Assessing the Potential of Inoculative Field Releases of Telenomus remus to Control Spodoptera frugiperda in Ghana
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
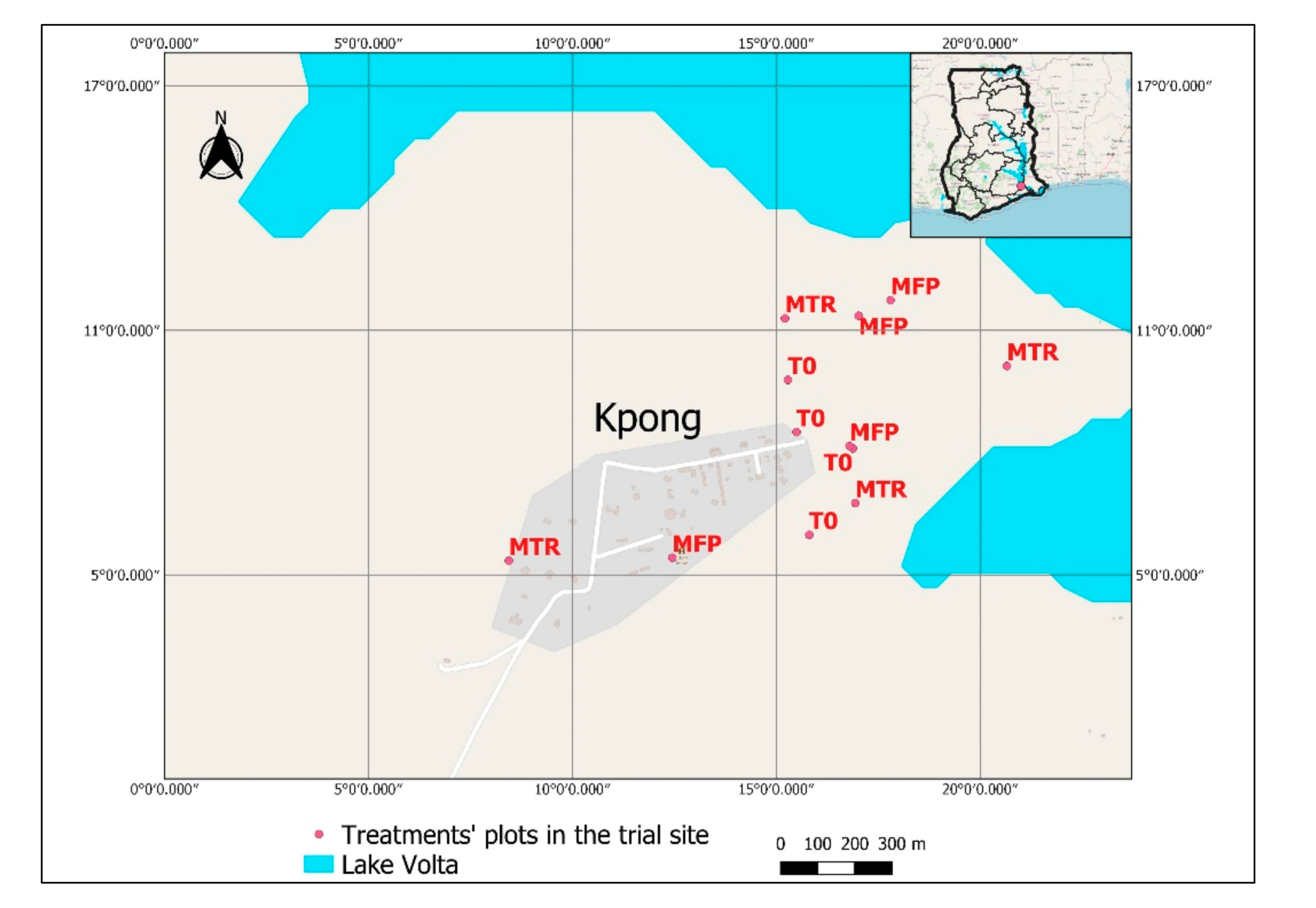
2.1. Study Site
2.2. Experimental Design
2.3. General Crop Management
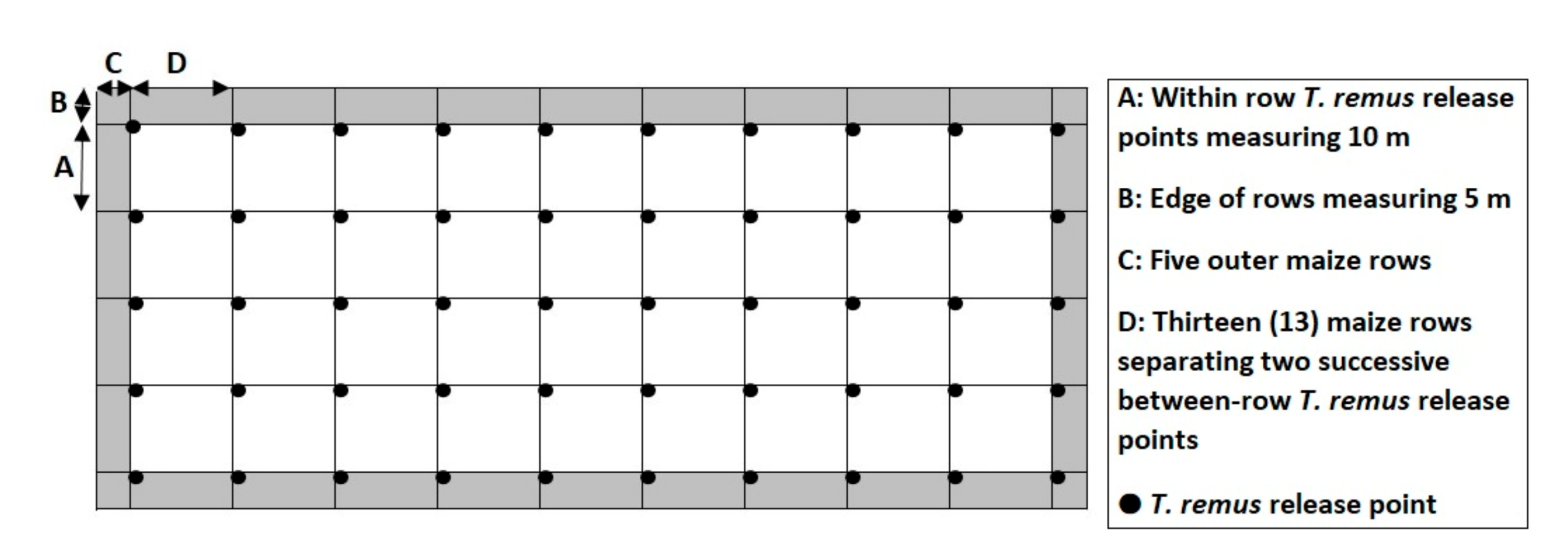
2.4. Telenomus Remus Releases
- -
- Non-sealed letter envelopes containing approximately 300 parasitized eggs were fastened on maize plants (or on sticks when the maize plants were less than three weeks old). They were spaced every 13th maize row (13 × 0.8 m = 10.4 m), without considering the 5 outer rows at both sides of the plot;
- -
- Within the maize row, the envelopes containing the ≈300 parasitized eggs were set up every 10 m, leaving 5 m at the ends of the rows.
2.5. Data Collection
2.6. Statistical Analysis
3. Results
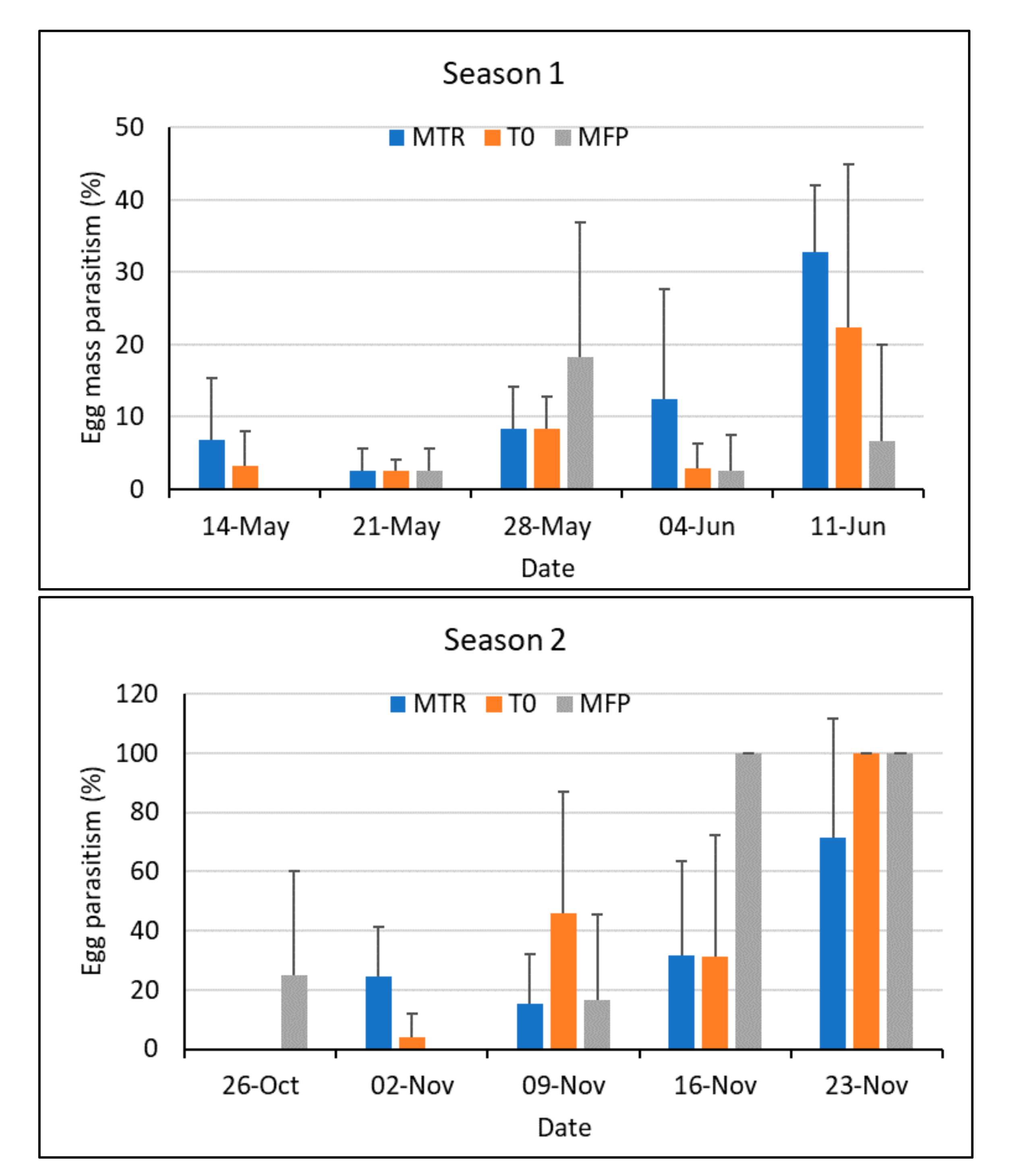
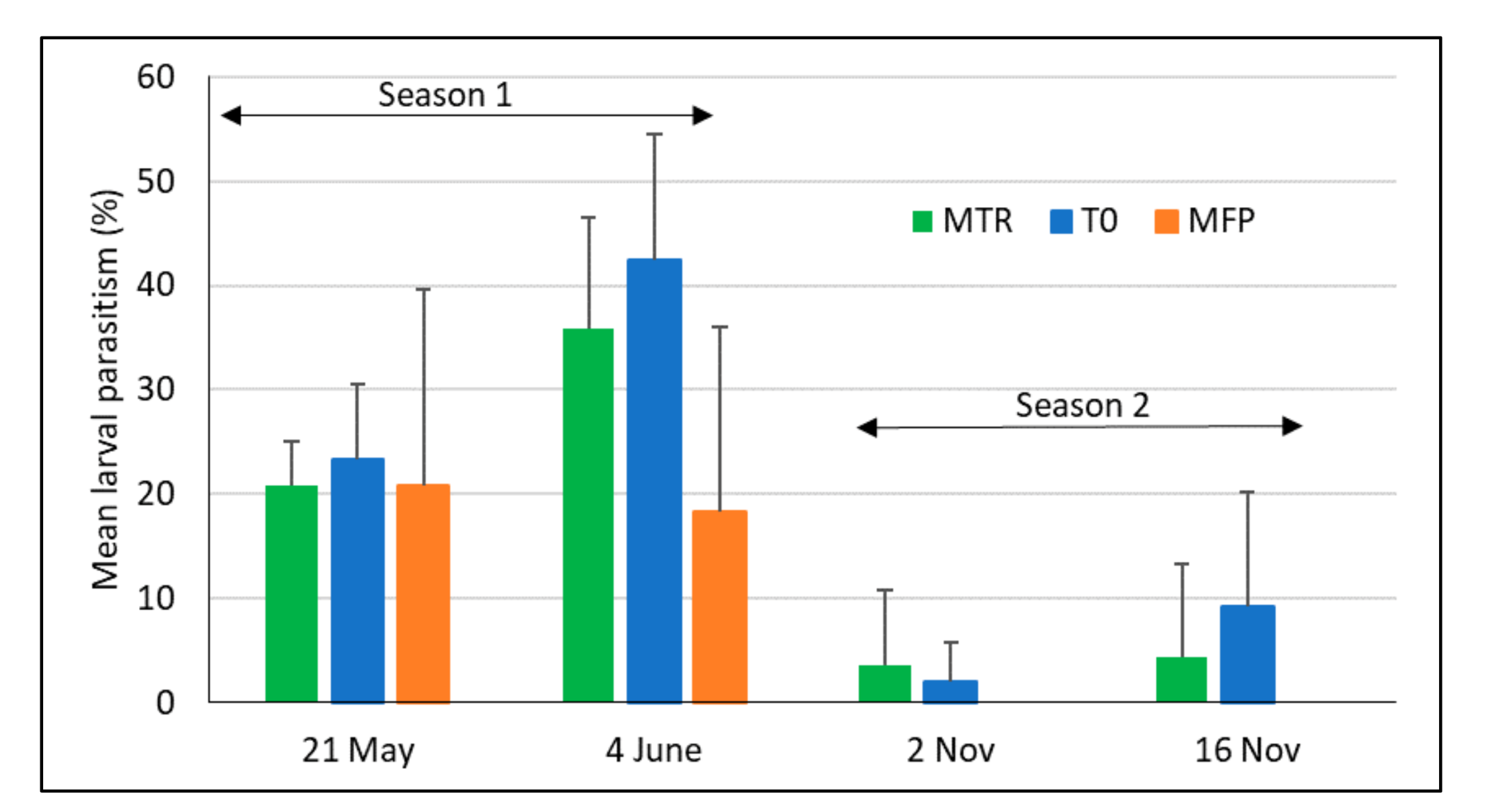
3.1. Parasitism Rate by T. remus during the First and Second Rainy Seasons
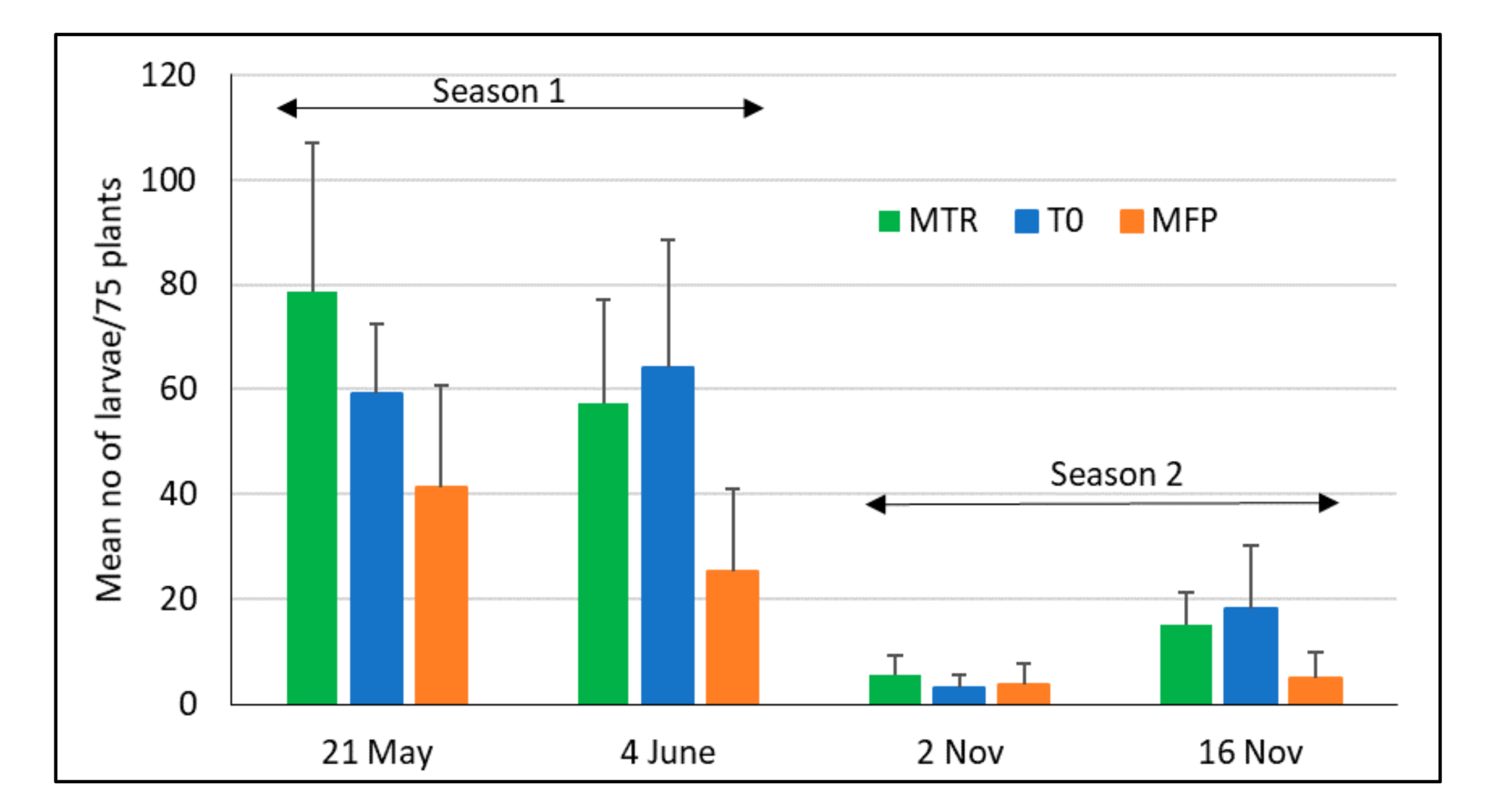
3.2. FAW Damage and Larval Density under Different Treatments
3.3. Seasonal Abundance of Larval Parasitoids and Their Parasitism Rate during the Trials
3.4. FAW Damage on Maize Tassels and Cobs, and Yield under Different Treatments
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goergen, G.; Kumar, P.L.; Sankung, S.B.; Togola, A.; Tamò, M. First report of outbreaks of the fall armyworm Spodoptera frugiperda (J E Smith) (Lepidoptera, Noctuidae), a new alien invasive pest in west and central africa. PLoS ONE 2016, 11, e0165632. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.agric.wa.gov.au/fall-armyworm-western-australia (accessed on 24 May 2021).
