Simple Summary
The egg parasitoid Aprostocetus brevipedicellus Yang and Cao (Eulophidae: Tetrastichinae) is one of the most promising biocontrol agents for forest pest control. Mass rearing of A. brevipedicellus is critical for large-scale field release programs, but the optimal rearing hosts are currently not documented. In this study, the parasitism of A. brevipedicellus and suitability of their offspring on Antheraea pernyi eggs with five different treatments were tested under laboratory conditions to determine the performance and suitability of A. brevipedicellus. Among the host egg treatments, A. brevipedicellus exhibited optimal parasitism on manually-extracted, unfertilized, and washed (MUW) eggs of A. pernyi, and A. brevipedicellus offspring emerging from MUW eggs had high egg load. The results indicate that MUW eggs are optimal for the mass production of A. brevipedicellus.
Abstract
Aprostocetus brevipedicellus, a eulophid gregarious egg parasitoid of lepidopterous pests, is a potential biological control agent for the control of many forest pests. A dominant factitious host, Antheraea pernyi, has been widely used for mass rearing several parasitoids in China. However, whether A. pernyi eggs are suitable for A. brevipedicellus rearing remains unclear. Here we evaluated A. brevipedicellus parasitism and fitness of their offspring on A. pernyi eggs with five different treatments, including manually-extracted, unfertilized and washed eggs (MUW), naturally-laid, unfertilized and washed eggs (NUW), naturally-laid, unfertilized, and unwashed (NUUW) eggs, naturally-laid, fertilized and washed eggs (NFW), and naturally-laid, fertilized and unwashed eggs (NFUW). The results showed that A. brevipedicellus could parasitize host eggs in all treatments but significantly preferred MUW eggs to other treatments. Moreover, A. brevipedicellus preferred unfertilized eggs to fertilized eggs and parasitized more washed eggs than unwashed. The pre-emergence time of parasitoid offspring emerging from fertilized eggs was shorter than that from unfertilized eggs. More parasitoid offspring emerged from unwashed eggs than that from washed eggs. The offspring emergence rate was high (>95%) and also female-biased (>85%) among all egg treatments. The egg load of female parasitoid offspring emerging from MUW and NUW eggs was 30–60% higher than the remaining treatments. Overall, MUW eggs of A. pernyi are the most suitable for the mass production of A. brevipedicellus.
1. Introduction
In China, forest insect pests have posed a serious threat to forest trees and agricultural crops. There are 5055 pest species accounting for damage in 11.4282 million ha of forestry in China [1]. The outbreaks of some pests often lead to a reduction in the yield of some economically important trees [2,3]. Management against crop and forest pests has mainly relied on insecticide applications [4,5]. However, long-term and extensive insecticide applications lead to environmental contamination and pest resistance development [6,7]. Furthermore, pesticide applications negatively impact non-target organisms such as beneficial arthropods (e.g., natural enemies of pests), leading to a resurgence of secondary pests, reducing biodiversity, and impacting overall ecosystem sustainability [7,8,9,10,11]. Therefore, it is necessary to develop effective and environmentally friendly control methods to control forest pests.
Biological control is a key method for pest management in forestry [12,13,14]. Among biological control agents that attack forest pests, parasitic wasps have received increasing attention due to their advantageous attributes such as host specificity, host feeding and parasitism, high efficiency, and easy mass rearing, etc. [15,16]. Eulophid parasitoids have been successfully used to control important forest pests in the United States [17], China [18], and worldwide [19,20]. Although biocontrol programs are promising, low abundance of natural enemies can lead to the failure of biological control programs, demonstrating a need to improve mass-rearing techniques for field augmentative release of natural enemies.
Aprostocetus brevipedicellus Yang and Cao (Eulophidae: Tetrastichinae) is an egg parasitoid first found in 2005 [21]. It can parasitize the eggs of lepidopterous pests such as Caligula japonica Moore, Dendrolimu spp., and Lebeda nobilis Walker and is one of the most promising biocontrol agents for forest pest control [22]. Many Aprostocetus parasitoids have successfully been used in forestry to control midge, beetle, and weevil pests [23,24,25,26,27,28].
Currently, mass rearing of Aprostocetus parasitoids relies on natural hosts for field release programs, such as mass rearing of Aprostocetus hagenowii Ratzeburg on the American cockroach Periplaneta americana L. for control [29]. To our knowledge, no mass rearing of A. brevipedicellus has been documented. The factitious eggs of Chinese oak silkworm Antheraea pernyi Guérin-Méneville are widely used for mass production of egg parasitoids such as Trichogramma and Anastatus because they are simple and cheap to mass-produce, and easy to transport [4,14,30,31,32,33]. Previous studies have demonstrated that the fertilization status of host eggs can be important for parasitism and fitness of the parasitoid offspring [34,35,36,37,38,39]. Generally, parasitoids prefer parasitizing fertilized host eggs [35,36,37,38]. However, this is not always the case [39]. The extracted eggs of A. pernyi from unfertilized females have been reported to use mass rearing of egg parasitoids [14]. A recent study demonstrated that Trichogramma parasitoids prefer parasitizing manually-extracted unfertilized washed A. pernyi eggs. Trichogramma offspring had increased fitness when reared on this factious host compared to natural hosts [34]. However, whether these unfertilized eggs can be used for A. brevipedicellus mass production remains unclear.
Additionally, host egg washing has been proved to influence parasitism preference and host orientation [34,40,41,42,43]. In this study, we explore the possibility of whether A. pernyi eggs can be used as a rearing host for A. brevipedicellus. We also evaluate the parasitism preference and fitness of A. brevipedicellus offspring on A. pernyi eggs under different host statuses. The objective of the current study was to determine the optimal A. pernyi egg treatment for mass production of A. brevipedicellus.
