Grape Phylloxera Genetic Structure Reveals Root–Leaf Migration within Commercial Vineyards
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
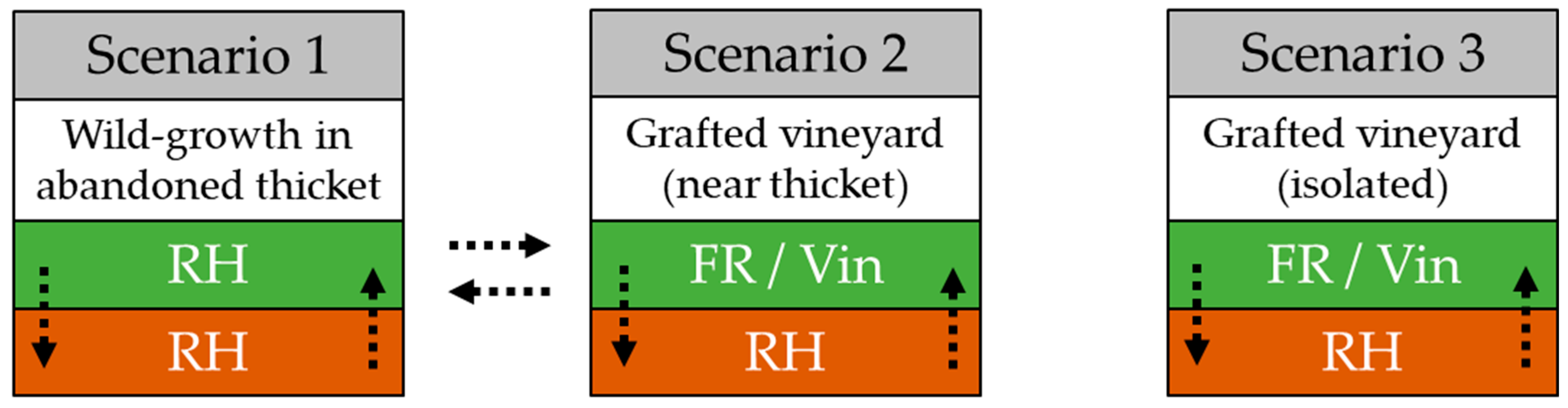
2.1. Phylloxera Sampling
2.2. DNA Isolation and Genotyping
2.3. Population Genetic Analyses
3. Results
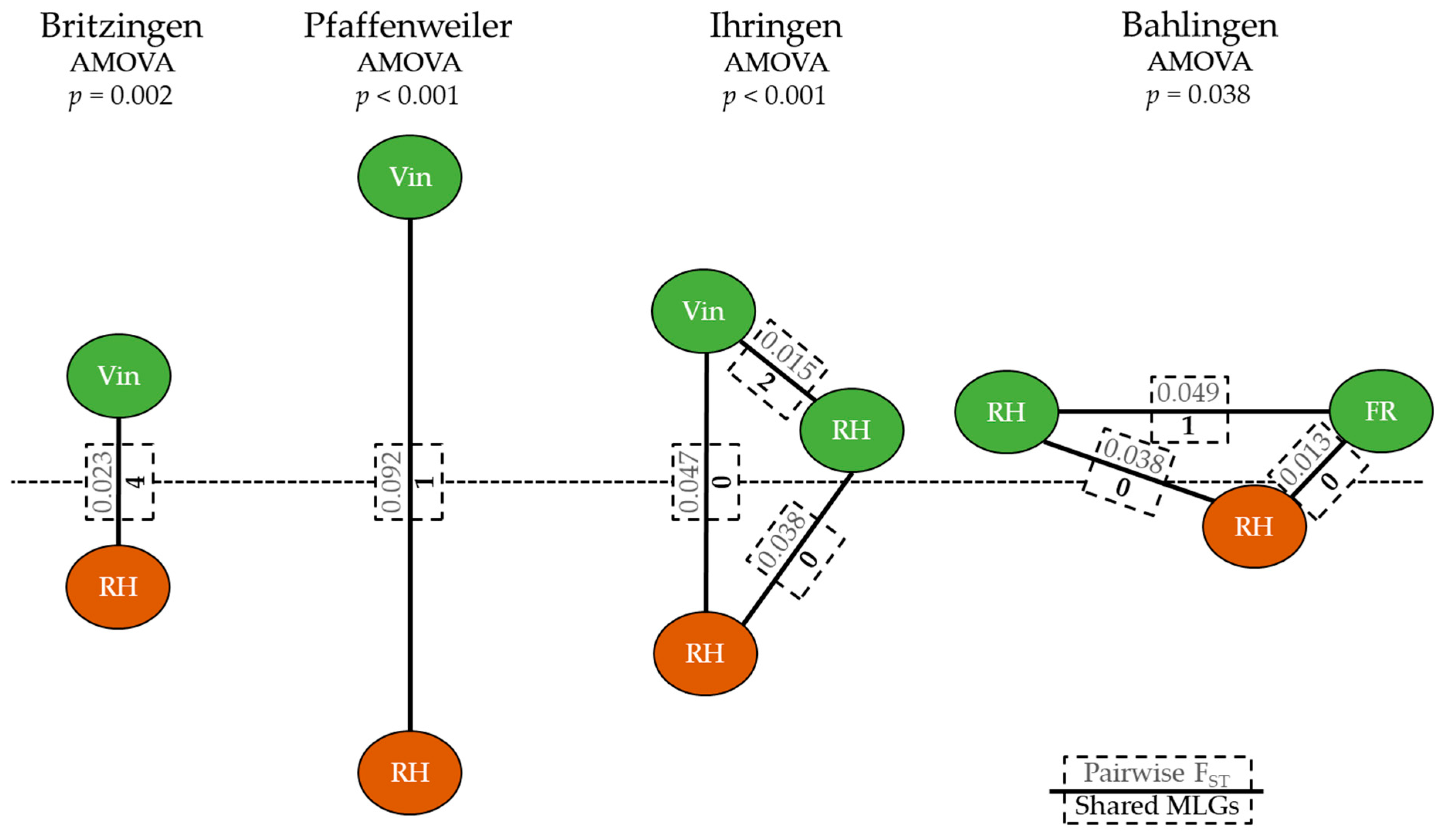
3.1. Population Structure of Vineyard Scenarios
3.2. Leaf Infestation Outbreaks
3.3. Reproduction and Genetic Diversity
4. Discussion
4.1. Sources of Vineyard Leaf Infestation
4.2. Population Bottlenecks
4.3. Critical Considerations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Powell, K.S.; Cooper, P.D.; Forneck, A. The Biology, Physiology and Host–Plant Interactions of Grape Phylloxera Daktulosphaira vitifoliae. In Advances in Insect Physiology; Academic Press: Cambridge, MA, USA, 2013; Volume 45, pp. 159–218. ISBN 9788578110796. [Google Scholar]
- Eitle, M.W.; Carolan, J.C.; Griesser, M.; Forneck, A. The salivary gland proteome of root-galling grape phylloxera (Daktulosphaira vitifoliae Fitch) feeding on Vitis spp. PLoS ONE 2019, 14, e0225881. [Google Scholar] [CrossRef] [PubMed]
- Granett, J.; Walker, M.A.; Kocsis, L.; Omer, A.D. Biology and management of grape Phylloxera. Annu. Rev. Entomol. 2001, 46, 387–412. [Google Scholar] [CrossRef] [PubMed]
- Forneck, A.; Huber, L. (A)sexual reproduction—A review of life cycles of grape phylloxera, Daktulosphaira vitifoliae. Entomol. Exp. Appl. 2009, 131, 1–10. [Google Scholar] [CrossRef]
- Schmid, J.; Sopp, E.; Rühl, E.H. Breeding rootstock varieties with complete phylloxera resistance. Acta Hortic. 1998, 473, 131–135. [Google Scholar] [CrossRef]
- Granett, J.; Walker, M.A. Nodosity populations on rootstocks are not a current threat to californian vineyards. Acta Hortic. 2009, 816, 23–28. [Google Scholar] [CrossRef]
- Kocsis, L.; Granett, J.; Walker, M.A. Performance of Hungarian phylloxera strains on Vitis riparia rootstocks. J. Appl. Entomol. 2002, 126, 567–571. [Google Scholar] [CrossRef]
- Wapshere, A.J.; Helm, K.F. Phylloxera and Vitis: An Experimentally Testable Coevolutionary Hypothesis. Am. J. Enol. Vitic. 1987, 38, 216–222. [Google Scholar]
- Mcleod, M.J. Damage Assessment and Biology of Foliar Rape Phylloxera (Homoptera: Phylloxeridae) in Ohio. Ph.D. Thesis, The Ohio State University, Columbus, OH, USA, 1990. [Google Scholar]
- Powell, K.S. Grape phylloxera: An overview. Root Feed. Ecosyst. Perspect. 2008, 96–114. [Google Scholar] [CrossRef]
- Mueller, N.; Bogenrieder, A. Verwilderte Reben an den Böschungen des Kaiserstuhls; University of Freiburg: Freiburg im Breisgau, Germany, 2010. [Google Scholar]
