Multiple Introductions of the Pestiferous Land Snail Theba pisana (Müller, 1774) (Gastropoda: Helicidae) in Southern California
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Collection
2.2. Specimen Preparation and Morphological Examination
2.3. Generation of Sequence Data
2.4. Sequence Comparison, Analysis, and Phylogenetic Inference
3. Results
3.1. Shell Morphology
3.2. Radular Morphology
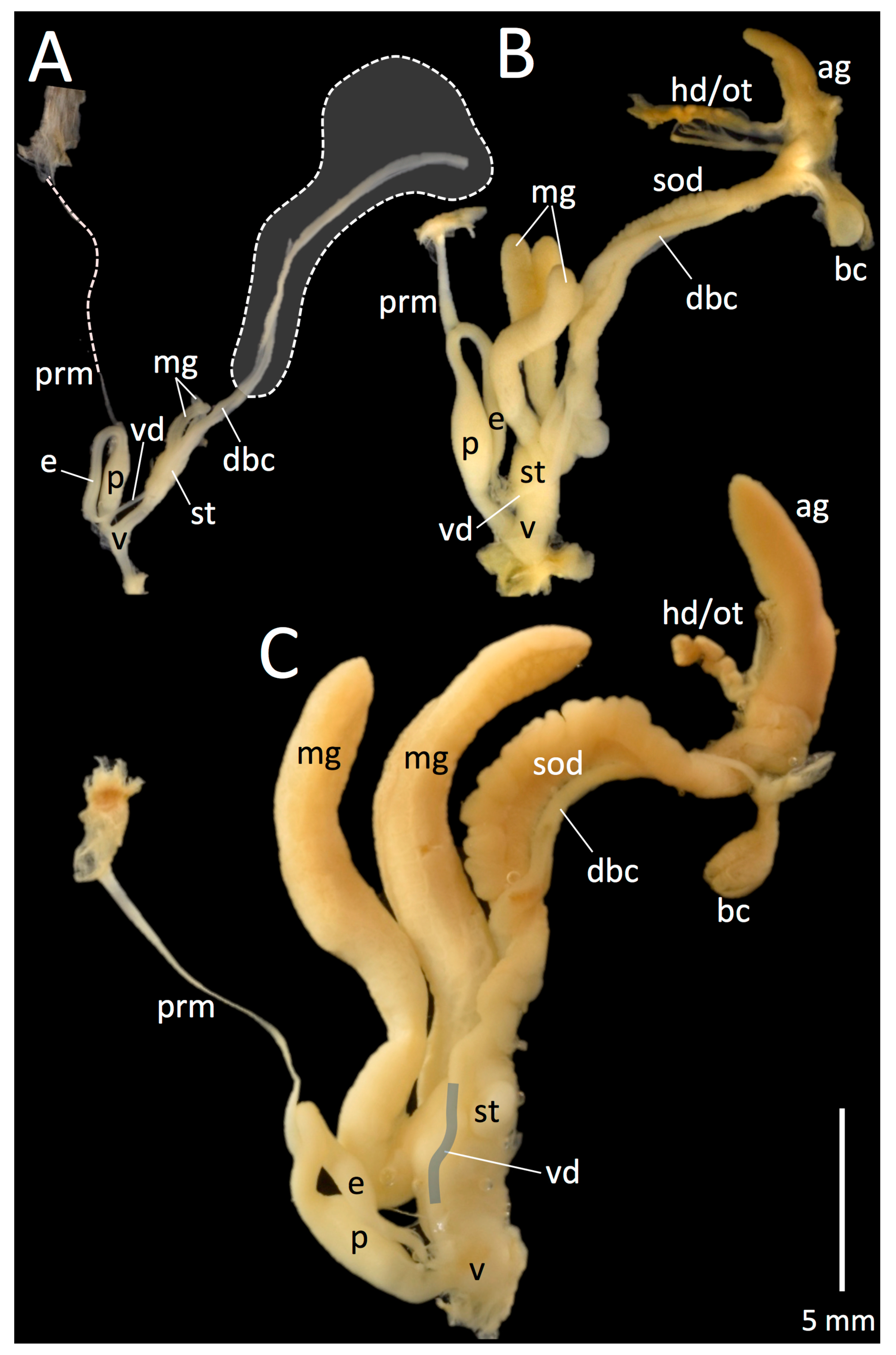
3.3. Reproductive Anatomy
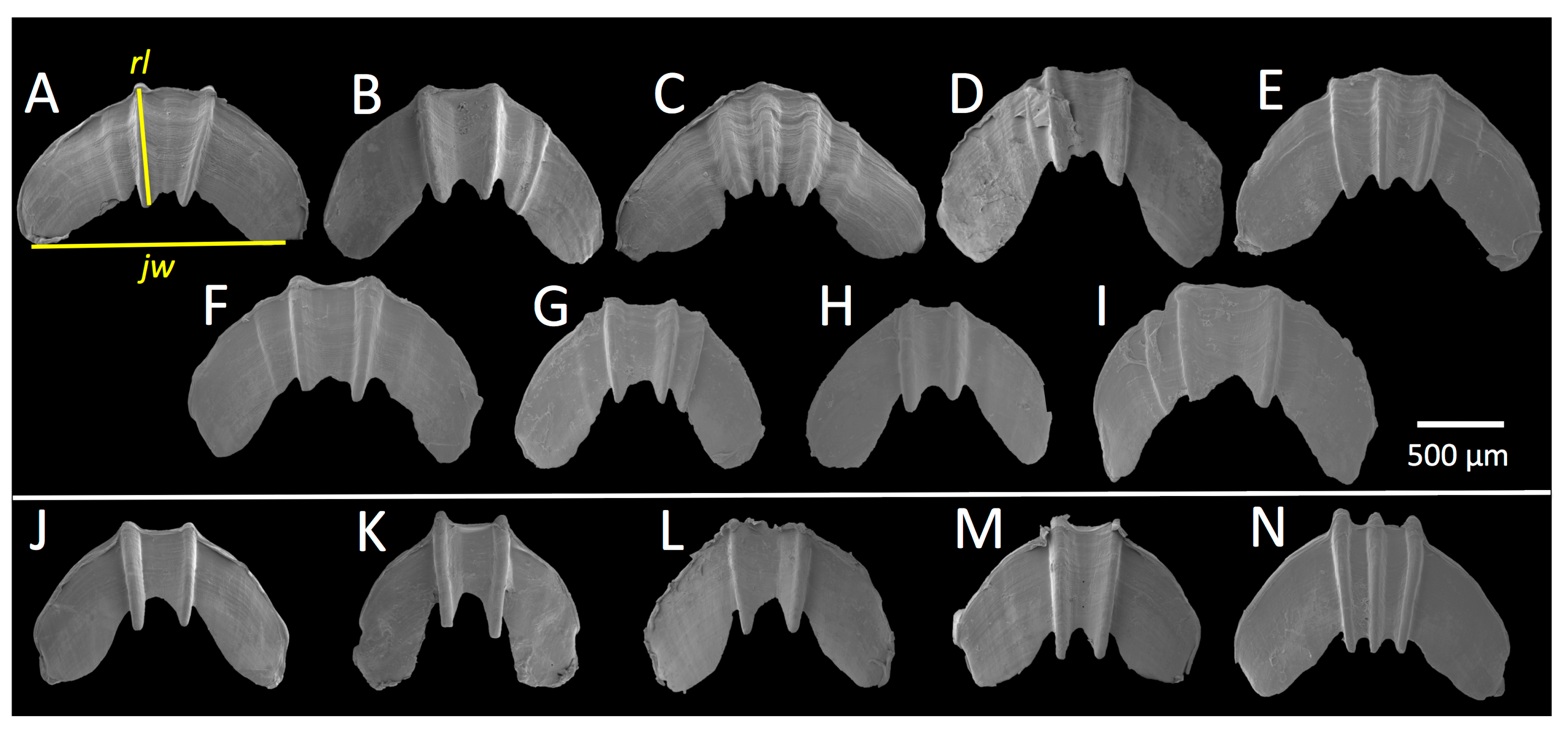
3.4. Jaw Morphology
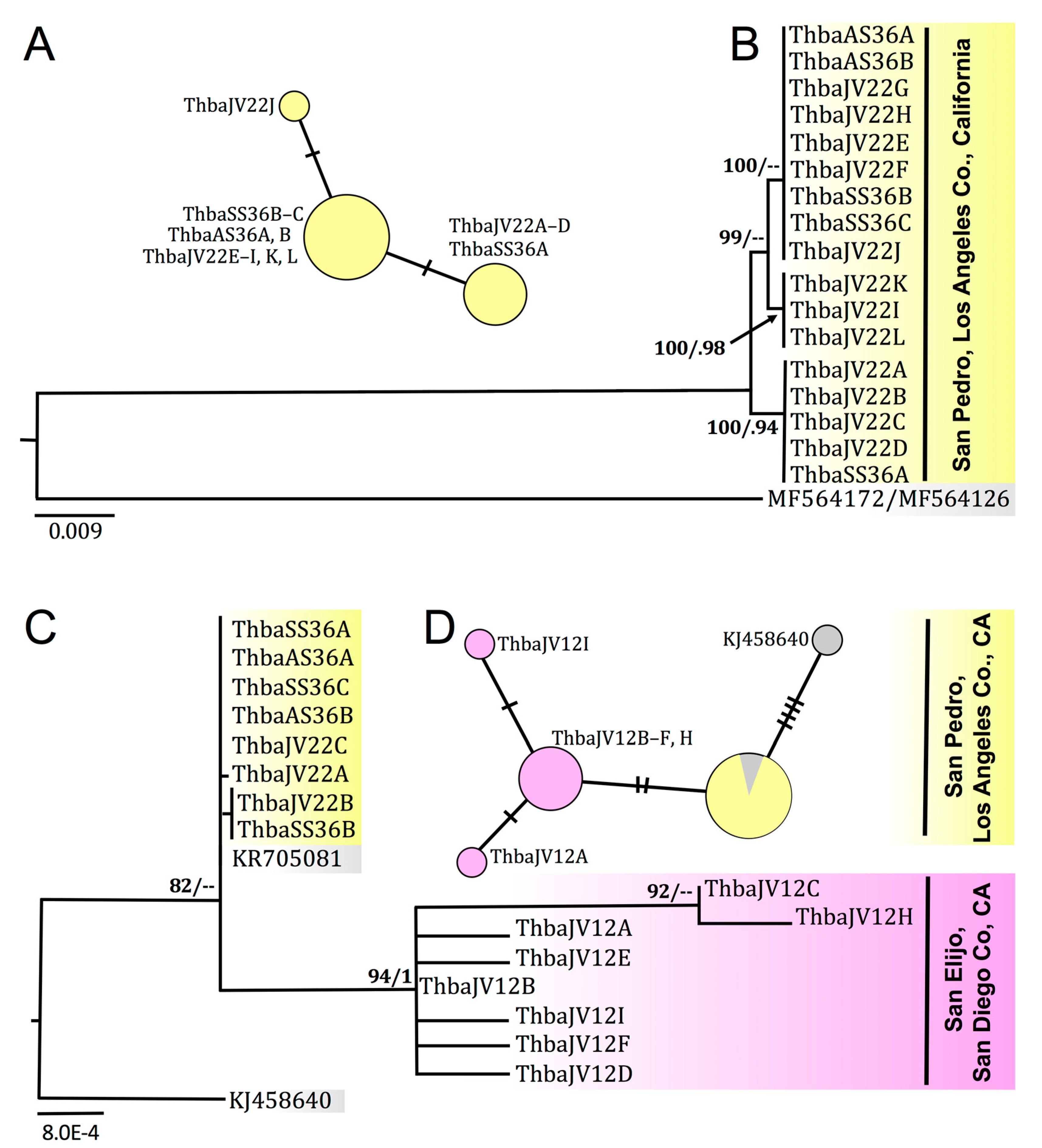
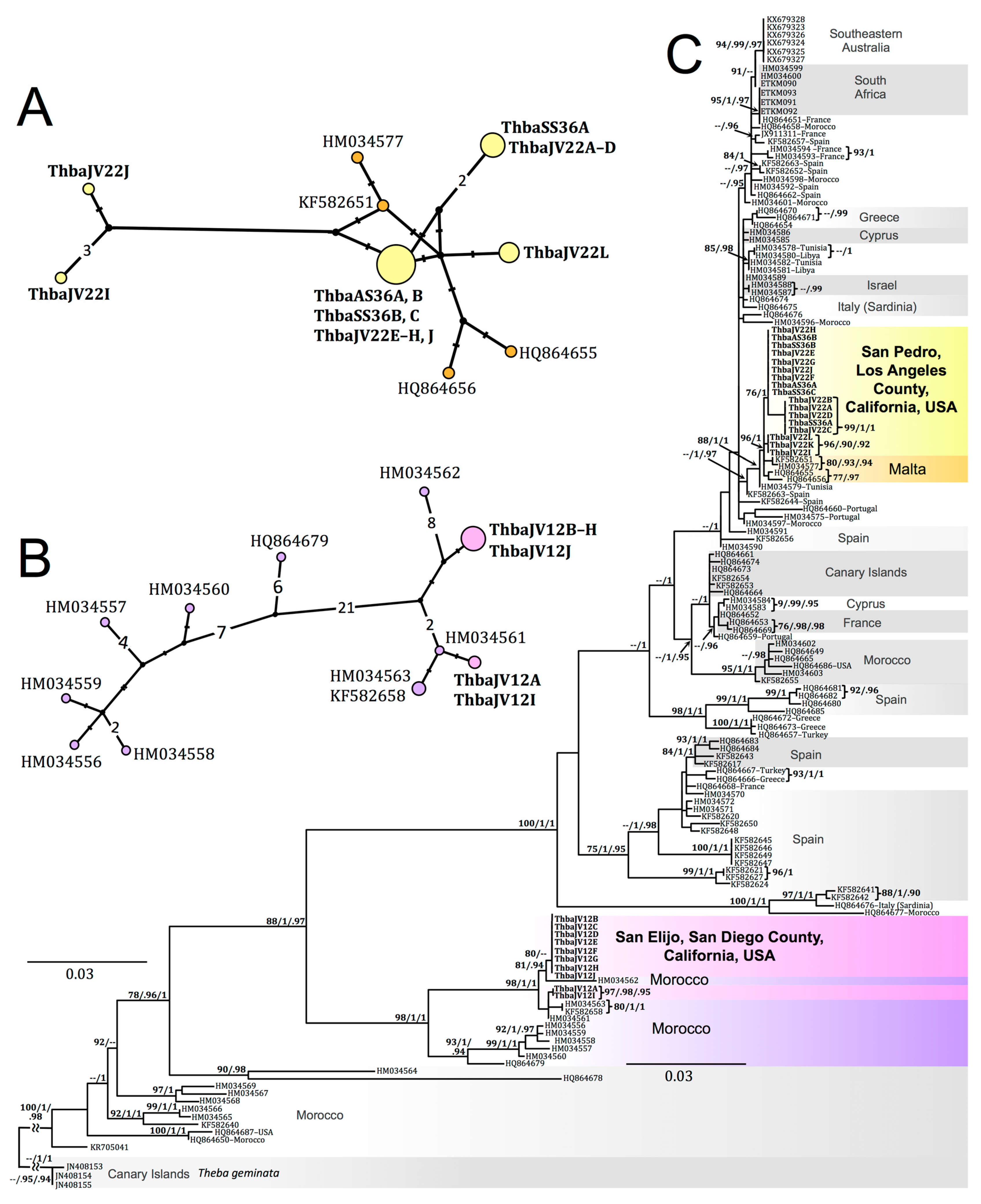
3.5. Molecular Sequence Data and Phylogenetic Systematics
4. Discussion