2. Materials and Methods
2.1. Parasitoids
Aprostocetus brevipedicellus was initially collected from parasitized eggs of Japanese giant silkworm Caligula japonica in walnut orchards in Kangxian (105–106° E, 32.9–33.7° N), Gansu Province, China, in 2017. The species were identified based on the morphological characteristics as described by Yao [21] and further confirmed by Dr. Gary Gibson in 2018 [5]. The voucher specimens of parasitoids were preserved in the Institute of Biological Control, Jilin Agricultural University, Changchun, Jilin province, China.
2.2. Host
Cocoons of Chinese oak silkworm, A. pernyi, were collected in Yongji City, Jilin Province, China, and then stored at 4 °C in the Institute of Biological Control, Jilin Agricultural University. After storage for 2–3 months, cocoons were transferred to an emergence room at 25 °C to allow adult emergence. Newly emerged adults (<6 h) were collected for the experiments.
2.3. Antheraea pernyi Egg Treatments
Egg treatments of A. pernyi were conducted to determine the optimal condition for the rearing of Aprostocetus brevipedicellus. The five treatments including (1) manually-extracted, unfertilized and washed eggs (MUW), (2) naturally-laid, unfertilized and washed eggs (NUW), (3) naturally-laid, unfertilized and unwashed eggs (NUUW), (4) naturally-laid, fertilized and washed eggs (NFW), and (5) naturally-laid, fertilized and unwashed eggs (NFUW). The preparation of the five egg treatments was similar to our previous study [34]. The MUW eggs were collected by dissecting the abdomen of unmated and mature female moths, and extracted eggs were subject to washing with distilled water immediately. Then washed eggs were air-dried under room temperature (about 1 h). When eggs dried, immature green eggs were removed, and the healthy eggs within 4 h after drying were used for the experiment. To prepare naturally-laid unfertilized eggs, the newly emerged female moths (<6 h) were collected and introduced to a screened cage (40 cm × 40 cm × 40 cm) to allow egg deposition. Our previous study found that 15% honey-water solution was suitable for a moth. Therefore, a 15% honey-water solution was also provided inside the cage for adult feeding. The cage was checked hourly, naturally-laid unfertilized eggs were divided into two groups; one group was washed with distilled water, air-dried under room temperature (NUW), and another group was not washed (NUUW). To prepare naturally-laid fertilized eggs, newly emerged female and male moths (<6 h) were collected and introduced to a screened cage (40 cm × 40 cm × 40 cm) to allow mating, with 15% honey-water solution provided as food. Newly laid fertilized eggs (<6 h) were divided into two groups, one was washed with distilled water and air-dried (NFW), and another group was not washed (NFUW). 0-day-old naturally-laid eggs were exposed to the parasitoids.
2.4. Suitability of A. pernyi Egg with Different Treatments on Parasitism by A. brevipedicellus
2.4.1. No-Choice Test
A no-choice experiment of A. brevipedicellus on treated A. pernyi eggs was conducted under laboratory conditions (25 ± 1 °C, 70 ± 5% RH, and 14 L: 10 D). Newly emerged A. brevipedicellus females (<1 h old) were collected and introduced with males in glass tubes provided with 10% honey-water solution as food (2.5 cm × 12 cm, diameter × height) to allow mating for 3 days before use in experiments. Based on our observations, one A. brevipedicellus female can parasitize up to 20 A. pernyi eggs within 24 h. Therefore, mated female parasitoids were individually exposed to 40 host eggs in a no-choice test for 24 h to allow a surplus supply of hosts. For each treatment, forty host eggs were glued onto a paper card (0.5 cm × 5.0 cm) using nontoxic glue and placed inside a glass tube (1.0 × 7.5 cm, diameter × height). One three-day-old, mated female A. brevipedicellus was also introduced into the glass tube containing an egg card. Our previous study found that a 10% honey-water solution was suitable for A. brevipedicellus. Therefore, a 10% honey-water solution was provided as food during the test. After 24 h, A. brevipedicellus was removed from the glass tube. The host eggs on the card were then cut out from the card and held individually in a glass tube, and maintained in an incubator under the conditions as described above. All parasitized eggs were checked daily until the emergence of all adults. After there was no further adult emergence, host eggs were individually dissected under a stereomicroscope to check the number of dead wasps left inside the chorion. For each egg treatment, the number of parasitized eggs (number of the host eggs with emergence hole + number of host eggs without emergence hole but containing parasitoids), pre-emergence time (d) (the number of days from exposure of host eggs to the parasitoid to the adult offspring emerging from host egg), the number of emerged adults per host egg (dead adults inside host eggs were excluded), the emergence rate (the number of eggs with emergence holes/total number of parasitized eggs × 100), percentage of female progeny (number of emerged females/total number of emerged females and males × 100), the number of dead parasitoids left inside the eggs, female offspring body size (left hind tibia length of female adults, HTL), and the egg load per female offspring were recorded. For HTL, thirty female adults from each treatment were selected randomly, and HTL was measured using an ultramicroscope (Keyence VHX-2000). To record the egg load per female offspring, thirty newly-emerged females were randomly selected from each treatment and then dissected under a stereomicroscope to examine the ovary to document the number of eggs. For each egg treatment, 30 female A. brevipedicellus were tested.
