Species Identification of Wireworms (Agriotes spp.; Coleoptera: Elateridae) of Agricultural Importance in Europe: A New “Horizontal Identification Table”
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
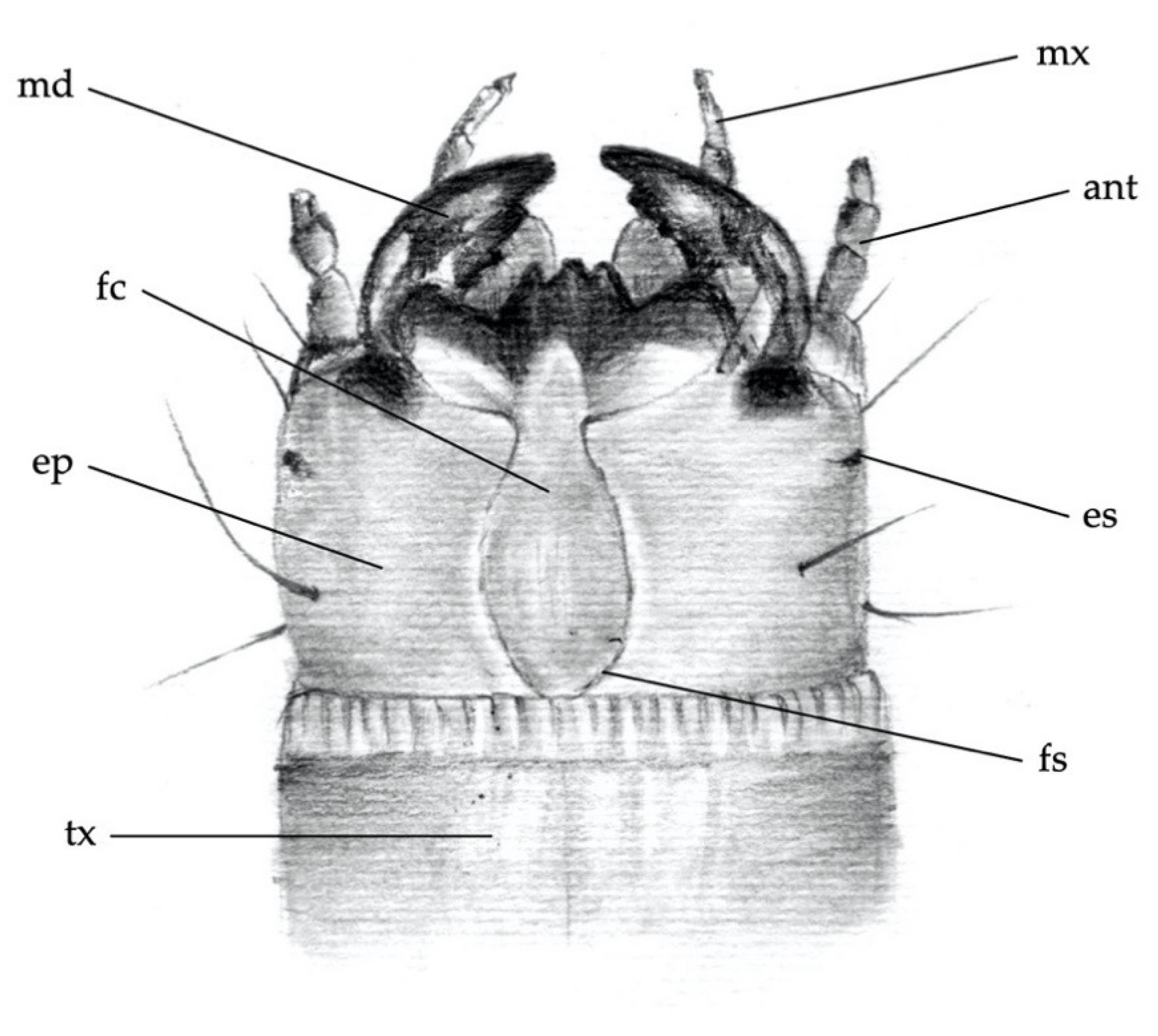
2.1. First Step: Larvae Description per Species
- Elateridae larvae collected in the field in several European countries (listed below) were reared until the adult stage in single vials, according to the method described by Furlan [36,37]. The rearing was performed in order to associate the larva (and its morphological