4.1. Theba pisana in Southern California
4.2. Non-Californian Theba pisana
4.3. Mucous Glands
4.4. Jaw
4.5. Shell and Radula
4.6. Pathway(s) of Introduction into California
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Däumer, C.; Greve, C.; Hutterer, R.; Misof, B.; Haase, M. Phylogeography of an invasive land snail: Natural range expansion versus anthropogenic dispersal in Theba pisana pisana. Biol. Invasions 2012, 14, 1665–1682. [Google Scholar] [CrossRef]
- Rumi, A.; Sánchez, J.; Ferrando, N.S. Theba pisana (Müller, 1774) (Gastropoda, Helicidae) and other alien land molluscs species in Argentina. Biol. Invasions 2010, 12, 2985–2990. [Google Scholar] [CrossRef]
- Gittenberger, E.; Ripken, T.E. The genus Theba (Mollusca: Gastropoda: Helicidae), systematics and distribution. Zool. Verh. Leiden 1987, 241, 1–62. [Google Scholar]
- Odendaal, L.J.; Haupt, T.M.; Griffiths, C.L. The alien invasive land snail Theba pisana in the West Coast National Park: Is there cause for concern? Koedoe 2008, 50, 93–98. [Google Scholar] [CrossRef]
- Herbert, D.G. The Introduced Terrestrial Mollusca of South Africa; South African National Biodiversity Institute, SANBI Biodiversity Series: Pretoria, South Africa, 2010; pp. 1–108. Available online: http://opus.sanbi.org/bitstream/20.500.12143/5678/1/BioSeries_15_2010.pdf (accessed on 24 April 2020).