2.4.2. Choice Test
A choice experiment was tested under laboratory conditions (25 ± 1 °C, 70 ± 5% RH and 14 L: 10 D) to determine the host preference of A. brevipedicellus for A. pernyi eggs among the five treatments previously listed. One three-day-old and mated female A. brevipedicellus, as described above, was introduced into a glass tube (1.0 × 7.5 cm, diameter × height) containing an egg card. The egg card carried forty A. pernyi eggs from all egg treatments, with eight eggs per treatment, and was randomly placed on the paper card (0.5 cm × 5.0 cm). 10% honey-water solution was also provided as food. After 24 h, parasitoids were removed, and host egg cards were cut out individually and kept in an incubator as described above for posttreatment observation. After 6 days, the eggs were examined under a stereomicroscope to document the number of parasitized eggs of each treatment. This experiment was replicated 30 times.
2.5. Statistical Analysis
For the no-choice bioassay, a one-way analysis of variance (ANOVA) was conducted to determine the effect of treatment on the number of parasitized eggs, pre-emergence time, number of emerged adults per egg, emergence rate, number of dead wasps left inside per egg, percentage of female progeny, hind tibia length and egg load of per female offspring. Tukey’s honestly significant difference (HSD) test was used to compare means at p < 0.05. All data were subject to a normality test (Shapiro–Wilk test) prior to ANOVA. All percentage data were arcsine square-root-transformed prior to the Shapiro–Wilk test. The analysis was performed on the transformed data, and untransformed means ± SE were presented. For the choice test, data were analyzed using the Friedman non-parametric analysis to determine the effect of treatment on the number of parasitized eggs. All data analyses were performed using SPSS 23.0 (SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Suitability of A. pernyi Egg with Different Treatments on Parasitism by A. brevipedicellus
3.1.1. No-Choice Test
Fertilization significantly affected the number of eggs parasitized (F1,116 = 8.10, p = 0.0052) regardless of washing or not. However, the interaction between fertilization and washing treatments did not affect the number of parasitized eggs (F1,116 = 0.81, p = 0.3686). Aprostocetus brevipedicellus successfully parasitized A. pernyi eggs in all egg treatments, and there was a significant difference in the number of parasitized eggs among treatments (F4,145 = 130.07, p < 0.0001) (Figure 1A). The manually-extracted egg treatment resulted in the maximum number of parasitized eggs, which was 63–242% higher than the remaining treatments.
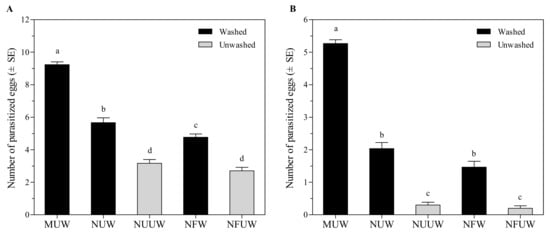
Figure 1.
Number of parasitized Antheraea pernyi eggs by Aprostocetus brevipedicellus with different treatments in no-choice test (A) and the choice test (B). MUW: manually-extracted, unfertilized and washed eggs; NUW: naturally-laid, unfertilized and washed eggs; NUUW: naturally-laid, unfertilized and unwashed eggs; NFW: naturally-laid, fertilized and washed eggs; NFUW: naturally-laid, fertilized and unwashed eggs. In the no-choice test, different lower-case letters on top of bars indicate significant differences among egg treatments based on Tukey’s multiple comparison (HSD) test (p < 0.05). In the choice test, different lower-case letters on bars indicate significant differences among egg treatments based on Friedman’s significant difference test (p < 0.05).
3.1.2. Choice Test
In the choice test, MUW eggs of A. pernyi resulted in the maximum number of parasitized eggs, which was 259–2533% higher than the remaining treatments (χ2 = 101.41, df = 4, p < 0.0001) (Figure 1B). Aprostocetus brevipedicellus parasitized 578% more NUW eggs than NUUW eggs (Z = 4.25, p < 0.0001), and parasitized 633% more NFW eggs than NFUW eggs (Z = 3.51, p < 0.0001). The number of parasitized eggs was equivalent between NUUW and NFUW treatments (Z = 0.37, p = 0.7130).
3.2. Suitability of A. pernyi Egg with Different Treatments on Development of A. brevipedicellus Offspring
3.2.1. Pre-Emergence Time
The pre-emergence time of A. brevipedicellus offspring was 6–10% shorter on NFW and NFUW treatments compared with MUW, NUW, and NUUW treatments (F4,735 = 36.83, p < 0.0001) (Figure 2A).
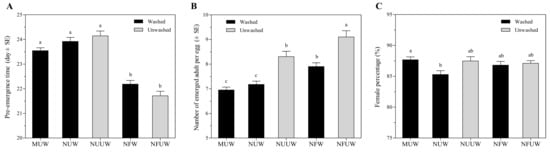
Figure 2.
Pre-emergence time (A), the number of emerged adults per egg (B), and female progeny percentage (C) of Aprostocetus brevipedicellus on Antheraea pernyi eggs with the five treatments. MUW: manually-extracted, unfertilized and washed eggs; NUW: naturally-laid, unfertilized and washed eggs; NUUW: naturally-laid, unfertilized and unwashed eggs; NFW: naturally-laid, fertilized and washed eggs; NFUW: naturally-laid, fertilized and unwashed eggs. Different lower-case letters on bars indicate significant differences among egg treatments based on Tukey’s multiple comparison (HSD) test (p < 0.05).
3.2.2. Emergence of Aprostocetus brevipedicellus
The number of emerged A. brevipedicellus adults per A. pernyi egg was 10–31% higher in the NFUW treatment compared with the remaining egg treatments (F4,735 = 26.38, p < 0.0001) (Figure 2B). MUW and NUW egg treatments resulted in about seven adults per host, significantly lower than the remaining treatments. Treatment had a significant effect on the percentage of female progeny (F4,735 = 3.14, p = 0.0141). Aprostocetus brevipedicellus offspring emerging from MUW egg treatment was 3% more female-biased than the NUW egg treatment (Figure 2C). Emergence rate did not significantly vary between all egg treatments (F4,761 = 0.28, p = 0.8940), and ranged from 95% to 97%. Similarly, there were no significant differences in the number of dead parasitoids per host egg between all egg treatments (F4,761 = 0.53, p = 0.7170). The number of dead parasitoids per egg was the highest in those from NFUW host eggs (0.35), followed by NUUW (0.23), NFW (0.22), MUW (0.17), and NUW host eggs (0.17).
