Recolonization by Slugs: Vertical and Horizontal Dispersal by the Field Slug, Deroceras reticulatum
Abstract
Simple Summary
Abstract
1. Introduction
- Most recolonization would be by horizontal dispersal.
- Vertical dispersal would make a small contribution to recolonization and over a short period.
2. Materials and Methods
2.1. Barriers Used for Trapping
- A “Temporary” square Defined Area Trap (DAT), 40 × 40 cm with sides 25 cm high made of galvanized steel sheet. The trap sides were pushed into the soil to a depth of 5–10 cm (depending on soil density). For a two-week period, slugs were collected in the morning from beneath a hardboard sheet that lay on the soil surface, fitting within the trap. After two weeks of trapping the DAT was removed for two weeks, to allow horizontal and vertical dispersing slugs to recolonize the site. The trapping was then repeated, returning the DAT to exactly the same location.
- A trap as in (1) was used, but was not removed (“Permanent”) between trapping periods so that only vertically dispersing slugs were able to recolonize the site.
- A trap as in (2), but the DAT was closed (“Covered”) with a close fitting lid made of fine mesh. The lid allowed air movement, but ensured that no slugs that climbed the wall of the DAT could enter or leave.
2.2. Site Description
2.3. Statistical Methods
3. Results
3.1. Slug Species
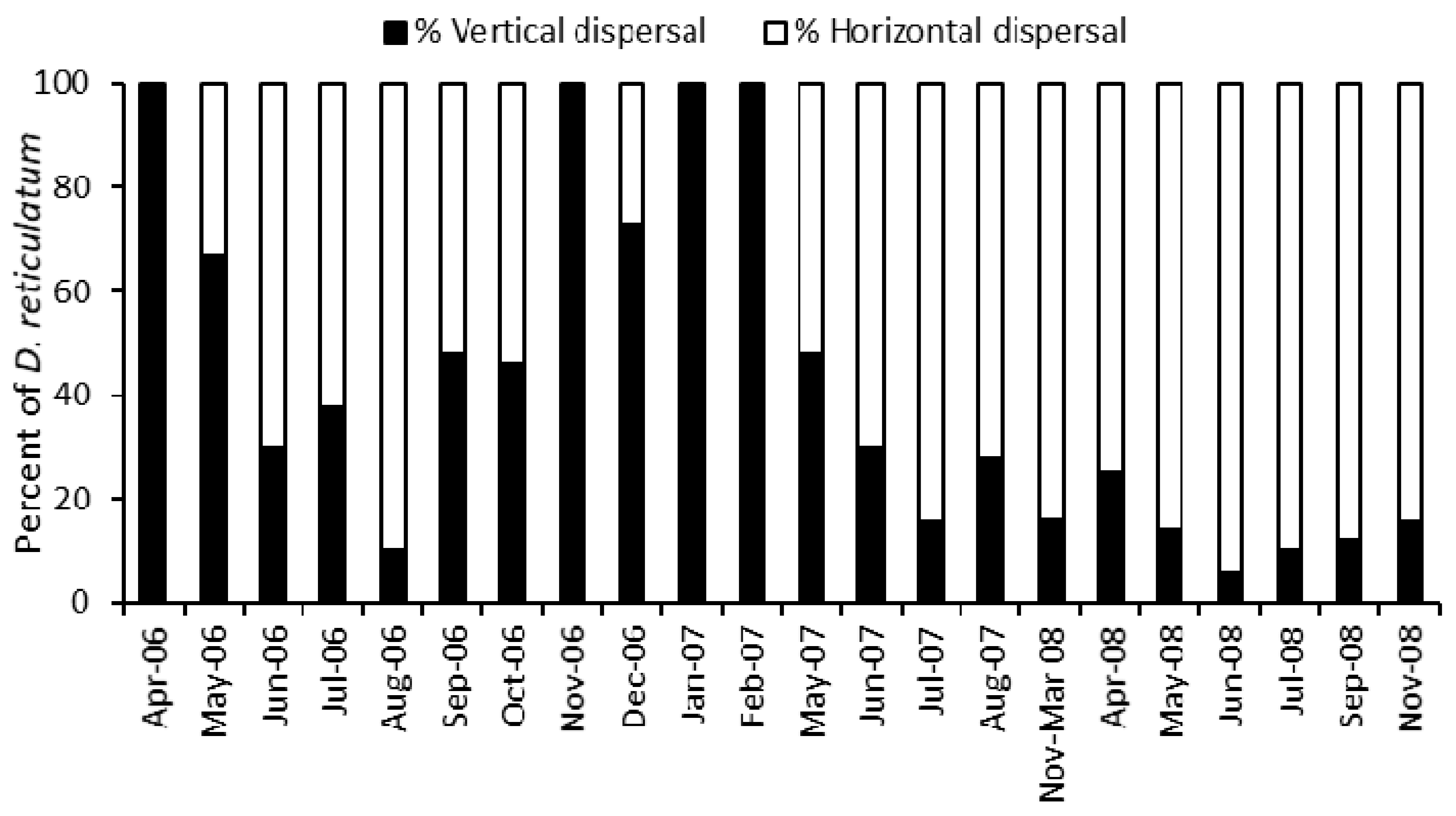
3.2. Trap Captures
- Those slugs dispersing vertically were estimated from the average capture in Covered and Permanent traps where horizontal movement was prevented.