- Könnecke, T.; Aigner, C.; Specht, S.; Lawo, N.C.; Forneck, A. A stepwise assessment of Daktulosphaira vitifoliae infested grapevines in a Viennese vineyard site. Acta Hortic. 2011, 904, 59–62. [Google Scholar] [CrossRef]
- Bao, L.V.; Scatoni, I.B.; Gaggero, C.; Gutiérrez, L.; Monza, J.; Walker, M.A. Genetic diversity of grape phylloxera leaf-galling populations on Vitis species in Uruguay. Am. J. Enol. Vitic. 2015, 66, 46–53. [Google Scholar] [CrossRef]
- Quirós, D.; Remaudiere, G.; Nieto Nafría, J. Contribución al Conocimiento de Aphididae y Phylloxeridae (Hemiptera: Sternorrhyncha) de Panamá. Neotrop. Entomol. 2009, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Forneck, A.; Powell, K.S.; Walker, M.A. Scientific opinion: Improving the definition of grape phylloxera biotypes and standardizing biotype screening protocols. Am. J. Enol. Vitic. 2016, 67, 371–376. [Google Scholar] [CrossRef][Green Version]
- Molnár, J.G.; Németh, C.; Májer, J.; Jahnke, G.G. Assessment of phylloxera leaf galling incidence on european grapevines in badacsony Hungary. Acta Hortic. 2009, 816, 97–104. [Google Scholar] [CrossRef]
- Fahrentrapp, J.; Müller, L.; Schumacher, P. Is there need for leaf-galling grape phylloxera control? Presence and distribution of Dactulosphaira vitifoliae in Swiss vineyards. Int. J. Pest Manag. 2015, 61, 340–345. [Google Scholar] [CrossRef]
- Kopf, A. Untersuchungen zur Abundanz der Reblaus (Dactylosphaera vitifolii Shimer) und zur Nodositätenbildung in Abhängigkeit von Umweltfaktoren. Ph.D. Thesis, University of Hohenheim, Stuttgart, Germany, 2000. [Google Scholar]
- Schilder, F.A. Die Blattreblaus auf der Edelrebe. Der Züchter 1949, 19, 184–187. [Google Scholar] [CrossRef]
- Stellwaag-Kittler, F. Über den Einfluß von Außenfaktoren auf den Reblausbefall. In Proceedings of the Verhandlungen der deutschen Gesellschaft für angewandte Entomologie e.V. auf der dreizehnten Mitgliederversammlung; Geisenheim HSB: Geisenheim, Germany, 1955; pp. 91–98. [Google Scholar]
- Forneck, A.; Mammerler, R.; Tello, J.; Breuer, M.; Müller, J.; Fahrentrapp, J. First European leaf-feeding grape phylloxera (Daktulosphaira vitifoliae Fitch) survey in Swiss and German commercial vineyards. Eur. J. Plant Pathol. 2019, 154, 1029–1039. [Google Scholar] [CrossRef]
- Corrie, A.M.; Crozier, R.H.; Van Heeswijck, R.; Hoffmann, A.A. Clonal reproduction and population genetic structure of grape phylloxera, Daktulosphaira vitifoliae, in Australia. Heredity 2002, 88, 203–211. [Google Scholar] [CrossRef][Green Version]