3.2.3. Female Hind Tibia Length and Egg Load of Aprostocetus brevipedicellus Offspring
Hind tibia length of A. brevipedicellus female offspring ranged from 410.50 μm to 428.10 μm, and no significant differences were found in the hind tibia length among all egg treatments (F4,145 = 0.80, p = 0.5277). Female offspring emerging from MUW egg treatment resulted in the longest hind tibia length, followed by NUW, NFW, NUUW, and NFUW. Aprostocetus brevipedicellus female offspring emerging from MUW egg treatment resulted in maximum egg load, which was 2–12% higher than the remaining treatments (F4,145 = 8.04, p < 0.0001) (Figure 3).
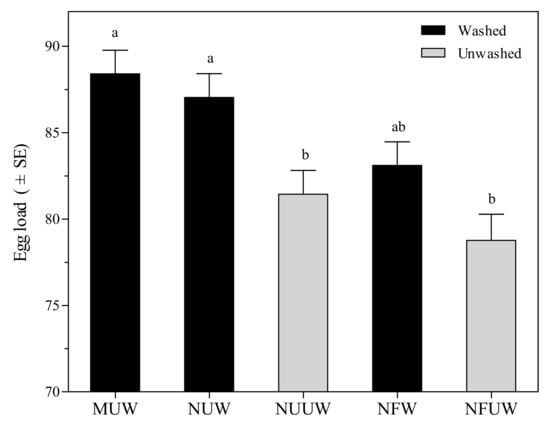
Figure 3.
Egg load of Aprostocetus brevipedicellus offspring from Antheraea pernyi eggs with five treatments. MUW: manually-extracted, unfertilized and washed eggs; NUW: naturally-laid, unfertilized and washed eggs; NUUW: naturally-laid, unfertilized and unwashed eggs; NFW: naturally-laid, fertilized and washed eggs; NFUW: naturally-laid, fertilized and unwashed eggs. Different lower-case letters on bars indicate significant differences among egg treatments based on Tukey’s multiple comparison test (HSD) (p < 0.05).
4. Discussion
The large eggs of the Chinese oak silkworm, A. pernyi, have been demonstrated as an excellent substitute host for mass-production for several egg parasitoids, especially Trichogramma and Anastatus [14,30,33,34]. In this study, we evaluated the parasitism of five A. pernyi egg treatments by A. brevipedicellus, and the fitness of resulting A. brevipedicellus offspring. The results showed that A. brevipedicellus successfully parasitized A. pernyi eggs in all egg treatments with varied parasitism and host suitability performance. Our study indicated that the MUW A. pernyi eggs showed great potential for mass production of A. brevipedicellus, since the results showed that this treatment resulted in the maximum number of parasitized host eggs in both the choice and no-choice experiments and high egg load in offspring from this treatment.
Previous studies have demonstrated that the host quality [14,44,45] and fertilization of host eggs can affect the parasitism rate, with most species preferring to parasitize fertilized eggs [35,36,37,38]. However, our results showed that A. brevipedicellus could recognize both fertilized and unfertilized host eggs and preferred parasitizing unfertilized eggs. Similar findings were also reported for T. japonicus and T. dendrolimi, which prefer unfertilized H. halys, and A. peryni eggs for mass rearing [34,39]. In addition, the present study found that the washing of host eggs influenced parasitoid preference, and A. brevipedicellus parasitized significantly more washed eggs than unwashed. Wang et al. found the same results for T. dendrolimi parasitizing A. pernyi eggs [34]. This may be attributable to changes in volatile profiles of A. pernyi eggs after washing treatments.
Furthermore, the washing treatment may soften and thin the egg chorion due to the friction between the eggs during washing. However, further studies are required to verify this phenomenon. Among the washed A. pernyi eggs, A. brevipedicellus parasitized significantly more manually-extracted eggs than naturally-laid eggs. The difference in preference may be due to the immature chorion of eggs, which is easier for A. brevipedicellus oviposition. The early manual extraction of eggs may have terminated the tanning process related to hardening the egg chorion [44]. Previous studies have demonstrated that the hardness and thickness of A. pernyi chorion is a limiting factor for some parasitoids [33,34]. Results presented here showed that a hard egg chorion can be an obstacle to parasitism and is not conducive to mass production.
The performance of A. brevipedicellus offspring was also affected by the fertilization of host eggs [34,35,37,38]. This study found that the pre-emergence time of A. brevipedicellus developing in fertilized eggs of A. pernyi was significantly shorter than that in unfertilized eggs. Similarly, Yang et al. reported that three Trichogramma species had shorter developmental time in fertilized eggs of Chilo suppressalis Walker than those in nonfertilized [37]. The embryonic development of fertilized host eggs can significantly influence the fitness of parasitoids [46]. The difference in the pre-emergence time between fertilized and unfertilized eggs seen in the current study may be attributable to the process of embryonic development, which increased accessibility of various nutrients within the host egg before exposure to the parasitoid. This increase in nutrient availability may have led to faster development of A. brevipedicellus offspring on fertilized eggs than unfertilized eggs. Other factors may also be involved, e.g., egg deposition period [47], but they were not measured in the present study.
