Encapsulation of Basil Essential Oil by Paste Method and Combined Application with Mechanical Trap for Oriental Fruit Fly Control
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Insects
- Experiment 1: Volatile analysis of the essential oils and B. dorsalis attractiveness
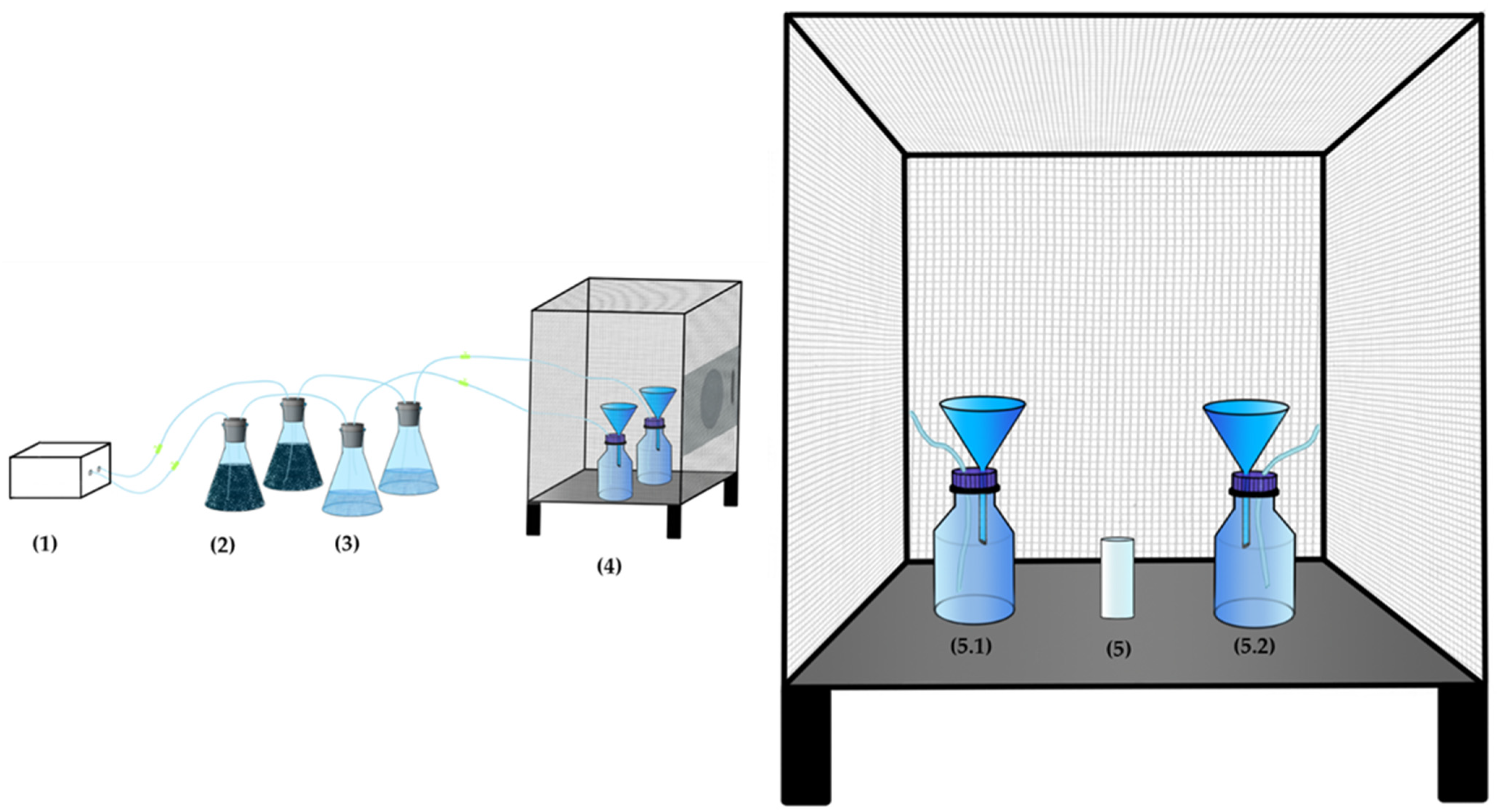
2.3. Essential Oil Extraction
2.4. Volatile Analyses
2.5. Attractiveness
2.6. Insect Olfactory-Chemosensory Responses
- Experiment 2: Paste matrix complexation with basil essential oil encapsulation
2.7. Paste Matrix Complexation
2.8. Encapsulation Characteristics
2.9. Fourier-Transform Infrared Spectroscopy (FTIR)
2.10. Controlled Release of the Capsules
- Experiment 3: Product efficacy in the field test study
2.11. The Experiment Site
2.12. Trap
2.13. Statistical Analysis
3. Results
- Experiment 1: Volatile analysis of the essential oils and B. dorsalis attractiveness (in-vitro study)
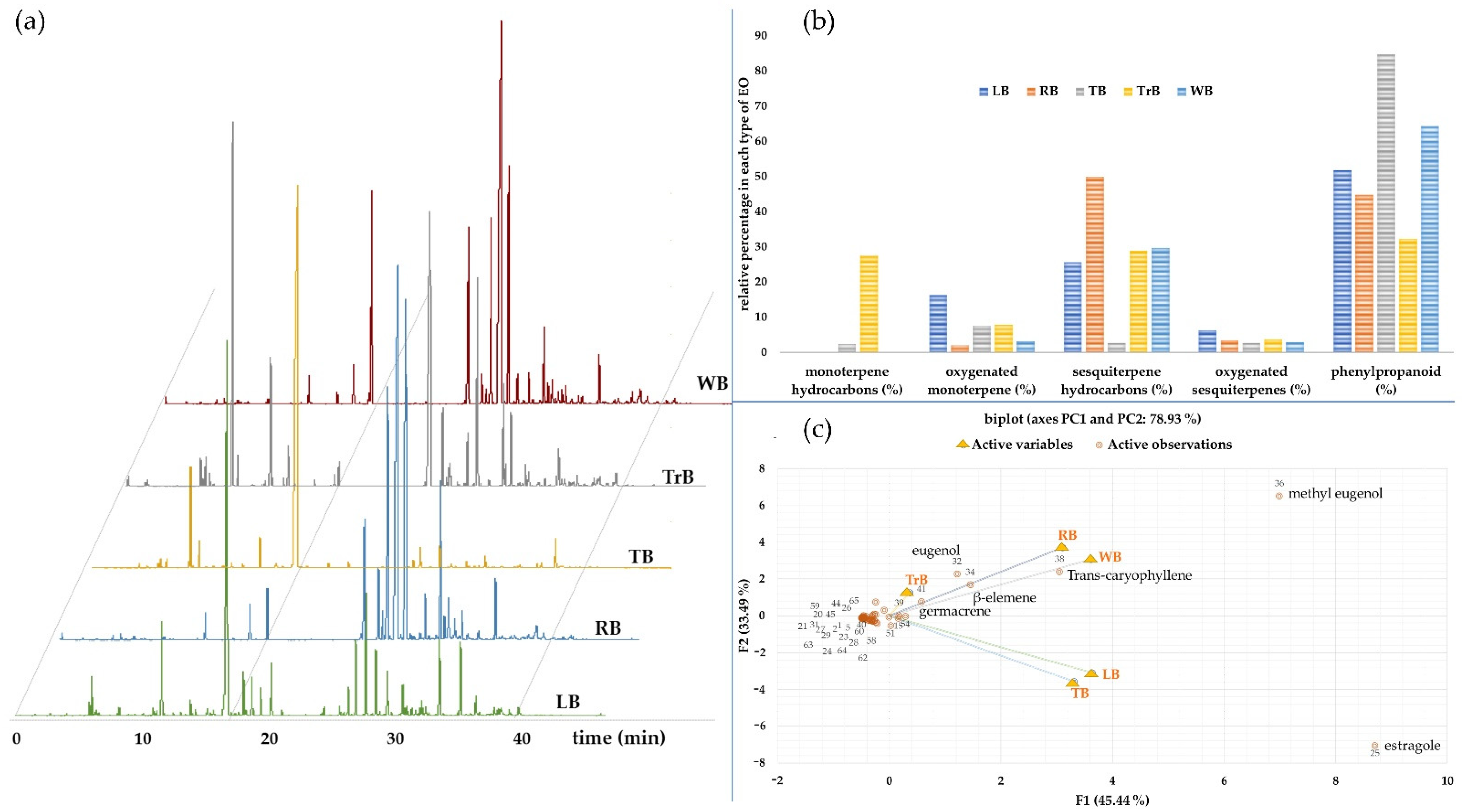
3.1. Chemical Compositions
3.2. Attractiveness
3.3. Insect Olfactory Chemosensory Responses
- Experiment 2: Paste matrix complexation with basil essential oil encapsulation
3.4. Emulsion Characteristic and Encapsulation Characteristics
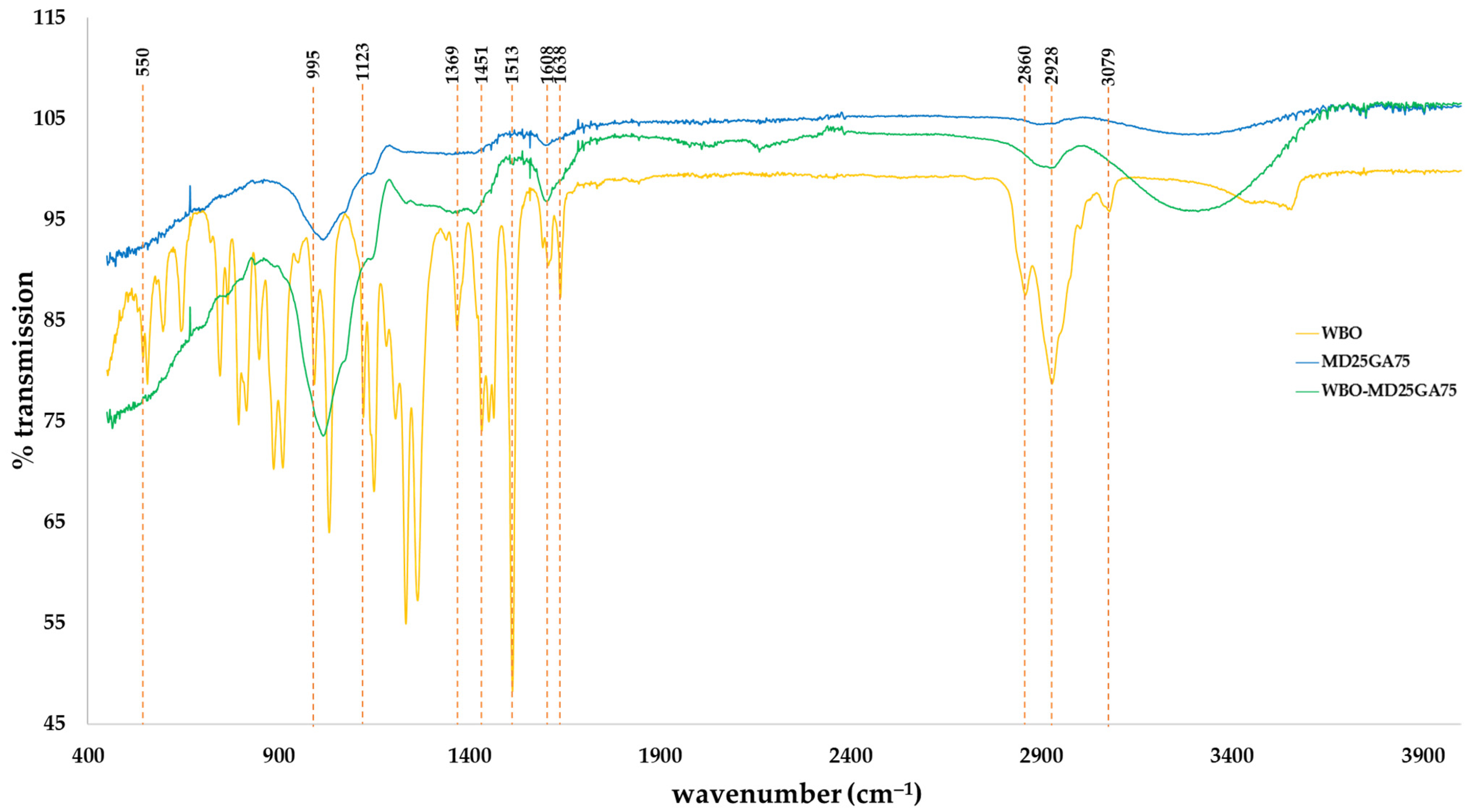
3.5. FTIR
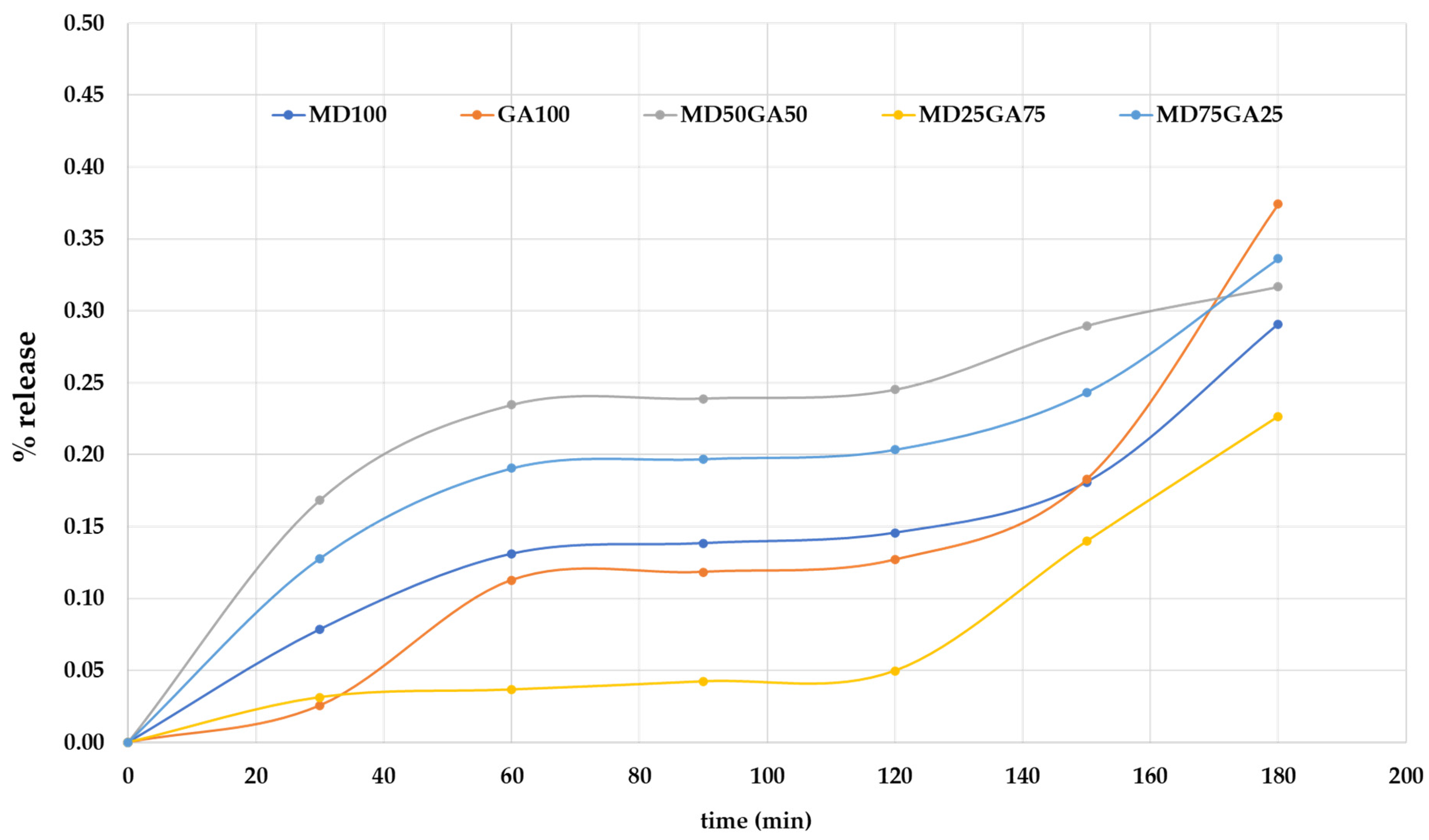
3.6. Controlled Release of the White Holy Basil-Encapsulate Product
- Experiment 3: WBO-encapsulated products efficacy in the field test study
3.7. Product Efficacy
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Canhanga, L.; De Meyer, M.; Cugala, D.; Massimiliano, V.; Maulid, M. Economic injury level of the Oriental fruit fly, Bactrocera dorsalis (Diptera: Tephritidae), on commercial mango farms in Manica Province, Mozambique. Afr. Entomol. 2020, 28, 278–289. [Google Scholar] [CrossRef]
- Orankanok, W.; Chinvinijkul, S.; Thanaphum, S.; Sitilob, P.; Enkerlin, W.R. Area-Wide Integrated Control of Oriental Fruit Fly Bactrocera Dorsalis and Guava Fruit Fly Bactrocera Correcta in Thailand; Springer: Dordrecht, The Netherlands, 2007; pp. 517–526. [Google Scholar]
- Fezza, T.J.; Shelly, T.E. Comparative dispersal and survival of male Oriental fruit flies (Diptera: Tephritidae) from wild and genetic sexing strains. Int. J. Trop. Insect Sci. 2020, 41, 751–757. [Google Scholar] [CrossRef]
- Chinajariyawong, A.; Kritsaneepaiboon, S.; Drew, R. Efficacy of protein bait sprays in controlling fruit flies (Diptera: Tephritidae) infesting angled luffa and bitter gourd in Thailand. Raffles Bull. Zool. 2003, 51, 7–16. [Google Scholar]
- Verghese, A.; Sreedevi, K.; Nagaraju, D. Pre and Postharvest IPM for the Mango Fruit Fly, Bactrocera dorsalis (Hendel). In Proceedings of the International Symposium on Fruit Flies of Economic Importance: From Basic to Applied Knowledge, Salvador, Brazil, 10–15 September 2006. [Google Scholar]
- Ji, Q.E.; Chen, J.H.; McInnis, D.O.; Guo, Q.L. The effect of methyl eugenol exposure on subsequent mating performance of sterile males of Bactrocera dorsalis. J. Appl. Entomol. 2013, 137, 238–243. [Google Scholar] [CrossRef]
- Chen, H.Y.; Chen, L.S.; Shen, Z.C.; Zhou, H.J.; Hao, L.; Xu, H.; Zhou, X.H. Synthesis of mesoporous silica post-loaded by methyl eugenol as an environment-friendly slow-release bio pesticide. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Vargas, R.I.; Leblanc, L.; Piñero, J.C.; Hoffman, K.M. Male Annihilation, Past, Present, and Future. In Trapping and the Detection, Control, and Regulation of Tephritid Fruit Flies; Springer: Berlin, Germany, 2014; pp. 493–511. [Google Scholar]
