Filtration of Nutritional Fluids in the German Wasp Vespula germanica (Vespidae, Hymenoptera)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
- None: No glass beads were visible inside the head, thorax, or abdomen. If glass beads were located on the mouthparts, it also was counted as not inside the head.
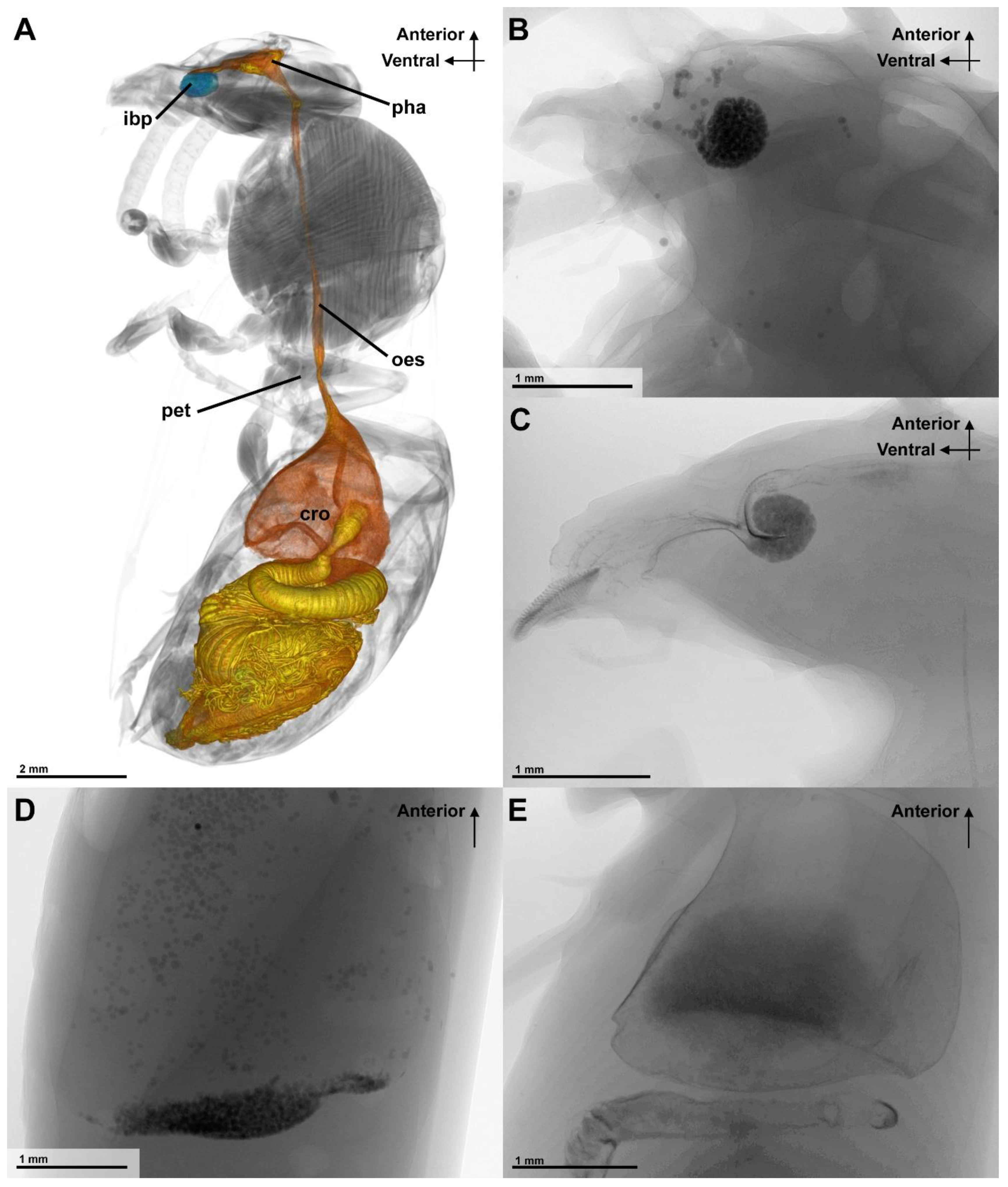
- Inside the infrabuccal pocket: Glass beads were visible inside the head as globular shadows in the infrabuccal pocket. The mandibular joint was used to determine if a glass bead was inside or outside the head, as it was viewed from a lateral direction, in a close position to the functional mouth opening.
- Abdomen: Glass beads were visible inside the anterior abdomen, where the crop is located.
- The head was removed from the body and the antennae were cut off. Then, a sagittal cut was performed using a stiff razorblade.
- The head was removed and a parasagittal cut close to the mandibular joint was performed. The musculature and cuticle structures such as the tentorium were removed, until it was possible to extract the pharynx with the infrabuccal pocket plus hypopharynx and epipharynx. The dorsal and ventral parts were separated carefully with the use of forceps, dissection needles, and microscissors.