- Day, R.; Abrahams, P.; Bateman, M.; Beale, T.; Clottey, V.; Cock, M.; Colmenarez, Y.; Corniani, N.; Early, R.; Godwin, J.; et al. Fall Armyworm: Impacts and Implications for Africa. Outlooks Pest Manag. 2017, 28, 196–201. [Google Scholar] [CrossRef]
- Nagoshi, R.N.; Ni Htain, N.; Boughton, D.; Zhang, L.; Xiao, Y.; Nagoshi, B.Y.; Mota-Sanchez, D. Southeastern Asia fall armyworms are closely related to populations in Africa and India, consistent with common origin and recent migration. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Kansiime, M.K.; Mugambi, I.; Rwomushana, I.; Nunda, W.; Lamontagne-Godwin, J.; Rware, H.; Phiri, N.A.; Chipabika, G.; Ndlovu, M.; Day, R. Farmer perception of fall armyworm (Spodoptera frugiderda J.E. Smith) and farm-level management practices in Zambia. Pest Manag. Sci. 2019, 75, 2840–2850. [Google Scholar] [CrossRef]
- Kenis, M.; Du Plessis, H.; Berg, J.V.D.; Ba, M.N.; Goergen, G.; Kwadjo, K.E.; Baoua, I.; Tefera, T.; Buddie, A.; Cafà, G.; et al. Telenomus remus, a candidate parasitoid for the biological control of Spodoptera frugiperda in Africa, is already Present on the Continent. Insects 2019, 10, 92. [Google Scholar] [CrossRef]
- Harrison, R.D.; Thierfelder, C.; Baudron, F.; Chinwada, P.; Midega, C.; Schaffner, U.; Berg, J.V.D. Agro-ecological options for fall armyworm (Spodoptera frugiperda JE Smith) management: Providing low-cost, smallholder friendly solutions to an invasive pest. J. Environ. Manag. 2019, 243, 318–330. [Google Scholar] [CrossRef]
- Murúa, M.G.; Molina-Ochoa, J.; Fidalgo, P. Natural Distribution of Parasitoids of Larvae of the Fall Armyworm, Spodoptera frugiperda, in Argentina. J. Insect Sci. 2009, 9, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Rios-Velasco, C.; Gallegos-Morales, G.; Cambero-Campos, J.; Cerna-Chávez, E.; Del Rincón-Castro, M.C.; Valenzuela-García, R. Natural Enemies of the Fall Armyworm Spodoptera frugiperda (Lepidoptera: Noctuidae) in Coahuila, México. Fla. Èntomol. 2011, 94, 723–726. [Google Scholar] [CrossRef]