- Roth, B.; Hertz, C.M.; Cerutti, R. White snails (Helicidae) in San Diego County, California. Festivus 1987, 19, 84–88. [Google Scholar]
- Baker, G.H.; Vogelzang, B.K. Life history, population dynamics and polymorphism of Theba pisana (Mollusca: Helicidae) in Australia. J. Appl. Ecol. 1988, 25, 867–887. [Google Scholar] [CrossRef]
- Baker, G.H. The population dynamics of the Mediterranean snails Cernuella virgata, Cochlicella acuta (Hygromiidae) and Theba pisana (Helicidae) in pasture-cereal rotations in South Australia: A 20-year study. Aust. J. Exp. Agric. 2008, 48, 1514–1522. [Google Scholar] [CrossRef]
- Cooperative Economic Insect Report (CEIR). Animal and Plant Health Inspection Service. In Plant Protection and Quarantine Programs; CEIR: Washington, DC, USA, 1966; Volume 16, p. 205. [Google Scholar]
- Hanna, G. Introduced molluscs of western North America. Occas. Pap. Calif. Acad. Sci. 1966, 48, 1–108. [Google Scholar]
- Cowie, R.; Robinson, D.G. Pathways of introduction of nonindigenous land and freshwater snails and slugs. In Invasive Species: Vectors and Management Strategies; Carlton, J., Ed.; Island Press: Washington, DC, USA, 2003; p. 30. [Google Scholar]
- Daily Wire AP. Snails Menace All Vegetation at La Jolla. The San Francisco Call, 5 May 1922; 111. [Google Scholar]
- Daily Wire AP. Snails Worry at La Jolla. Los Angeles Times, 6 May 1922. [Google Scholar]
- Dean, J.D. Land mollusca in the Vale of Glamorgan. Cardiff Nat. Soc. Rep. Trans. 1915, 48, 50–58. [Google Scholar]
- Hodel, R.; Arakelian, G.; Wilen, C. The White Garden Snail. An old but potentially series pest of landscape ornamentals. PalmArbor 2018, 3, 1–20. [Google Scholar]
- Tandingan De Ley, I.; Schurkman, J.; Wilen, C.; Dillman, A.R. Mortality of the invasive white garden snail Theba pisana exposed to three US isolates of Phasmarhabditis spp. (P. hermaphrodita, P. californica, and P. papillosa). PLoS ONE 2020, 15, e0228244. [Google Scholar] [CrossRef]
- Basinger, A.J. The White Snail (Helix pisana) at La Jolla, California. J. Econ. Entomol. 1923, 16, 522–526. [Google Scholar] [CrossRef]
- Cockerell, T.D.A. Helix pisana in California. Am. Nat. 1924, 58, 190–192. [Google Scholar] [CrossRef]
- Gammon, E.T. Helicid snails in California. Bull. Calif. Dep. Agric. 1943, 32, 173–187. [Google Scholar]
- Snail Cleanup at La Jolla. Bl. Trib. 1922, 33, 1.
- Mishler, M. Archivist Notes. Timekeeper Newsl. Jolla Hist. Soc. 2012, 21, 6. [Google Scholar]
- Daily Wire AP. Snails Menace La Jolla’s Gardens. La Jolla Journal, 28 April 1922. [Google Scholar]
- White Snail Fight on at San Diego. Los Angeles Times, 6 August 1924.
- Miller, E.; Swails, S.; Swails, D.; Olson, F.; Staen, R.T. White garden snail (Theba pisana Müller) Efficacy of selected bait and sprayable molluscicides. J. Agric. Entomol. 1988, 5, 189–197. [Google Scholar]
- Norris, M. Pests last seen in 1922: Destructive White Snails Back in Country. Los Angeles Times, 17 August 1985; A3. [Google Scholar]
- Hanna, G.D. A new outbreak of Helix pisana in California. Nautilus 1933, 46, 139–140. [Google Scholar]
- Pilsbry, H. Land Mollusca of North America (north of Mexico). Acad. Nat. Sci. Phila. Mono. 1939, 3, 1–573. [Google Scholar]
- Basinger, A.J. Measuring the Efficiency of Materials Used for Snail Control. J. Econ. Entomol. 1935, 28, 903–905. [Google Scholar] [CrossRef]
- War on Pests Calls for Heroic Campaign. Los Angeles Times, 17 June 1935; A8.
- Cooperative Economic Insect Report (CEIR). Animal and Plant Health Inspection Service. Plant Protection and Quarantine Programs; CEIR: Washington, DC, USA, 1968; Volume 18.
- Cooperative Economic Insect Report (CEIR). Animal and Plant Health Inspection Service. Plant Protection and Quarantine Programs; CEIR: Washington, DC, USA, 1971; Volume 21.
- Dees, L.T. Edible land snails in the United States; US Department of the Interior, Fish and Wildlife Service, Bureau of Sport Fisheries and Wildlife: U.S. Government Printing Office: Washington, DC, USA, 1970; Volume 91, p. 8.
- Smart, R. Theba pisana Observation. iNaturalist: San Pedro, CA, USA, 2013. Available online: https://www.inaturalist.org/observations/274431 (accessed on 24 April 2021).
- NatureinLA. Theba pisana Observation. iNaturalist: San Pedro, CA, USA, 2015. Available online: https://www.inaturalist.org/observations/1769498 (accessed on 24 April 2021).
- Littlejohn, D. Snails Run Amok: San Pedro Nature Preserve Reluctant Host to Invasive Creatures. Daily Breeze, 26 July 2019. Available online: https://www.dailybreeze.com/2019/07/26/snails-run-amok-san-pedro-nature-preserve-reluctant-host-to-invasive-creatures/ (accessed on 12 March 2021).