3. Results
3.1. Fluid Filtration
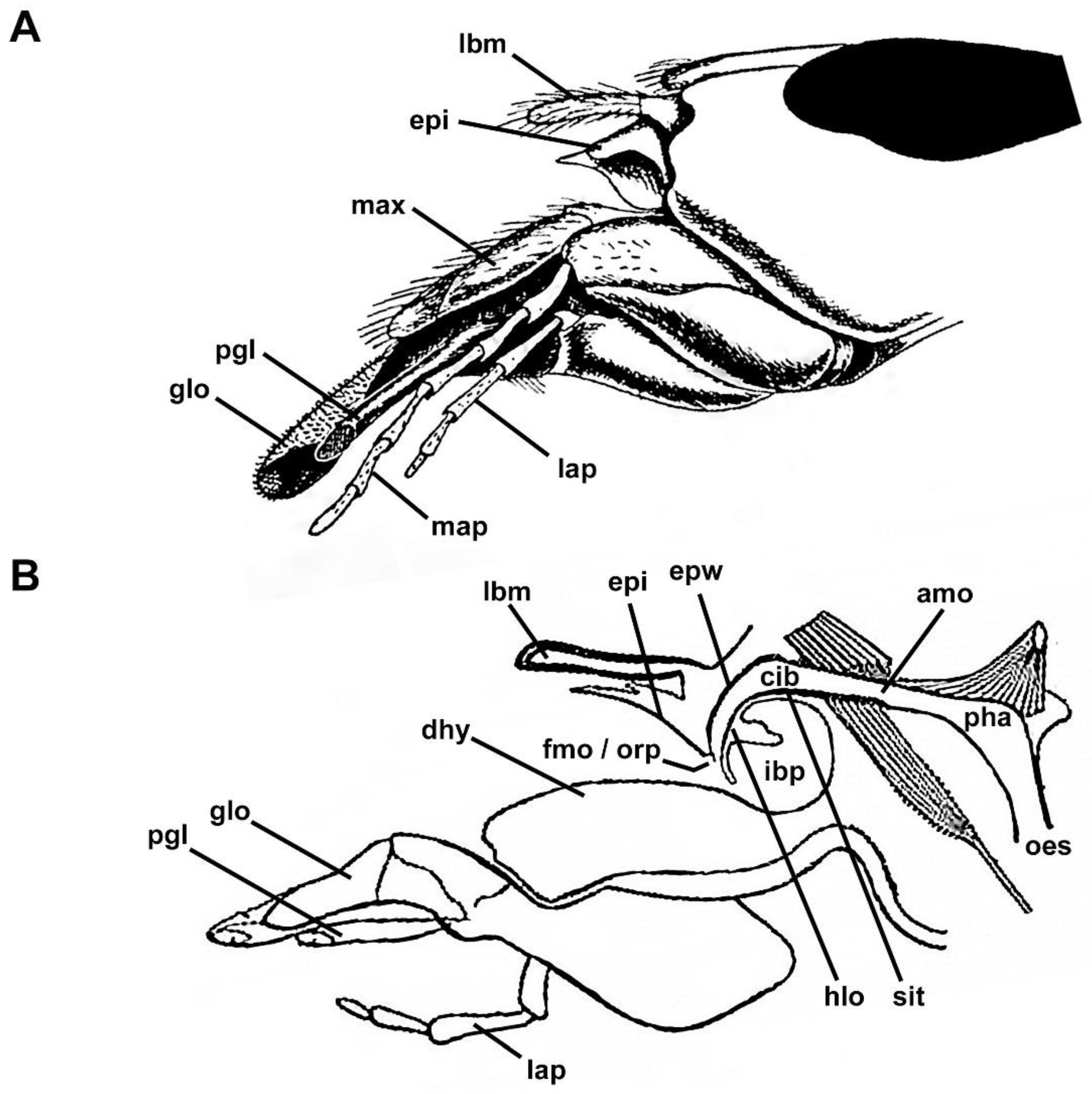
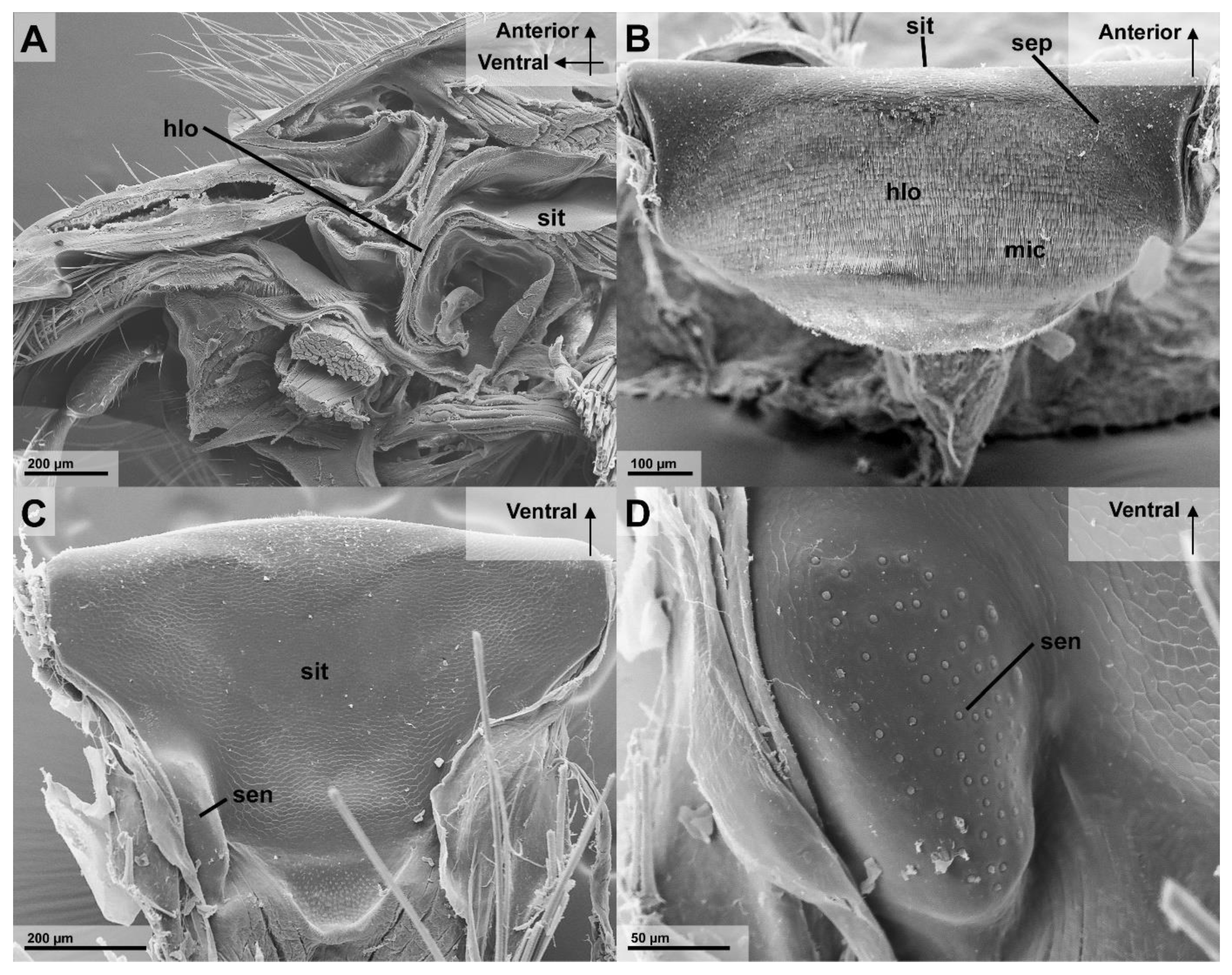
3.2. Filtering Structures
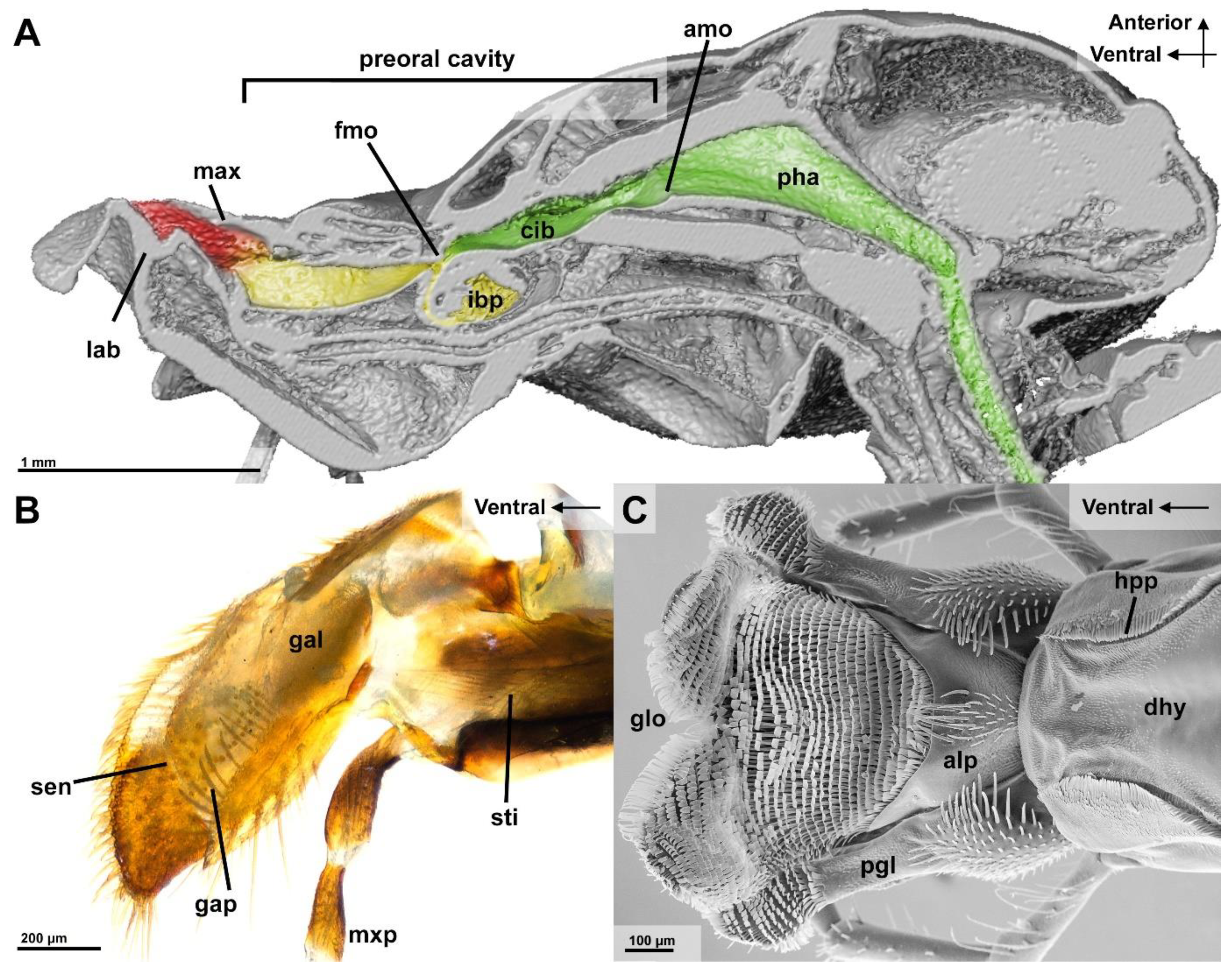
3.2.1. Epipharyngeal Wall
3.2.2. Infrabuccal Pocket
3.2.3. Cibarium and Sitophore
4. Discussion
4.1. First Filtration Step
4.2. Second Filtration Step
4.3. Function of the Infrabuccal Pocket
4.4. Staining and Testing Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Abbreviation | Term | Concept | URI | References | Synonym |
|---|---|---|---|---|---|
| amo | anatomical mouth | Opening of pharynx, always below frontal ganglion; with attachment of the anterior precerebral frontopharyngeal dilator, often enclosed by strongly developed ring muscle. | Beutel, R.G., Friedrich, F., Yang, X.K., & Ge, S.Q. Insect morphology and phylogeny: a textbook for students of entomology. Walter de Gruyter 2013. DOI:10.1515/9783110264043. (*) | ||
| app | anterior ligula plate | Anterior sclerotised plate at the base of the ligula | Duncan, C.D. A Contribution to the biology of North American Vespine Wasps, Stanford University Press: Stanford, USA, 1939. edited by the author (*) | ||
| cardo | The sclerite that is articulated with the cranium at the cranio-cardinal articulation, is connected to the stipes distolaterally via the stipitocardinal hinge, and receives the site of attachment of the occipito-cardinal muscle. | http://purl.obolibrary.org/obo/HAO_0000187 | Miko, I. 2009. -2019 Curator. Hymenoptera Anatomy Ontology.; Karlsson, D., and F. Ronquist. 2012. Skeletal Morphology of Opius dissitus and Biosteres carbonarius, with a Discussion of Terminology and Morphological Variation in Opiinae (Hymenoptera: Braconidae). PLoS ONE 7:1–38. | ||
| cib | cibarium | The anatomical space that is the anteriormost part of the alimentary canal and is delimited proximally by the proximomedian margin of the sitophore and distally by the functional mouth. | http://purl.obolibrary.org/obo/HAO_0000201 | Deans, A. R. 2009. HAO curator.. | |
| cro | crop | Crop-like extended part of the posterior foregut | Beutel, R.G., Friedrich, F., Yang, X.K., & Ge, S.Q. Insect morphology and phylogeny: a textbook for students of entomology. Walter de Gruyter 2013. DOI:10.1515/9783110264043. (*) | ingluvies | |
| cuticle | The acellular anatomical structure that is the external layer of the integument (covers the entire body surface as well as lines ectodermal invaginations such as the stomodeum, proctodeum, and tracheae) and produced by the epidermal cells. | http://purl.obolibrary.org/obo/HAO_0000240 | Nichols, S. W. (eds.). 1989. The Torre-Bueno Glossary of Entomology. New York Entomological Society and the American Museum of Natural History, New York.; Miko, I. 2009. -2019 Curator. Hymenoptera Anatomy Ontology. | ||
| dhy | distal hypopharynx | The area that is located on the anterior surface of the hypopharyngeal wall and is delimited proximally by the infrabuccal pouch or the distal margin of the sitophore, distally by the salivarial orifice, and laterally by the lateral parts of the prementum and the hypopharyngeal rods. | http://purl.obolibrary.org/obo/HAO_0001575 | Vilhelmsen, L., and I. Miko. 2010. Curators/Head. | labial portion of the hypopharynx |
| epw | epipharyngeal wall | The conjunctiva that extends between the distal margin of the labrum and the proximal boundary of the cibarium. | http://purl.obolibrary.org/obo/HAO_0000300 | Vilhelmsen, L. 1996. The preoral cavity of lower Hymenoptera (Insecta): comparative morphology and phylogenetic significance. Zoologica Scripta 25:143–170. | |