- Wang, W.; He, P.; Zhang, Y.; Liu, T.; Jing, X.; Zhang, S. The Population Growth of Spodoptera frugiperda on Six Cash Crop Species and Implications for Its Occurrence and Damage Potential in China. Insects 2020, 11, 639. [Google Scholar] [CrossRef] [PubMed]
- Sisay, B.; Simiyu, J.; Mendesil, E.; Likhayo, P.; Ayalew, G.; Mohamed, S.; Subramanian, S.; Tefera, T. Fall Armyworm, Spodoptera frugiperda Infestations in East Africa: Assessment of Damage and Parasitism. Insects 2019, 10, 195. [Google Scholar] [CrossRef]
- Agboyi, L.K.; Goergen, G.; Beseh, P.; Mensah, S.A.; Clottey, V.A.; Glikpo, R.; Buddie, A.; Cafà, G.; Offord, L.; Day, R.; et al. Parasitoid Complex of Fall Armyworm, Spodoptera frugiperda, in Ghana and Benin. Insects 2020, 11, 68. [Google Scholar] [CrossRef]
- Agboyi, L.K.; Mensah, S.A.; Clottey, V.A.; Beseh, P.; Glikpo, R.; Rwomushana, I.; Day, R.; Kenis, M. Evidence of Leaf Consumption Rate Decrease in Fall Armyworm, Spodoptera frugiperda, Larvae Parasitized by Coccygidium luteum. Insects 2019, 10, 410. [Google Scholar] [CrossRef] [PubMed]
- Durocher-Granger, L.; Mfune, T.; Musesha, M.; Lowry, A.; Reynolds, K.; Buddie, A.; Cafà, G.; Offord, L.; Chipabika, G.; Dicke, M.; et al. Factors influencing the occurrence of fall armyworm parasitoids in Zambia. J. Pest Sci. 2020, 1–14. [Google Scholar] [CrossRef]
- De Clercq, P.; Mason, P.G.; Babendreier, D. Benefits and risks of exotic biological control agents. BioControl 2011, 56, 681–698. [Google Scholar] [CrossRef]
- Cave, R.D. Biology, ecology and use in pest management of Telenomus remus. Biocontrol News Inf. 2000, 21, 21–26. [Google Scholar]
