-Sex Life Table Analysis; National Chung Hsing University: Taichung, Taiwan, 2021; Available online: http://140120197173/Ecology/ (accessed on 1 August 2021).
- Euler, L. Recherches généales sur la mortalité et la multiplication du genre humain. Mémoires de L’académie des Sci. de Berl. 1760, 16, 144–164. [Google Scholar]
- Lotka, A.J. Studies on the mode of growth of material aggregates. Am. J. Sci. 1907, 24, 199–216. [Google Scholar] [CrossRef]
- Goodman, D. Optimal life histories, optimal notation, and the value of reproductive value. Am. Nat. 1982, 119, 803–823. [Google Scholar] [CrossRef]
- Birch, L.C. The intrinsic rate of natural increase of an insect population. J. Anim. Ecol. 1948, 17, 15–26. [Google Scholar] [CrossRef]
- Efron, B.; Tibshirani, R.J. An Introduction to the Bootstrap; Chapman and Hall: New York, NY, USA, 1993. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.W.; Chi, H.; Smith, C.L. Linking demography and consumption of Henosepilachna vigintioctopunctata (Coleoptera: Coccinellidae) fed on Solanum photeinocarpum: With a new method to project the uncertainty of population growth and consumption. J. Econ. Entomol. 2018, 111, 1–9. [Google Scholar] [CrossRef]
- Hesterberg, T.; Moore, D.S.; Monaghan, S.; Clipson, A.; Epstein, R. Bootstrap methods and permutation tests. Chapter 14. In The Practice of Business Statistics, 2nd ed.; Moore, D.S., McCabe, G.P., Duckworth, W.M., Sclove, S.L., Eds.; W. H. Freeman and Company: New York, NY, USA, 2005; pp. 14.1–14.70. [Google Scholar]
- Lucas, É.; Giroux, S.; Demougeot, S.; Duchesne, R.M.; Coderre, D. Compatibility of a natural enemy, Coleomegilla maculata lengi (Col., Coccinellidae) and four insecticides used against the Colorado potato beetle (Col., Chrysomelidae). J. Appl. Entomol. 2004, 128, 233–239. [Google Scholar] [CrossRef]
- Garzón, A.; Medina, P.; Amor, F.; Viñuela, E.; Budia, F. Toxicity and sublethal effects of six insecticides to last instar larvae and adults of the biocontrol agents Chrysoperla carnea (Stephens) (Neuroptera: Chrysopidae) and Adalia bipunctata (L.) (Coleoptera: Coccinellidae). Chemosphere 2015, 132, 87–93. [Google Scholar] [CrossRef] [Green Version]
- Yao, F.-L.; Zheng, Y.; Zhao, J.-W.; Desneux, N.; He, Y.-X.; Weng, Q.-Y. Lethal and sublethal effects of thiamethoxam on the whitefly predator Serangium japonicum (Coleoptera: Coccinellidae) through different exposure routes. Chemosphere 2015, 128, 49–55. [Google Scholar] [CrossRef]
- Jan, N.A.; Saber, M. Sublethal effects of imidacloprid and pymetrozine on the functional response of the aphid parasitoid, Lysiphlebus fabarum. Entomol. Gen. 2018, 38, 173–190. [Google Scholar]
- Vincent, C.; Ferran, A.; Guige, L.; Gambier, J.; Brun, J. Effects of imidacloprid on Harmonia axyridis (Coleoptera: Coccinellidae) larval biology and locomotory behaviour. Eur. J. Entomol. 2000, 97, 501–506. [Google Scholar] [CrossRef] [Green Version]
