Nectar Uptake of a Long-Proboscid Prosoeca Fly (Nemestrinidae)—Proboscis Morphology and Flower Shape
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
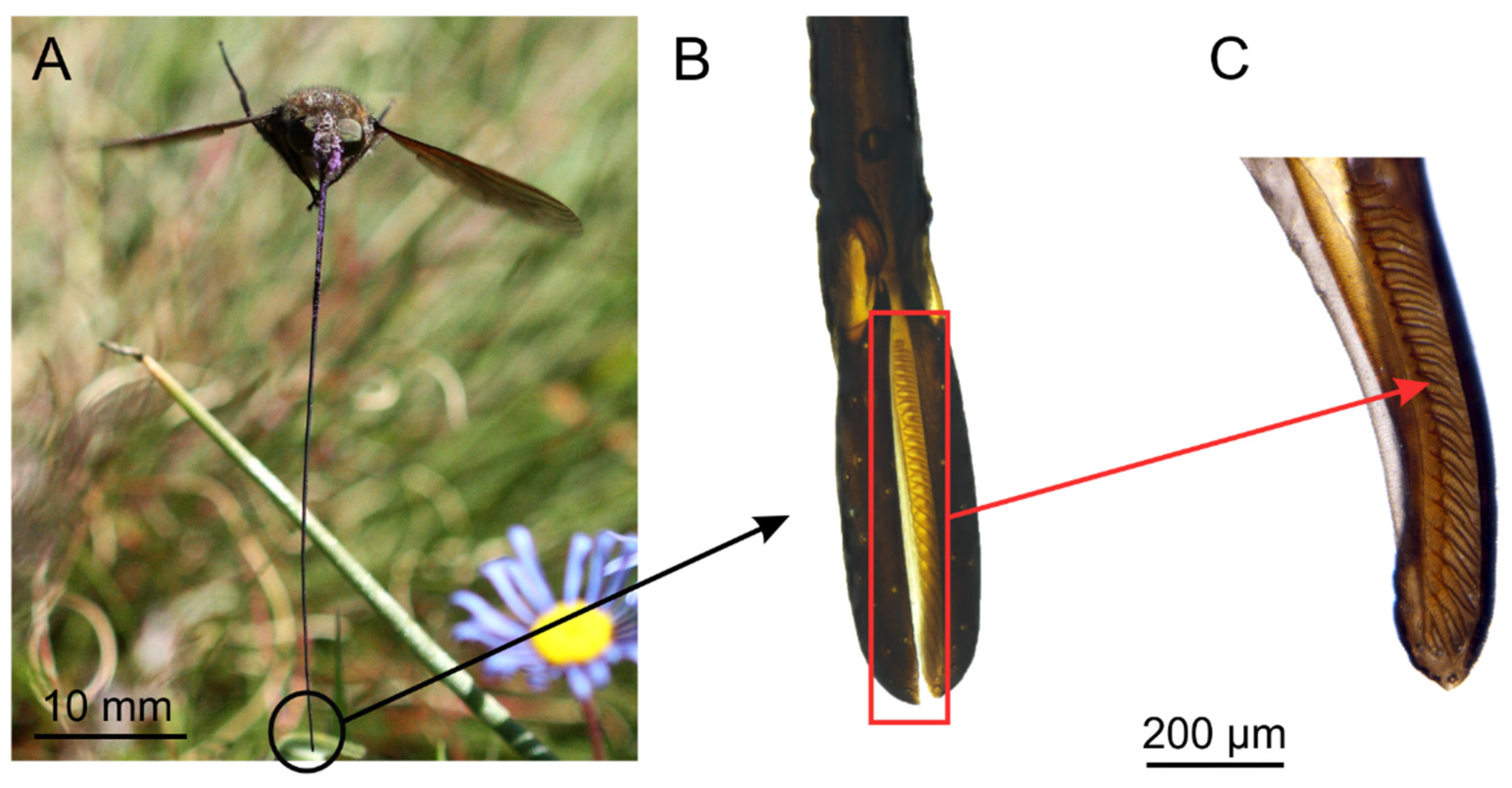
2.2. Proboscis Morphometry
2.3. Nectar Host Plant and Nectar Sampling
3. Results
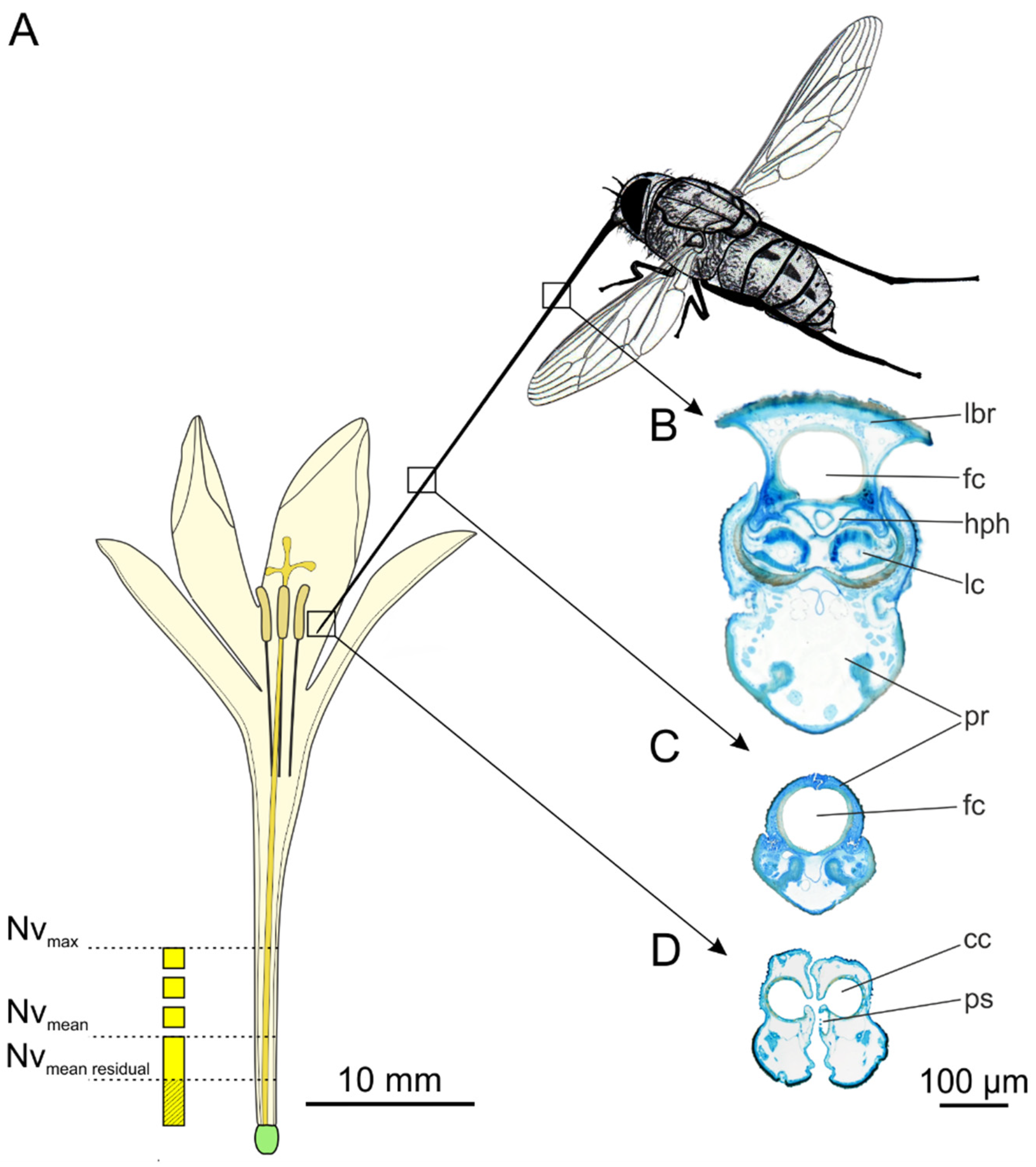
3.1. Flower Visiting Behaviour and Proboscis Morphology
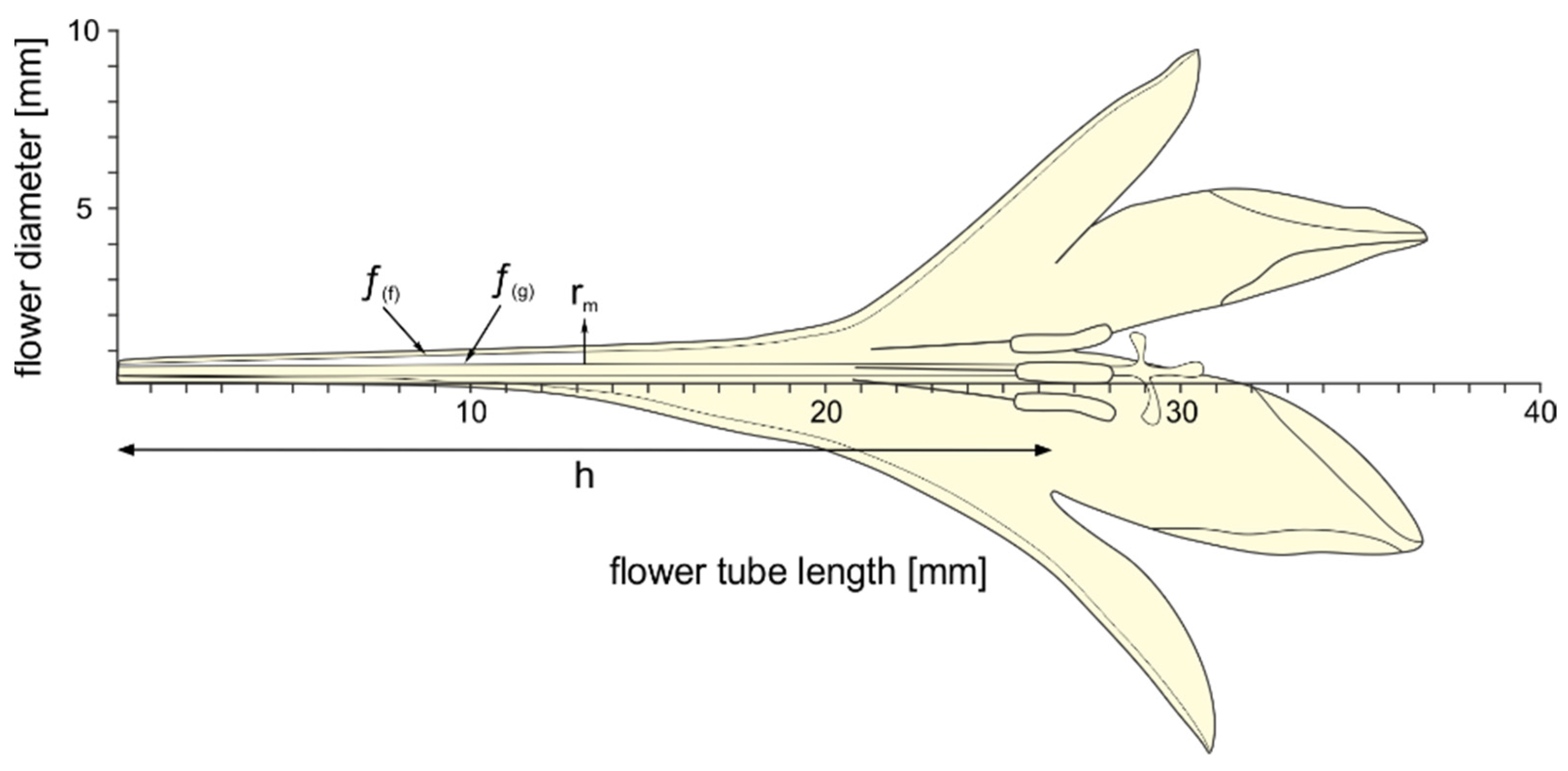
3.2. Flower Shape and Nectar Availability
4. Discussion
4.1. Functional Morphology of the Proboscis
4.2. Nectar Supply
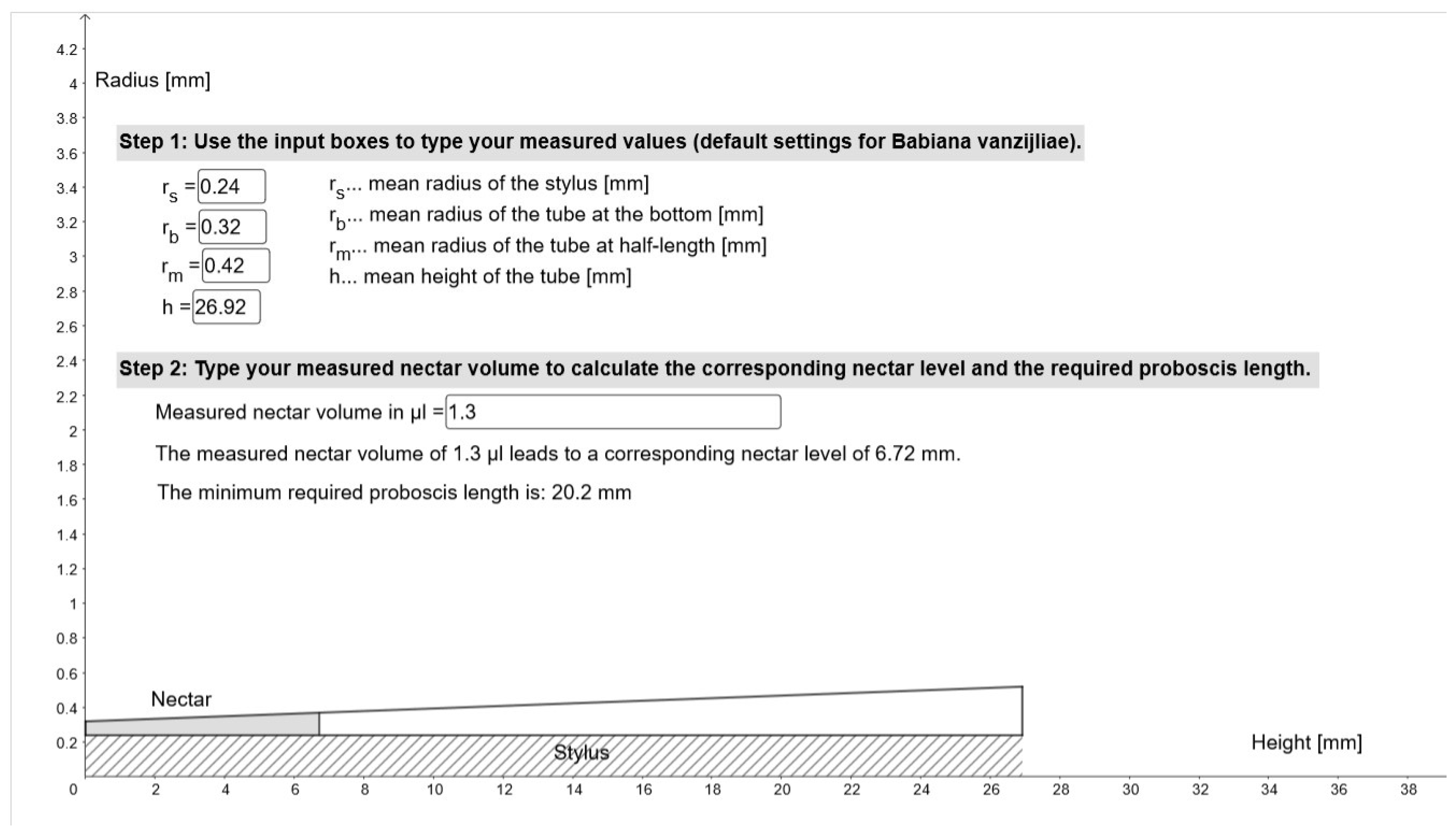
4.3. Nectar Accessibility
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Krenn, H.W.; Plant, J.D.; Szucsich, N.U. Mouthparts of flower-visiting insects. Arthropod Struct. Dev. 2005, 34, 1–40. [Google Scholar] [CrossRef]
- Krenn, H.W. Fluid-Feeding Mouthparts. In Insect Mouthparts Form, Function, Development and Performance. Zoological Monographs 5; Krenn, H.W., Ed.; Springer Nature: Cham, Switzerland, 2019; pp. 47–99. [Google Scholar]
- Proctor, M.; Yeo, P.; Lack, A. The Natural History of Pollination; Harper; Collins Publishers: London, UK, 1997. [Google Scholar]
- Wäckers, F.L.; Romeis, J.; Van Rijn, P. Nectar and pollen feeding by insect herbivores and implications for multitrophic interactions. Annu. Rev. Entomol. 2007, 52, 301–323. [Google Scholar] [CrossRef]
- Ssymank, A.; Kearns, C.A.; Pape, T.; Thompson, F.C. Pollinating Flies (Diptera): A major contribution to plant diversity and agricultural production. Biodiversity 2008, 9, 86–89. [Google Scholar] [CrossRef]
- Vlašánková, A.; Padyšáková, E.; Bartoš, M.; Mengual, X.; Janečková, P.; Janeček, Š. The nectar spur is not only a simple specialization for long-proboscid pollinators. New Phytol. 2017, 215, 1574–1581. [Google Scholar] [CrossRef]