Many female parasitoids can assess host quality and quantity to determine suitability for offspring development [46,47,48,49]. Our data showed that the number of emerged adults per host egg was significantly affected by the fertilization and washing treatment, and more A. brevipedicellus adults emerged per host egg from fertilized eggs than from unfertilized eggs. Similarly, egg parasitoids Gonatocerus morrilli Howard and Telenomus coloradensis Crawford parasitizing on Homalodisca vitripennis Germar eggs had higher mortality on unfertilized host eggs [35]. This is likely due to the increased accessibility of various nutrients associated with the fertilized A. pernyi eggs. We suspect that embryonic development of fertilized A. pernyi egg improves accessibility to nutrients in the host egg for the parasitoid. Therefore, when parasitizing fertilized eggs, the parasitoids might lay the larger number of eggs per host egg. A recent study on Trichogramma parasitizing A. pernyi eggs also indicated that the number of emerged adults per fertilized host egg was significantly higher than per unfertilized host egg [34]. There was a significantly higher number of emerged adults per egg on unwashed eggs than that on washed for the host egg washing treatments. We suspect that, compared with the washed eggs, it is difficult for parasitoids to parasitize unwashed eggs with the thicker and harder chorion. Therefore, parasitoids will expect to lay a larger number of offspring inside if they successfully parasitize an unwashed egg. This may be a parasitic strategy when parasitoids are confronted with disadvantage conditions [50,51].
Results showed that A. brevipedicellus offspring was strongly female-biased (>85% females) in all treatments. The percentage of female progeny under the natural field conditions was 77% [5], and smaller than that under laboratory conditions. Previous studies have demonstrated that it is preferable to have a female biased sex ratio of the parasitoids in biocontrol programs [29,52]. There is often a positive relationship between fitness and body size [46,53,54,55], and the body size of parasitoids is often positively correlated to their fecundity [56,57]. For example, Wang et al. found that the body size of female Ooencyrtus kuvanae Howard positively affects its fecundity [58]. Host quality can also influence the egg load of parasitoids [14,59]. Li et al. reported that the number of emerged Oomyzus sokolowskii Kurdjumov adults per host was negatively correlated with egg load [60]. Although there were no significant differences in parasitoid body size between treatments, female offspring emerging from MUW and NUW eggs had the highest egg load. NUW resulted in 39–61% less parasitism than the MUW treatment, suggesting that MUW A. pernyi eggs are the most optimal host for the mass rearing of A. brevipedicellus.
5. Conclusions
In conclusion, MUW eggs of A. pernyi were most suitable for oviposition based on host preference, parasitism, and parameters of offspring fitness. Our results provide evidence to support that MUW eggs of A. pernyi are desirable for mass production of A. brevipedicellus. They also provide the possibility for large-scale application of A. brevipedicellus to control the forest pests effectively. However, further studies are needed to optimize the rearing ratio of A. brevipedicellus to MUW eggs and their biocontrol efficiency against target pests in field release programs.
Author Contributions
Conceptualization, L.-S.Z. and X.-B.Y.; methodology, J.W., Y.-M.C. and L.-S.Z.; validation, L.-S.Z., X.-B.Y. and N.D.; formal analysis, L.-S.Z., Y.-M.C., X.-B.Y. and N.D.; investigation, J.W.; resources, L.-S.Z., Y.-M.C. and R.-E.L.; data curation, J.W., Y.-M.C. and L.-S.Z.; writing—original draft preparation, J.W., Y.-M.C. and L.-S.Z.; writing—review and editing, L.-S.Z., X.-B.Y. and N.D.; visualization, L.-S.Z., X.-B.Y. and N.D.; supervision, L.-S.Z., X.-B.Y. and N.D.; project administration, L.-S.Z.; funding acquisition, L.-S.Z. and R.-E.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key R&D Program of China (2017YFE0104900), Program of Introducing Talents to Chinese Universities (111 Program, D20023), and Key R&D Programs (Agriculture) of Gansu Province (20YF3NK030).
Data Availability Statement
Not applicable.
Acknowledgments
We thank Gary Gibson (Honorary Research Associate, Agriculture and Agri-Food Canada, Canadian National Collection of insects) for the species confirmation of Aprostocetus brevipedicellus.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Song, Y.S.; Dong, Y.Q.; Cui, D.Y.; Yue, F.Z.; Zhu, N.B.; Bai, H.Y. Species analysis of forest pest in China, V. Insect. Forest Pest. Dis. 2019, 38, 26–30. (In Chinese) [Google Scholar]