- Manoukis, N.C.; Vargas, R.I.; Carvalho, L.; Fezza, T.; Wilson, S.; Collier, T.; Shelly, T.E. A field test on the effectiveness of male annihilation technique against Bactrocera dorsalis (Diptera: Tephritidae) at varying application densities. PLoS ONE 2019, 14, e0213337. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.H.; Nishida, R.; Jang, E.B.; Shelly, T.E. Pheromones, Male Lures, and Trapping of Tephritid Fruit Flies. In Trapping and the Detection, Control, and Regulation of Tephritid Fruit Flies: Lures, Area-Wide Programs, and Trade Implications; Shelly, T., Epsky, N., Jang, E.B., Reyes-Flores, J., Vargas, R., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 15–74. [Google Scholar] [CrossRef]
- Mirani, Z. Study of diffusion and adoption of sustainable agricultural practice (Male Annihilation Technique). Int. J. Educ. Dev. Using ICT 2007, 3, 89–99. [Google Scholar]
- Manrakhan, A.; Daneel, J.-H.; Beck, R.; Love, C.N.; Gilbert, M.J.; Virgilio, M.; De Meyer, M. Effects of male lure dispensers and trap types for monitoring of Ceratitis capitata and Bactrocera dorsalis (Diptera: Tephritidae). Pest Manag. Sci. 2021, 77, 2219–2230. [Google Scholar] [CrossRef] [PubMed]
- Santos Sánchez, N.; Salas-Coronado, R.; Hernandez-Carlos, B.; Villanueva, C. Shikimic Acid Pathway in Biosynthesis of Phenolic Compounds. In Plant Physiological Aspects of Phenolic Compounds; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Christenson, L. The Male Annihilation Technique in the Control of Fruit Flies; ACS Publications: Washington, DC, USA, 1963. [Google Scholar]
- Ndlela, S.; Mohamed, S.; Ndegwa, P.; Ong’Amo, G.; Ekesi, S. Male annihilation technique using methyl eugenol for field suppression of Bactrocera dorsalis (Hendel)(Diptera: Tephritidae) on mango in Kenya. Afr. Entomol. 2016, 24, 437–447. [Google Scholar] [CrossRef]
- Tan, K.H.; Nishida, R. Methyl eugenol: Its occurrence, distribution, and role in nature, especially in relation to insect behavior and pollination. J. Insect Sci. 2012, 12, 56. [Google Scholar] [CrossRef] [PubMed]
- Kah-Wei Hee, A.; Tan, K.-H. Transport of methyl eugenol-derived sex pheromonal components in the male fruit fly, Bactrocera dorsalis. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2006, 143, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Law, J.H.; Regnier, F.E. Pheromones. Annu. Rev. Biochem. 1971, 40, 533–548. [Google Scholar] [CrossRef] [PubMed]
- Janssen, A.; Scheffer, J.; Ntezurubanza, L.; Svendsen, A.B. Antimicrobial activities of some Ocimum species grown in Rwanda. J. Ethnopharmacol. 1989, 26, 57–63. [Google Scholar] [CrossRef]
- Zoghbi, M.d.G.B.; Oliveira, J.; Andrade, E.H.A.; Trigo, J.R.; Fonseca, R.C.M.; Rocha, A.E.S. Variation in volatiles of Ocimum campechianum Mill. and Ocimum gratissimum L. cultivated in the North of Brazil. J. Essent. Oil Bear. Plants 2007, 10, 229–240. [Google Scholar] [CrossRef]
- Awasthi, P.; Dixit, S. Chemical Compositions of Ocimum sanctum Shyama and Ocimum sanctum Rama Oils from the plains of Northern India. J. Essent. Oil Bear. Plants 2007, 10, 292–296. [Google Scholar] [CrossRef]
- Muráriková, A.; Ťažký, A.; Neugebauerová, J.; Planková, A.; Jampílek, J.; Mučaji, P.; Mikuš, P.J.M. Characterization of essential oil composition in different basil species and pot cultures by a GC-MS method. Molecules 2017, 22, 1221. [Google Scholar] [CrossRef]
- Hossain, M.S.; Sarkar, B.C.; Hossain, M.M.; Mian, M.Y.; Rajotte, E.G.; Muniappan, R.; O’Rourke, M.E. Comparison of biorational management approaches against mango fruit fly (Bactrocera dorsalis Hendel) in Bangladesh. Crop. Prot. 2020, 135, 104807. [Google Scholar] [CrossRef]
- Pandey, A.K.; Singh, P.; Tripathi, N.N. Chemistry and bioactivities of essential oils of some Ocimum species: An overview. Asian Pac. J. Trop. Biomed. 2014, 4, 682–694. [Google Scholar] [CrossRef]