- Baker, H.G.; Baker, I. Floral nectar sugars constituents in relation to pollinator type. In Handbook of Experimental Pollination Biology; Jones, C.E., Little, R.J., Eds.; Van Nostrand Reinhold: New York, NY, USA, 1983; pp. 117–141. [Google Scholar]
- Perret, M.; Chautems, A.; Spichiger, R.; Peixoto, M.; Savolainen, V. Nectar sugar composition in relation to pollination syndromes in Sinningieae (Gesneriaceae). Ann. Bot. 2001, 87, 267–273. [Google Scholar] [CrossRef]
- Nicolson, S.W.; Thornburg, R.W. Nectar chemistry. In Nectaries and Nectar; Nicolson, S.W., Nepi, M., Pacini, E., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 215–264. [Google Scholar]
- Zimmerman, M. Patchiness in the dispersion of nectar resources: Probable causes. Oecologia 1981, 49, 154–157. [Google Scholar] [CrossRef]
- Galetto, L.; Bernardello, G. Floral nectaries, nectar production dynamics and chemical composition in six Ipomoea species (Convolvulaceae) in relation to pollinators. Ann. Bot. 2004, 94, 269–280. [Google Scholar] [CrossRef]
- Wolff, D. Nectar sugar composition and volumes of 47 species of Gentianales from a southern Ecuadorian montane forest. Ann. Bot. 2006, 97, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, S.W.; Nepi, M.; Pacini, E. Nectaries and Nectar; Springer: Dordrecht, The Netherlands, 2007. [Google Scholar]
- Manning, J.C.; Goldblatt, P. The Prosoeca peringueyi (Diptera: Nemestrinidae) Pollination Guild in Southern Africa: Long-tongued Flies and Their Tubular Flowers. Ann. Mo. Bot. Gard. 1996, 83, 67–86. [Google Scholar] [CrossRef]
- Goldblatt, P.; Manning, J.C. The Long-Proboscid Fly Pollination System in Southern Africa. Ann. Mo. Bot. Gard. 2000, 87, 146–170. [Google Scholar] [CrossRef]