- Cowie, R.H.; Dillon, R.T.; Robinson, D.G.; Smith, J.W. Alien non-marine snails and slugs of priority quarantine importance in the United States: A preliminary risk assessment. Am. Malacol. Bull. 2009, 27, 113–132. [Google Scholar] [CrossRef]
- Cowie, R.H. Microhabitat choice and high temperature tolerance in the land snail Theba pisana (Mollusca: Gastropoda). J. Zool. 1985, 207, 201–211. [Google Scholar] [CrossRef]
- Johnson, M.S. Founder effects and geographic variation in the land snail Theba pisana. Heredity 1988, 61, 133–142. [Google Scholar] [CrossRef]
- Greve, C.; Hutterer, R.; Groh, K.; Haase, M.; Misof, B. Evolutionary diversification of the genus Theba (Gastropoda: Helicidae) in space and time: A land snail conquering islands and continents. Mol. Phylogenetics Evol. 2010, 57, 572–584. [Google Scholar] [CrossRef]
- Haase, M.; Greve, C.; Hutterer, R.; Misof, B. Amplified fragment length polymorphisms, the evolution of the land snail genus Theba (Stylommatophora: Helicidae), and an objective approach for relating fossils to internal nodes of a phylogenetic tree using geometric morphometrics. Zool. J. Linn. Soc. 2014, 171, 92–107. [Google Scholar] [CrossRef]
- Brown, B.; Borkent, A.; Wetzer, R.; Pentcheff, D. New types of inventories at the Natural History Museum of Los Angeles County. Am. Entomol. 2014, 60, 231–232. [Google Scholar] [CrossRef][Green Version]
- Moran, S. Weather-and population density-induced infantilism in the landsnail Theba pisana in a semi-arid climate. Int. J. Biometeorol. 1989, 33, 101–108. [Google Scholar] [CrossRef]
- Koene, J.M.; Schulenburg, H. Shooting darts: Co-evolution and counter-adaptation in hermaphroditic snails. BMC Evol. Biol. 2005, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Holyoak, D.T.; Holyoak, G.A. Reassessment of the keeled subspecies of Theba pisana (Gastropoda: Helicidae) from the sand dunes of south-western Portugal. Iberus 2016, 34, 19–39. [Google Scholar]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar] [PubMed]
- Geller, J.; Meyer, C.; Parker, M.; Hawk, H. Redesign of PCR primers for mitochondrial cytochrome c oxidase subunit I for marine invertebrates and application in all-taxa biotic surveys. Mol. Ecol. Resour. 2013, 13, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Palumbi, S.R. Nucleic acids II: The polymerase chain reaction. In Molecular Systematics; Hillis, D.M., Moritz, C., Mable, B.K., Eds.; Sinauer & Associates Inc.: Sunderland, MA, USA, 1996; pp. 205–247. [Google Scholar]
- Wade, C.M.; Mordan, P.B. Evolution within the gastropod molluscs; using the ribosomal RNA gene-cluster as an indicator of phylogenetic relationships. J. Molluscan Stud. 2000, 66, 565–570. [Google Scholar] [CrossRef]
- Vendetti, J.; Burnett, E.; Carlton, L.; Curran, A.T.; Lee, C.; Matsumoto, R.; Mc Donnell, R.; Reich, I.; Willadsen, O. The introduced terrestrial slugs Ambigolimax nyctelius (Bourguignat, 1861) and Ambigolimax valentianus (Férussac, 1821) (Gastropoda: Limacidae) in California, with a discussion of taxonomy, systematics, and discovery by citizen science. J. Nat. Hist. 2019, 53, 1607–1632. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Böckers, A.; Greve, C.; Hutterer, R.; Misof, B.; Haase, M. Testing heterogeneous base composition as potential cause for conflicting phylogenetic signal between mitochondrial and nuclear DNA in the land snail genus Theba Risso 1826 (Gastropoda: Stylommatophora: Helicoidea). Org. Divers. Evol. 2016, 16, 835–846. [Google Scholar] [CrossRef]
- Villesen, P. FaBox: An online toolbox for fasta sequences. Mol. Ecol. Notes 2007, 7, 965–968. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evo. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Darriba, D.; Taboada, G.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Darriba, D.; Posada, D.; Kozlov, A.M.; Stamatakis, A.; Morel, B.; Flouri, T. ModelTest-NG: A new and scalable tool for the selection of DNA and protein evolutionary models. Mol. Biol. Evo. 2020, 37, 291–294. [Google Scholar] [CrossRef]
- Miller, M.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010; pp. 1–8. [Google Scholar]
- Bouaziz-Yahiatene, H.; Pfarrer, B.; Medjdoub-Bensaad, F.; Neubert, E. Revision of Massylaea Möllendorff, 1898 (Stylommatophora, Helicidae). ZooKeys 2017, 694, 109–133. [Google Scholar] [CrossRef]
- Razkin, O.; Gómez-Moliner, B.J.; Prieto, C.E.; Martínez-Ortí, A.; Arrébola, J.R.; Muñoz, B.; Chueca, L.J.; Madeira, M.J. Molecular phylogeny of the western Palaearctic Helicoidea (Gastropoda, Stylommatophora). Mol. Phylogenet. Evol. 2015, 83, 99–117. [Google Scholar] [CrossRef]
- Neiber, M.; Hausdorf, B. Molecular phylogeny reveals the polyphyly of the snail genus Cepaea (Gastropoda: Helicidae). Mol. Phylogenetics Evol. 2015, 93, 143–149. [Google Scholar] [CrossRef]
- Bandelt, H.; Forster, P.; Röhl, A. Median-joining networks for inferring intraspecific phylogenies. Mol. Biol. Evol. 1999, 16, 37–48. [Google Scholar] [CrossRef]
- Leigh, J.W.; Bryant, D. Popart: Full-feature software for haplotype network construction. Methods Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Ronquist, F.; Huelsenbeck, J. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [PubMed]
- Pattengale, N.D.; Alipour, M.; Bininda-Emonds, O.R.; Moret, B.M.; Stamatakis, A. How many bootstrap replicates are necessary? J. Comput. Biol. 2010, 17, 337–354. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A. FigTree v1. 3.1. 2009. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 12 March 2021).
- Hesse, P. Genus Euparypha Hartm. In Iconographie der Land- & Süsswasser-Mollusken mit Vorzüglicher Berücksichtigung der Europäischen Noch Nicht Abgebildeten Arten; Band, N.F., Ed.; Berlin & Wiesbaden: Wiesbaden, Germany, 1915; pp. 1–72, [In German]; Available online: http://biodiversitylibrary.org/page/280865 (accessed on 13 March 2020).