| epi | epipharynx | The lobe that is situated posterior to the labrum and is the distalmost part of the epipharyngeal wall. | http://purl.obolibrary.org/obo/HAO_0000301 | Miko, I. 2009. -2019 Curator. Hymenoptera Anatomy Ontology. | epipharyngeal lobe |
| fmo | functional mouth | The anatomical space that is delimited posteriorly by the distal part of the sitophore and anteriorly by the tormae. | http://purl.obolibrary.org/obo/HAO_0000361 | Curators, H. A. O. 2009. The Hymenoptera Anatomy Ontology Curation Team. Hymenoptera Anatomy Ontology.; Snodgrass, R. E. 1935. Principles of insect morphology. McGraw-Hill Book Co., Inc., New York & London 667 pp. | |
| gal | galea | The lobe that is located on the stipes at the distal part of the posterior stipital sclerite distolateral to the lacinia. | http://purl.obolibrary.org/obo/HAO_0000368 | Miko, I. 2009. -2019 Curator. Hymenoptera Anatomy Ontology.; Karlsson, D., and F. Ronquist. 2012. Skeletal Morphology of Opius dissitus and Biosteres carbonarius, with a Discussion of Terminology and Morphological Variation in Opiinae (Hymenoptera: Braconidae). PLoS ONE 7:1–38. | |
| gap | galeal pecten | The row of setae that is located on the medial wall of the galeo-lacinial complex proximal to the coeloconic sensilla of galea. | http://purl.obolibrary.org/obo/HAO_0002243 | Duncan, C. D. 1939. A contribution to the biology of North American vespine wasps. Stanford University Publications, Biological Sciences 8:1–233.; Crosskey, R. W. 1951. The morphology, taxonomy, and biology of the British Evanioidea (Hymenoptera). Transactions of the Royal Entomological Society of London 102:247–301. | galeal comb |
| glo | glossa | The lobe of the labium that is limited posteroproximally by the prementum, anteroproximally by the fold traversing the salivary orifice, and laterally by the paraglossae. | http://purl.obolibrary.org/obo/HAO_0000376 | Snodgrass, R. E. 1935. Principles of insect morphology. McGraw-Hill Book Co., Inc., New York & London 667 pp.; Deans, A. R. 2009. HAO curator..; Karlsson, D., and F. Ronquist. 2012. Skeletal Morphology of Opius dissitus and Biosteres carbonarius, with a Discussion of Terminology and Morphological Variation in Opiinae (Hymenoptera: Braconidae). PloS ONE 7:1–38.; Prentice, M. A. 1998. The comparative morphology and phylogeny of Apoid wasps (Hymenoptera: Apoidea) Thesis (Ph D in Entomology)—University of California, Berkeley..; Vilhelmsen, L. 1996. The preoral cavity of lower Hymenoptera (Insecta): comparative morphology and phylogenetic significance. Zoologica Scripta 25:143–170. | |
| hli | hypopharyngeal lip | Transverse ridge on the proximal margin of the distal hypopharynx, with a change in cuticular structure, at the entrance to the infrabuccal pocket | Author (*) | ||
| hlo | hypopharyngeal lobe | The lobe that is situated distally of the sitophore and proximally of the infrabuccal pouch. | http://purl.obolibrary.org/obo/HAO_0001565 | Vilhelmsen, L. 1996. The preoral cavity of lower Hymenoptera (Insecta): comparative morphology and phylogenetic significance. Zoologica Scripta 25:143–170. | |
| hpp | hypopharyngeal pecten | The anterolateral area of the distal hypopharynx that is adjacent with the proximal part of the hypopharyngeal rod proximal to the hypopharyngeal button and is covered with acanthae. | http://purl.obolibrary.org/obo/HAO_0002214 | Duncan, C. D. 1939. A contribution to the biology of North American vespine wasps. Stanford University Publications, Biological Sciences 8:1–233. | |
| hypopharynx | The area that is delimited proximally by the proximal margin of the sitophore, distally by the salivarial orifice. | http://purl.obolibrary.org/obo/HAO_0000409 | Vilhelmsen, L., and I. Miko. 2010. Curators/Head.. | hypopharyngeal wall | |