- Patel, R.P.; Singh, R.; Rao, B.R.R.; Singh, R.R.; Srivastava, A.; Lal, R.K. Differential response of genotype × environment on phenology, essential oil yield and quality of natural aroma chemicals of five Ocimum species. Ind. Crop. Prod. 2016, 87, 210–217. [Google Scholar] [CrossRef]
- Juntachote, T.; Berghofer, E.; Siebenhandl, S.; Bauer, F. The antioxidative properties of holy basil and galangal in cooked ground pork. Meat Sci. 2006, 72, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, N.; Rawal, S.; Verma, M.; Poddar, M.; Alok, S. A phytopharmacological overview on Ocimum species with special emphasis on Ocimum sanctum. Biomed. Prev. Nutr. 2013, 3, 185–192. [Google Scholar] [CrossRef]
- Grayer, R.J.; Kite, G.C.; Goldstone, F.J.; Bryan, S.E.; Paton, A.; Putievsky, E. Infraspecific taxonomy and essential oil chemotypes in sweet basil, Ocimum basilicum. Phytochemistry 1996, 43, 1033–1039. [Google Scholar] [CrossRef]
- Al-Maskri, A.Y.; Hanif, M.A.; Al-Maskari, M.Y.; Abraham, A.S.; Al-sabahi, J.N.; Al-Mantheri, O. Essential Oil from Ocimum basilicum (Omani Basil): A Desert Crop. Nat. Prod. Commun. 2011, 6. [Google Scholar] [CrossRef]
- Saran, P.L.; Tripathy, V.; Saha, A.; Kalariya, K.A.; Suthar, M.K.; Kumar, J. Selection of superior Ocimum sanctum L. accessions for industrial application. Ind. Crop. Prod. 2017, 108, 700–707. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Callahan, A.; Cantrell, C.L. Yield and oil composition of 38 basil (Ocimum basilicum L.) accessions grown in Mississippi. J. Agric. Food Chem. 2008, 56, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Avetisyan, A.; Markosian, A.; Petrosyan, M.; Sahakyan, N.; Babayan, A.; Aloyan, S.; Trchounian, A. Chemical composition and some biological activities of the essential oils from basil Ocimum different cultivars. BMC Complement. Altern. Med. 2017, 17, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jentzsch, P.V.; Ramos, L.A.; Ciobotă, V. Handheld Raman spectroscopy for the distinction of essential oils used in the cosmetics industry. Cosmetics 2015, 2, 162–176. [Google Scholar] [CrossRef]
- Tangpao, T.; Chung, H.-H.; Sommano, S.R. Aromatic profiles of essential oils from five commonly used Thai basils. Foods 2018, 7, 175. [Google Scholar] [CrossRef] [PubMed]
- Verghese, A.; Sreedevi, K.; Nagaraju, D.; Mala, B. A farmer-friendly trap for the management of the fruit fly Bactrocera spp. (Tephritidae: Diptera). Pest Manag. Hortic. Ecosyst. 2006, 12, 164–167. [Google Scholar]
- Chowdhary, K.; Kumar, A.; Sharma, S.; Pathak, R.; Jangir, M. Ocimum sp.: Source of biorational pesticides. Ind. Crop. Prod. 2018, 122, 686–701. [Google Scholar] [CrossRef]
- Ling Chang, C.; Kyu Cho, I.; Li, Q.X. Insecticidal activity of basil oil, trans-anethole, estragole, and linalool to adult fruit flies of Ceratitis capitata, Bactrocera dorsalis, and Bactrocera cucurbitae. J. Econ. Entomol. 2009, 102, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Turek, C.; Stintzing, F.C. Stability of essential oils: A Review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Maes, C.; Bouquillon, S.; Fauconnier, M.-L. Encapsulation of essential oils for the development of biosourced pesticides with controlled release: A review. Molecules 2019, 24, 2539. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, M.; Ho, T.M.; Bhandari, B.R. Encapsulation of tea tree oil by amorphous beta-cyclodextrin powder. Food Chem. 2017, 221, 1474–1483. [Google Scholar] [CrossRef] [PubMed]