- Gutierrez-Martinez, A.; Tolón-Becerra, A.; Lastra-Bravo, X.B. Biological control of spodoptera frugiperda eggs using Telenomus remus nixon in maize-bean-squash polyculture. Am. J. Agric. Biol. Sci. 2012, 7, 285–292. [Google Scholar] [CrossRef]
- Figueiredo, M.; Lucia, T.; Cruz, I. Effect of Telenomus remus Nixon (Hymenoptera: Scelionidae) Density on Control of Spodoptera frugiperda (Smith) (Lepidoptera: Noctuidae) egg masses upon release in a maize Field. Rev. Bras. Milho Sorgo 2002, 1, 12–19. [Google Scholar] [CrossRef]
- Salazar-Mendoza, P.; Rodriguez-Saona, C.; Fernandes, O.A. Release density, dispersal capacity, and optimal rearing conditions for Telenomus remus, an egg parasitoid of Spodoptera frugiperda, in maize. Biocontrol Sci. Technol. 2020, 30, 1040–1059. [Google Scholar] [CrossRef]
- Ferrer, F. Biological control of agricultural insect pests in Venezuela; advances, achievements, and future perspectives. Biocontrol News Inf. 2001, 22, 67–74. [Google Scholar]
- Laminou, S.A.; Ba, M.N.; Karimoune, L.; Doumma, A.; Muniappan, R. Parasitism of locally recruited egg parasitoids of the fall armyworm in Africa. Insects 2020, 11, 430. [Google Scholar] [CrossRef]
- Fening, K.O.; Forchibe, E.E.; Afreh-Nuamah, K. Neem as a cost-effective and potent biopesticide against the diamondback moth Plutella xylostella L. (Lepidoptera: Plutellidae) and the cabbage webworm Hellula undalis F. (Lepidoptera: Crambidae). West Afr. J. Appl. Ecol. 2020, 28, 52–63. [Google Scholar]
- Nkrumah, F.; Klutse, N.A.B.; Adukpo, D.C.; Owusu, K.; Quagraine, K.A.; Owusu, A.; Gutowski, W., Jr. Rainfall variability over Ghana: Model versus rain gauge observation. Int. J. Geosci. 2014, 5, 673–683. [Google Scholar] [CrossRef]