- Tello, J.; Forneck, A. Use of dna markers for grape phylloxera population and evolutionary genetics: From rapds to ssrs and beyond. Insects 2019, 10, 317. [Google Scholar] [CrossRef]
- Riaz, S.; Lund, K.T.; Granett, J.; Walker, M.A. Population diversity of grape phylloxera in California and evidence for sexual reproduction. Am. J. Enol. Vitic. 2017, 68, 218–227. [Google Scholar] [CrossRef]
- Arancibia, C.; Riaz, S.; Agüero, C.; Ramirez-Corona, B.; Alonso, R.; Buscema, F.; Martínez, L.; Walker, M.A. Grape phylloxera (Daktulosphaira vitifoliae Fitch) in Argentina: Ecological associations to diversity, population structure and reproductive mode. Aust. J. Grape Wine Res. 2018, 24, 284–291. [Google Scholar] [CrossRef]
- Pedneault, K.; Provost, C. Fungus resistant grape varieties as a suitable alternative for organic wine production: Benefits, limits, and challenges. Sci. Hortic. 2016, 208, 57–77. [Google Scholar] [CrossRef]
- Riaz, S.; Lund, K.T.; Lin, H.; Walker, M.A. Development and characterization of a large set of microsatellite markers for grape phylloxera (Daktulosphaira vitifoliae Fitch). Vitis 2014, 53, 95–101. [Google Scholar]
- Vorwerk, S.; Forneck, A. Reproductive mode of grape phylloxera (Daktulosphaira vitifoliae, Homoptera: Phylloxeridae) in Europe: Molecular evidence for predominantly asexual populations and a lack of gene flow between them. Genome 2006, 49, 678–688. [Google Scholar] [CrossRef] [PubMed]
- Forneck, A.; Dockner, V.; Mammerler, R.; Powell, K.S.; Kocsis, L.; Papura, D.; Fahrentrapp, J.; Riaz, S.; Walker, M.A. PHYLLI—An international database for grape phylloxera (Daktulosphaira vitifoliae Fitch). Integr. Prot. Prod. Vitic. 2017, 128, 45–51. [Google Scholar]
- Lin, H.; Walker, M.A.; Hu, R.; Granett, J. New simple sequence repeat loci for the study of grape phylloxera (Daktulosphaira vitifoliae) genetics and host adaptation. Am. J. Enol. Vitic. 2006, 57, 33–40. [Google Scholar]
- Tello, J.; Mammerler, R.; Čajić, M.; Forneck, A. Major Outbreaks in the Nineteenth Century Shaped Grape Phylloxera Contemporary Genetic Structure in Europe. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Dorken, M.E.; Eckert, C.G. Severely reduced sexual reproduction in northern populations of a clonal plant, Decodon verticillatus (Lythraceae). J. Ecol. 2001, 89, 339–350. [Google Scholar] [CrossRef]
- Raymond, M.; Rousset, F. GENEPOP (version 1.2): Population genetics software for exact tests and ecumenicism. Heredity 1995, 86, 248–249. [Google Scholar] [CrossRef]
- Goudet, J. FSTAT (Version 1.2): A Computer Program to Calculate F-Statistics. J. Hered. 1995, 86, 485–486. [Google Scholar] [CrossRef]