- Sriwichai, T.; Junmahasathien, T.; Sookwong, P.; Potapohn, N.; Sommano, S. Evaluation of the optimum harvesting maturity of makhwaen fruit for the perfumery industry. Agriculture 2019, 9, 78. [Google Scholar] [CrossRef]
- De Lira, C.S.; Pontual, E.V.; de Albuquerque, L.P.; Paiva, L.M.; Paiva, P.M.G.; de Oliveira, J.V.; Napoleão, T.H.; Navarro, D.M.d.A.F. Evaluation of the toxicity of essential oil from Alpinia purpurata inflorescences to Sitophilus zeamais (maize weevil). Crop. Prot. 2015, 71, 95–100. [Google Scholar] [CrossRef]
- Biasazin, T.D.; Chernet, H.T.; Herrera, S.L.; Bengtsson, M.; Karlsson, M.F.; Lemmen-Lechelt, J.K.; Dekker, T. Detection of volatile constituents from food lures by Tephritid fruit flies. Insects 2018, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- McKinney, R.M.; Vernier, C.; Ben-Shahar, Y. The neural basis for insect pheromonal communication. Curr. Opin. Insect Sci. 2015, 12, 86–92. [Google Scholar] [CrossRef][Green Version]
- Bhandari, B.R.; D’Arc, B.R.; Padukka, I. Encapsulation of lemon oil by paste method using β-cyclodextrin: Encapsulation efficiency and profile of oil volatiles. J. Agric. Food Chem. 1999, 47, 5194–5197. [Google Scholar] [CrossRef]
- Carneiro, H.C.F.; Tonon, R.V.; Grosso, C.R.F.; Hubinger, M.D. Encapsulation efficiency and oxidative stability of flaxseed oil microencapsulated by spray drying using different combinations of wall materials. J. Food Eng. 2013, 115, 443–451. [Google Scholar] [CrossRef]
- Zhang, T.; Luo, Y.; Wang, M.; Chen, F.; Liu, J.; Meng, K.; Zhao, H. Double-layered microcapsules significantly improve the long-term effectiveness of essential oil. Polymers 2020, 12, 1651. [Google Scholar] [CrossRef]
- Lopez, M.D.; Maudhuit, A.; Pascual-Villalobos, M.J.; Poncelet, D. Development of formulations to improve the controlled-release of linalool to be applied as an insecticide. J. Agric. Food Chem. 2012, 60, 1187–1192. [Google Scholar] [CrossRef] [PubMed]
- Broumas, T.; Haniotakis, G.E. Comparative field studies of various traps and attractants of the olive fruit fly, Bactrocera oleae. Entomol. Exp. Appl. 1994, 73, 145–150. [Google Scholar] [CrossRef]
- Sutaphanit, P.; Chitprasert, P. Optimisation of microencapsulation of holy basil essential oil in gelatin by response surface methodology. Food Chem. 2014, 150, 313–320. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Ristivojevic, P.; Gegechkori, V.; Litvinova, T.M.; Morton, D.W. Essential oil quality and purity evaluation via ft-ir spectroscopy and pattern recognition techniques. Appl. Sci. 2020, 10, 7294. [Google Scholar] [CrossRef]
- Khalid, K.A. Influence of water stress on growth, essential oil, and chemical composition of herbs (Ocimum sp.). Int. Agrophysics 2006, 20, 289–296. [Google Scholar]
- Abdollahi Mandoulakani, B.; Eyvazpour, E.; Ghadimzadeh, M. The effect of drought stress on the expression of key genes involved in the biosynthesis of phenylpropanoids and essential oil components in basil (Ocimum basilicum L.). Phytochemistry 2017, 139, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rezaie, R.; Mandoulakani, B.A.; Fattahi, M. Cold stress changes antioxidant defense system, phenylpropanoid contents and expression of genes involved in their biosynthesis in Ocimum basilicum L. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.K. Chemical composition and antimicrobial activity of the essential oil of Ocimum basilicum L. (sweet basil) from Western Ghats of North West Karnataka, India. Anc. Sci. Life 2014, 33, 151. [Google Scholar] [CrossRef]
- Kothari, S.K.; Bhattacharya, A.K.; Ramesh, S. Essential oil yield and quality of methyl eugenol rich Ocimum tenuiflorum L.f. (syn. O. sanctum L.) grown in south India as influenced by method of harvest. J. Chromatogr. A 2004, 1054, 67–72. [Google Scholar] [CrossRef]