- Fening, K.O.; MacCarthy, D.S.; Tegbe, R.E. The Effect of intercropping and soil amendment on the population dynamics of pests and natural enemies of white cabbage. West Afr. J. Appl. Ecol. 2020, 28, 96–112. [Google Scholar]
- Davis, F.M.; Ng, S.S.; Williams, W.P. Visual rating scales for screening whorl-stage corn for resistance to fall armyworm. Miss Agric. For. Exp. Stn. Tech. Bull. 1992, 186, 1–9. [Google Scholar]
- Prasanna, B.M.; Huesing, J.E.; Eddy, R.; Peschke, V.M. Fall Armyworm in Africa: A Guide for Integrated Pest Management, 3rd ed.; CIMMYT: Mexico City, Mexico, 2018; pp. 11–106. [Google Scholar]
- Liao, Y.-L.; Yang, B.; Xu, M.-F.; Lin, W.; Wang, D.-S.; Chen, K.-W.; Chen, H.-Y. First report of Telenomus remus parasitizing Spodoptera frugiperda and its field parasitism in southern China. J. Hymenopt. Res. 2019, 73, 95–102. [Google Scholar] [CrossRef]
- Tefera, T.; Goftishu, M.; Ba, M.; Muniappan, R. A Guide to Biological Control of Fall Armyworm in Africa Using Egg Parasitoids, 1st ed.; ICIPE: Nairobi, Kenya, 2019. [Google Scholar]
- Elibariki, N.; Bajracharya, A.S.R.; Bhat, B.; Tefera, T.; Mottern, J.L.; Evans, G.; Muniappan, R.; Yubak, D.G.; Pallangyo, B.; Likhayo, P. Candidates for augmentative biological control of Spodoptera frugiperda (J E smith) in Kenya, Tanzania and Nepal. Indian J. Èntomol. 2020, 82, 606–608. [Google Scholar] [CrossRef]
- Pomari-Fernandes, A.; Bueno, A.D.F.; De Bortoli, S.A.; Favetti, B.M. Dispersal capacity of the egg parasitoid Telenomus remus Nixon (Hymenoptera: Platygastridae) in maize and soybean crops. Biol. Control 2018, 126, 158–168. [Google Scholar] [CrossRef]
- Hernández, D.; Ferrer, F.; Linares, B. Introducion de Telenomus remus Nixon (Hym.: Scelionidae) para controlar Spodoptera frugiperda (Lep.: Noctuidae) en Yaritagua-Venezuela. Agron. Trop. 1989, 39, 199–205. [Google Scholar]