| ibp | infrabuccal pocket | The pouch that is situated on the hypopharyngeal wall distally of the sitophore. | http://purl.obolibrary.org/obo/HAO_0001563 | Vilhelmsen, L., and I. Miko. 2010. Curators/Head.. | infrabuccal pouch |
| labio-maxillary complex | The anatomical cluster that is composed of the labium and maxillae. | http://purl.obolibrary.org/obo/HAO_0000452 | Deans, A. R. 2009. HAO curator.. | maxillo-labial complex | |
| lab | labium | The appendage that is encircled by the area that is proximally delimited by the lateral margins of the cardo and the posterior stipital sclerite laterally, and the anatomical line that is tangential to the salivary duct and traverses the salivary orifice anteriorly. | http://purl.obolibrary.org/obo/HAO_0000453 | Deans, A. R. 2009. HAO curator..; Kirby, W., and W. Spencer. 1828. An introduction to Entomology: or elements of the natural history of insects. Longman, Rees, Orme, Brown & Green, London 732 pp. | |
| ligula | The anatomical cluster that is composed of the glossa and paraglossae. | http://purl.obolibrary.org/obo/HAO_0000496 | Miko, I. 2009. -2019 Curator. Hymenoptera Anatomy Ontology.; Prentice, M. A. 1998. The comparative morphology and phylogeny of Apoid wasps (Hymenoptera: Apoidea) Thesis (Ph D in Entomology)—University of California, Berkeley..; Duncan, C. D. 1939. A contribution to the biology of North American vespine wasps. Stanford University Publications, Biological Sciences 8:1–233.; Snodgrass, R. E. 1942. The skeleto-muscular mechanisms of the honey bee. Smithsonian Miscellaneous Collections 103:1–120. | ||
| max | maxilla | The appendage that is encircled by the area that is proximally delimited by the hypostoma posteriorly, the median margin of the mandible laterally, the labrum anterolaterally, and the labium medially. | http://purl.obolibrary.org/obo/HAO_0000513 | Gibson, G. A. P., J. D. Read, and R. Fairchild. 1998. Chalcid wasps (Chalcidoidea): illustrated glossary of positional and morphological terms; Miko, I. 2009. -2019 Curator. Hymenoptera Anatomy Ontology. | |
| mxp | maxillary palpus | The palp that is located on the maxilla articulating laterally on the stipes | http://purl.obolibrary.org/obo/HAO_0000515 | Vilhelmsen, L., and I. Miko. 2010. Curators/Head.. | |
| mentum | The sclerite that articulates basally with the submentum and apically with the prementum. | http://purl.obolibrary.org/obo/HAO_0000534 | Goulet, H., and J. T. Huber. 1993. Hymenoptera of the World: An Identification Guide to Families. Research Branch, Agriculture Canada Publication 1894/E., Ottawa, ON 668 pp.; Miko, I. 2009. -2019 Curator. Hymenoptera Anatomy Ontology.; Vilhelmsen, L. 1996. The preoral cavity of lower Hymenoptera (Insecta): comparative morphology and phylogenetic significance. Zoologica Scripta 25:143–170. | ||
| mic | microtrichium | Hair without articulation, usually solid; several microtrichia are formed by one hypodermal cell | Beutel, R.G., Friedrich, F., Yang, X.K., & Ge, S.Q. Insect morphology and phylogeny: a textbook for students of entomology. Walter de Gruyter 2013. DOI:10.1515/9783110264043. (*) | ||
| mouthparts | The anatomical cluster that is composed of the labrum, epipharyngeal wall, hypopharyngeal wall (including the sitophore), mandibles, maxillae, labium, and conjunctivae connecting them. | http://purl.obolibrary.org/obo/HAO_0000639 | Goulet, H., and J. T. Huber. 1993. Hymenoptera of the World: An Identification Guide to Families. Research Branch, Agriculture Canada Publication 1894/E., Ottawa, ON 668 pp.; Deans, A. R. 2009. HAO curator.. | ||