- Nishida, R.; Tan, K.H.; Serit, M.; Lajis, N.H.; Sukari, A.M.; Takahashi, S.; Fukami, H. Accumulation of phenylpropanoids in the rectal glands of males of the Oriental fruit fly, Dacus dorsalis. Experientia 1988, 44, 534–536. [Google Scholar] [CrossRef]
- Gomez-Diaz, C.; Martin, F.; Garcia-Fernandez, J.M.; Alcorta, E. The two main olfactory receptor families in Drosophila, ORs and IRs: A comparative approach. Front. Cell. Neurosci. 2018, 12, 253. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Smagghe, G.; Lei, Z.; Wang, J.-J. Identification of male- and female-specific olfaction genes in antennae of the Oriental fruit fly (Bactrocera dorsalis). PLoS ONE 2016, 11, e0147783. [Google Scholar] [CrossRef] [PubMed]
- Leal, W.S. Odorant Reception in Insects: Roles of receptors, binding proteins, and degrading enzymes. Annu. Rev. Entomol. 2013, 58, 373–391. [Google Scholar] [CrossRef] [PubMed]
- Benton, R.; Sachse, S.; Michnick, S.W.; Vosshall, L.B. Atypical membrane topology and heteromeric function of drosophila odorant receptors in vivo. PLoS Biol. 2006, 4, e20. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xu, P.; Sanchez, S.; Duran, P.; Andreazza, F.; Isaacs, R.; Dong, K. Behavioral and physiological responses of Drosophila melanogaster and D. suzukii to volatiles from plant essential oils. Pest Manag. Sci 2021, 77, 3698–3705. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, R.L.; Metcalf, E.R.; Mitchell, W.C.; Lee, L.W. Evolution of olfactory receptor in Oriental fruit fly Dacus dorsalis. Proc. Natl. Acad. Sci. USA 1979, 76, 1561–1565. [Google Scholar] [CrossRef]
- Akdeniz, B.; Sumnu, G.; Sahin, S. Microencapsulation of phenolic compounds extracted from onion (Allium cepa) skin. J. Food Process. Preserv. 2018, 42, e13648. [Google Scholar] [CrossRef]
- Ravichandran, K.; Palaniraj, R.; Saw, N.M.M.T.; Gabr, A.M.M.; Ahmed, A.R.; Knorr, D.; Smetanska, I. Effects of different encapsulation agents and drying process on stability of betalains extract. J. Food Sci. Technol. 2014, 51, 2216–2221. [Google Scholar] [CrossRef]
- Robert, P.; Gorena, T.; Romero, N.; Sepulveda, E.; Chavez, J.; Saenz, C. Encapsulation of polyphenols and anthocyanins from pomegranate (Punica granatum) by spray drying. Int. J. Food Sci. Technol. 2010, 45, 1386–1394. [Google Scholar] [CrossRef]
- Hosseini, A.; Jafari, S.M.; Mirzaei, H.; Asghari, A.; Akhavan, S. Application of image processing to assess emulsion stability and emulsification properties of Arabic gum. Carbohydr. Polym. 2015, 126, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Akhavan Mahdavi, S.; Jafari, S.M.; Assadpoor, E.; Dehnad, D. Microencapsulation optimization of natural anthocyanins with maltodextrin, gum Arabic and gelatin. Int. J. Biol. Macromol. 2016, 85, 379–385. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, L.M.; Araújo, A.; Mendes, N.H.D.; de Souza, J.M.G.A.; Menezes, A.A.L. The temporal pattern of mating behavior of the fruit fly, Anastrepha zenildae in the laboratory. J. Insect Sci. 2011, 11, 151. [Google Scholar] [CrossRef] [PubMed]
- Dukas, R. Natural history of social and sexual behavior in fruit flies. Sci. Rep. 2020, 10, 21932. [Google Scholar] [CrossRef] [PubMed]
- Poramarcom, R.; Boake, C.R.B. Behavioural influences on male mating success in the Oriental fruit fly, Dacus dorsalis Hendel. Anim. Behav. 1991, 42, 453–460. [Google Scholar] [CrossRef]
- Zhang, S.X.; Miner, L.E.; Boutros, C.L.; Rogulja, D.; Crickmore, M.A. Motivation, perception, and chance converge to make a binary decision. Neuron 2018, 99, 376–388.e376. [Google Scholar] [CrossRef] [PubMed]