- Barker, G.M.; Efford, M.G. Predatory Gastropods as Natural Enemies of Terrestrial Gastropods and Other Invertebrates. In Natural Enemies of Terrestrial Molluscs; CABI Publishing: Wallingford, UK, 2004; pp. 279–403. [Google Scholar]
- Satoh, S.S.; Ikeda, S.; Yamazaki, Y. Multiple introduction events and artificial long-distance dispersal of the exotic slug Ambigolimax valentianus in Japan. Molluscan Res. 2020, 40, 276–281. [Google Scholar] [CrossRef]
- Pinceel, J.; Jordaens, K.; Van Houtte, N.; Bernon, G.; Backeljau, T. Population genetics and identity of an introduced terrestrial slug: Arion subfuscus sl in the north-east USA (Gastropoda, Pulmonata, Arionidae). Genetica 2005, 125, 155–171. [Google Scholar] [CrossRef]
- Mc Donnell, R.J.; Rugman-Jones, P.; Backeljau, T.; Breugelmans, K.; Jordaens, K.; Stouthamer, R.; Paine, T.; Gormally, M. Molecular identification of the exotic slug Arion subfuscus sensu stricto (Gastropoda: Pulmonata) in California, with comments on the source location of introduced populations. Biol. Invasions 2011, 13, 61–66. [Google Scholar] [CrossRef]
- Grindon, A.J.; Davison, A. Irish Cepaea nemoralis land snails have a cryptic Franco-Iberian origin that is most easily explained by the movements of Mesolithic humans. PLoS ONE 2013, 8, e65792. [Google Scholar] [CrossRef] [PubMed]
- Layton, K.K.; Warne, C.P.K.; Nicolai, A.; Ansart, A.; deWaard, J.R. Molecular evidence for multiple introductions of the banded grove snail (Cepaea nemoralis) in North America. Can. J. Zool. 2019, 97, 392–398. [Google Scholar] [CrossRef]
- Guiller, A.; Martin, M.C.; Hiraux, C.; Madec, L. Tracing the invasion of the Mediterranean land snail Cornu aspersum aspersum becoming an agricultural and garden pest in areas recently introduced. PLoS ONE 2012, 7, e49674. [Google Scholar] [CrossRef]
- Gaitán-Espitia, J.D.; Scheihing, R.; Poulin, E.; Artacho, P.; Nespolo, R.F. Mitochondrial phylogeography of the land snail Cornu aspersum: Tracing population history and the impact of human-mediated invasion in austral South America. Evol. Ecol. Res. 2013, 15, 61–78. [Google Scholar]
- Sherpa, S.; Ansart, A.; Madec, L.; Martin, M.C.; Dréano, S.; Guiller, A. Refining the biogeographical scenario of the land snail Cornu aspersum aspersum: Natural spatial expansion and human-mediated dispersal in the Mediterranean basin. Mol. Phylogenetics Evol. 2018, 120, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, J.; Schlitt, B.; Kořínková, T.; Reise, H.; Barker, G.M. Genetic evidence illuminates the origin and global spread of the slug Deroceras invadens. J. Molluscan Stud. 2020, 86, 306–322. [Google Scholar] [CrossRef]
- Korábek, O.; Kosová, T.; Dolejš, P.; Petrusek, A.; Neubert, E.; Juřičková, L. Geographic isolation and human-assisted dispersal in land snails: A Mediterranean story of Helix borealis and its relatives (Gastropoda: Stylommatophora: Helicidae). Zool. J. Linn. Soc. 2021, 26, zlaa186. [Google Scholar] [CrossRef]
- Fontanilla, I.K.C.; Maria, I.M.P.S.; Garcia, J.R.M.; Ghate, H.; Naggs, F.; Wade, C.M. Restricted genetic variation in populations of Achatina (Lissachatina) fulica outside of East Africa and the Indian Ocean Islands points to the Indian Ocean Islands as the earliest known common source. PLoS ONE 2014, 9, e105151. [Google Scholar] [CrossRef]
- Ayyagari, V.S.; Sreerama, K. Evaluation of haplotype diversity of Achatina fulica (Lissachatina) [Bowdich] from Indian sub-continent by means of 16S rDNA sequence and its phylogenetic relationships with other global populations. 3 Biotech 2017, 7, 252. [Google Scholar] [CrossRef]
- Capinha, C.; Essl, F.; Seebens, H.; Moser, D.; Pereira, H.M. The dispersal of alien species redefines biogeography in the Anthropocene. Science 2015, 348, 1248–1251. [Google Scholar] [CrossRef]
- Ruiz, G.; Carlton, J. Invasion vectors: A conceptual framework for management. In Invasive Species: Vectors and Management Strategies; Carlton, J., Ruiz, G., Eds.; Island Press: Washington, DC, USA, 2003; pp. 459–504. [Google Scholar]
- Greve, C.; Haase, M.; Hutterer, R.; Rödder, D.; Ihlow, F.; Misof, B. Snails in the desert: Species diversification of Theba (Gastropoda: Helicidae) along the Atlantic coast of NW Africa. Ecol. Evol. 2017, 7, 5524–5538. [Google Scholar] [CrossRef]
- Yang, Q.Q.; Liu, S.W.; He, C.; Yu, X.P. Distribution and the origin of invasive apple snails, Pomacea canaliculata and P. maculata (Gastropoda: Ampullariidae) in China. Sci. Rep. 2018, 8, 1185. [Google Scholar] [CrossRef]
- Xue, D.X.; Graves, J.; Carranza, A.; Sylantyev, S.; Snigirov, S.; Zhang, T.; Liu, J.X. Successful worldwide invasion of the veined rapa whelk, Rapana venosa, despite a dramatic genetic bottleneck. Biol. Invasions 2018, 20, 3297–3314. [Google Scholar] [CrossRef]
- Van Elden, S.; Miranda, N.A.; Perissinotto, R.; Adams, J.B. Plant selection and grazing activity of the invasive snail Theba pisana in coastal Algoa Bay, South Africa. Afr. Zool. 2015, 50, 227–231. [Google Scholar] [CrossRef]
- Shik, J.Z.; Dussutour, A. Nutritional dimensions of invasive success. Trends Ecol. Evol. 2020, 35, 691–703. [Google Scholar] [CrossRef]
