Influence of Plant Physical and Anatomical Characteristics on the Ovipositional Preference of Orius sauteri (Hemiptera: Anthocoridae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Predator Rearing
2.2. Plant Material
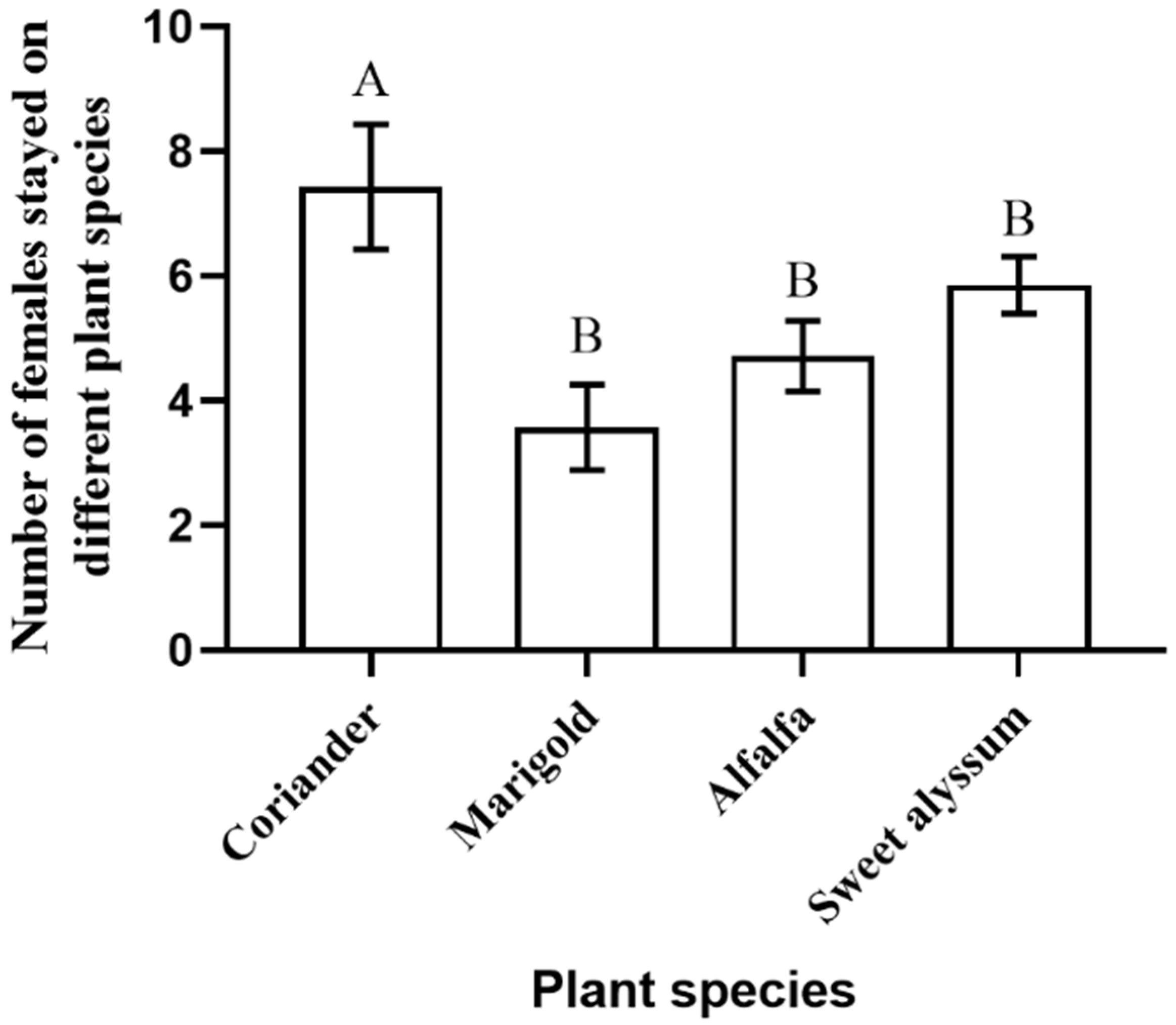