- Yang, Z.-Q.; Wang, X.-B.Y.; Zhang, Y.-N. Recent advances in biological control of important native and invasive forest pests in China. Biol. Control 2014, 68, 117–128. [Google Scholar] [CrossRef]
- Xu, M.; Xu, F.Y.; Liu, Y.P.; Pan, Y.S.; Wu, X.Q. Assessment of Metarhizium anisopliae (Clavicipitaceae) and its vector, Scleroderma guani (Hymenoptera: Bethylidae), for the control of Monochamus alternatus (Coleoptera: Cerambycidae). Can. Entomol. 2015, 147, 628–634. [Google Scholar] [CrossRef]
- Li, D.S.; Liao, C.Y.; Zhang, B.X.; Song, Z.W. Biological control of insect pests in litchi orchards in China. Biol. Control. 2014, 68, 23–36. [Google Scholar] [CrossRef]
- Chen, Y.M.; Gibson, G.A.P.; Peng, L.F.; Iqbal, A.; Zang, L.S. Anastatus Motschulsky (Hymenoptera, Eupelmidae): Egg parasitoids of Caligula japonica Moore (Lepidoptera, Saturniidae) in China. ZooKeys 2019, 881, 109–134. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Schuler, M.A.; Berenbaum, M.R. Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annu. Rev. Entomol. 2007, 52, 231–253. [Google Scholar] [CrossRef]
- Desneux, N.; Decourtye, A.; Delpuech, J.M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef] [PubMed]
- Desneux, N.; Wajnberg, E.; Fauvergue, X.; Privet, S.; Kaiser, L. Oviposition behaviour and patch-time allocation in two aphid parasitoids exposed to deltamethrin residues. Entomol. Exp. Appl. 2004, 112, 227–235. [Google Scholar] [CrossRef]
- Desneux, N.; Ramirez-Romero, R.; Kaiser, L. Multistep bioassay to predict recolonization potential of emerging parasitoids after a pesticide treatment. Environ. Toxicol. Chem. 2006, 25, 2675–2682. [Google Scholar] [CrossRef] [Green Version]
- Taning, C.N.T.; Vanommeslaeghe, A.; Smagghe, G. With or without foraging for food, field-realistic concentrations of sulfoxaflor are equally toxic to bumblebees (Bombus terrestris). Entomol. Gen. 2019, 39, 151–155. [Google Scholar] [CrossRef]
- Varikou, K.; Garantonakis, N.; Birouraki, A. Exposure of Bombus terrestris L. to three different active ingredients and two application methods for olive pest control. Entomol. Gen. 2019, 39, 53–60. [Google Scholar] [CrossRef]
- Desneux, N.; Wajnberg, E.; Wyckhuys, K.A.G.; Burgio, G.; Arpaia, S.; Narváez-Vasquez, C.A.; González-Cabrera, J.; Ruescas, D.C.; Tabone, E.; Frandon, J.; et al. Biological invasion of European tomato crops by Tuta absoluta: Ecology, geographic expansion and prospects for biological control. J. Pest. Sci. 2010, 83, 197–215. [Google Scholar] [CrossRef]
- Huang, N.X.; Jaworski, C.C.; Desneux, N.; Zhang, F.; Yang, P.Y.; Wang, S. Long-term and large-scale releases of Trichogramma promote pesticide decrease in maize in northeastern China. Entomol. Gen. 2020, 40, 331–335. [Google Scholar] [CrossRef]
- Zang, L.S.; Wang, S.; Zhang, F.; Desneux, N. Biological control with Trichogramma in China: History, present status and perspectives. Annu. Rev. Entomol. 2021, 66, 463–484. [Google Scholar] [CrossRef]
- Chan, M.S.; Godfray, H.C.J. Host-feeding strategies of parasitoid wasps. Evol. Ecol. 1993, 7, 593–604. [Google Scholar] [CrossRef]
- Pennacchio, F.; Strand, M.R. Evolution of developmental strategies in parasitic Hymenoptera. Annu. Rev. Entomol. 2006, 49, 233–258. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.J.; Bauer, L.S.; Abell, K.J.; Lelito, J.P.; Van Driesche, R. Establishment and abundance of Tetrastichus planipennisi (Hymenoptera: Eulophidae) in Michigan: Potential for success in classical biocontrol of the invasive emerald ash borer (Coleoptera: Buprestidae). J. Econ. Entomol. 2013, 106, 1145–1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.H.; Che, P.F.; Yang, X.B.; Song, L.W.; Zhang, C.R.; Benelli, G.; Desneux, N.; Zang, L.S. Optimized pupal age of Tenebrio molitor L. (Coleoptera: Tenebrionidae) enhanced mass rearing efficiency of Chouioia cunea Yang (Hymenoptera: Eulophidae). Sci. Rep. 2019, 9, 3229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morais, W.C.C.; Plata-Rueda, A.; Martínez, L.C.; Zanuncio, A.J.V.; Fernandes, F.L.; Wilcken, C.F.; Zanuncio, J.C.; Serrão, J.E. Potential of Diaphania hyalinata and Tenebrio molitor as alternative host for mass rearing of Palmistichus elaeisis (Hymenoptera: Eulophidae). Entomol. Gen. 2019, 39, 285–294. [Google Scholar] [CrossRef]
- Voegele, J.M. Biological control of Brontispa longissima in Western Samoa: An ecological and economic evaluation. Agric. Ecosyst. Environ. 1989, 27, 315–329. [Google Scholar] [CrossRef]
- Yao, Y.X. Taxonomic Study on Chalcidoids Parasitizing Forest Defoliators in China (Hymenoptera). Ph.D. Thesis, Chinese Academy of Forestry Sciences, Beijing, China, 2005. (In Chinese). [Google Scholar]
- Yang, Z.Q.; Yao, Y.X.; Cao, L.M. Chalcidoidea Parasitizing Forest Defoliators (Hymenoptera); Science Press: Beijing, China, 2015; p. 283. (In Chinese) [Google Scholar]