features) with the adult. Images were identified by Giuseppe Platia, a leading expert in Palearctic Elateridae [38];
- rearing cages were built and used to house identified beetles for egg deposition [36];
- click beetles were collected in the field in Germany, identified by keys [39,40] and wireworms reared from these beetles following the method by Kölliker et al. [41]. The larval specimens from known species were then checked for consistency with existing keys [7,27,30,31] and also compared with field-collected larvae, mainly from Germany.
2.2. Second Step: Discriminating Characters
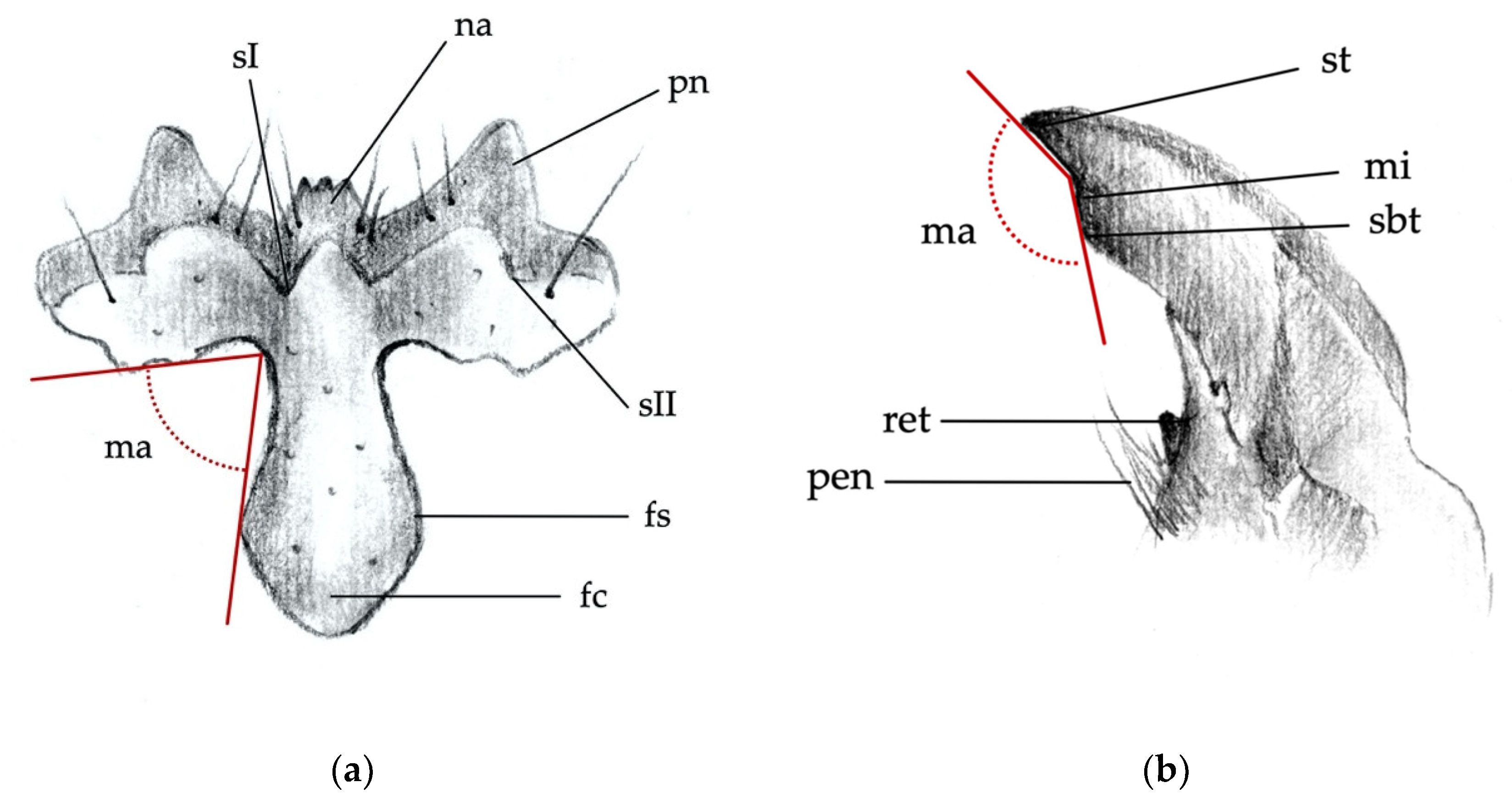
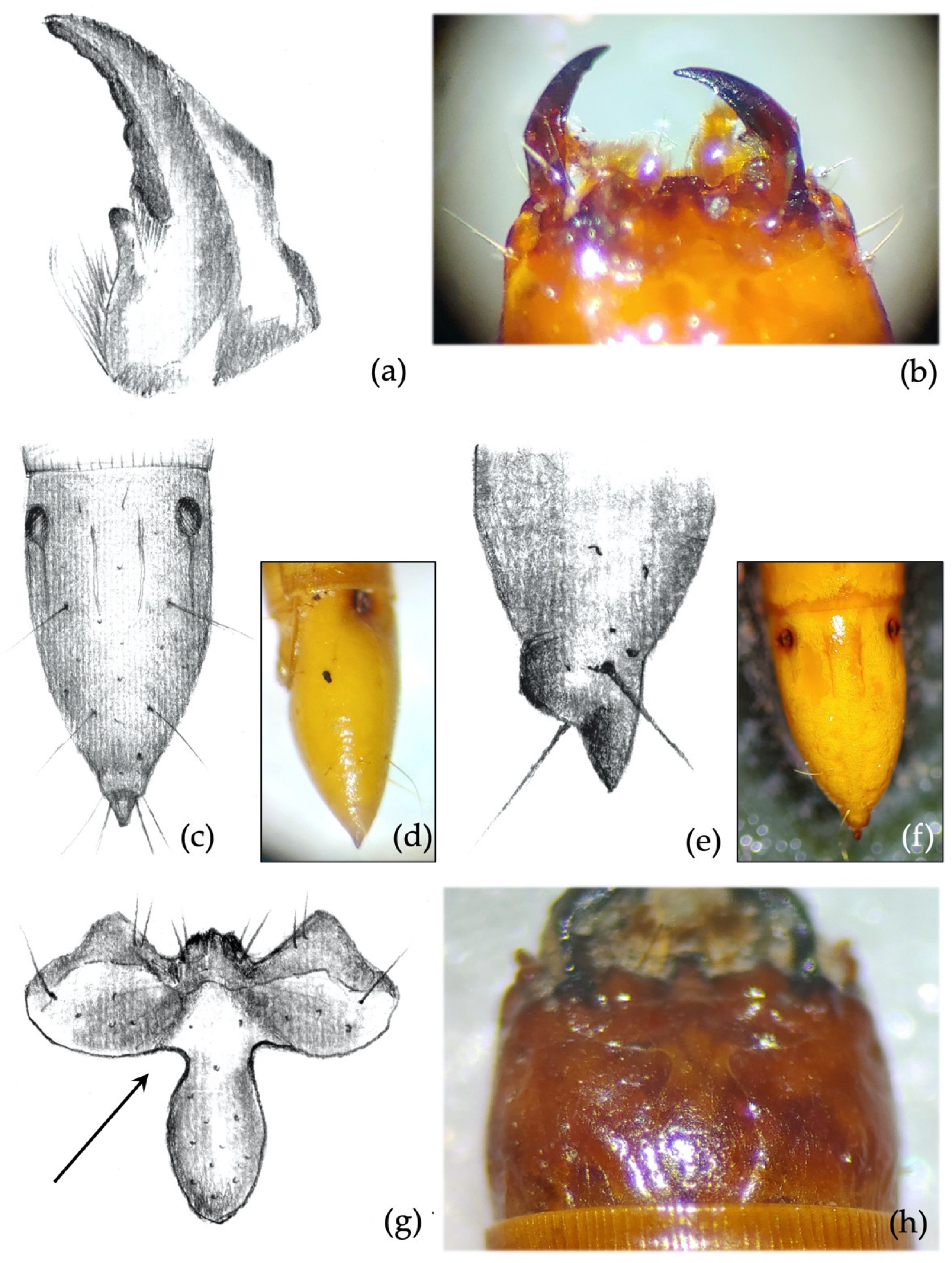
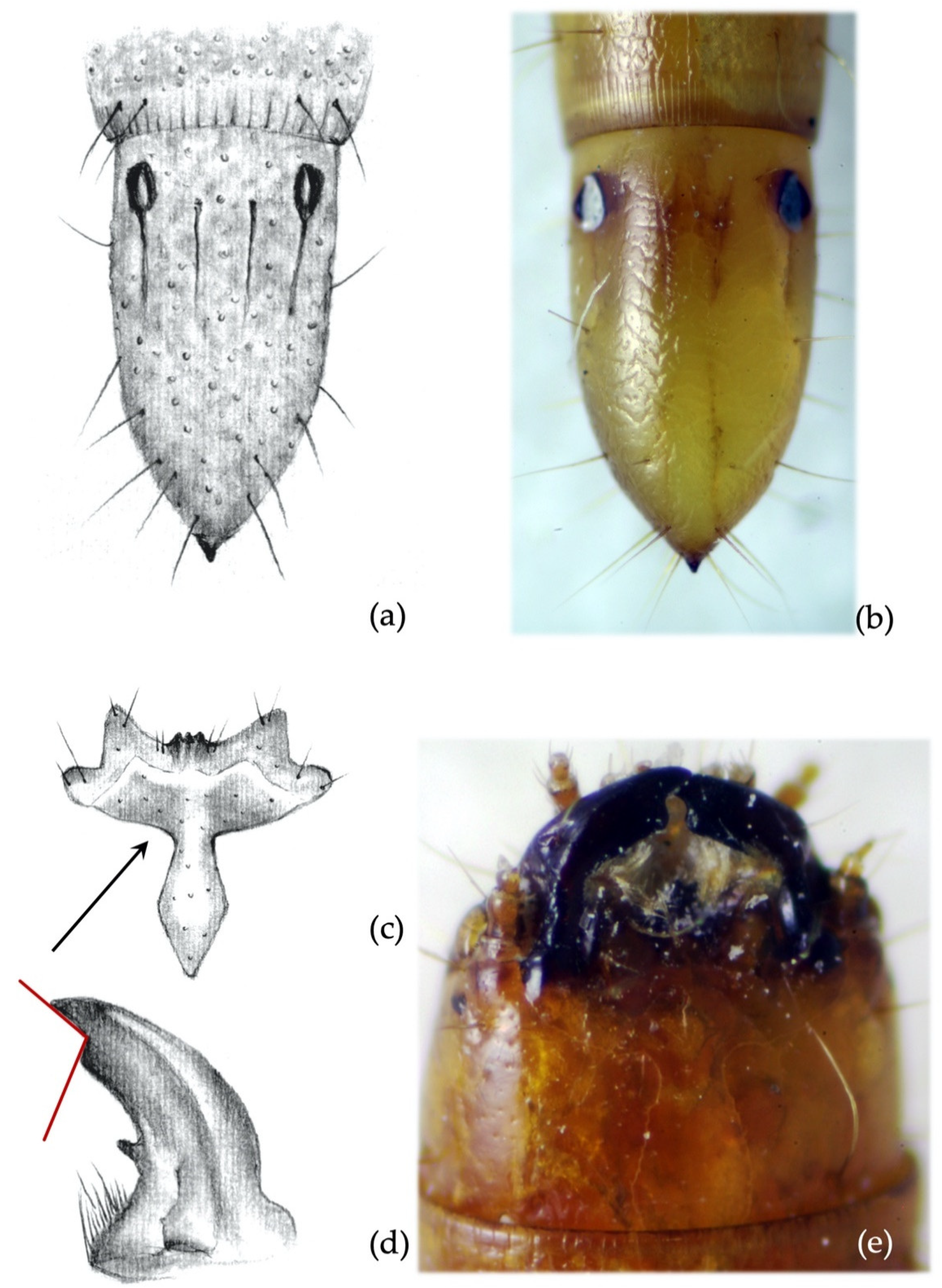
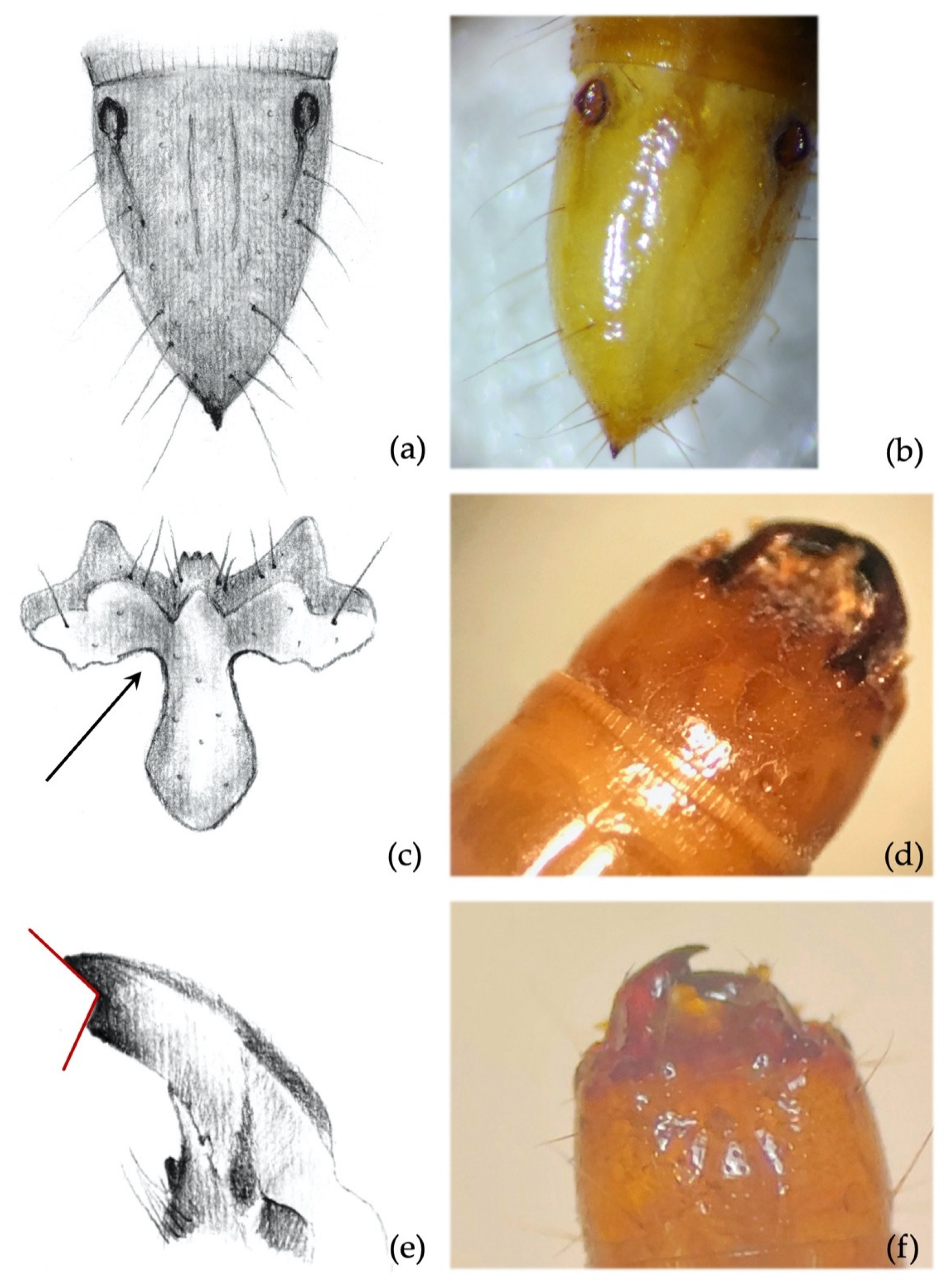
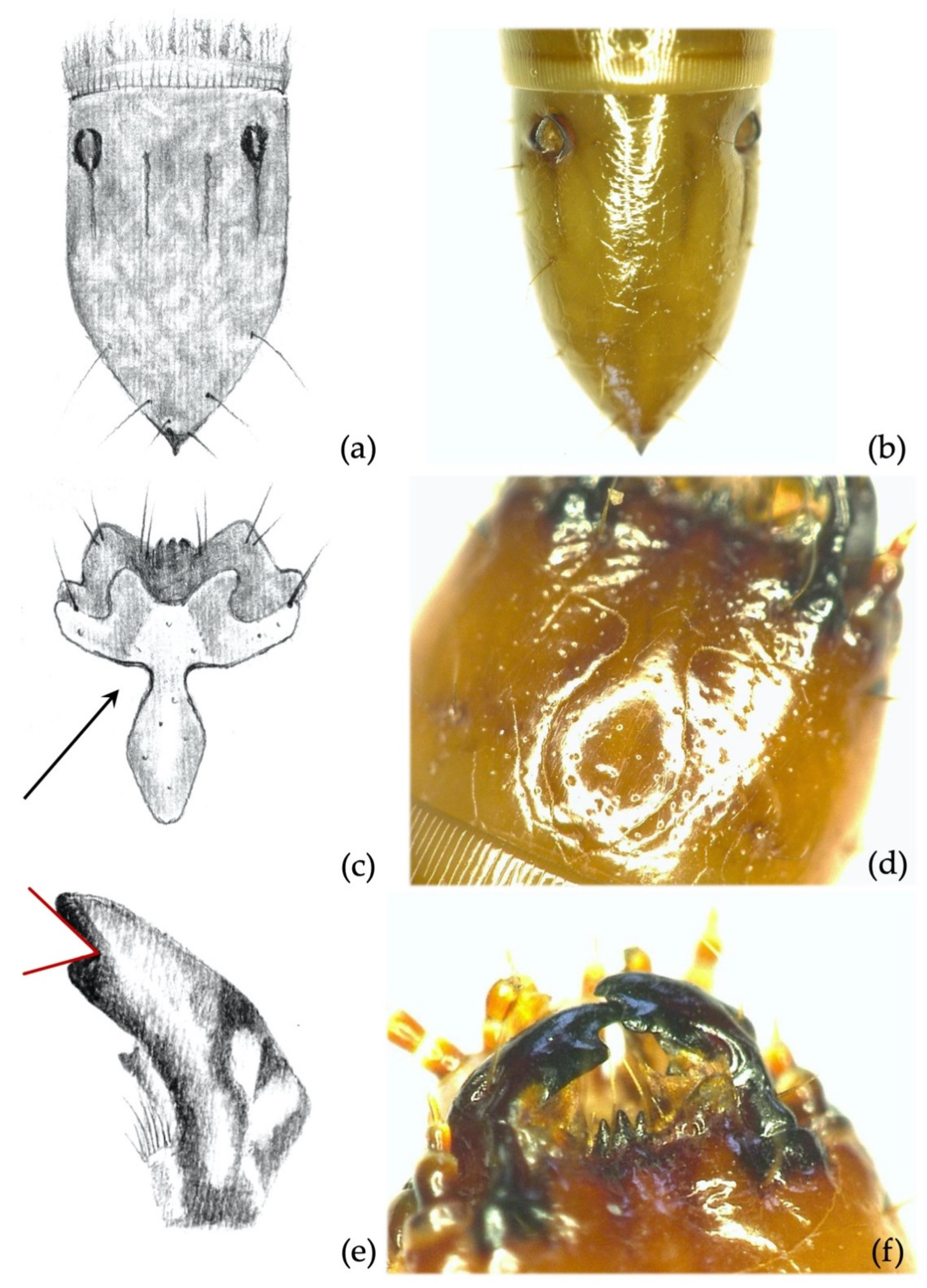
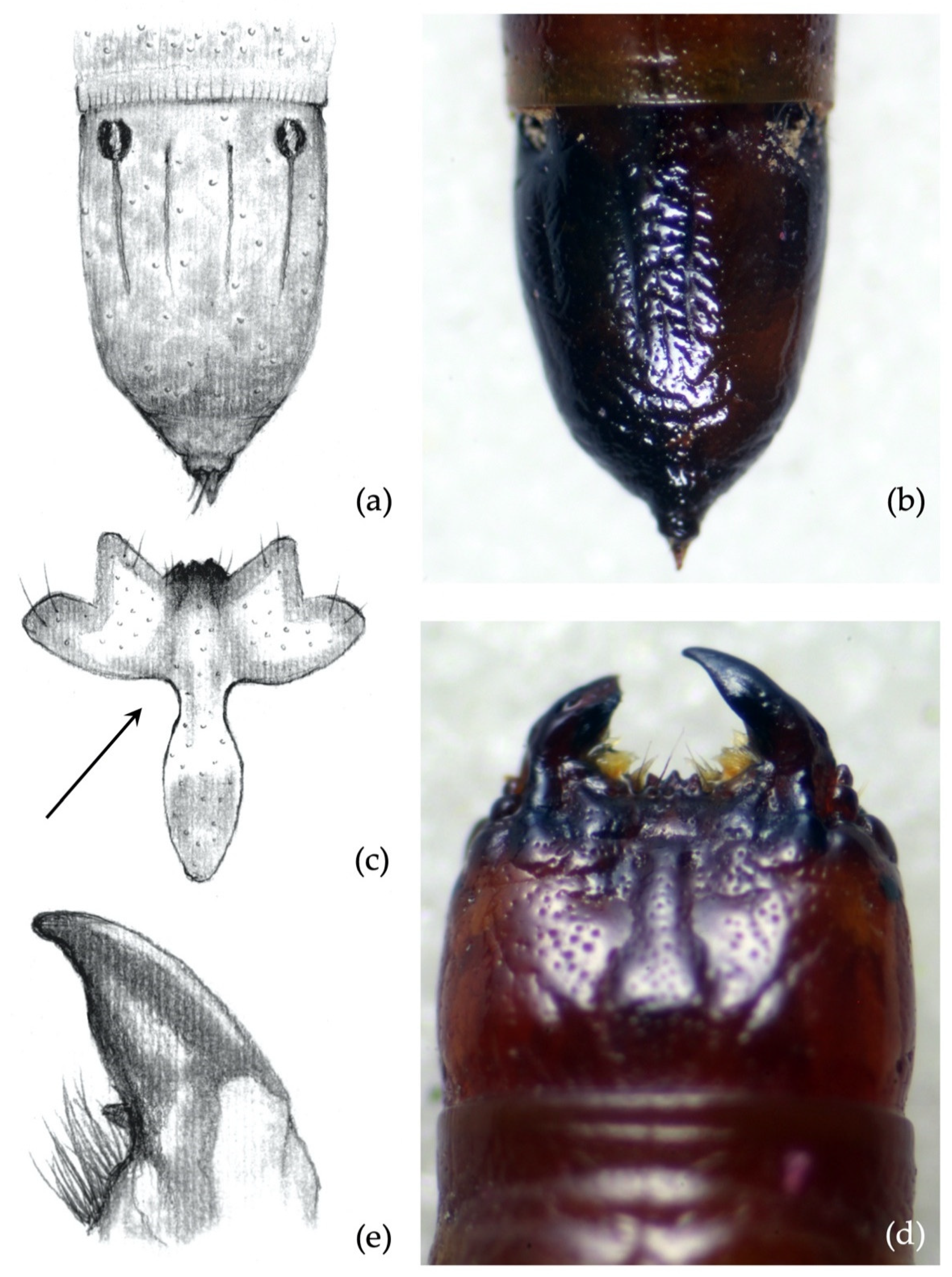
- Clypeus: absence/presence and density of the punctures on the clypeus; amplitude of the measured angle (ma) (Figure 2a). Some taxa present a certain level of intraspecific variability in the amplitude of the angle, with a certain overlap in amplitude being found in a few species.