- Landis, D.A.; Wratten, S.; Gurr, G. Habitat Management to Conserve Natural Enemies of Arthropod Pests in Agriculture. Annu. Rev. Èntomol. 2000, 45, 175–201. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, M.L.C.; Cruz, I.; Della Lucia, T.M.C. Controle integrado de Spodoptera frugiperda (Smith and Abbott) utilizando-se o parasitóide Telenomus remus Nixon. Pesqui. Agropecu. Bras. 1999, 34, 1975–1982. [Google Scholar] [CrossRef]
- Gao, S.; Tang, Y.; Wei, K.; Wang, X.; Yang, Z.; Zhang, Y. Relationships between Body Size and Parasitic Fitness and Offspring Performance of Sclerodermus pupariae Yang et Yao (Hymenoptera: Bethylidae). PLoS ONE 2016, 11, e0156831. [Google Scholar] [CrossRef]
- West, S.; Flanagan, K.; Godfray, H. The Relationship between Parasitoid Size and Fitness in the Field, a Study of Achrysocharoides zwoelferi (Hymenoptera: Eulophidae). J. Anim. Ecol. 1996, 65, 631. [Google Scholar] [CrossRef]
- Sisay, B.; Simiyu, J.; Malusi, P.; Likhayo, P.; Mendesil, E.; Elibariki, N.; Wakgari, M.; Ayalew, G.; Tefera, T. First report of the fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae), natural enemies from Africa. J. Appl. Èntomol. 2018, 142, 800–804. [Google Scholar] [CrossRef]
- Otim, M.; Aropet, S.A.; Opio, M.; Kanyesigye, D.; Opolot, H.N.; Tay, W.T. Parasitoid Distribution and Parasitism of the Fall Armyworm Spodoptera frugiperda (Lepidoptera: Noctuidae) in Different Maize Producing Regions of Uganda. Insects 2021, 12, 121. [Google Scholar] [CrossRef]
- Earl, S.L. Competitive interactions between Chelonus insularis Cresson and Telenomus remus Nixon, Two Parasitoids of Spodoptera exigua Hubner. Master’s Thesis, University of Arizona, Tucson, AZ, USA, 1983; 59p. [Google Scholar]