- Those slugs dispersing horizontally were estimated from the Temporary traps, where both horizontal and vertical dispersal were possible, after subtracting the number estimated to have dispersed vertically.
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Glen, D.-M.; Wiltshire, C.-W.; Butler, R.-C. Slug population changes following molluscicide treatment in relation to distance from edge of treated area. Crop. Prot. 1991, 10, 408–412. [Google Scholar] [CrossRef]
- Knop, E.; Rindlisbacher, N.; Ryser, S.; Grüebler, M.U. Locomotor activity of two sympatric slugs: Implication for the invasion success of terrestrial invertebrates. Ecosphere 2013, 4, 1–8. [Google Scholar] [CrossRef]
- Watz, J.; Nyqvist, D. Artificial barriers against arionid slug movement. Crop. Prot. 2021, 142, 105525. [Google Scholar] [CrossRef]
- Glen, D.M.; Wiltshire, C.W.; Bohan, D.A. Abundance and Vertical Distribution of Slugs in Soil Following Cultivation; Home-Grown Cereals Authority: London, UK, 2006. [Google Scholar]
- Schuder, I.; Port, G.; Bennison, J. Barriers, repellents and antifeedants for slug and snail control. Crop. Prot. 2003, 22, 1033–1038. [Google Scholar] [CrossRef]
- Clements, R.O.; Murray, P.J. Comparison between defined-area slug traps and other methods of trapping slugs in cereal fields. Crop. Prot. 1991, 10, 152–154. [Google Scholar] [CrossRef]
- Ferguson, C.M.; Hanks, C.B. Evaluation of defined-area trapping for estimating the density of the field slug Deroceras reticulatum (Muller). Ann. Appl. Biol. 1990, 117, 451–454. [Google Scholar] [CrossRef]
- Cameron, R.A.D.; Jackson, N.; Eversham, B. A field key to the slugs of the British Isles. Field Stud. 1983, 5, 807–824. [Google Scholar]
- Cleveland, R.B.; Cleveland, W.S.; McRae, J.E.; Terpenning, I. STL: A seasonal-trend decomposition procedure based on loess. J. Off. Stat. 1990, 6, 3–73. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. 2020. Available online: https://www.R-project.org/ (accessed on 20 May 2021).