- Paudel, B.R.; Shrestha, M.; Dyer, A.G.; Zhu, X.; Abdusalam, A.; Li, Q. Out of Africa: Evidence of the obligate mutualism between long corolla tubed plant and long-tongued fly in the Himalayas. Ecol. Evol. 2015, 5, 5240–5251. [Google Scholar] [CrossRef]
- Bauder, J.A.-S.; Karolyi, F. Superlong Proboscises as Co-adaptations to Flowers. In Insect Mouthparts Form, Function, Development and Performence. Zoological Monographs 5; Krenn, H.W., Ed.; Springer Nature: Cham, Switzerland, 2019; pp. 479–527. [Google Scholar]
- Johnson, S.D.; Steiner, K.E. Long-Tongued Fly Pollination and Evolution of Floral Spur Length in the Disa draconis Complex (Orchidaceae). Evolution 1997, 51, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Goldblatt, P.; Manning, J.C. The long-proboscid fly Pollination System in Gladiolus (Iridaceae). Ann. Mo. Bot. Gard. 1999, 86, 758–774. [Google Scholar] [CrossRef]
- Goldblatt, P.; Manning, J.C. Radiation of pollination systems in the Iridaceae of sub-Saharan Africa. Ann. Bot. 2006, 97, 317–344. [Google Scholar] [CrossRef] [PubMed]
- Goldblatt, P.; Manning, J.C. Floral Biology of Babiana (Iridaceae: Crocoideae): Adaptive floral radiation and pollination. Ann. Mo. Bot. Gard. 2007, 94, 709–733. [Google Scholar] [CrossRef]
- Potgieter, C.J.; Edwards, T.J. The Stenobasipteron wiedmanni (Diptera, Nemestrinidae) pollination guild in Eastern Southern Africa. Ann. Mo. Bot. Gard. 2005, 92, 254–267. [Google Scholar]
- Anderson, B.; Johnson, S.D. The geographical mosaic of coevolution in a plant-pollinator mutualism. Evolution 2008, 62, 220–225. [Google Scholar] [CrossRef]
- Anderson, B.; Johnson, S.D. Geographical covariation and local convergence of flower depth in a guild of fly-pollinated plants. New Phytol. 2009, 182, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Pauw, A.; Stofberg, J.; Waterman, R.J. Flies and Flowers in Darwins’s Race. Evolution 2009, 63, 268–279. [Google Scholar] [CrossRef]