- Ożgo, M.; Bogucki, Z. Colonization, stability, and adaptation in a transplant experiment of the polymorphic land snail Cepaea nemoralis (Gastropoda: Pulmonata) at the edge of its geographical range. Biol. J. Linn. Soc. Lond. 2011, 104, 462–470. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Y.; Ren, Y.; Wang, H.; Li, S.; Jiang, F.; Yin, L.; Qiao, X.; Zhang, G.; Qian, W.; et al. The genome of the golden apple snail Pomacea canaliculata provides insight into stress tolerance and invasive adaptation. Gigascience 2018, 7, giy101. [Google Scholar] [CrossRef]
- Köhler, H.R.; Capowiez, Y.; Mazzia, C.; Eckstein, H.; Kaczmarek, N.; Bilton, M.C.; Burmester, J.K.; Capowiez, L.; Chueca, L.J.; Favilli, L.; et al. Experimental simulation of environmental warming selects against pigmented morphs of land snails. Ecol. Evol. 2021, 11, 1111–1130. [Google Scholar] [CrossRef] [PubMed]
- Hebert, P.D.; Ratnasingham, S.; De Waard, J.R. Barcoding animal life: Cytochrome c oxidase subunit 1 divergences among closely related species. Proc. Royal Soc. B 2003, 270, S96–S99. [Google Scholar] [CrossRef]
- Wall-Palmer, D.; Hegmann, M.; Goetze, E.; Peijnenburg, K.T. Resolving species boundaries in the Atlanta brunnea species group (Gastropoda, Pterotracheoidea). ZooKeys 2019, 899, 59–84. [Google Scholar] [CrossRef]
- Bodon, M.; Cianfanelli, S.; Chueca, L.J.; Pfenninger, M. Candidula conglomeratica sp. nov. from the northern Apennines, Italy (Gastropoda: Eupulmonata: Geomitridae). Arch. Molluskenkd. 2020, 149, 203–220. [Google Scholar] [CrossRef]
- Prevot, V.; Jordaens, K.; Sonet, G.; Backeljau, T. Exploring species level taxonomy and species delimitation methods in the facultatively self-fertilizing land snail genus Rumina (Gastropoda: Pulmonata). PLoS ONE 2013, 8, e60736. [Google Scholar] [CrossRef]
- Breugelmans, K.; Jordaens, K.; Adriaens, E.; Remon, J.P.; Cardona, J.Q.; Backeljau, T. DNA barcodes and phylogenetic affinities of the terrestrial slugs Arion gilvus and A. ponsi (Gastropoda, Pulmonata, Arionidae). ZooKeys 2013, 365, 83–104. [Google Scholar]
- Davison, A.; Blackie, R.L.; Scothern, G.P. DNA barcoding of stylommatophoran land snails: A test of existing sequences. Mol. Ecol. Resour. 2009, 9, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A. Der geschlechtsapparat der Stylommatophoren in taxonomischer hinsicht. In Abhandlungen des Naturwissenschaftlichen Vereins für Sachsen und Thüringen in Halle; 1855; Volume 1, pp. 1–52. Available online: https://www.biodiversitylibrary.org/bibliography/11495 (accessed on 13 April 2021).
- Moquin-Tandon, A. Histoire Naturelle des Mollusques terrestres et Fluviatiles de France, Contenant des Études Générales Sur Leur Anatomie et Leur Physiologie et la Description Particulière des Genres, des Espèces et des Varieties; Baillière, J.-B., Ed.; Paris, France, 1855; Volume 2, Available online: https://www.biodiversitylibrary.org/item/100085#page/42/mode/1up (accessed on 13 April 2021).
- Cain, A.J. Genetics of some morphs in the land snail Theba pisana. Malacologia 1984, 25, 381–411. [Google Scholar]
- Cowie, R.H. The life-cycle and productivity of the land snail Theba pisana (Mollusca: Helicidae). J. Anim. Ecol. 1984, 53, 311–325. [Google Scholar] [CrossRef]
- List of Intercepted Plant Pests; USDA, Plant Quarantine and Control Administration: Hayattsville, MD, USA, 1931.
- List of Intercepted Plant Pests; USDA, Plant Quarantine and Control Administration: Hayattsville, MD, USA, 1948.
- List of Intercepted Plant Pests; USDA, Plant Quarantine and Control Administration: Hayattsville, MD, USA, 1960.
- List of Intercepted Plant Pests; USDA, Plant Quarantine and Control Administration: Hayattsville, MD, USA, 1982.
- List of Intercepted Plant Pests; USDA, Plant Quarantine and Control Administration: Hayattsville, MD, USA, 1987.
- Cooperative Economic Insect Report. US. APHIS; Plant Pest Control Division: Hayattsville, MD, USA, 1987; Volume 10.
- Philippi, R. Enumeratio molluscorum Siciliae cum viventium tum in tellure tertiaria fossilium quae in itinere suo observavit. Berolini, S. Schroppii. 1844, Volume 2, p. 303. Available online: https://www.biodiversitylibrary.org/item/180011#page/7/mode/1up (accessed on 14 April 2021).
- Une société de naturalistes, and d’agriculteurs. Nouveau Dictionnaire d’histoire Naturelle: Appliquée aux arts, à l’Agriculture, à L’économie Rurale et Domestique, à la Médecine, etc; Chez Deterville: Paris, France, 1818; Volume 22, p. 618. Available online: https://www.biodiversitylibrary.org/item/60124#page/7/mode/1up (accessed on 1 May 2021).
- Editorial Staff. Palermitani orfani del Festino, ma il 14 luglio non sarà senza “babbaluci”. [it Italian]. Sapori di Sicilia Magazine, 12 July 2020. Available online: https://www.saporidisiciliamagazine.it/ (accessed on 11 March 2021).
- Ditta, D. Dalla “calia” ai “babbaluci”, senza scordare lo sfincione: La mappa del cibo al Festino. [it Italian]. Palermo Today, 13 July 2018. Available online: https://www.palermotoday.it/attualita/mappa-cibo-festino-santa-rosalia-2018.html (accessed on 11 March 2021).