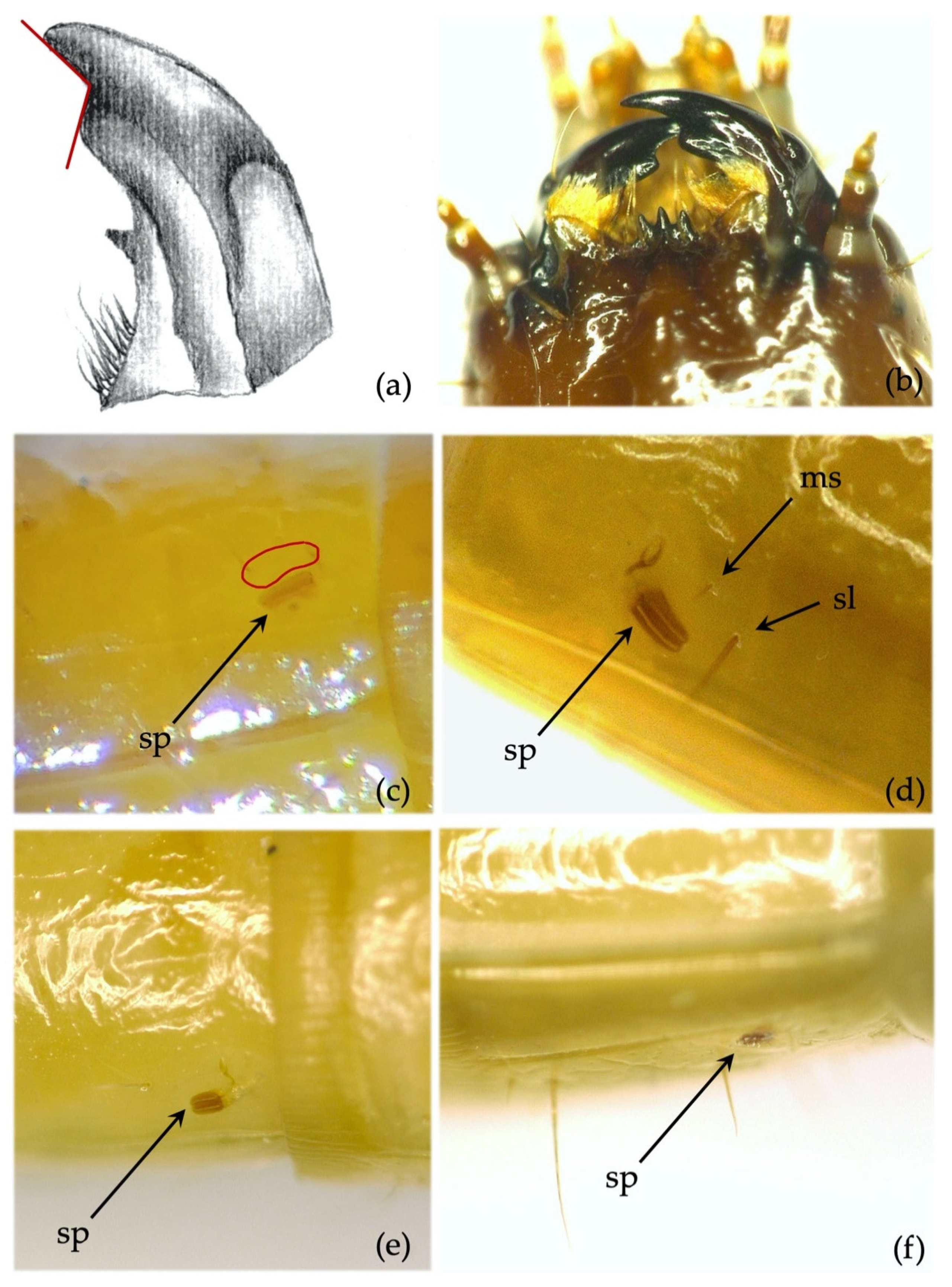
- Mandibles: absence/presence of the subapical tooth on the inner margin of the mandible. Amplitude of the measured angle (ma) between the subapical tooth (sbt) and the apex of the mandible (st) (Figure 2b). This character is often difficult to observe because the tooth tends to erode due to larval feeding; consequently, only recently formed mandibles on post-molting larvae, or larvae with slightly worn mandibles, can be reliably identified.
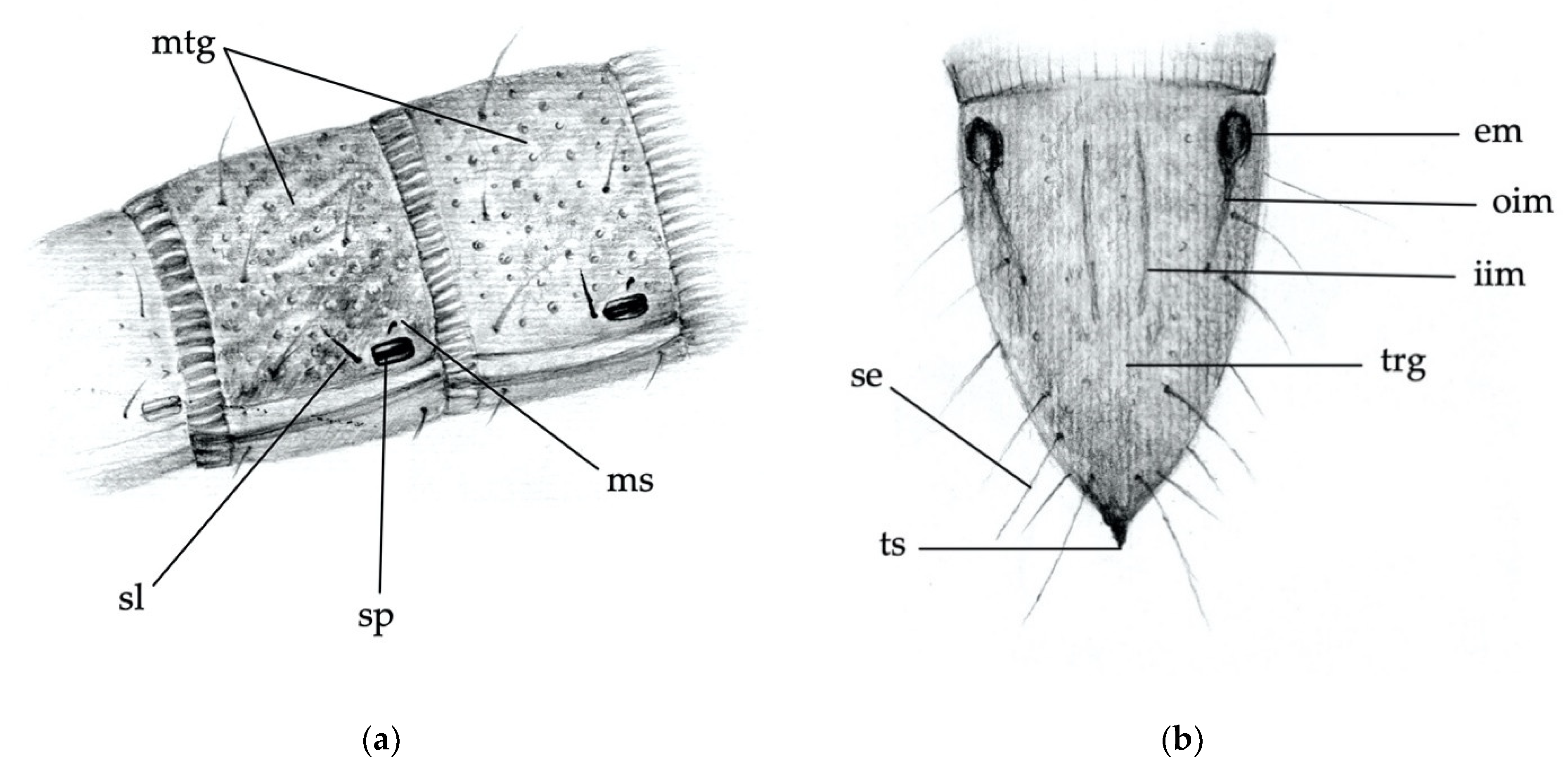
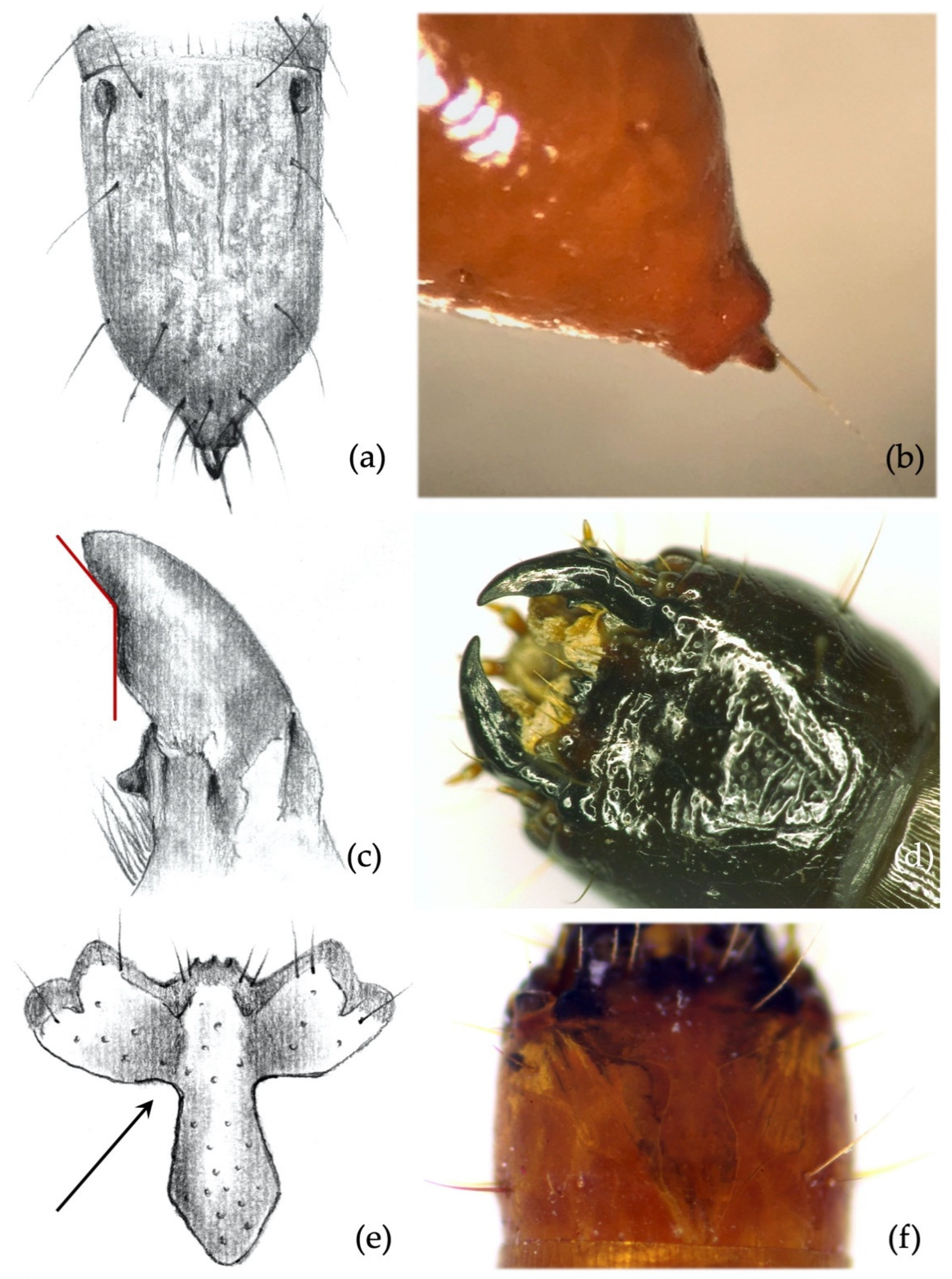
- Abdominal segments: absence/presence of granules and punctures on the tergites; length/width ratio of spiracles; presence/absence of minus setae (ms) (Figure 3a).
- Ninth abdominal segment: general shape; absence/presence of the bulge at the apex of the segment; profile and proportion of the bulge when present; absence/presence of dark elevated setiferous tubercles; presence and shape of terminal spike (ts) (Figure 3b).