- Barraclough, D.A. An overview of the South African tangle-veined flies (Diptera: Nemestrinidae), with an annotated key to the genera and a checklist of species. Zootaxa 2006, 1277, 39–63. [Google Scholar] [CrossRef]
- Barraclough, D.A.; Colville, J.F.; Karolyi, F.; Krenn, H.W. A striking new species of Prosoeca Schiner, 1867 (Diptera: Nemestrinidae): An important pollinator from the Bokkeveld Plateau, Northern Cape Province, South Africa. Zootaxa 2018, 4497, 411–421. [Google Scholar] [CrossRef]
- Theron, G.L.; Grenier, F.; Anderson, B.C.; Ellis, A.G.; Johnson, S.D.; Midgley, J.M.; van der Niet, T. Key long-proboscid fly pollinator overlooked: Morphological and molecular analyses reveal a new Prosoeca (Nemestrinidae) species. Biol. J. Linn. Soc. 2020, 131, 26–38. [Google Scholar] [CrossRef]
- Manning, J.; Goldblatt, P. Plants of The Greater Cape Floristic Region 1: The Core Cape Flora. Strelitzia 2012, 29, xiv, 846. [Google Scholar]
- Cowling, R.M.; Esler, K.J.; Rundel, P.W. Namaqualand, South Africa-An overview of a unique winter-rainfall desert ecosystem. Plant Ecol. 1999, 142, 3–21. [Google Scholar] [CrossRef]
- Karolyi, F.; Szucsich, N.U.; Colville, J.F.; Krenn, H.W. Adaptations for nectar-feeding in the mouthparts of long-proboscid flies (Nemestrinidae: Prosoeca). Biol. J. Linn. Soc. 2012, 107, 414–424. [Google Scholar] [CrossRef]
- Karolyi, F.; Morawetz, L.; Colville, J.F.; Handschuh, S.; Metscher, B.D.; Krenn, H.W. Time management and nectar flow: Flower handling and suction feeding in long-proboscid flies (Nemestrinidae: Prosoeca). Naturwissenschaften 2013, 100, 1083–1093. [Google Scholar] [CrossRef]
- Mucina, L.; Rutherford, M.C. The vegetation of South Africa, Lesotho and Swaziland. Strelitzia 2007, 19, 801. [Google Scholar]