- Wolfert, P. The Food of Morocco; A&C Black: London, UK, 2012; p. 528. [Google Scholar]
- Meineke, E.K.; Davies, T.J.; Daru, B.H.; Davis, C.C. Biological collections for understanding biodiversity in the Anthropocene. Philos. Trans. R. Soc. B. 2018, 374, 20170386. [Google Scholar] [CrossRef] [PubMed]
- Shultz, A.J.; Adams, B.J.; Bell, K.C.; Ludt, W.B.; Pauly, G.B.; Vendetti, J.E. Natural history collections are critical resources for contemporary and future studies of urban evolution. Evol. Appl. 2021, 14, 233–247. [Google Scholar] [CrossRef]
- Lothrop, G. Italians of Los Angeles: An Historical Overview. South. Calif. Q. 2003, 85, 249–300. [Google Scholar] [CrossRef]
- Gatto, M. Los Angeles’s Little Italy; Arcadia Publishing: San Francisco, CA, USA, 2009; p. 128. [Google Scholar]
- Littlejohn, D. Little Italy district coming to San Pedro, dividing public opinion. Daily Breeze. 27 September 2019. Available online: https://www.dailybreeze.com/2019/09/27/little-italy-district-coming-to-san-pedro-dividing-public-opinion/ (accessed on 12 March 2021).




| LACM Lot Number | Collection Date | Isolate Name | GenBank Accession Number | Reproductive System | Collection Locality | |||
|---|---|---|---|---|---|---|---|---|
| CO1 | ITS2 | 16S | ||||||
| 182358 | 1-Jun-2019 | ThbaS36A | MW831635 | MW832220 | MW847212 | 100% Immature (n = 5) | San Pedro, Los Angeles County, California | White Point Nature Preserve, BioSCAN site, south entrance 33.716, −18.316 |
| ThbaSS36B | MW831647 | MW832226 | MW847224 | |||||
| ThbaSS36C | MW831648 | MW832227 | MW847225 | |||||
| ThbaAS36A | MW831640 | MW832224 | MW847217 | |||||
| ThbaAS36B | MW831641 | MW832225 | MW847218 | |||||
| 182330 | 22-Jun-2020 | ThbaJV22A | MW831636 | MW832221 | MW847213 | 100% Immature (n = 15) | Marine Mammal Care Center, 33.712, −118.295 | |
| ThbaJV22B | MW831637 | MW832222 | MW847214 | |||||
| ThbaJV22C | MW831638 | MW832223 | MW847215 | |||||
| ThbaJV22D | MW831639 | – | MW847216 | |||||
| 182329 | 22-Jun-2020 | ThbaJV22E | MW831642 | – | MW847219 | 100% Immature (n = 15) | White Point Nature Preserve, south entrance, 33.716, −118.316 | |
| ThbaJV22F | MW831643 | – | MW847220 | |||||
| ThbaJV22G | MW831644 | – | MW847221 | |||||
| ThbaJV22H | MW831645 | – | MW847222 | |||||
| 182331 | 22-Jun-2020 | ThbaJV22I | MW831651 | – | MW847228 | 100% Immature (n = 15) | White Point Nature Preserve, native plant garden, 33.716, −118.315 | |
| ThbaJV22J | MW831646 | – | MW847223 | |||||
| ThbaJV22K | MW831650 | – | MW847227 | |||||
| ThbaJV22L | MW831649 | – | MW847226 | |||||
| 182342 | 17-Oct-2020 | – | – | – | – | 7% Immature 93% Mature (n = 15) | White Point Nature Preserve, grassland loop trail, 33.715, −118.311 | |
| 182335 | 12-Sept-2020 | ThbaJV12A | MW831652 | MW832228 | – | 20% Immature 80% Intermediate? (n = 15) | Solano Beach/Encinitas, San Diego County, California | San Elijo Lagoon Ecological Reserve, Santa Carina trail, 33.0077, −117.2596 |
| ThbaJV12B | MW831654 | MW832230 | – | |||||
| ThbaJV12C | MW831655 | MW832231 | – | |||||
| ThbaJV12D | MW831656 | MW832232 | – | |||||
| ThbaJV12E | MW831657 | MW832233 | – | |||||
| ThbaJV12F | MW831658 | MW832234 | – | |||||
| ThbaJV12G | MW831659 | – | – | |||||
| ThbaJV12H | MW831660 | MW832235 | – | |||||
| ThbaJV12I | MW831653 | MW832229 | – | |||||
| ThbaJV12J | MW831661 | – | – | |||||
| San Pedro T. pisana | San Elijo T. pisana | All T. pisana | |
|---|---|---|---|
| San Pedro T. pisana (n = 17) | 0.004 | ||
| San Elijo T. pisana (n = 10) | 0.131 | 0.004 | |
| all other T. pisana (n = 128) | 0.059 | 0.127 | 0.087 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vendetti, J.E.; Sandig, K.; Sahakyan, A.; Granados, A. Multiple Introductions of the Pestiferous Land Snail Theba pisana (Müller, 1774) (Gastropoda: Helicidae) in Southern California. Insects 2021, 12, 662. https://doi.org/10.3390/insects12080662
Vendetti JE, Sandig K, Sahakyan A, Granados A. Multiple Introductions of the Pestiferous Land Snail Theba pisana (Müller, 1774) (Gastropoda: Helicidae) in Southern California. Insects. 2021; 12(8):662. https://doi.org/10.3390/insects12080662
Chicago/Turabian StyleVendetti, Jann E., Kimiko Sandig, Armenuhi Sahakyan, and Alyana Granados. 2021. "Multiple Introductions of the Pestiferous Land Snail Theba pisana (Müller, 1774) (Gastropoda: Helicidae) in Southern California" Insects 12, no. 8: 662. https://doi.org/10.3390/insects12080662
APA StyleVendetti, J. E., Sandig, K., Sahakyan, A., & Granados, A. (2021). Multiple Introductions of the Pestiferous Land Snail Theba pisana (Müller, 1774) (Gastropoda: Helicidae) in Southern California. Insects, 12(8), 662. https://doi.org/10.3390/insects12080662