- Excoffier, L.; Laval, G.; Schneider, S. Arlequin ver. 3.0: An integrated software package for population genetics data analysis. Evol. Bioinform. Online 2005, 1, 47–50. [Google Scholar] [CrossRef]
- Excoffier, L.; Smouse, P.E.; Quattro, J.M. Analysis of Molecular Variance Inferred From Metric Distances among DNA Haplotypes Application to Human Mitochondrial DNA Restriction Data. Genetics 1992, 131, 479–491. [Google Scholar] [CrossRef]
- Stenberg, P.; Lundmark, M.; Saura, A. MLGsim: A program for detecting clones using a simulation approach. Mol. Ecol. Notes 2003, 3, 329–331. [Google Scholar] [CrossRef]
- Rispe, C.; Legeai, F.; Nabity, P.D.; Fernández, R.; Arora, A.K.; Baa-Puyoulet, P.; Banfill, C.R.; Bao, L.; Barberà, M.; Bouallègue, M.; et al. The genome sequence of the grape phylloxera provides insights into the evolution, adaptation, and invasion routes of an iconic pest. BMC Biol. 2020, 18, 1–25. [Google Scholar] [CrossRef]
- Lund, K.T.; Riaz, S.; Walker, M.A. Population structure, diversity and reproductive mode of the grape phylloxera (Daktulosphaira vitifoliae) across its native range. PLoS ONE 2017, 12, e0170678. [Google Scholar] [CrossRef][Green Version]
- Corrie, A.M.; van Heeswijck, R.; Hoffmann, A.A. Evidence for host-associated clones of grape phylloxera Daktulosphaira vitifoliae (Hemiptera: Phylloxeridae) in Australia. Bull. Entomol. Res. 2003, 93, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Corrie, A.M.; Hoffmann, A.A. Fine-scale genetic structure of grape phylloxera from the roots and leaves of Vitis. Heredity 2004, 92, 118–127. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Harrison, J.S.; Mondor, E.B. Evidence for an invasive aphid “superclone”: Extremely low genetic diversity in oleander aphid (Aphis nerii) populations in the Southern United States. PLoS ONE 2011, 6, e017524. [Google Scholar] [CrossRef]
- Loxdale, H.D. The nature and reality of the aphid clone: Genetic variation, adaptation and evolution. Agric. For. Entomol. 2008, 10, 81–90. [Google Scholar] [CrossRef]
- Forneck, A.; Powell, K.S.; Walker, M.A. Reblaus-Biotypen: Stand der Forschung. Der Dtsch. Weinbau 2015, 25, 18–21. [Google Scholar]
- Forneck, A.; Walker, M.A.; Blaich, R. Genetic structure of an introduced pest, grape phylloxera (Daktulosphaira vitifoliae Fitch), in Europe. Genome 2000, 43, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Savoi, S.; Eitle, M.W.; Berger, H.; Curto, M.; Meimberg, H.; Griesser, M.; Forneck, A. Comparative transcriptome analysis of two root- feeding grape phylloxera (D. Vitifoliae) lineages feeding on a rootstock and V. Vinifera. Insects 2020, 11, 691. [Google Scholar] [CrossRef] [PubMed]