- Krenn, H.W.; Karolyi, F.; Melin, A.; Colville, J.F. Flowers fuel flies–supply of nectar for long-proboscid flies (Prosoeca sp., Nemestrinidae). In Proceedings of the 9th International Congress of Dipterology, Windhoek, Namibia, 25–30 November 2018; Kirk-Spriggs, A.H., Muller, B.S., Eds.; p. 141. [Google Scholar]
- Pernstich, A.; Krenn, H.W.; Pass, G. Preparation of serial sections of arthropods using 2,2-dimethoxypropane dehydration and epoxy resin embedding under vacuum. Biotech. Histochem. 2003, 78, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Goldblatt, P.; Manning, J.C. A Revision of the Southern African Genus Babiana (Iridaceae: Crocoideae). Strelitzia 2007, 18, 99. [Google Scholar]
- Lehnert, M.S.; Monaenkova, D.; Andrukh, T.; Beard, C.E.; Adler, P.H.; Kornev, K.K. Hydrophobic-hydrophilic dichotomy of the butterfly proboscis. J. R. Soc. Interface 2013, 10, 20130336. [Google Scholar] [CrossRef] [PubMed]
- Lehnert, M.S.; Bennett, A.; Reiter, K.E.; Gerard, P.D.; Wei, Q.-H.; Byler, M.; Yan, H.; Lee, W.-K. Mouthpart conduit sizes of fluid-feeding insects determine the ability to feed from pores. Proc. R. Soc. B Biol. Sci. 2017, 284, 20162026. [Google Scholar] [CrossRef]
- Karolyi, F.; Colville, J.F.; Handschuh, S.; Metscher, B.D.; Krenn, H.W. One proboscis, two tasks: Adaptations to blood-feeding and nectar-extracting in long-proboscid horse flies (Tabanidae, Philoliche). Arthropod Struct. Dev. 2014, 43, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Nagatomi, A.; Soroida, K. The structure of the mouthparts of the orthorraphous Brachycera (Diptera) with special reference to blood-sucking. Beiträge Entomol. Contrib. Entomol. 1985, 35, 263–368. [Google Scholar]