- Jacas, J.A.; Peña, J.E.; Duncan, R.E. Successful oviposition and reproductive biology of Aprostocetus vaquitarum (Hymenoptera: Eulophidae): A predator of Diaprepes abbreviatus (Coleoptera: Curculionidae). Biol. Control 2005, 33, 352–359. [Google Scholar] [CrossRef]
- Ulmer, B.J.; Jacas, J.A.; Peña, J.E.; Duncan, R.E.; Castillo, J. Effect of temperature on life history of Aprostocetus vaquitarum (Hymenoptera: Eulophidae), an egg parasitoid of Diaprepes abbreviatus (Coleoptera: Curculionidae). Biol. Control 2006, 39, 19–25. [Google Scholar] [CrossRef]
- Li, J.Q.; Yang, Y.; Wang, S.X.; Feng, H.C.; Huang, D.Z.; Jin, Y.J. Host selection and location behavior of Aprostocetus prolixus LaSalle et Huang (Hymenoptera: Eulophidae), an egg parasitoid of Apriona germari (Hope) (Coleoptera: Cerambycidae). Acta Entomol. Sin. 2007, 50, 1122–1128. (In Chinese) [Google Scholar]
- Nacro, S.; Nénon, J.P. Female reproductive biology of Platygaster diplosisae (Hymenoptera: Platygastridae) and Aprostocetus procerae (Hymenoptera: Eulophidae), two parasitoids associated with the African rice gall midge, Orseolia oryzivora (Diptera: Cecidomyiidae). Entomol. Sci. 2008, 11, 231–237. [Google Scholar] [CrossRef]
- Ouattara, D.; Nacro, S.; Latévi, K.; Coulibaly, A. Ecology of Platygaster Diplosisae (Hymenoptera: Platygasteridae) and Aprostocetus Procerae (Hymenoptera: Eulophidae), parasitoids of Orseolia Oryzivora (Diptera: Cecidomyiidae). Int. J. Curr. Adv. Res. 2019, 8, 20482–20487. [Google Scholar]
- Sampson, B.J.; Roubos, C.R.; Stringer, S.J.; Marshall, D.; Liburd, O.E. Biology and efficacy of Aprostocetus (Eulophidae: Hymenoptera) as a parasitoid of the blueberry gall midge complex: Dasineura oxycoccana and Prodiplosis vaccinii (Diptera: Cecidomyiidae). J. Econ. Entomol. 2013, 106, 73–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tee, H.S.; Saad, A.R.; Lee, C.Y. Suitability of heat- and freeze-killed oothecae of the American Cockroach (Dictyoptera: Blattidae) as hosts for an Oothecal parasitoid, Aprostocetus hagenowii (Hymenoptera: Eulophidae). J. Econ. Entomol. 2010, 103, 1770–1774. [Google Scholar] [CrossRef]
- Liu, P.C.; Men, J.; Zhao, B.; Wei, J.R. Fitness-related offspring sex allocation of Anastatus disparis, a gypsy moth egg parasitoid, on different-sized host species. Entomol. Exp. Appl. 2017, 163, 281–286. [Google Scholar] [CrossRef]
- Li, T.H.; Tian, C.Y.; Zang, L.S.; Hou, Y.Y.; Ruan, C.C.; Yang, X.B.; Lucie, M.; Desneux, N. Multiparasitism with Trichogramma dendrolimi on egg of Chinese oak silkworm, Antheraea pernyi, enhances emergence of Trichogramma ostriniae. J. Pest. Sci. 2019, 92, 707–713. [Google Scholar] [CrossRef]
- Li, X.-B.Y.; Lei, Q.; Hua, H.Q.; Song, H.F.; Wang, S.; Ramirez-Romero, R.; Dai, H.J.; Li, J.T.; Li, Y.X. Impact of host suitability on oviposition preference toward fertilized and unfertilized host eggs in two Trichogramma parasitoid species. Entomol. Gen. 2019, 39, 313–323. [Google Scholar] [CrossRef]
- Iqbal, A.; Chen, Y.M.; Hou, Y.Y.; Zhang, L.S.; Desneux, N.; Zang, L.S. Factitious host species impact on the outcome of multiparasitism between egg parasitoids. J. Pest. Sci. 2019, 92, 1261–1269. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, Z.P.; Hou, Y.Y.; Yang, X.B.; Wang, S.; Dai, H.J.; Xu, Y.Y.; Zang, L.S. Manually-extracted unfertilized eggs of Chinese oak silkworm, Antheraea pernyi, enhance mass production of Trichogramma parasitoids. Entomol. Gen. 2020, 40, 397–406. [Google Scholar] [CrossRef]
- Krugner, R. Suitability of non-fertilized eggs of Homalodisca vitripennis for the egg parasitoid Gonatocerus morrilli. BioControl 2014, 59, 167–174. [Google Scholar] [CrossRef]
- Xu, J.; Yang, X.B.; Lin, Y.; Zang, L.S.; Tian, C.Y.; Ruan, C.C. Effect of fertilized, unfertilized, and UV-irradiated hosts on parasitism and suitability for Trichogramma parasitoids. Entomol. Exp. Appl. 2016, 161, 50–56. [Google Scholar] [CrossRef]
- Yang, X.; Qu, Y.L.; Wu, Z.Y.; Lin, Y.; Ruan, C.C.; Desneux, N.; Zang, L.S. Parasitism and suitability of fertilized and nonfertilized eggs of the rice striped stem borer, Chilo suppressalis (Lepidoptera: Crambidae), for Trichogramma parasitoids. J. Econ. Entomol. 2016, 109, 1524–1528. [Google Scholar] [CrossRef]
- Du, W.M.; Xu, J.; Hou, Y.Y.; Lin, Y.; Zang, L.S.; Yang, X.B.; Zhang, J.J.; Ruan, C.C.; Desneux, N. Trichogramma parasitoids can distinguish between fertilized and unfertilized host eggs. J. Pest. Sci. 2018, 91, 771–780. [Google Scholar] [CrossRef]
- Yang, S.Y.; Zhan, H.X.; Zhang, F.; Dirk, B.; Zhong, Y.Z.; Lou, Q.Z.; Zhong, Y.; Zhang, J.P. Development and fecundity of Trissolcus japonicus on fertilized and unfertilized eggs of the brown marmorated stink bug, Halyomorpha halys. J. Pest. Sci. 2018, 91, 1–9. [Google Scholar] [CrossRef]