- Qu, Y.; Xiao, D.; Li, J.Y.; Chen, Z.; Biondi, A.; Desneux, N.; Gao, X.; Song, D. Sublethal and hormesis effects of imidacloprid on the soybean aphid Aphis glycines. Ecotoxicology 2015, 24, 479–487. [Google Scholar] [CrossRef]
- He, F.; Sun, S.; Tan, H.; Sun, X.; Shang, D.; Yao, C.; Qin, C.; Ji, S.; Li, X.; Zhang, J.; et al. Compatibility of chlorantraniliprole with the generalist predator Coccinella septempunctata L. (Coleoptera: Coccinellidae) based toxicity, life-cycle development and population parameters in laboratory microcosms. Chemosphere 2019, 225, 182–190. [Google Scholar] [CrossRef]
- Wang, P.; Zhou, L.-L.; Yang, F.; Liu, X.-M.; Wang, Y.; Lei, C.-L.; Si, S.-Y. Lethal and behavioral sublethal side effects of thiamethoxam on the predator Harmonia axyridis. Entomol. Exp. Appl. 2018, 166, 703–712. [Google Scholar] [CrossRef]
- Yu, C.; Lin, R.; Fu, M.; Zhou, Y.; Zong, F.; Jiang, H.; Lv, N.; Piao, X.; Zhang, J.; Liu, Y.; et al. Impact of imidacloprid on life-cycle development of Coccinella septempunctata in laboratory microcosms. Ecotoxicol. Environ. Saf. 2014, 110, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Grafton-Cardwell, E.E.; Gu, P. Conserving vedalia beetle, Rodolia cardinalis (Mulsant) (Coleoptera: Coccinellidae), in citrus: A continuing challenge as new insecticides gain registration. J. Econ. Entomol. 2003, 96, 1388–1398. [Google Scholar] [CrossRef]
- Papachristos, D.P.; Milonas, P.G. Adverse effects of soil applied insecticides on the predatory coccinellid Hippodamia undecimnotata (Coleoptera: Coccinellidae). Biol. Control 2008, 47, 77–81. [Google Scholar] [CrossRef]
- Xie, J.; De Clercq, P.; Pan, C.; Li, H.; Zhang, Y.; Pang, H. Larval nutrition-induced plasticity affects reproduction and gene expression of the ladybeetle, Cryptolaemus montrouzieri. BMC Evol. Biol. 2015, 15, 276. [Google Scholar] [CrossRef] [Green Version]
- Xiang, X.; Liu, S.H.; Wang, X.G.; Zhang, Y.M.; Gong, C.W.; Chen, L.; Zhang, S.; Shen, L. Sublethal effects of sulfoxaflor on population projection and development of the white-backed planthopper, Sogatella furcifera (Hemiptera: Delphacidae). Crop Prot 2019, 120, 97–102. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, F.; Mu, W.; Wang, Q.; Li, H.; Chen, C. Life table study of the effects of sublethal concentrations of thiamethoxam on Bradysia odoriphaga Yang and Zhang. Pestic. Biochem. Physiol. 2014, 111, 31–37. [Google Scholar] [CrossRef]
- Devine, G.J.; Harling, Z.K.; Scarr, A.W.; Devonshire, A.L. Lethal and sublethal effects of imidacloprid on nicotine-tolerant Myzus nicotianae and Myzus persicae. Pestic. Sci. 1996, 48, 57–62. [Google Scholar] [CrossRef]
| Insecticide | N a | Dose ng a.i. Insect −1 (95% Confidence Limits)−1 | Slope ± SE | χ2 | p | df | ||
|---|---|---|---|---|---|---|---|---|
| LD10 | LD30 | LD50 | ||||||
| Imidacloprid | 320 | 10.68 (7.60–13.74) | 20.95 (16.70–25.34) | 33.41 (27.75–40.21) | 2.588 ± 0.256 | 1.164 | 0.948 | 5 |
| Deltamethrin | 400 | 0.77 (0.54–1.02) | 1.76 (1.38–2.16) | 3.10 (2.53–3.81) | 2.128 ± 0.189 | 2.913 | 0.893 | 7 |