- Borkent, C.J.; Gillung, J.P.; Winterton, S.L. Jewelled spider flies of North America: A revision and phylogeny of Eulonchus Gerstaecker (Diptera, Acroceridae). Zookeys 2016, 619, 103–146. [Google Scholar] [CrossRef]
- Szucsich, N.U.; Krenn, H.W. Morphology and function of the proboscis in Bombyliidae (Diptera, Brachycera) and implications for proboscis evolution in Brachycera. Zoomorphology 2000, 120, 79–90. [Google Scholar] [CrossRef]
- Szucsich, N.U.; Krenn, H.W. Flies and concealed nectar sources: Morphological innovations in the proboscis of Bombyliidae (Diptera). Acta Zool. 2002, 83, 183–192. [Google Scholar] [CrossRef]
- Reich, D.; Berger, A.; Balthazar, M.; Chartier, M.; Sherafati, M.; Schönenberger, J.; Manafzadeh, S.; Staedler, Y.M. Modularity and evolution of flower shape: The role of function, development, and spandrels in Erica. New Phytol. 2020, 226, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Pauw, A.; Cocucci, A.A.; Sérsic, A.N. The least effective pollinator principle: Specialized morphology despite generalized ecology. Plant. Biol. 2020, 22, 924–931. [Google Scholar] [CrossRef]
- Willmer, P.G.; Stone, G.N. Behavioral, Ecological, and Physiological Determinants of the Activity Patterns of Bees. Adv. Study Behav. 2004, 34, 347–466. [Google Scholar]
- Kevan, P.G.; Baker, H.G. Insects as Flower Visitors and Pollinators. Annu. Rev. Entomol. 1983, 28, 407–453. [Google Scholar] [CrossRef]
- Haber, W.A.; Frankie, G.W. A Tropical Hawkmoth Community: Costa Rican Dry Forest Sphingidae. Biotropica 1989, 21, 155–172. [Google Scholar] [CrossRef]
- Stiles, F.G.; Freeman, C.E. Patterns in Floral Nectar Characteristics of some Bird-Visited Plant Species from Costa Rica. Biotropica 1993, 25, 191–205. [Google Scholar] [CrossRef]