| oes | oesophagus | Part of foregut fllowing the pharynx; with one pair of thin dilators or completely lacking dilator muscles; with thin ring muscle layer | Beutel, R.G., Friedrich, F., Yang, X.K., & Ge, S.Q. Insect morphology and phylogeny: a textbook for students of entomology. Walter de Gruyter 2013. DOI:10.1515/9783110264043. (*) | esophagus | |
| orp | oral pecten | A fringe of setae projecting over the slit-like true mouth = (functional mouth); *Attached at the epharyngeal wall, close to the functional mouth opening | Duncan, C.D. A Contribution to the biology of North American Vespine Wasps, Stanford University Press: Stanford, USA, 1939. with additon by the author (*) | ||
| pgl | paraglossa | The lobe that is connected to the distal margin of the prementum posteroproximally, to the premental hypopharynx proximolaterally and anteroproximally, to the glossa proximomedially, and bears the basiparaglossal brush and the paraglossal sclerite. | http://purl.obolibrary.org/obo/HAO_0000686 | Deans, A. R. 2009. HAO curator..; Karlsson, D., and F. Ronquist. 2012. Skeletal Morphology of Opius dissitus and Biosteres carbonarius, with a Discussion of Terminology and Morphological Variation in Opiinae (Hymenoptera: Braconidae). PLoS ONE 7:1–38. | |
| pet | petiolus | Part of the metasoma, usually metasomal segment 1; the usually narrow, parallel-sided stalk joining the rest of the metasoma to the propodeum. | http://purl.obolibrary.org/obo/HAO_0000020 | Miko, I. 2009. -2019 Curator. Hymenoptera Anatomy Ontology. | |
| pha | pharynx | The anatomical space that is located proximal to the cibarium. | http://purl.obolibrary.org/obo/HAO_0001740 | Vilhelmsen, L., and I. Miko. 2010. Curators/Head.. | |
| prementum | The sclerite that is median, is connected via conjunctiva along its proximolateral margins to the stipites, is articulated with the labial palps, is continuous along its distal margin with the ligula and distolateral margins with the distal hypopharynx, and receives the site of attachments of the extrinsic labial palp muscles. | http://purl.obolibrary.org/obo/HAO_0000804 | Snodgrass, R. E. 1935. Principles of insect morphology. McGraw-Hill Book Co., Inc., New York & London 667 pp.; Gibson, G. A. P., J. D. Read, and R. Fairchild. 1998. Chalcid wasps (Chalcidoidea): illustrated glossary of positional and morphological terms; Miko, I. 2009. -2019 Curator. Hymenoptera Anatomy Ontology.; Karlsson, D., and F. Ronquist. 2012. Skeletal Morphology of Opius dissitus and Biosteres carbonarius, with a Discussion of Terminology and Morphological Variation in Opiinae (Hymenoptera: Braconidae). PLoS ONE 7:1–38. | ||
| preoral cavity | The anatomical cluster that includes the labrum, hypopharynx, and labium. | http://purl.obolibrary.org/obo/HAO_0000809 | Vilhelmsen, L. 1996. The preoral cavity of lower Hymenoptera (Insecta): comparative morphology and phylogenetic significance. Zoologica Scripta 25:143–170.; Yoder, M. J. 2009. Curator. Hymenoptera Anatomy Ontology. | ||
| sep | secretory pore | The anatomical space that corresponds to the distal end of an exocrine gland. | http://purl.obolibrary.org/obo/HAO_0001966 | Hopper, K., J. Woolley, K. Hoelmer, K. Wu, G. Qiao, and S. Lee. 2012. An identification key to species in the mali complex of Aphelinus (Hymenoptera: Chalcidoidea) with descriptions of three new species. Journal of Hymenoptera Research 26:73–96. | |
| segment | An anatomical structure that is metameric and is connected to other metameric subdivisions by muscles and is delimited by its sclerites. | http://purl.obolibrary.org/obo/HAO_0000929 | Miko, I. 2009. -2019 Curator. Hymenoptera Anatomy Ontology. | ||