- Primary characters: high stability (up to 100% of the observed specimens): present in just one or a few species only.
- Secondary characters: lower stability and specificity, but useful for confirming identification when combined with primary and other secondary characters.
3. Results
3.1. Species Characters
3.1.1. Agriotes ustulatus (Schäller 1783)
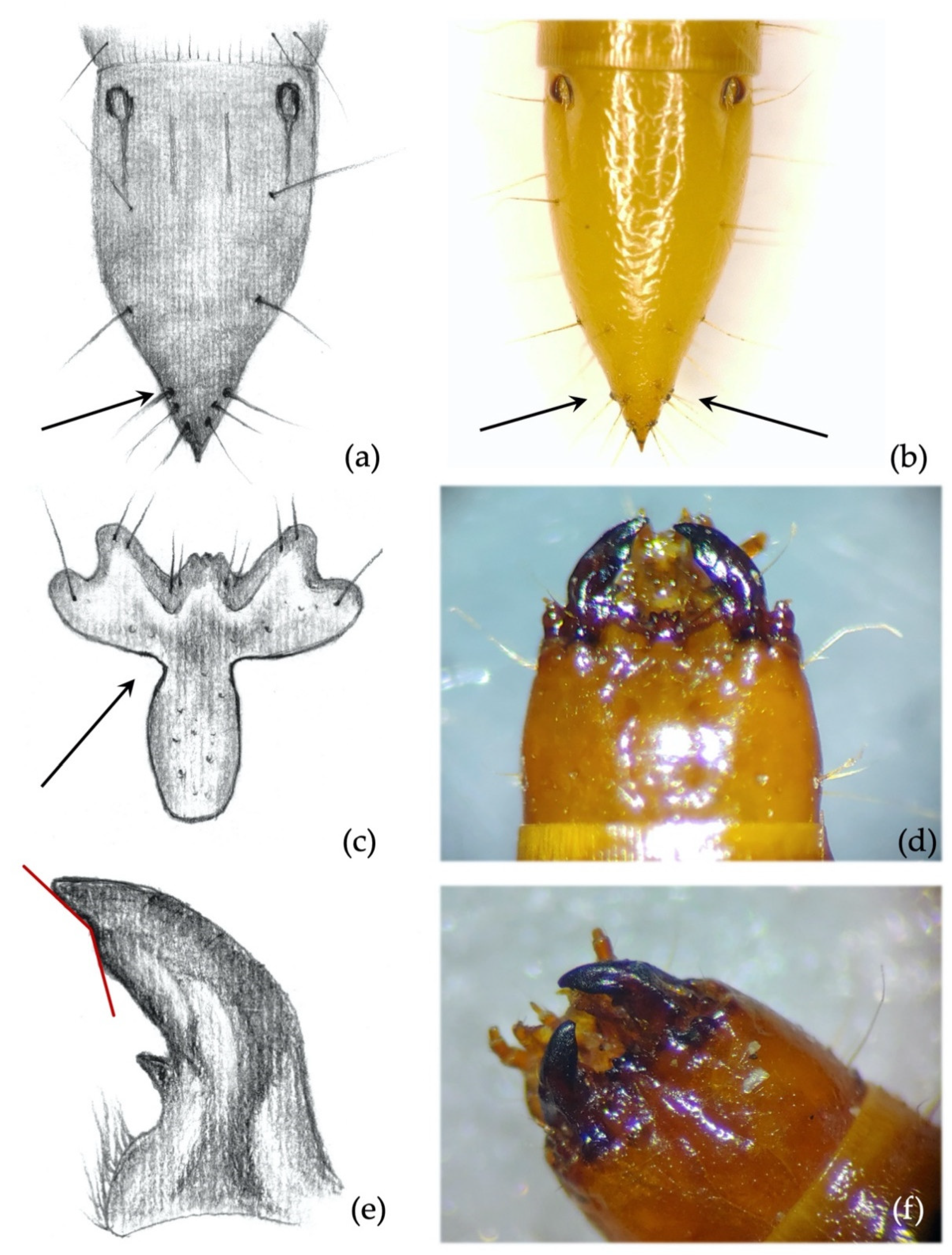
- Ninth abdominal segment. Setiferous tubercles present and well demarked (feature unique to this species) (Figure 4a,b): by looking from the apex of the dorsal part of the 9th segment and moving towards its base, each seta arises from an elevated dark tubercle. From a lateral view, it appears as a dark elevation around the base of the seta, while from above it resembles a circle.
- Ninth abdominal segment. Segment subconical in both dorsal and lateral view, suddenly shrinking in the apical third (Figure 4a,b).