- Bjorksten, T.A.; Hoffmann, A.A. Plant cues influence searching behaviour and parasitism in the egg parasitoid Trichogramma nr. brassicae. Ecol. Entomol. 1998, 23, 355–362. [Google Scholar] [CrossRef]
- DeLury, N.C.; Gries, R.; Gries, G.; Judd, G.J.R.; Khaskin, G. Moth scale-derived kairomones used by egg-larval parasitoid Ascogaster quadridentatato locate eggs of its host, Cydia pomonella. J. Chem. Ecol. 1999, 25, 2419–2431. [Google Scholar] [CrossRef]
- Meiners, T.; Westerhaus, C.; Hilker, M. Specificity of chemical cues used by a specialist egg parasitoid during host location. Entomol. Exp. Appl. 2000, 95, 151–159. [Google Scholar] [CrossRef]
- Boyle, S.M.; Weber, D.C.; Hough-Goldstein, J.; Hoelmer, K.A. Host kairomones influence searching behavior of Trissolcus japonicus (Hymenoptera: Scelionidae), a parasitoid of Halyomorpha halys (Heteroptera: Pentatomidae). Environ. Entomol. 2020, 49, 15–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pak, G.A.; van Dalen, A.; Kaashoek, N.; Dijkman, H. Host egg chorion structure influencing host suitability for the egg parasitoid Trichogramma Westwood. J. Insect Physiol. 1990, 36, 869–875. [Google Scholar] [CrossRef]
- Karamaouna, F.; Copland, M.J.W. Host suitability, quality and host size preference of Leptomastix epona and Pseudaphycus flavidulus, two endoparasitoids of the mealybug Pseudococcus viburni, and host size effect on parasitoid sex ratio and clutch size. Entomol. Exp. Appl. 2000, 96, 149–158. [Google Scholar] [CrossRef]
- Godfray, H.C.J. Parasitoids. Behavior and Evolutionary Ecology; Princeton University Press: Princeton, NJ, USA, 1994. [Google Scholar]
- Desneux, N.; Barta, R.J.; Hoelmer, K.A.; Hopper, K.R.; Heimpel, G.E. Multifaceted determinants of host specificity in an aphid parasitoid. Oecologia 2009, 160, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Biondi, A.; Desneux, N.; Amiens-Desneux, E.; Siscaro, G.; Zappalà, L. Biology and developmental strategies of the palaearctic parasitoid Bracon nigricans (Hymenoptera: Braconidae) on the neotropical moth Tuta absoluta (Lepidoptera: Gelechiidae). J. Econ. Entomol. 2013, 106, 1638–1647. [Google Scholar] [CrossRef]
- Monticelli, L.S.; Nguyen, L.T.; Amiens-Desneux, E.; Luo, C.; Lavoir, A.V.; Gatti, J.L.; Desneux, N. The preference-performance relationship as a means of classifying parasitoids according to their specialization degree. Evol. Appl. 2019, 12, 1626–1640. [Google Scholar] [CrossRef]
- Van Alphen, J.J.M.; Visser, M.E. Superparasitism as an adaptive strategy for insect parasitoids. Annu. Rev. Entomol. 1990, 35, 59–79. [Google Scholar] [CrossRef]
- Gu, H.; Wang, Q.; Dorn, S. Superparasitism in Cotesia glomerata: Response of hosts and consequences for parasitoids. Ecol. Entomol. 2003, 28, 422–431. [Google Scholar] [CrossRef]
- King, B.H. Offspring sex ratio and number in response to proportion of host sizes and ages in the parasitoid wasp Spalangia cameroni (Hymenoptera: Pteromalidae). Environ. Entomol. 2002, 31, 505–508. [Google Scholar] [CrossRef] [Green Version]
- Visser, M.E. The importance of being large: The relationship between size and fitness in females of the parasitoid Aphaereta minuta (Hymenoptera: Brachonidae). J. Anim. Ecol. 1994, 63, 963–978. [Google Scholar] [CrossRef]
- West, S.A.; Flanagan, K.E.; Godfray, H.C.J. The relationship between parasitoid size and fitness in the field, a study of Achrysocharoides zwoelferi (Hymenoptera: Eulophidae). J. Anim. Ecol. 1996, 65, 631–639. [Google Scholar] [CrossRef]
- Ellers, J.; Van Alphen, J.J.M.; Sevenster, J.G. A field study of size-fitness relationships in the parasitoid Asobara tabida. J. Anim. Ecol. 1998, 67, 318–324. [Google Scholar] [CrossRef]
- Durocher-Granger, L.; Martel, V.; Boivin, G. Gamete number and size correlate with adult size in the egg parasitoid Trichogramma euproctidis. Entomol. Exp. Appl. 2011, 140, 262–268. [Google Scholar] [CrossRef]
- Yanagi, S.; Tuda, M. Female size constrains egg size via the influence of reproductive organ size and resource storage in the seed beetle Callosobruchus chinensis. J. Insect Physiol. 2012, 58, 1432–1437. [Google Scholar] [CrossRef]
- Wang, J.J.; Liu, X.B.; Zhang, Y.A.; Wen, C.; Wei, J.R. The reproductive capability of Ooencyrtus kuvanae reared on eggs of the factitious host Antheraea pernyi. J. Appl. Entomol. 2014, 138, 267–272. [Google Scholar] [CrossRef]
- Drost, Y.C.; Cardé, R.T. Influence of host deprivation on egg load and oviposition behaviour of Brachymeria intermedia, a parasitoid of gypsy moth. Physiol. Entomol. 1992, 17, 230–234. [Google Scholar] [CrossRef]
- Li, X.W.; Zhu, L.T.; Meng, L.; Li, B.P. Brood size and sex ratio in response to host quality and wasp traits in the gregarious parasitoid Oomyzus sokolowskii (Hymenoptera: Eulophidae). PeerJ 2017, 5, e2919. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).