- Haq, I.u.; Cáceres, C.; Meza, J.S.; Hendrichs, J.; Vreysen, M.J.B. Different methods of methyl eugenol application enhance the mating success of male Oriental fruit fly (Dipera: Tephritidae). Sci. Rep. 2018, 8, 6033. [Google Scholar] [CrossRef]


| Treatments | Ratio | Emblem |
|---|---|---|
| Maltodextrin (MD) | 100 | MD100 |
| Gum Arabic (GA) | 100 | GA100 |
| MD–GA | 50–50 | MD50GA50 |
| MD–GA | 75–25 | MD75GA25 |
| MD–GA | 25–75 | MD25GA75 |
| No. | RI | Compounds | Volatile Types | Amount of Chemical (µg/mL Essential Oils ± SE) | ||||
|---|---|---|---|---|---|---|---|---|
| LB | RB | TB | TrB | WB | ||||
| 1 | 785 | methyl 2-methylbutanoate | others | nd | nd | nd | 2.56 ± 0.07 | nd |
| 2 | 818 | (E)-hex-2-enal | others | nd | nd | nd | 1.04 ± 0.12 | nd |
| 3 | 974 | β-pinene | monoterpene | nd | nd | 2.72 ± 0.06 | nd | nd |
| 4 | 976 | 1-octen-3-ol | others | 3.64 ± 0.12 | nd | nd | 7.58 ± 0.27 | nd |
| 5 | 981 | 6-methyl-5-hepten-2-one | others | 11.02 ± 0.41 | nd | nd | 2.25 ± 0.08 | nd |
| 6 | 987 | myrcene | monoterpene | nd | nd | 3.15 ± 0.11 | 7.89 ± 0.21 | nd |
| 7 | 991 | 6-methyl-5-Hepten-2-ol | others | 1.25 ± 0.06 | nd | nd | nd | nd |
| 8 | 996 | 3-octanol | others | nd | nd | nd | 4.29 ± 0.14 | nd |
| 9 | 1127 | l-limonene | monoterpene | nd | nd | 2.23 ± 0.08 | nd | nd |
| 10 | 1129 | 1,8-cineole | monoterpenoid | 2.41 ± 0.09 | nd | 37.91 ± 1.28 | nd | nd |
| 11 | 1136 | trans-ocimene | monoterpene | nd | nd | nd | 169.23 ± 4.57 | nd |
| 12 | 1144 | (E)-3,7-dimethylocta-1,3,6-triene | monoterpene | nd | nd | 9.42 ± 0.34 | nd | nd |
| 13 | 1144 | ocimene | monoterpene | nd | nd | nd | 7.98 ± 0.26 | nd |
| 14 | 1185 | fenchone | monoterpenoid | 2.64 ± 0.09 | nd | nd | nd | nd |
| 15 | 1198 | linalool | monoterpenoid | 32.64 ± 1.33 | 6.95 ± 0.21 | 1.77 ± 0.07 | 45.01 ± 1.90 | 7.41 ± 0.41 |
| 16 | 1201 | 6-methyl-hepta-3,5-dien-2-one | others | nd | nd | nd | 2.00 ± 0.12 | nd |
| 17 | 1219 | (3E,5E)-2,6-dimethyl-1,3,5,7-octatetraene | monoterpene | nd | nd | nd | 2.42 ± 0.06 | nd |
| 18 | 1226 | 2,6-dimethyl-2,4,6-octatriene | monoterpene | nd | nd | nd | 20.29 ± 0.51 | nd |
| 19 | 1237 | trans-epoxyocimene | monoterpenoid | nd | nd | nd | 0.89 ± 0.05 | nd |
| 20 | 1242 | camphor | monoterpenoid | 5.48 ± 0.22 | nd | 13.57 ± 0.57 | nd | 3.25 ± 0.13 |
| 21 | 1249 | nerol oxide | monoterpenoid | 2.01 ± 0.11 | nd | nd | nd | nd |
| 22 | 1267 | l-borneol | monoterpenoid | nd | 10.50 ± 0.32 | nd | nd | 11.99 ±0.42 |
| 23 | 1268 | p-Mentha-1,5-dien-8-ol | monoterpenoid | nd | nd | nd | 2.13 ± 0.10 | nd |
| 24 | 1292 | fenchol | monoterpenoid | 13.05 ± 0.30 | nd | 1.48 ± 0.04 | nd | 3.65 ± 0.10 |
| 25 | 1294 | estragole | phenylpropanoid | 316.87 ± 15.12 | 14.98 ± 0.47 | 609.47 ± 20.00 | nd | 84.17 ± 2.81 |
| 26 | 1299 | (E,E)-2,6-dimethyl-3,5,7-octatrien-2-ol | monoterpenoid | nd | nd | nd | 10.96 ±0.43 | nd |
| 27 | 1323 | nerol | monoterpenoid | 15.35 ± 0.66 | nd | nd | nd | nd |
| 28 | 1335 | neral | monoterpenoid | 13.43 ± 0.55 | nd | nd | nd | nd |
| 29 | 1349 | geraniol | monoterpenoid | 9.67 ± 0.35 | nd | nd | nd | nd |
| 30 | 1365 | geranial | monoterpenoid | 18.74 ± 0.76 | nd | nd | nd | nd |
| 31 | 1380 | bornyl acetate | others | 2.48 ± 0.10 | nd | nd | nd | nd |
| 32 | 1447 | eugenol | phenylpropanoid | 3.15 ± 0.12 | 48.84 ± 1.28 | nd | 224.24 ± 9.97 | 83.04 ± 2.79 |
| 33 | 1471 | α-copaene | sesquiterpene | 3.57 ± 0.18 | 24.67 ± 0.13 | nd | 25.06 ± 1.15 | 8.86 ± 0.31 |
| 34 | 1478 | β-elemene | sesquiterpene | 9.38 ± 0.35 | 119.06 ± 3.99 | nd | 2.97 ± 0.19 | 68.52 ± 2.89 |
| 35 | 1479 | β-bourbonene | sesquiterpene | nd | nd | nd | 2.78 ±0.13 | nd |