- Clypeus. Agriotes ustulatus is the only species in which the measured angle is consistently >90° (Figure 4c). This angle was >90° on both sides of the clypeus in about 99.5% of the specimens observed (100% when we consider only one of the two sides), while it was equal to 90° in the remaining 0.5%. The same character was found in A. sputator although with a lower consistency (75% of specimens).
- Mandibles. Subapical tooth present, forming a measured angle clearly >90° (Figure 4e,f) with the apex of the mandible; about 15% of the specimens possess an angle of approximately 90°. Depending on the larva’s origin area, the angle varies between approximately 100° and 150°, with an average of 120°. The measured angle is generally more obtuse in A. ustulatus than in A. brevis.
3.1.2. Agriotes sordidus (Illiger 1807)
- Ninth abdominal segment. Segment subconical in both dorsal and lateral view, suddenly shrinking in the apical fourth part (Figure 5a); terminal spike of the segment presenting a large square protrusion (bulge) at the base (Figure 5b). Proportions and size of the bulge vary among individuals, but it is always present and easy to spot. The peculiar bulge illustrated identifies A. sordidus with certainty in the regions of its distribution.
- Mandibles. The subapical tooth forms a measured angle far greater than 90° (Figure 5c,d); this angle differs from all the other species with the only exceptions being A. rufipalpis and some A. ustulatus (average angle of A. sordidus: 148°; range: 130–170°).
- Clypeus. Measured angles of the frontoclypeus form a right angle (Figure 5e,f); this feature is fairly consistent among individuals.
- Other secondary characters. Rarely, there can be granules on thoracic sternites between coxae, but smaller and weaker than in A. sputator.
3.1.3. Agriotes litigiosus (Rossi 1792)
- Mandibles. Almost falciform, without the subapical tooth on the inner margin. The character is 100% consistent and can be observed even in significantly worn specimens (Figure 6a,b).
- Ninth abdominal segment. Segment oblong, 2× longer than wide at the base. Apical part of the segment pointed (spike) and symmetrical; some specimens present a small asymmetrical caudal enlargement (bulge) prior to the terminal spike (Figure 6c–f). Character 100% consistent.
- Clypeus. Measured angles of the frontoclypeus of 90° or <90°. This character is fairly consistent among the specimens observed (Figure 6g,h).
3.1.4. Agriotes sputator (L. 1758)
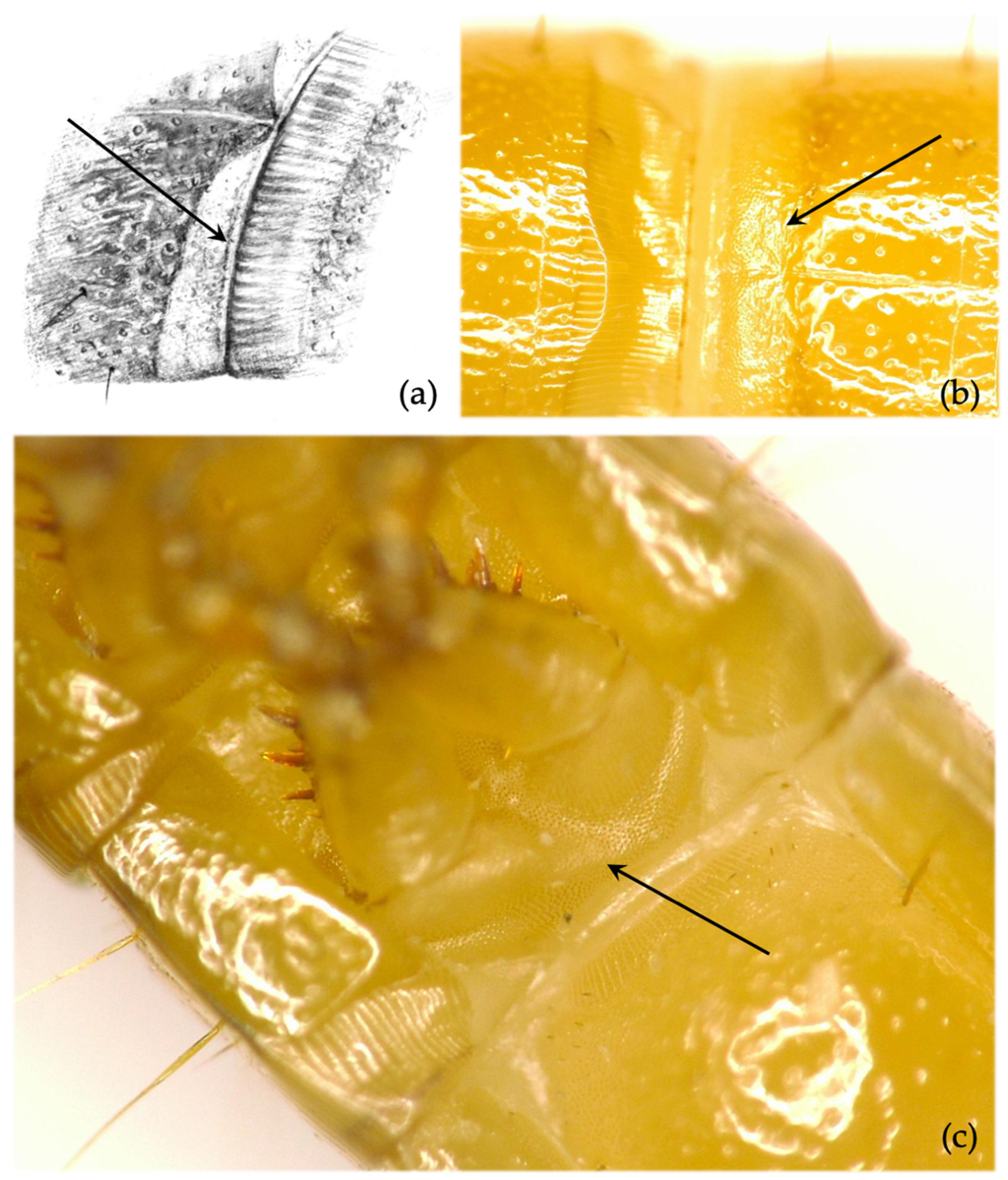
- Thoracic sterna. Thoracic sternites between the coxae present distinct granules (Figure 7c). The character is stable, but its observation is time-consuming and often complex. Generally, this character is most clearly visible on the last leg-bearing segment (3rd). Note: in one local population in northwest Germany, this character was missing in multiple specimens, which were identified as A. sputator by their other morphological traits and PCR results.
- Clypeus. Measured angles slightly >90°, on at least one side (>75% of the cases observed) (Figure 8c,d).
- Mandibles. Subapical tooth present (Figure 8d,e); measured angle ranging from 90° to 100° in Italy (in Germany, average angle: 95.6°; range: 85–112°).
- Size. A fully grown larva of this species is substantially smaller than other species, with its head capsule usually <1.5 mm wide.
3.1.5. Agriotes brevis (Candèze 1863)
- Ninth abdominal segment. Inner longitudinal lines (iim) longer than outers (oim) and 0.5× the length of the whole segment (Figure 9a,b). The character was 100% consistent for at least one of the two inner longitudinal lines; it applied to both the inner lines in more than 80% of specimens.
- Ninth abdominal segment. Terminal spike of the segment longer than wide (Figure 9a,b).
- Clypeus. Measured angle variable: 90° in 17% of specimens, most commonly <90° (80% of specimens) or slightly <90° (3%) (Figure 9c,d).
- Mandibles. The subapical tooth is present, forming a measured angle slightly more than 90° (Figure 9e,f).
- Size. Mature larvae of this species are similar to those of A. sputator; however, A. brevis never exceed 20 mm in length and the width of the head capsule is <1.5 mm.
3.1.6. Agriotes obscurus (L. 1758)
- Ninth abdominal segment. Inner longitudinal lines (iim) as long as outers (oim) and shorter than half of the segment length (Figure 11a,b). Segment 1.5–1.8× longer than wide at the base. Terminal spike of the segment as long as it is wide.
- Clypeus. Measured angles of approximately 90° (Figure 11c,d). Integument strongly punctured.
- Mandibles. Subapical tooth is present (Figure 10a,b), measured angle from 90–100° in Italy (in Germany, average angle: 101.3°; range: 86–128°).
- Other secondary characters. Spiracles on thorax and abdominal segments (1–8) 1.7× longer than wide (range: 1.3–2.1 in Germany in recent measurements). Minus setae present (Figure 10c,d). Rarely, there can be granules on thoracic sternites between coxae, but smaller and weaker than in A. sputator.
- Body color. On average, darker than A. lineatus, but this character can be difficult or even impossible to evaluate in a single individual.
3.1.7. Agriotes lineatus (L. 1767)
- Ninth abdominal segment. Inner longitudinal lines (iim) as long as the outers (oim) and shorter than half the segment length (Figure 12a,b). Terminal spike of the segment as long as it is wide.
- First to eighth abdominal segment. Minus setae is usually absent on the abdominal segments (1–8) (Figure 10e,f). This character had a 97% consistency in our records (all or most of the abdominal segments did not have a minus setae), slightly higher than that reported by Lehmhus and Niepold [26] (93% average). However, this character can vary among populations and a high percentage of local specimens may occasionally possess the minus setae.