- Liu, Y.; Li, X.; Zhou, C.; Liu, F.; Mu, W. Toxicity of nine insecticides on four natural enemies of Spodoptera exigua. Sci. Rep. 2016, 6, 39060. [Google Scholar] [CrossRef] [PubMed]
- Tambo, J.A.; Day, R.K.; Lamontagne-Godwin, J.; Silvestri, S.; Beseh, P.K.; Oppong-Mensah, B.; Phiri, N.A.; Matimelo, M. Tackling fall armyworm (Spodoptera frugiperda) outbreak in Africa: An analysis of farmers’ control actions. Int. J. Pest Manag. 2020, 66, 298–310. [Google Scholar] [CrossRef]
- Tang, S.; Tang, G.; Cheke, R.A. Optimum timing for integrated pest management: Modelling rates of pesticide application and natural enemy releases. J. Theor. Biol. 2010, 264, 623–638. [Google Scholar] [CrossRef]
- Baudron, F.; Zaman-Allah, M.A.; Chaipa, I.; Chari, N.; Chinwada, P. Understanding the factors influencing fall armyworm (Spodoptera frugiperda J.E. Smith) damage in African smallholder maize fields and quantifying its impact on yield. A case study in Eastern Zimbabwe. Crop Prot. 2019, 120, 141–150. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, D.P.; Tiwana, U.S. Forage yield compensation in maize with differential seed rates against insect herbivory of Chilo partellus (Swinhoe.). Range Manag. Agrofor. 2020, 41, 81–86. [Google Scholar]
- Liu, G.; Yang, Y.; Liu, W.; Guo, X.; Xue, J.; Xie, R.; Ming, B.; Wang, K.; Hou, P.; Li, S. Leaf Removal Affects Maize Morphology and Grain Yield. Agronomy 2020, 10, 269. [Google Scholar] [CrossRef]



| Season | Species (Order: Family) | Host Stage Attacked/Killed | Relative Abundance (%) * n1 = 184; ** n2 = 22 |
|---|---|---|---|
| Major rainy season | Chelonus bifoveolatus Szépligeti (Hym.: Braconidae) | Egg–larval | 63.04 |
| Chelonus cf. curvimaculatus Cameron | 0.54 | ||