| 36 | 1497 | methyl eugenol | phenylpropanoid | 28.12 ± 1.08 | 335.58 ± 11.99 | 9.57 ± 1.30 | 1.11 ± 0.01 | 372.57 ± 9.49 |
| 37 | 1509 | rotundene | sesquiterpene | nd | 1.48 ± 0.02 | nd | nd | nd |
| 38 | 1515 | trans-caryophyllene | sesquiterpene | 51.20 ± 2.17 | 193.96 ± 6.54 | nd | 19.11 ± 0.95 | 99.38 ± 3.20 |
| 39 | 1532 | trans-α-bergamotene | sesquiterpene | 27.55 ± 1.18 | nd | 10.91 ± 0.61 | 83.64 ± 3.58 | 10.08 ±0.38 |
| 40 | 1553 | α-humulene | sesquiterpene | 18.49 ± 0.76 | 14.82 ± 0.41 | nd | 2.68 ± 0.30 | 9.95 ± 0.38 |
| 41 | 1581 | germacrene | sesquiterpene | 13.31 ± 0.54 | 60.35 ± 2.12 | 2.83 ± 0.15 | 37.65 ± 1.77 | 25.13 ± 0.87 |
| 42 | 1585 | trans-β-farnesene | sesquiterpene | 2.71 ± 0.11 | nd | nd | 7.40 ± 0.39 | nd |
| 43 | 1589 | β-selinene | sesquiterpene | 3.53 ± 0.13 | 7.18 ± 0.20 | nd | nd | 6.21 ± 0.26 |
| 44 | 1596 | (Z,E)-α-farnesene | sesquiterpene | nd | nd | nd | 24.55 ±1.03 | nd |
| 45 | 1596 | α-selinene | sesquiterpene | 2.96 ± 0.12 | 13.85 ± 0.33 | nd | nd | 7.79 ± 0.39 |
| 46 | 1603 | α-bulnesene | sesquiterpene | nd | nd | 1.06 ± 0.01 | nd | 1.46 ± 0.06 |
| 47 | 1611 | trans-α-bisabolene | sesquiterpene | 2.89 ± 0.12 | nd | nd | 0.68 ± 0.03 | nd |
| 48 | 1614 | β-copaene | sesquiterpene | 5.66 ± 0.27 | nd | 4.97 ± 0.28 | nd | 4.28 ± 0.17 |
| 49 | 1619 | δ-cadinene | sesquiterpene | 3.31 ± 0.13 | 9.37 ± 0.20 | nd | 7.21 ± 0.37 | 5.39 ± 0.25 |
| 50 | 1625 | β-sesquiphellandrene | sesquiterpene | nd | nd | nd | 5.49 ±0.30 | nd |
| 51 | 1641 | cis-α-bisabolene | sesquiterpene | 37.24 ± 1.47 | nd | nd | nd | 2.06 ± 0.07 |
| 52 | 1647 | elemol | sesquiterpenoid | nd | 2.94 ± 0.02 | nd | nd | 2.30 ± 0.19 |
| 53 | 1671 | spathulenol | sesquiterpenoid | nd | nd | 1.36 ±0.03 | 3.27 ±0.16 | nd |
| 54 | 1676 | caryophyllene oxide | sesquiterpenoid | 30.16 ± 1.09 | 21.31 ± 0.44 | nd | 13.26 ± 0.65 | 15.71 ± 0.61 |
| 55 | 1682 | lanceol | sesquiterpenoid | nd | nd | nd | 1.58 ± 0.02 | nd |
| 56 | 1686 | salvial-4(14)-en-1-one | sesquiterpenoid | nd | nd | nd | 2.63 ± 0.27 | nd |
| 57 | 1801 | humulene epoxide II | sesquiterpenoid | 7.94 ± 0.29 | nd | 2.11 ± 0.00 | nd | nd |
| 58 | 1808 | cubenol | sesquiterpenoid | nd | nd | 2.21 ± 0.14 | nd | nd |
| 59 | 1809 | ledene oxide-(II) | sesquiterpenoid | nd | nd | nd | 3.99 ± 0.04 | nd |
| 60 | 1835 | τ-cadinol | sesquiterpenoid | 3.28 ± 0.11 | nd | 14.08 ± 0.79 | nd | 3.45 ± 0.16 |
| 61 | 1842 | 8-methylene-dispiro [2.1.2.4] undecane | others | 1.78 ± 0.04 | nd | nd | nd | nd |
| 62 | 1847 | β-eudesmol | sesquiterpenoid | 1.25 ± 0.03 | nd | nd | nd | nd |
| 63 | 1849 | α-cadinol | sesquiterpenoid | nd | nd | nd | 2.99 ± 0.05 | nd |
| 64 | 1850 | juniper camphor | sesquiterpenoid | nd | 5.73 ± 1.50 | nd | nd | 2.59 ± 0.12 |
| 65 | 1880 | α-bisabolol | sesquiterpenoid | 2.37 ± 0.07 | nd | nd | nd | nd |
| Percent yield essential oils from dried plant materials | 0.86 ± 0.32 | 1.02 ± 0.21 | 1.13 ± 0.32 | 0.26 ± 0.02 | 1.04 ± 0.06 | |||
| Compounds detected | 36 | 17 | 18 | 35 | 23 | |||
| Monoterpene hydrocarbons (%) | 0.0 | 0.0 | 2.4 | 27.4 | 0.0 | |||
| Oxygenated monoterpene (%) | 16.2 | 2.0 | 7.5 | 7.8 | 3.1 | |||
| Sesquiterpene hydrocarbons (%) | 25.6 | 49.9 | 2.7 | 28.9 | 29.7 | |||
| Oxygenated sesquiterpenes (%) | 6.3 | 3.4 | 2.7 | 3.7 | 2.9 | |||
| Others (%) | 51.8 | 44.8 | 84.7 | 32.3 | 64.3 | |||
| Volatile Organic Compounds | Average of Number of Trapped Insects | ||
|---|---|---|---|
| Treatment (n ± SE/100) | Control (n ± SE/100) | ||
| LB | 5.00 ± 1.68 a | 0 | |
| 95.00%♂ | 5.00%♀ | ||
| RB | 25.00 ± 2.48 bc | 0.5 ± 0.25 | |
| 90.00%♂ | 10.00%♀ | ||
| TB | 4.00 ± 1.47 a | 0.25 ± 0.22 | |