- Mandibles. Subapical tooth is present; measured angle usually ≤90° (Figure 12e,f); variable character due to abrasion of subapical tooth and apex of mandible.
- Other secondary characters. Spiracles on thorax and abdominal segments (1–8) on average 1.4× longer than wide.
- Body color. Lighter yellow than in the other species, but beware of freshly molted specimens from other species (lighter in color).
3.1.8. Agriotes proximus (Schwarz 1891) and Agriotes rufipalpis (Brullé 1832)
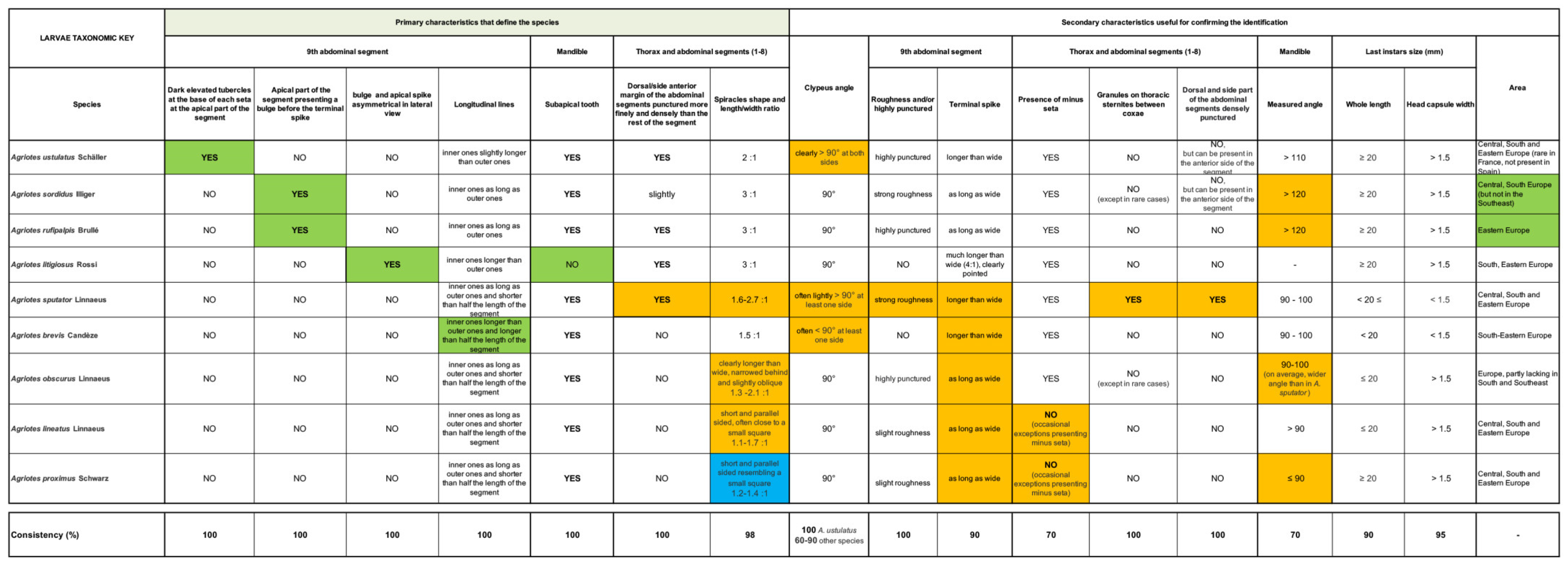
3.2. Horizontal Identification Table
3.3. Horizontal Identification Table—Usage
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Veres, A.; Wyckhuys, K.A.; Kiss, J.; Tóth, F.; Burgio, G.; Pons, X.; Avilla, C.; Vidal, S.; Razinger, J.; Bazok, R.; et al. An update of the Worldwide Integrated Assessment (WIA) on systemic pesticides. Part 4: Alternatives in major cropping systems. Environ. Sci. Pollut. Res. 2020, 27, 29867–29899. [Google Scholar] [CrossRef]
- Furlan, L.; Tóth, M. Occurrence of click beetle pest spp. (Coleoptera, Elateridae) in Europe as detected by pheromone traps: Survey results of 1998–2006. IOBC/WPRS Bull. 2007, 30, 19–25. [Google Scholar]
- Parker, W.E.; Howard, J.J. The biology and management of wireworms (Agriotes spp.) on potato with particular reference to the U.K. Agric. For. Entomol. 2001, 3, 85–98. [Google Scholar] [CrossRef]
- Lehmhus, J.; Niepold, F. New finds of the click beetle Agriotes sordidus (Illiger, 1807) and an overview on its current distribution in Germany. J. Kult. 2013, 65, 309–314. [Google Scholar] [CrossRef]
- Furlan, L.; Curto, G.; Ferrari, R.; Boriani, L.; Bourlot, G.; Turchi, A. Wireworm species damaging crops in Po Valley (Le specie di elateridi dannose alle colture agrarie nella Pianura Padana). Inf. Fitopatol. 2000, 5, 53–59. [Google Scholar]
- Furlan, L.; Di Bernardo, A.; Maini, S.; Ferrari, R.; Boriani, L.; Boriani, M.; Nobili, P.; Bourlot, G.; Turchi, A.; Vacante, V.; et al. First practical results of click beetle trapping with pheromone traps in Italy. In Proceedings of the XXI IWGO Conference, Legnaro, Italy, 27 October–3 November 2001; pp. 277–282. [Google Scholar]
- Cocquempot, C.; Martinez, M.; Courbon, R.; Blanchet, A.; Caruhel, P. Nouvelles donnees sur l’identification des larves de taupins (Coleoptera: Elateridae): Une aide a la connaissance biologique et a la cartographie des especes nuisibles. In Proceedings of the Cinquieme Conference INTERNATIONALE sur le Ravageurs en Agriculture, Montpellier, Paris, France, 7–9 December 1999; pp. 477–486. [Google Scholar]
- Furlan, L.; Contiero, B.; Chiarini, F.; Colauzzi, M.; Sartori, E.; Benvegnù, I.; Fracasso, F.; Giandon, P. Risk assessment of maize damage by wireworms (Coleoptera: Elateridae) as the first step in implementing IPM and in reducing the environmental impact of soil insecticides. Environ. Sci. Pollut. Res. 2017, 24, 236–251. [Google Scholar] [CrossRef]
- Furlan, L.; Tóth, M.; Yatsinin, V.; Ujvary, I. The project to implement IPM strategies against Agriotes species in Europe: What has been done and what is still to be done. In Proceedings of the XXI IWGO Conference, Legnaro, Italia, 27 October–3 November 2001; pp. 253–262. [Google Scholar]
- Furlan, L.; Tóth, M.; Parker, W.E.; Ivezić, M.; Pančić, S.; Brmez, M.; Dobrinĉić, R.; Barĉić, J.I.; Muresan, F.; Subchev, M.; et al. The efficacy of the new Agriotes sex pheromone traps in detecting wireworm population levels in different European countries. In Proceedings of the XXI IWGO, Legnaro, Italia, 27 October–3 November 2001; pp. 293–303. [Google Scholar]
- Masler, V. Skodlivé druhy kovácikovitych (Coleoptera, Elateridae) na Slovensku a ochrana proti nim. Polnohospodárska Veda 1982, 3, 126. [Google Scholar]
- Gomboc, S.; Milevoj, L.; Furlan, L.; Tóth, M.; Bitenc, P.; Bobnar, A.; Celar, F. Two-years of monitoring Click Beetles and Wireworms in Slovenia. In Proceedings of the XXI WGO Conference, Legnaro, Italy, 27 October–3 November 2001; pp. 283–292. [Google Scholar]
- Subchev, M.; Toshova, T.; Tóth, M.; Furlan, L. Click Beetles (Coleoptera: Elateridae) and their swarming period as established by pheromone traps in different plant habitats in Bulgaria: 1. Meadow. Acta Zool. Bulg. 2004, 56, 187–198. [Google Scholar]
- Leseigneur, L. Coléoptères Elateridae de la faune de France continentale et de Corse. Bull. Mens. Société Linnéenne Lyon 1972, 41, 327. [Google Scholar] [CrossRef]
- Furlan, L. The biology of Agriotes ustulatus Schäller (Col., Elateridae). I. Adults and oviposition. J. Appl. Entomol. 1996, 120, 269–274. [Google Scholar] [CrossRef]
- Karabatsas, K.; Tsakiris, V.; Zarpas, K.; Tsitsipis, J.A.; Furlan, L.; Tóth, M. Seasonal fluctuation of adult and larvae Agriotes spp. In Proceedings of the XXI IWGO Conference, Legnaro, Italy, 27 October–3 November 2001; pp. 269–276. [Google Scholar]
- Kosmacevskij, A.S. Nekotoryje voprosy biologii i ekologii scelkunov. Uc. Zap. Krasnodar. Gos. Ped. Inst. 1955, 14, 3–22. [Google Scholar]
- Tóth, M.; Imrei, Z.; Szarukán, I.; Korosi, R.; Furlan, L. First results of click beetle trapping with pheromone traps in Hungary 1998–2000. In Proceedings of the XXI IWGO Conference, Legnaro, Italy, 27 October–3 November 2001; pp. 263–268. [Google Scholar]
- Furlan, L. IPM thresholds for Agriotes wireworm species in maize in Southern Europe. J. Pest. Sci 2014, 87, 609–617. [Google Scholar] [CrossRef]
- Furlan, L.; Contiero, B.; Chiarini, F.; Benvegnù, I.; Tóth, M. The use of click-beetle pheromone traps to optimize the risk assessment of wireworm (Coleptera: Elateridae) maize damage. Sci. Rep. 2020, 10, 8780. [Google Scholar] [CrossRef]
- Furlan, L.; Bonetto, C.; Costa, B.; Finotto, A.; Lazzeri, L.; Malaguti, L.; Patalano, G.; Parker, W. The efficacy of biofumigant meals and plants to control wireworm populations. Ind. Crops Prod. 2010, 31, 245–254. [Google Scholar] [CrossRef]
- Furlan, L. Management and biological control of wireworm populations in Europe: Current possibilities and future perspectives. IOBC/WPRS Bull. 2007, 30, 11–16. [Google Scholar]