| Coccygidium luteum (Brullé) (Hym.: Braconidae) | Larval | 20.65 | |
| Charops sp. (Hym.: Ichneumonidae) | 5.98 | ||
| Cotesia icipe Fernandez-Triana and Fiaboe (Hym.: Braconidae) | Larval | 3.26 | |
| Undetermined specimens | Larval | 7.07 | |
| Minor rainy season | Chelonus bifoveolatus | Egg–larval | 31.82 |
| Chelonus cf. curvimaculatus | Egg–larval | 9.09 | |
| Coccygidium luteum | Larval | 22.73 | |
| Charops sp. | Larval | 31.82 | |
| Undetermined specimens | Larval | 4.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agboyi, L.K.; Layodé, B.F.R.; Fening, K.O.; Beseh, P.; Clottey, V.A.; Day, R.; Kenis, M.; Babendreier, D. Assessing the Potential of Inoculative Field Releases of Telenomus remus to Control Spodoptera frugiperda in Ghana. Insects 2021, 12, 665. https://doi.org/10.3390/insects12080665
Agboyi LK, Layodé BFR, Fening KO, Beseh P, Clottey VA, Day R, Kenis M, Babendreier D. Assessing the Potential of Inoculative Field Releases of Telenomus remus to Control Spodoptera frugiperda in Ghana. Insects. 2021; 12(8):665. https://doi.org/10.3390/insects12080665
Chicago/Turabian StyleAgboyi, Lakpo Koku, Babatoundé Ferdinand Rodolphe Layodé, Ken Okwae Fening, Patrick Beseh, Victor Attuquaye Clottey, Roger Day, Marc Kenis, and Dirk Babendreier. 2021. "Assessing the Potential of Inoculative Field Releases of Telenomus remus to Control Spodoptera frugiperda in Ghana" Insects 12, no. 8: 665. https://doi.org/10.3390/insects12080665
APA StyleAgboyi, L. K., Layodé, B. F. R., Fening, K. O., Beseh, P., Clottey, V. A., Day, R., Kenis, M., & Babendreier, D. (2021). Assessing the Potential of Inoculative Field Releases of Telenomus remus to Control Spodoptera frugiperda in Ghana. Insects, 12(8), 665. https://doi.org/10.3390/insects12080665