- Rispe, C.; Legeai, F.; Papura, D.; Bretaudeau, A.; Hudaverdian, S.; Le Trionnaire, G.; Tagu, D.; Jaquiéry, J.; Delmotte, F. De novo transcriptome assembly of the grapevine phylloxera allows identification of genes differentially expressed between leaf- and root-feeding forms. BMC Genom. 2016, 17, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kimberling, D.N.; Scott, E.R.; Price, P.W. Testing a new hypothesis: Plant vigor and phylloxera distribution on wild grape in Arizona. Oecologia 1990, 84, 1–8. [Google Scholar] [CrossRef]
- Steffan, H.; Rilling, G. Der Einfluss von Blatt-und Wurzelgallen der Reblaus (Dactylosphaera vfitifolii Shimer) auf des Verteilungsmuster der Assimitate in Reben (Vitis rupestris 187 G.). Vitis 1981, 20, 146–155. [Google Scholar]
- Kimberling, D.N.; Price, P.W. Competition, leaf morphology, and host clone effects on leaf-galling grape phylloxera (Homoptera: Phylloxeridae). Environ. Entomol. 1996, 25, 1147–1153. [Google Scholar] [CrossRef]
- King, P.D.; Buchanan, G.A. The Dispersal of Phylloxera Crawlers and Spread of Phylloxera Infestations in New Zealand and Australian Vineyards. Am. J. Enol. Vitic. 1986, 37, 26–33. [Google Scholar]
- Edwards, J.; Norng, S.; Powell, K.S.; Granett, J. Relationships between grape phylloxera abundance, fungal interactions and grapevine decline. Acta Hortic. 2007, 733, 151–157. [Google Scholar] [CrossRef]
- Savi, T.; Herrera, J.C.; Forneck, A. Leaf vs. Whole-Plant Biotic Attack: Does Vine Physiological Response Change? Water 2021, 13, 1429. [Google Scholar] [CrossRef]
- Yang, J.W.; Yi, H.S.; Kim, H.; Lee, B.; Lee, S.; Ghim, S.Y.; Ryu, C.M. Whitefly infestation of pepper plants elicits defence responses against bacterial pathogens in leaves and roots and changes the below-ground microflora. J. Ecol. 2011, 99, 46–56. [Google Scholar] [CrossRef]
- Nabity, P.D.; Haus, M.J.; Berenbaum, M.R.; DeLucia, E.H. Leaf-galling phylloxera on grapes reprograms host metabolism and morphology. Proc. Natl. Acad. Sci. USA 2013, 110, 16663–16668. [Google Scholar] [CrossRef]
- Omer, A.D.; Thaler, J.S.; Granett, J.; Karban, R. Jasmonic Acid Induced Resistance in Grapevines to a Root and Leaf Feeder. J. Econ. Entomol. 2009, 93, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Eitle, M.W.; Griesser, M.; Vankova, R.; Dobrev, P.; Aberer, S.; Forneck, A. Grape phylloxera (D. vitifoliae) manipulates SA/JA concentrations and signaling pathways in root galls of Vitis spp. Plant Physiol. Biochem. 2019, 144, 85–91. [Google Scholar] [CrossRef] [PubMed]