| 93.75%♂ | 6.25%♀ | ||
| TrB | 2.00 ± 1.41 a | 0.25 ± 0.22 | |
| 100.00%♂ | 0.00%♀ | ||
| WB | 18.75 ± 2.69 b | 0 | |
| 94.66%♂ | 5.44%♀ | ||
| ME | 28.75 ± 2.87 c | 0.5 ± 0.25 | |
| 89.56%♂ | 10.44%♀ | ||
| Volatile Organic Compounds | Frequency of Movement | |||
|---|---|---|---|---|
| Male | Female | |||
| Antennae | Forelegs | Antennae | Forelegs | |
| LB | + | ++ | ++ | ++ |
| RB | +++ | +++ | +++ | +++ |
| TB | +++ | ++++ | +++ | +++ |
| TrB | ++ | + | ++ | + |
| WB | ++++ | ++++ | ++ | +++ |
| ME | ++ | +++++ | ++ | ++ |
| Ethanol | + | ++ | + | +++ |
| Wall Materials | Emulsion | WBO-Encapsulated Products | ||
|---|---|---|---|---|
| Viscosity (cP) | Stability (%Separation) | Oil Loading (µL/0.2 g) * | Encapsulation Efficiency (%) | |
| MD100 | 150.0 | >1 | 0.163 ± 0.0218 a | 4.27 |
| GA100 | 2976.0 | - | 0.303 ± 0.0362 ab | 7.92 |
| MD50GA50 | 631.0 | - | 0.313 ± 0.0728 ab | 8.18 |
| MD25GA75 | 1348.0 | - | 0.360 ± 0.0678 b | 9.39 |
| MD75GA25 | 422.0 | >1 | 0.235 ± 0.0493 ab | 6.14 |
| The Structure of Processed Maltodextrin and Gum Arabic | |
|---|---|
| MD100 | GA100 |
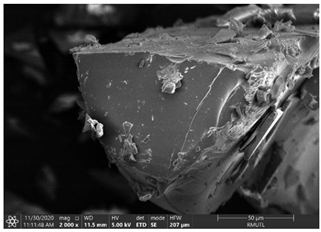 | 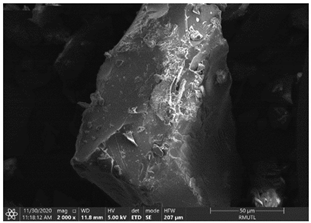 |
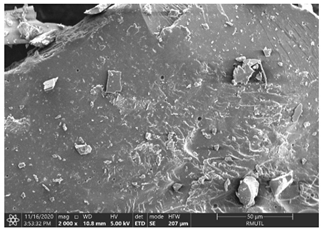
| The Structure of White Holy Basil Essential Oil (WBO) Loaded Capsules | |
| WBO-MD100 | WBO-GA100 |
 | 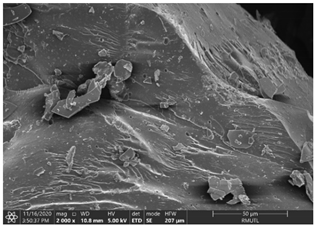 |
| WBO-MD50GA50 | WBO-MD25GA75 |
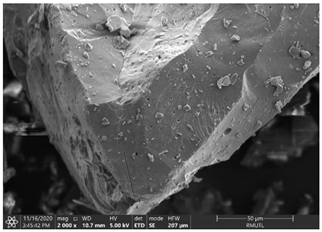 |  |
| WBO-MD75GA25 | |
 | |
| Product Formulas (Treatments) | The Average Number of Oriental Fruit Fly (Fly/Trap) | ||||
|---|---|---|---|---|---|
| Time of Exposure in the Field | |||||
| Mins | h | ||||
| 30 | 60 | 120 | 24 | 72 | |
| MD100 | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.25 ± 0.46 a | 0.75 ± 0.89 b |
| GA100 | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.25 ± 0.46 a | 0.50 ± 0.92 ab |
| MD50GA50 | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.31 ± 0.33 a | 0.31 ± 0.33 a |
| MD25GA75 | 0.25 ± 0.46 ab | 0.25 ± 0.46 a | 0.25 ± 0.46 a | 1.00 ± 0.75 bc | 1.00 ± 0.75 b |
| MD75GA25 | 0.75 ± 1.39 b | 1.00 ± 1.30 b | 1.25 ± 1.16 b | 1.50 ± 0.92 c | 2.00 ± 0.00 c |
| White holy basil essential oil | 0.00 ± 0.00 a | 0.25 ± 0.46 a | 0.25 ± 0.46 a | 0.50 ± 0.53 b | 0.50 ± 0.53 ab |
| Control (CTR) | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tangpao, T.; Krutmuang, P.; Kumpoun, W.; Jantrawut, P.; Pusadee, T.; Cheewangkoon, R.; Sommano, S.R.; Chuttong, B. Encapsulation of Basil Essential Oil by Paste Method and Combined Application with Mechanical Trap for Oriental Fruit Fly Control. Insects 2021, 12, 633. https://doi.org/10.3390/insects12070633
Tangpao T, Krutmuang P, Kumpoun W, Jantrawut P, Pusadee T, Cheewangkoon R, Sommano SR, Chuttong B. Encapsulation of Basil Essential Oil by Paste Method and Combined Application with Mechanical Trap for Oriental Fruit Fly Control. Insects. 2021; 12(7):633. https://doi.org/10.3390/insects12070633
Chicago/Turabian StyleTangpao, Tibet, Patcharin Krutmuang, Wilawan Kumpoun, Pensak Jantrawut, Tonapha Pusadee, Ratchadawan Cheewangkoon, Sarana Rose Sommano, and Bajaree Chuttong. 2021. "Encapsulation of Basil Essential Oil by Paste Method and Combined Application with Mechanical Trap for Oriental Fruit Fly Control" Insects 12, no. 7: 633. https://doi.org/10.3390/insects12070633
APA StyleTangpao, T., Krutmuang, P., Kumpoun, W., Jantrawut, P., Pusadee, T., Cheewangkoon, R., Sommano, S. R., & Chuttong, B. (2021). Encapsulation of Basil Essential Oil by Paste Method and Combined Application with Mechanical Trap for Oriental Fruit Fly Control. Insects, 12(7), 633. https://doi.org/10.3390/insects12070633