| Treatments | N | Development Time of Fourth Instar Larva (Days) | N | Development Time of Pupa (Days) |
|---|---|---|---|---|
| Control | 33 | 4.06 ± 0.12 c | 33 | 4.85 ± 0.09 ab |
| Imidacloprid LD10 | 37 | 5.22 ± 0.21 a | 36 | 4.75 ± 0.15 ab |
| Imidacloprid LD30 | 35 | 5.31 ± 0.13 a | 32 | 5.03 ± 0.11 a |
| Deltamethrin LD10 | 30 | 4.53 ± 0.13 b | 30 | 4.77 ± 0.12 ab |
| Deltamethrin LD30 | 35 | 4.66 ± 0.14 b | 32 | 4.75 ± 0.08 b |
| Treatments | N a | Fecundity (Eggs/Female) | APOP (Days) | TPOP (Days) | Female Adult Longevity (Days) | N b | Male Adult Longevity (Days) |
|---|---|---|---|---|---|---|---|
| Control | 17 | 773.24 ± 82.15 a | 4.76 ± 0.28 bc | 22.18 ± 0.40 c | 86.53 ± 8.86 a | 16 | 78.69 ± 8.17 a |
| Imidacloprid LD10 | 16 | 591.13 ± 63.80 ab | 5.88 ± 0.54 b | 24.31 ± 0.45 b | 71.06 ± 3.88 ab | 20 | 64.10 ± 7.49 ab |
| Imidacloprid LD30 | 15 | 476.60 ± 87.41 b | 9.00 ± 1.01 a | 28.27 ± 1.07 a | 67.27 ± 4.19 b | 17 | 61.88 ± 6.40 ab |
| Deltamethrin LD10 | 14 | 723.79 ± 132.05 ab | 4.43± 0.37 c | 22.00 ± 0.47 c | 79.79 ± 10.81 ab | 16 | 59.06 ± 4.41 b |
| Deltamethrin LD30 | 15 | 674.20± 131.06 ab | 4.47± 0.34 c | 22.73 ± 0.40 c | 72.08 ± 12.92 ab | 17 | 61.41 ± 4.98 ab |
| Treatments | N | Intrinsic Rate of Increase (r) (Day)−1 | Net Reproductive Rate (R0) (Offspring/Individual) | Mean Generation Time (T) (Days) | Finite Rate of Increase (λ) (Day)−1 |
|---|---|---|---|---|---|
| Control | 40 | 0.1590 ± 0.0078 a | 328.63 ± 69.51 a | 36.43 ± 1.03 c | 1.1724 ± 0.0091 a |
| Imidacloprid LD10 | 63 | 0.1229 ± 0.0079 b | 150.13 ± 36.07 bc | 40.78 ± 1.23 b | 1.1308 ± 0.0079 b |
| Imidacloprid LD30 | 80 | 0.0934 ± 0.0075 c | 89.36 ± 26.13 c | 48.11 ± 1.98 a | 1.0979 ± 0.0082 c |
| Deltamethrin LD10 | 45 | 0.1431 ± 0.010 ab | 225.18 ± 63.79 ab | 37.87 ± 1.35 bc | 1.1538 ± 0.0115 ab |
| Deltamethrin LD30 | 80 | 0.1234 ± 0.0091 b | 126.41 ± 37.90 bc | 39.24 ± 1.85 bc | 1.1313 ± 0.0103 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skouras, P.J.; Darras, A.I.; Mprokaki, M.; Demopoulos, V.; Margaritopoulos, J.T.; Delis, C.; Stathas, G.J. Toxicity, Sublethal and Low Dose Effects of Imidacloprid and Deltamethrin on the Aphidophagous Predator Ceratomegilla undecimnotata (Coleoptera: Coccinellidae). Insects 2021, 12, 696. https://doi.org/10.3390/insects12080696
Skouras PJ, Darras AI, Mprokaki M, Demopoulos V, Margaritopoulos JT, Delis C, Stathas GJ. Toxicity, Sublethal and Low Dose Effects of Imidacloprid and Deltamethrin on the Aphidophagous Predator Ceratomegilla undecimnotata (Coleoptera: Coccinellidae). Insects. 2021; 12(8):696. https://doi.org/10.3390/insects12080696
Chicago/Turabian StyleSkouras, Panagiotis J., Anastasios I. Darras, Marina Mprokaki, Vasilios Demopoulos, John T. Margaritopoulos, Costas Delis, and George J. Stathas. 2021. "Toxicity, Sublethal and Low Dose Effects of Imidacloprid and Deltamethrin on the Aphidophagous Predator Ceratomegilla undecimnotata (Coleoptera: Coccinellidae)" Insects 12, no. 8: 696. https://doi.org/10.3390/insects12080696