- Benefer, C.M.; van Herk, W.G.; Ellis, J.S.; Blackshaw, R.P.; Vernon, R.S.; Knight, M.E. The molecular identification and genetic diversity of economically important wireworm species (Coleoptera: Elateridae) in Canada. J. Pest. Sci. 2013, 86, 19–27. [Google Scholar] [CrossRef]
- Etzler, F.E. Identification of Economic Wireworms Using Traditional and Molecular Methods. Master’s Thesis, Montana State University, Bozeman, MT, USA, 2013; 286p. [Google Scholar]
- Staudacher, K.; Pitterl, P.; Furlan, L.; Cate, P.C.; Traugott, M. PCR based species identification of Agriotes larvae. Bull. Entomol. Res. 2011, 101, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Lehmhus, J.; Niepold, F. Identification of Agriotes wireworms-are they always what they appear to be. J. Kult. 2015, 67, 129–138. [Google Scholar]
- Dolin, G. Litschinki Zhuchov-Stschelkunov (Provolotschniki) Evropeiski Tschasti SSSR; Urozhaj: Kijev, Ukraine, 1964; 206p. (In Russian) [Google Scholar]
- Dolin, G. Handbook for the Identification of the Larvae of the Elateridae of URSSD; Urozhaj: Kijev, Ukraine, 1978. (In Russian) [Google Scholar]
- Van Emden, F.I. Larvae of British beetles—5. Elateridae. Entomol. Mon. Mag. 1945, 81, 13–37. [Google Scholar]
- Klausnitzer, B. Familie Elateridae. In Die Larven der Käfer Mitteleuropas, Band 2, Myxophaga/Polyphaga, Teil 1; Gustav Fischer Verlag: Jena, Germany, 1994; pp. 118–189. [Google Scholar]
- Palm, T. Die scandinavischen Elateriden-Larven (Coleoptera). Ent. Scand. 1972, 1 (Suppl. 2), 52–53. [Google Scholar]
- Rudolph, K. Contribution to the knowledge of wireworms in the fauna of the GDR and the FRG (A morphologic-taxonomic study). Zool. Jb. Syst. 1974, 101, 1–151. (In German) [Google Scholar]
- Eidt, D. A Description of the Larva of Agriotes mancus (Say), with a Key Separating the Larvae of A. lineatus (L.), A. mancus, A. obscurus (L.), and A. sputator (L.) from Nova Scotia. Can. Entomol. 1954, 86, 481–494. [Google Scholar] [CrossRef]
- Oehlke, J. Zur Larvalsystematik der Gattung Agriotes Esch. (Col. Elateridae). Dtsch. Entomol. Z. 1962, 9, 336–349. [Google Scholar] [CrossRef]
- Stehr, F.W. Immature Insects, 1st ed.; Kendall-Hunt Publishing Company: Dubuque, IA, USA, 1991; p. 570. [Google Scholar]
- Furlan, L. The biology of Agriotes ustulatus Schäller (Col., Elateridae). II. Larval development, pupation, whole cycle description and practical implications. J. Appl. Entomol. 1998, 122, 71–78. [Google Scholar] [CrossRef]
- Furlan, L. The biology of Agriotes sordidus Illiger (Col., Elateridae). J. Appl. Entomol. 2004, 128, 696–706. [Google Scholar] [CrossRef]
- Platia, G. Coleoptera Elateridae. In Fauna d’Italia; Edizioni Calderini: Bologna, Italy, 1994. [Google Scholar]
- Lohse, G.A. 44. Familie: Elateridae. In Die Käfer Mitteleuropas, Bd. 6, Diversicornia; Freude, H., Harde, K.W., Lohse, G.A., Eds.; Goecke & Evers: Krefeld, Germany, 1979; pp. 103–186. [Google Scholar]
- Laibner, S. Elateridae of the Czech and Slovak Republics; Ing. Vit Kabourek: Zlin, Czech Republic, 2000; pp. 1–292. [Google Scholar]
- Kölliker, U.; Jossi, W.; Kuske, S. Optimized protocol for wireworm rearing. IOBC/WPRS Bull. 2009, 45, 457–460. [Google Scholar]
- Tóth, M.; Furlan, L.; Xavier, A.; Vuts, J.; Toshova, T.; Subchev, M.; Szarukán, I.; Yatsynin, V. New sex attractant composition for the click beetle Agriotes proximus: Similarity to the pheromone of Agriotes lineatus. J. Chem. Ecol. 2008, 34, 107–111. [Google Scholar] [CrossRef]
- Vuts, J.; Tillash, T.; Furlan, L.; Csonka, E.B.; Felföldi, T.; Marialigeti, K.; Toshova, T.B.; Subchev, M.; Xavier, A.; Tóth, M. Agriotes proximus and A. lineatus (Coleoptera: Elateridae): A comparative study on the pheromone composition and cytochrome c oxidase subunit I gene sequence. Chemoecology 2012, 22, 23–28. [Google Scholar] [CrossRef]
- Tóth, M.; Furlan, L.; Szarukán, I.; Ujvary, I. Geranyl hexanoate attracting males of click beetles Agriotes rufipalpis Brullè and A. sordidus Illiger (Coleoptera: Elateridae). J. Appl. Entomol. 2002, 126, 312–314. [Google Scholar] [CrossRef]
- Subchev, M.; Toshova, T.; Mladenov, E.; Furlan, L.; Tóth, M. Click Beetles (Coleoptera: Elateridae) and their swarming as established by pheromone traps in different plant habitats in Bulgaria: 4. Tobacco. Acta Zool. Bulg. 2010, 62, 187–192. [Google Scholar]
- Furlan, L.; Tóth, M.; Cooperators. Evaluation of the effectiveness of the new Agriotes sex pheromone traps in different European countries. In Proceedings of the XX IWGO Conference, Adana, Turkey, 4–10 September 1999; pp. 171–175. [Google Scholar]
- Korschefsky, R. Bestimmungstabelle der bekanntesten deutschen Elateridenlarven. Arbeiten Über Morph. Taxon. Ent. AUS Berlin-Dahlem. 1941, 8, 217–230. [Google Scholar]
- Subklew, W. Agriotes lineatus L. und obscurus L. (Ein Beitrag zu ihrer Biologie und Morphologie). Zt. Angew. Entom. 1935, 21, 96–122. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furlan, L.; Benvegnù, I.; Bilò, M.F.; Lehmhus, J.; Ruzzier, E. Species Identification of Wireworms (Agriotes spp.; Coleoptera: Elateridae) of Agricultural Importance in Europe: A New “Horizontal Identification Table”. Insects 2021, 12, 534. https://doi.org/10.3390/insects12060534
Furlan L, Benvegnù I, Bilò MF, Lehmhus J, Ruzzier E. Species Identification of Wireworms (Agriotes spp.; Coleoptera: Elateridae) of Agricultural Importance in Europe: A New “Horizontal Identification Table”. Insects. 2021; 12(6):534. https://doi.org/10.3390/insects12060534
Chicago/Turabian StyleFurlan, Lorenzo, Isadora Benvegnù, María Fabiana Bilò, Jörn Lehmhus, and Enrico Ruzzier. 2021. "Species Identification of Wireworms (Agriotes spp.; Coleoptera: Elateridae) of Agricultural Importance in Europe: A New “Horizontal Identification Table”" Insects 12, no. 6: 534. https://doi.org/10.3390/insects12060534
APA StyleFurlan, L., Benvegnù, I., Bilò, M. F., Lehmhus, J., & Ruzzier, E. (2021). Species Identification of Wireworms (Agriotes spp.; Coleoptera: Elateridae) of Agricultural Importance in Europe: A New “Horizontal Identification Table”. Insects, 12(6), 534. https://doi.org/10.3390/insects12060534