- Nicolson, S.W. Nectar consumers. In Nectaries and Nectar; Nicolson, S.W., Nepi, M., Pacini, E., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 289–342. [Google Scholar]
- Klumpers, S.G.T.; Stang, M.; Klinkhamer, P.G.L. Foraging efficiency and size matching in a plant–pollinator community: The importance of sugar content and tongue length. Ecol. Lett. 2019, 22, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Borrell, B.; Krenn, H. Nectar Feeding in Long-Proboscid Insects. In Ecology and Biomechanics; Herrel, A., Speck, T., Rowe, N.P., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 183–212. [Google Scholar]
- Feinsinger, P. Ecological Interactions between Plants and Hummingbirds in a Successional Tropical Community. Ecol. Monogr. 1978, 48, 269–287. [Google Scholar] [CrossRef]
- Southwick, E.E.; Loper, G.M.; Sadwick, S.E. Nectar Production, Composition, Energetics and Pollinator Attractiveness in Spring Flowers of Western New York. Am. J. Bot. 1981, 68, 994–1002. [Google Scholar] [CrossRef]
- Galetto, L.; Bernardello, G. Chapter 5.3 Nectar. In Practical Pollination Biology; Dafni, A., Kevan, P.G., Husband, B.C., Eds.; Enviroquest Ltd.: Cambridge, ON, Canada, 2005; pp. 261–313. [Google Scholar]
- Morrant, D.S.; Schumann, R.; Petit, S. Field methods for sampling and storing nectar from flowers with low nectar volumes. Ann. Bot. 2009, 103, 533–542. [Google Scholar] [CrossRef] [PubMed]

| Prosoeca marinusi Flies | Min–Max [mm] | Mean ± s.d. [mm] |
|---|---|---|
| Body length (n = 80) | 16.21–21.96 | 19.14 ± 1.25 |
| Proboscis length (n = 80) | 23.68–38.86 | 32.63 ± 2.93 |
| Labellum length (n = 10) 1 | 0.79–0.89 | 0.82 ± 0.04 |
| Proximal proboscis (n = 10), height 1 | 0.5–0.63 | 0.65 ± 0.04 |
| Width 1 | 0.31–0.44 | 0.37 ± 0.04 |
| Distal proboscis (n = 10), diameter 1 | 0.24–0,19 | 0.21 ± 0.02 |