| sen | sensillum | A sense organ embedded in the integument and consisting of one or a cluster of sensory neurons and associated sensory structures, support cells, and glial cells forming a single organized unit with a largely bona fide boundary. | http://purl.obolibrary.org/obo/HAO_0000933 | Tweedie, S., M. Ashburner, K. Falls, P. Leyland, P. McQuilton, S. Marygold, G. Millburn, D. Osumi-Sutherland, A. Schroeder, R. Seal, H. Zhang, and The FlyBase Consortium. 2009. FlyBase: enhancing Drosophila Gene Ontology annotations. Nucleic Acids Research 37:D555-D559 | |
| sit | sitophore | The sclerite that is located in the proximal part of the hypopharygeal wall delimited distally by the functional mouth and proximally by the proximal boundary of the cibarium. The tentorio-hypopharyngeal muscle inserts on the proximal margin of the sitophore. | http://purl.obolibrary.org/obo/HAO_0000939 | Vilhelmsen, L. 1996. The preoral cavity of lower Hymenoptera (Insecta): comparative morphology and phylogenetic significance. Zoologica Scripta 25:143–170.; Deans, A. R. 2009. HAO curator.. | |
| spi | spine-like microtrichium | See Microtrichium, shorter hair-like process | Author (*) | ||
| sti | stipes | The appendage that is connected posteroproximally to the hypostoma, anteroproximally and lateroproximally to the mandible, and medioproximally to the labium and the hypopharynx via conjunctiva, is connected to the cranium via muscles and that bears the maxillary palp. | http://purl.obolibrary.org/obo/HAO_0000958 | Miko, I. 2009. -2019 Curator. Hymenoptera Anatomy Ontology.; Gibson, G. A. P., J. D. Read, and R. Fairchild. 1998. Chalcid wasps (Chalcidoidea): illustrated glossary of positional and morphological terms; Snodgrass, R. E. 1956. Anatomy of the Honey Bee. Comstock Publishing Associates, Ithaca 334 pp.; Karlsson, D., and F. Ronquist. 2012. Skeletal Morphology of Opius dissitus and Biosteres carbonarius, with a Discussion of Terminology and Morphological Variation in Opiinae (Hymenoptera: Braconidae). PLoS ONE 7:1–38. | |
| submentum | The interstipital sclerite that is connected proximally to the postarticular portion of the postmentum. | http://purl.obolibrary.org/obo/HAO_0002150 | Goulet, H., and J. T. Huber. 1993. Hymenoptera of the World: An Identification Guide to Families. Research Branch, Agriculture Canada Publication 1894/E., Ottawa, ON 668 pp. | prearticular portion of the postmentum |
References
- Krenn, H.W.; Plant, J.D.; Szucsich, N.U. Mouthparts of flower-visiting insects. Arthropod Struct. Dev. 2005, 34, 1–40. [Google Scholar] [CrossRef]
- Krenn, H.W. Fluid-Feeding Mouthparts. In Insect Mouthparts Form, Function, Development and Performance; Krenn, H.W., Ed.; Zoological Monographs Volume 5; Springer Nature: Cham, Switzerland, 2019; pp. 47–99. [Google Scholar]
- Mauss, V.; Kuba, K.; Krenn, H.W. Evolution of the Multifunctional Mouthparts of Adult Vespidae. In Insect Mouthparts Form, Function, Development and Performance; Krenn, H.W., Ed.; Zoological Monographs Volume 5; Springer Nature: Cham, Switzerland, 2019; pp. 154–196. [Google Scholar] [CrossRef]
- Vilhelmsen, L. The preoral cavity of lower Hymenoptera (Insecta): Comparative morphology and phylogenetic significance. Zool. Scr. 1996, 25, 143–170. [Google Scholar] [CrossRef]
- Edwards, R. Social Wasps Their Biology and Control, 1st ed.; Rentokil Limited: East Grinstead, UK, 1980; pp. 106–303. [Google Scholar]
- Matsuura, M.; Yamane, S. Biology of the Vespine Wasps; Springer: Berlin, Germany, 1990; pp. 99–113. [Google Scholar]
- Weyrauch, W. Wie ein Wespennest entsteht. Die Nat. 1939, 4, 4–57. [Google Scholar]
- Duncan, C.D. A Contribution to the Biology of North American Vespine Wasps; Stanford University Press: Stanford, CA, USA, 1939. [Google Scholar]
- Richter, M.R. Social Wasp (Hymneoptera: Vespidae) Foraging Behavior. Annual. Rev. Entomol. 2000, 45, 121–150. [Google Scholar] [CrossRef]