- Campbell, A. Better Use of Molluscicide Pellets for Improved Management of Slugs. Unpublished. Ph.D. Thesis, University of Newcastle upon Tyne, Newcastle upon Tyne, UK, 2021. [Google Scholar]
- Forbes, E.; Back, M.A.; Brooks, A.; Petrovskaya, N.B.; Petrovskii, S.V.; Pope, T.W.; Walters, K.F. Locomotor behaviour promotes stability of the patchy distribution of slugs in arable fields: Tracking the movement of individual Deroceras reticulatum. Pest. Manag. Sci. 2020, 76, 2944–2952. [Google Scholar] [CrossRef] [PubMed]
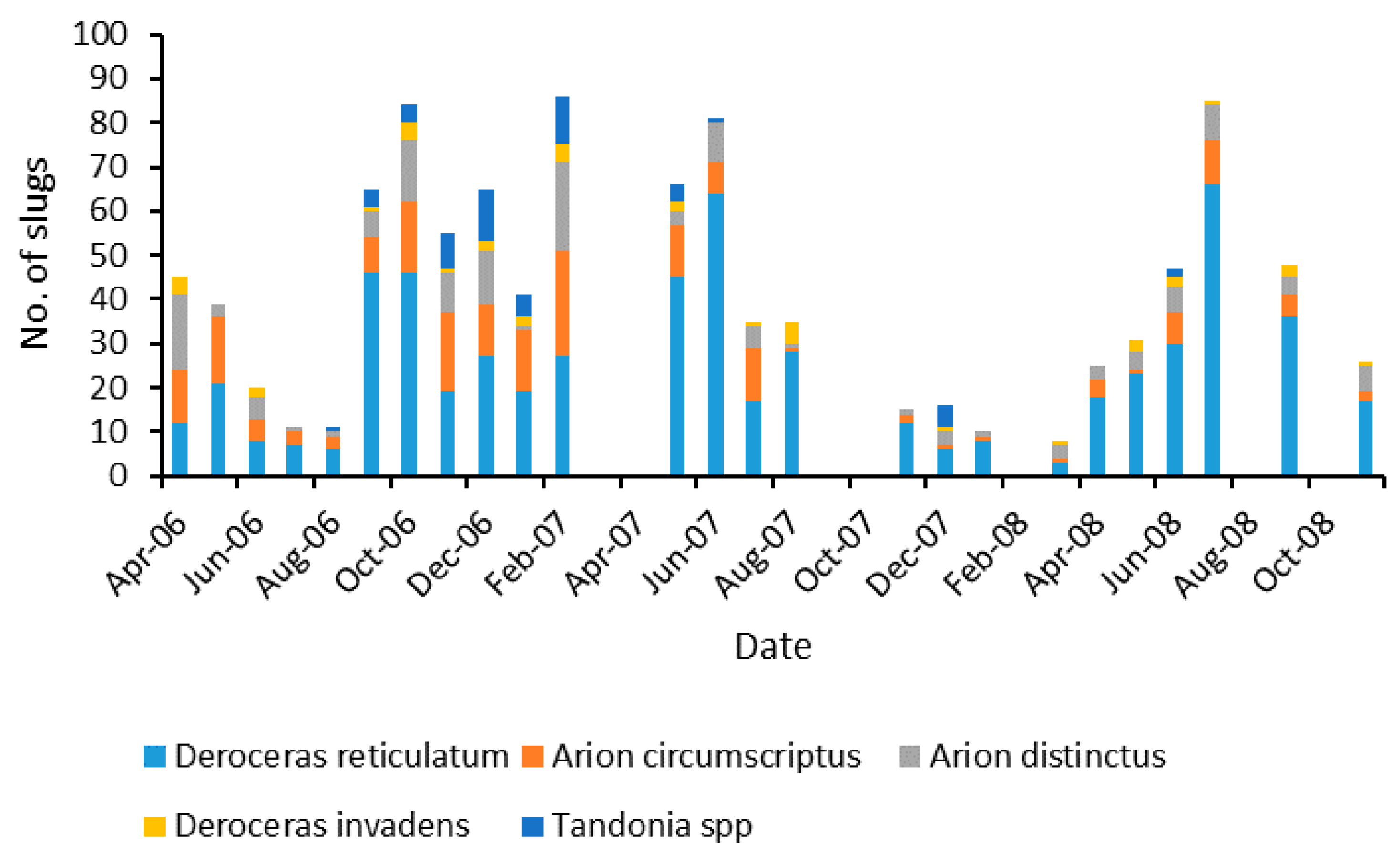
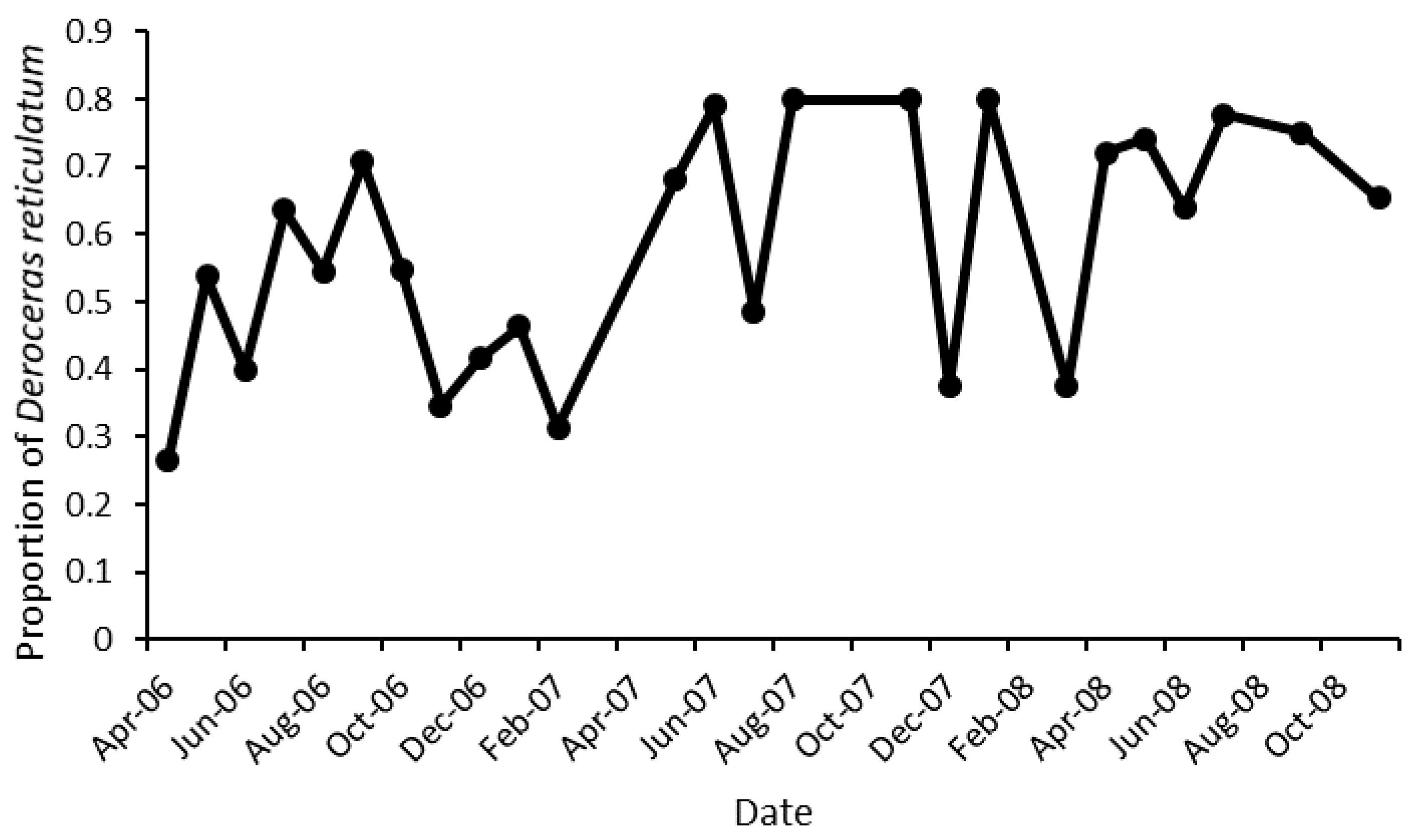
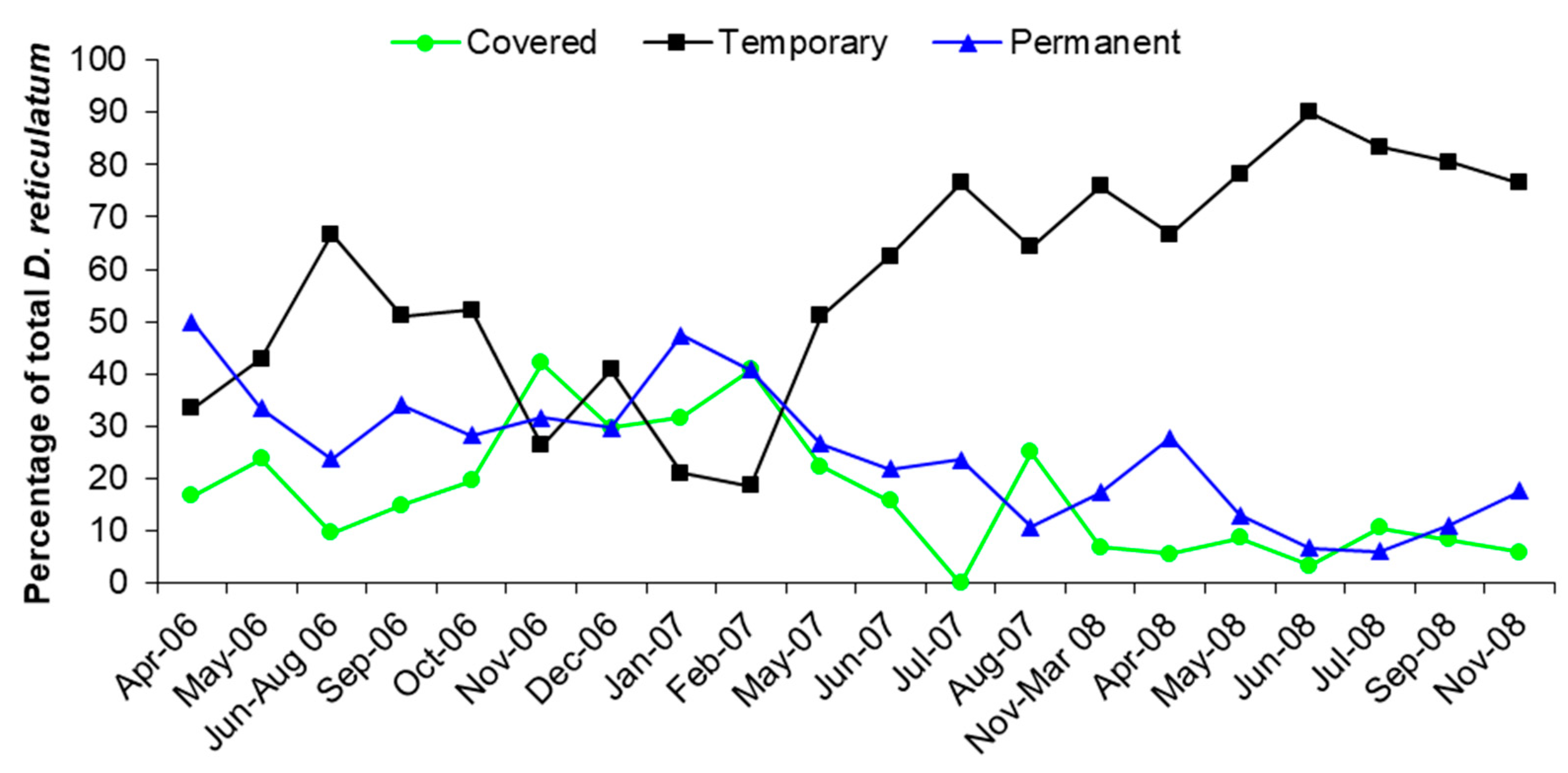
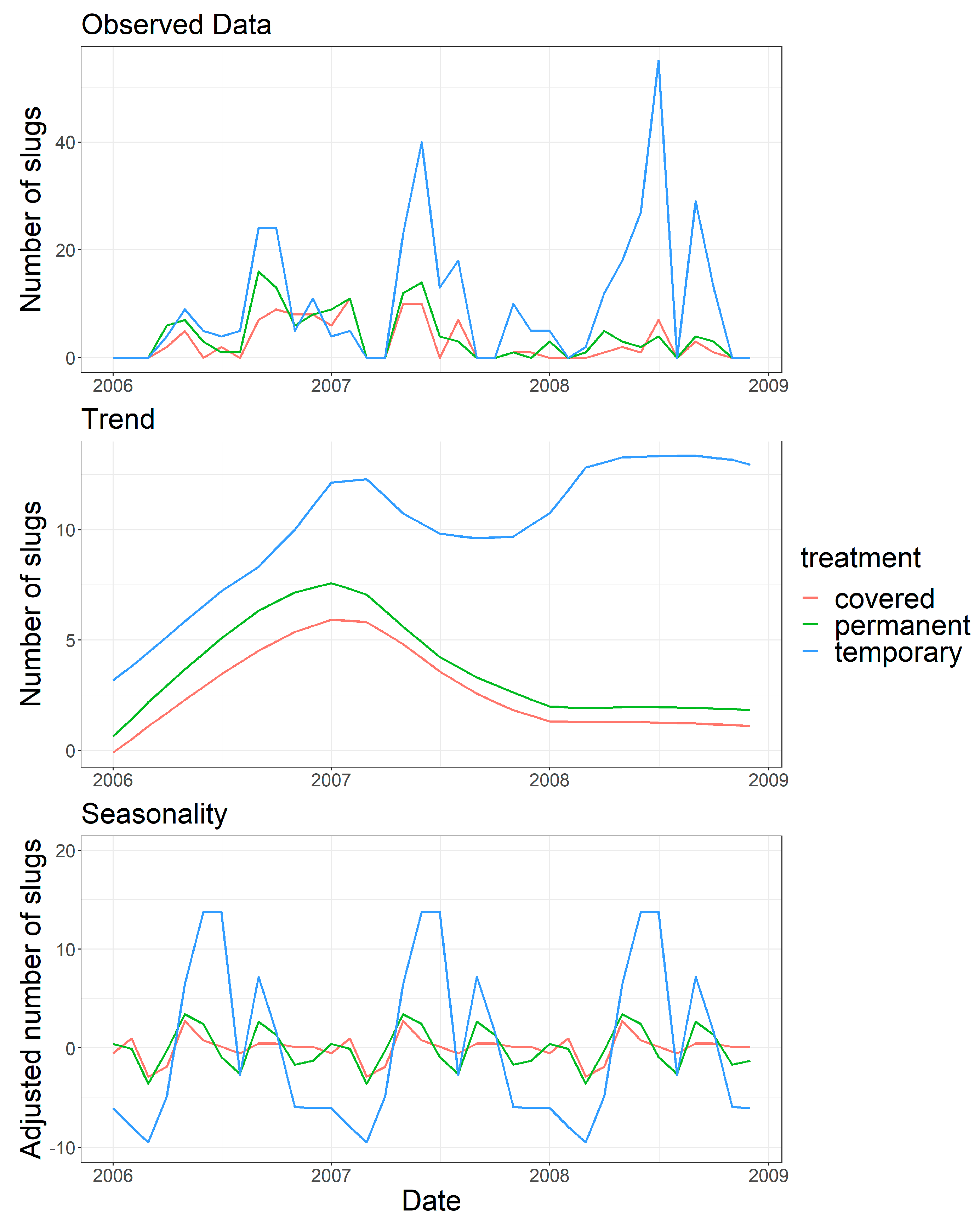

| Slug Species | Number |
|---|---|
| Deroceras reticulatum (Müller) | 611 |
| Arion circumscriptus silvaticus Lohmander | 196 |
| Arion distinctus Mabille | 146 |
| Tandonia spp. | 57 |
| Deroceras invadens Reise, Hutchinson, Schunack &Schlitt | 40 |
| Arion circumscriptus circumscriptus Johnston | 11 |
| Arion fasciatus (Nilsson) | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Port, G.; Craig, A.; Shirley, M. Recolonization by Slugs: Vertical and Horizontal Dispersal by the Field Slug, Deroceras reticulatum. Insects 2021, 12, 531. https://doi.org/10.3390/insects12060531
Port G, Craig A, Shirley M. Recolonization by Slugs: Vertical and Horizontal Dispersal by the Field Slug, Deroceras reticulatum. Insects. 2021; 12(6):531. https://doi.org/10.3390/insects12060531
Chicago/Turabian StylePort, Gordon, Alan Craig, and Mark Shirley. 2021. "Recolonization by Slugs: Vertical and Horizontal Dispersal by the Field Slug, Deroceras reticulatum" Insects 12, no. 6: 531. https://doi.org/10.3390/insects12060531
APA StylePort, G., Craig, A., & Shirley, M. (2021). Recolonization by Slugs: Vertical and Horizontal Dispersal by the Field Slug, Deroceras reticulatum. Insects, 12(6), 531. https://doi.org/10.3390/insects12060531