| Sampling Type and Year | Vineyard Location | Vin + RH | FR + RH | RH (Thicket) | Total | |||
|---|---|---|---|---|---|---|---|---|
| Leaf | Root | Leaf | Root | Leaf | Root | |||
| Vineyard-wide sampling; 2018 | Britzingen | 100 | 85 | - | - | - | - | 185 |
| Pfaffenweiler | 39 | 49 | - | - | - | - | 88 | |
| Ihringen | 71 | 65 | - | - | 45 | - | 181 | |
| Bahlingen | 9 | 8 | 85 | 13 | 25 | - | 140 | |
| Single-plant sampling; 2019 | Bahlingen E1 | - | - | 50 | 55 | - | - | 105 |
| Bahlingen E2 | - | - | 60 | 63 | - | - | 123 | |
| Britzingen | Pfaffenweiler | Ihringen | Bahlingen | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| L | R | L | R | L | R | T | L | R | T | |
| Individuals (n) | 86 | 65 | 30 | 36 | 38 | 39 | 44 | 52 | 8 | 18 |
| Distinct MLGs | 39 | 36 | 16 | 19 | 36 | 28 | 37 | 46 | 8 | 12 |
| Gen. div. (R) | 0.45 | 0.55 | 0.52 | 0.51 | 0.95 | 0.71 | 0.84 | 0.88 | 1.00 | 0.65 |
| Clonal MLGs 1 | 21/23 | 9/11 | 7/7 | 9/9 | 4/4 | 7/7 | 6/6 | 5/5 | 0/0 | 2/2 |
| Unique alleles | 0/25 | 5/33 | 0/23 | 0/25 | 0/28 | 1/35 | 3/37 | 0/32 | 0/20 | 0/27 |
| p (HWE) | <0.001 | <0.001 | <0.001 | <0.001 | 0.427 | <0.001 | 0.005 | 0.17 | 0.587 | <0.001 |
| FIS multi-locus | −0.05 | −0.05 | −0.13 | −0.05 | −0.05 | −0.12 | 0.08 | −0.04 | 0.05 | −0.09 |
| Single-Plant Sampling | Vineyard Sampling | Native Habitat Sampling [39] | |
|---|---|---|---|
| Individuals (n) | 152 | 416 | 41 |
| Distinct MLGs | 45 | 186 | 38 |
| Gen. div. (R) | 0.29 | 0.45 | 0.93 |
| Clonal MLGs 1 | 18/25 | 52/76 | 2/3 |
| Unique alleles | 0/17 | 8/28 | 22/28 |
| p (HWE) | <0.001 | <0.001 | <0.001 |
| FIS multi-locus | −0.168 | 0.013 | 0.160 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilmink, J.; Breuer, M.; Forneck, A. Grape Phylloxera Genetic Structure Reveals Root–Leaf Migration within Commercial Vineyards. Insects 2021, 12, 697. https://doi.org/10.3390/insects12080697
Wilmink J, Breuer M, Forneck A. Grape Phylloxera Genetic Structure Reveals Root–Leaf Migration within Commercial Vineyards. Insects. 2021; 12(8):697. https://doi.org/10.3390/insects12080697
Chicago/Turabian StyleWilmink, Jurrian, Michael Breuer, and Astrid Forneck. 2021. "Grape Phylloxera Genetic Structure Reveals Root–Leaf Migration within Commercial Vineyards" Insects 12, no. 8: 697. https://doi.org/10.3390/insects12080697
APA StyleWilmink, J., Breuer, M., & Forneck, A. (2021). Grape Phylloxera Genetic Structure Reveals Root–Leaf Migration within Commercial Vineyards. Insects, 12(8), 697. https://doi.org/10.3390/insects12080697