- Spradbery, J.P. Wasps: An Account of the Biology and Natural History of Solitary and Social Wasps; University of Washington Press: Seattle, WA, USA, 1973; pp. 40–291. [Google Scholar]
- Greene, A. Dolichovespula and Vespula. In The Social Biology of Wasps; Ross, K.G., Matthews, R.W., Eds.; Cornell University Press: New York, NY, USA, 1991; pp. 263–305. [Google Scholar]
- Janet, C. Études Sur Les Fourmis, Les Guêpes Et Les Abeilles. 9, Sur Vespa Crabro L.—Histoire D’un Nid Depuis Son Origine. Mém. Soc. Zool. de Fr. 1895, 8, 1–140. [Google Scholar]
- Hunt, J.H. Nourishment and the evolution of the social Vespidae. In The Social Biology of Wasps; Ross, K.G., Matthews, R.W., Eds.; Cornell University Press: New York, NY, USA, 1991; pp. 426–450. [Google Scholar]
- Baranek, B.; Kuba, K.; Bauder, J.; Krenn, H.W. Mouthpart dimorphism in male and female wasps of Vespula vulgaris and Vespula germanica (Vespidae, Hymenoptera). Dtsch. Entomol. Z. 2018, 65, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Kirmayer, R. Bau und Entwicklung der Mundteile bei Vespula vulgaris. In Gegenbaurs Morphologisches Jahrbuch; Ruge, G., Ed.; Verlag von Wilhelm Engelmann: Leipzig, Germany, 1909; Volume 39, pp. 1–31. [Google Scholar]
- Silveira, O.T.; Dos Santos Junior, J.N.A. Comparative morphology of the mandibles of female polistine social wasps (Hymenoptera, Vespidae, Polistinae). Rev. Bras. De Entomol. 2011, 55, 479–500. [Google Scholar] [CrossRef] [Green Version]
- Seifert, G. Entomologisches Praktikum, 3rd ed.; Georg Thieme Verlag: Stuttgart, Germany, 1995; pp. 209–214. [Google Scholar]
- Popovici, O.A.; Mikó, I.; Seltmann, K.C.; Deans, A.R. The maxillo-labial complex of Sparasion (Hymenoptera, Platygastroidea). J. Hymenopt. Res. 2014, 111, 77–111. [Google Scholar] [CrossRef] [Green Version]
- Popovici, O.A.; Vilhelmsen, L.; Masner, L.; Mikó, I.; Johnsom, N. Maxillolabial complex in scelionids (Hymenoptera: Platygastroidea): Morphology and phylogenetic implications. Insect Syst. Evol. 2017, 48, 315–439. [Google Scholar] [CrossRef]
- Jervis, M. Functional and evolutionary aspects of mouthpart structure in parasitoid wasps. Biol. J. Linn. Soc. 1998, 63, 461–493. [Google Scholar] [CrossRef]
- Snodgrass, R.E. The skeleto-muscular mechanisms of the honey bee. Smithson. Misc. Collect. 1942, 103, 4–32. [Google Scholar]
- Jervis, M.; Vilhelmsen, L. Mouthpart evolution in adults of the basal, “symphytan”, hymenopteran lineages. Biol. J. Linn. Soc. 2000, 70, 121–146. [Google Scholar] [CrossRef]
- Matsuda, R. Morphology of the Head of a Sawfly, Macrophya pluricincta Norton (Hymenoptera, Tenthredinidae). J. Kans. Entomol. Soc. 1957, 30, 99–108. [Google Scholar]
- Bugnion, E. Notes relatives a la terminologie des organes buccaux des insectes. Bull. Société Zool. Fr. 1925, 50, 352–358. [Google Scholar]
- Gotwald, W.H. Comparative morphological studies of the ants, with particular reference to the mouthparts (Hymenoptera: Formicidae). Cornell Univ. Agric. Exp. Stn. Mem. 1969, 408, 1–150. [Google Scholar] [CrossRef]
- Wang, C.; Billen, J.; Pan, X.; He, H. Morphology and ultrastructure of the infrabuccal pocket and its lining epithelium in workers of Ectomomyrmex javanus (Hymenoptera: Formicidae). Micron 2018, 115, 50–53. [Google Scholar] [CrossRef]
- Eisner, T.; Happ, G.M. The Infrabuccal Pocket of a Formicine Ant: A Social Filtration Device. Psyche 1962, 69, 107–116. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Menzel, R.; Sandoz, J.; Giurfa, M. Revisiting olfactory classical conditioning of the proboscis extension response in honey bees: A step toward standardized procedures. J. Neurosci. Methods 2012, 211, 159–167. [Google Scholar] [CrossRef]