| Labella (n = 10), diameter 1 | 0.24–0.18 | 0.22 ± 0.01 |
| Babiana vanzijliae Flowers | Min–Max [mm] | Mean ± s.d. [mm] |
|---|---|---|
| Perianth tube length (n = 41) | 19–38.25 | 26.92 ± 5.75 |
| Perianth tube external radius (n = 41) | 0.75–1.43 | 0.95 ± 0.12 |
| Perianth tube internal radius (n = 9), mid 1 | 0.37–0.47 | 0.42 ± 0.03 |
| bottom 1 | 0.28–0.37 | 0.32 ± 0.04 |
| Stylus radius (n = 9), bottom 1 | 0.22–0.27 | 0.25 ± 0.02 |
| Floral wall thickness (n = 9) 1 | 0.43–0.60 | 0.53 ± 0.07 |
| Nectar Per Flower | Volume [µm]Min–Max, Mean ± s.d. | Height [mm] 2Min–Max, Mean ± s.d. |
|---|---|---|
| Before fly activity (n = 44) 1 | 0.1–5.3, 1.54 ± 0.94 | 0.73–10.37, 5.12 ± 2.63 |
| After flower visit (n = 39) | 0.0–2.0, 0.52 ± 0.63 | 0.0–8.87, 2.67 ± 3.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krenn, H.W.; Karolyi, F.; Lampert, P.; Melin, A.; Colville, J.F. Nectar Uptake of a Long-Proboscid Prosoeca Fly (Nemestrinidae)—Proboscis Morphology and Flower Shape. Insects 2021, 12, 371. https://doi.org/10.3390/insects12040371
Krenn HW, Karolyi F, Lampert P, Melin A, Colville JF. Nectar Uptake of a Long-Proboscid Prosoeca Fly (Nemestrinidae)—Proboscis Morphology and Flower Shape. Insects. 2021; 12(4):371. https://doi.org/10.3390/insects12040371
Chicago/Turabian StyleKrenn, Harald W., Florian Karolyi, Peter Lampert, Annalie Melin, and Jonathan F. Colville. 2021. "Nectar Uptake of a Long-Proboscid Prosoeca Fly (Nemestrinidae)—Proboscis Morphology and Flower Shape" Insects 12, no. 4: 371. https://doi.org/10.3390/insects12040371
APA StyleKrenn, H. W., Karolyi, F., Lampert, P., Melin, A., & Colville, J. F. (2021). Nectar Uptake of a Long-Proboscid Prosoeca Fly (Nemestrinidae)—Proboscis Morphology and Flower Shape. Insects, 12(4), 371. https://doi.org/10.3390/insects12040371