- Megibow, A.J.; Bosniak, M.A. Dilute barium as a contrast agent for abdominal CT. Am. J. Roentgenol. 1980, 134, 1273–1274. [Google Scholar] [CrossRef] [Green Version]
- Davey, K.G.; Treherne, J.E. Studies on crop function in the cockroach (Periplaneta americana L.). J. Exp. Biol. 1963, 40, 763–773. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Metscher, B.D. MicroCT for comparative morphology: Simple staining methods allow high-contrast 3D imaging of diverse non-mineralized animal tissues. BMC Physiol. 2009, 9, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lösel, P.D.; Kamp, T.; Van De Jayme, A.; Ershov, A.; Faragó, T.; Pichler, O.; Heuveline, V. Introducing Biomedisa as an open-source online platform for biomedical image segmentation. Nat. Commun. 2020, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Cardona, A. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glancey, B.M.; Vander Meer, R.K.; Glover, A.; Lofgren, C.S.; Vinson, S.B. Filtration of microparticles from liquids ingested by the red imported fire ant Solenopsis invicta Buren. Insectes Sociaux 1981, 28, 395–401. [Google Scholar] [CrossRef]
- Quinlan, R.J.; Cherrett, J.M. Studies on the role of the infrabuccal pocket of the leaf-cutting ant Acromyrmex octospinosus (Reich) (Hym., Formicidae). Insectes Sociaux 1978, 25, 237–245. [Google Scholar] [CrossRef]
- Paul, J.; Roces, F. Comparative Functional Morphology of Ant Mouthparts and Significance for Liquid Food Intake. In Insect Mouthparts Form, Function, Development and Performance; Krenn, H.W., Ed.; Zoological Monographs Volume 5; Springer Nature: Cham, Switzerland, 2019; Volume 1, pp. 154–196. [Google Scholar] [CrossRef]
- Zimmermann, D.; Randolf, S.; Mauss, V. Morphological adaptations to silk production by adult females in the pollen wasp genus Quartinia (Masarinae, Vespidae)—A keystone character for ground nesting in dry sand habitats. Arthropod Struct. Dev. 2021, 62, 101045. [Google Scholar] [CrossRef]
- Hansen, L.D.; Spangenberg, W.J.; Gaver, M.M. The infrabuccal chamber of Camponotus modoc (Hymenoptera: Formicidae): Ingestion, digestion and survey of bacteria. In Proceedings of the 3rd International Conference on Urban Pests; Robinson, W.H., Rettich, F., Rambo, G.W., Eds.; Graficke Zavody: Hronov, Czeck Republic, 1999; pp. 211–219. [Google Scholar]


| Glass Bead Size (µm) | Number of Experiments |
|---|---|
| <152 | n = 19 |
| 152–212 | n = 10 |
| 212–300 | n = 13 |
| 450–600 | n = 5 |
| <152–212 | n = 10 |
| <152–600 | n = 5 |
| Glass Bead Sizes | Number of Workers | Glass Beads in Infrabuccal Pocket 1 | Glass Beads in Crop 2 | ||
|---|---|---|---|---|---|
| (µm) | (n) | Yes | No | Yes | No |
| <152 | 19 | 100% | 0% | 63% | 37% |
| 152–212 | 10 | 60% | 40% | 0% | 100% |
| 212–300 | 13 | 0% | 100% | 0% | 100% |
| 450–600 | 5 | 0% | 100% | 0% | 100% |
| <152–212 | 10 3 | 100% | 0% | 40% | 50% |
| <152–600 | 5 | 100% | 0% | 60% | 40% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuba, K.; Krenn, H.W. Filtration of Nutritional Fluids in the German Wasp Vespula germanica (Vespidae, Hymenoptera). Insects 2022, 13, 185. https://doi.org/10.3390/insects13020185
Kuba K, Krenn HW. Filtration of Nutritional Fluids in the German Wasp Vespula germanica (Vespidae, Hymenoptera). Insects. 2022; 13(2):185. https://doi.org/10.3390/insects13020185
Chicago/Turabian StyleKuba, Kenneth, and Harald W. Krenn. 2022. "Filtration of Nutritional Fluids in the German Wasp Vespula germanica (Vespidae, Hymenoptera)" Insects 13, no. 2: 185. https://doi.org/10.3390/insects13020185
APA StyleKuba, K., & Krenn, H. W. (2022). Filtration of Nutritional Fluids in the German Wasp Vespula germanica (Vespidae, Hymenoptera). Insects, 13(2), 185. https://doi.org/10.3390/insects13020185