2.3. Behavioral Preferences of Minute Pirate Bug for Different Plant Species
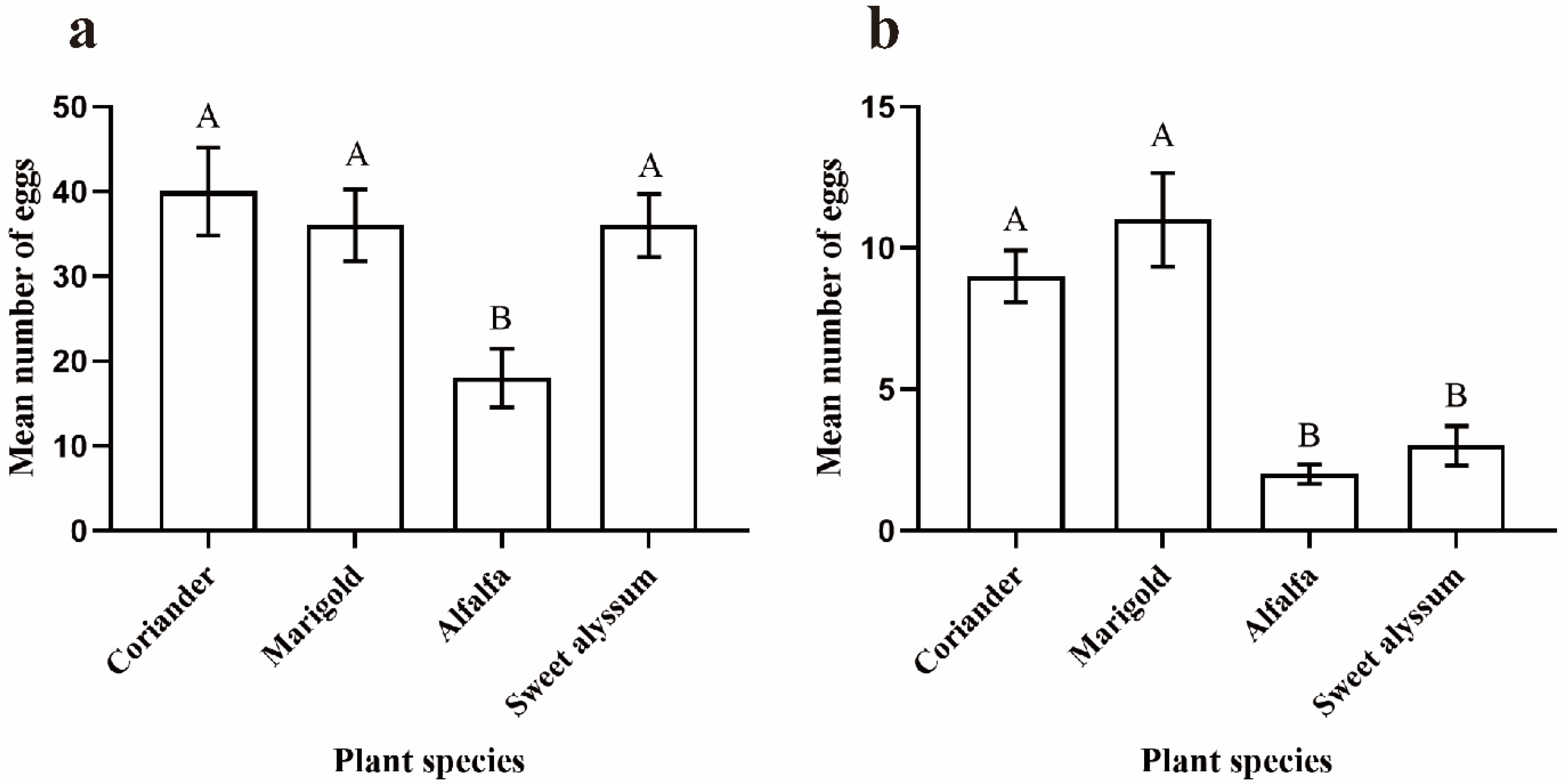
2.4. Plant Acceptability and Oviposition Preferences
2.4.1. Plant Acceptability
2.4.2. Oviposition Preferences
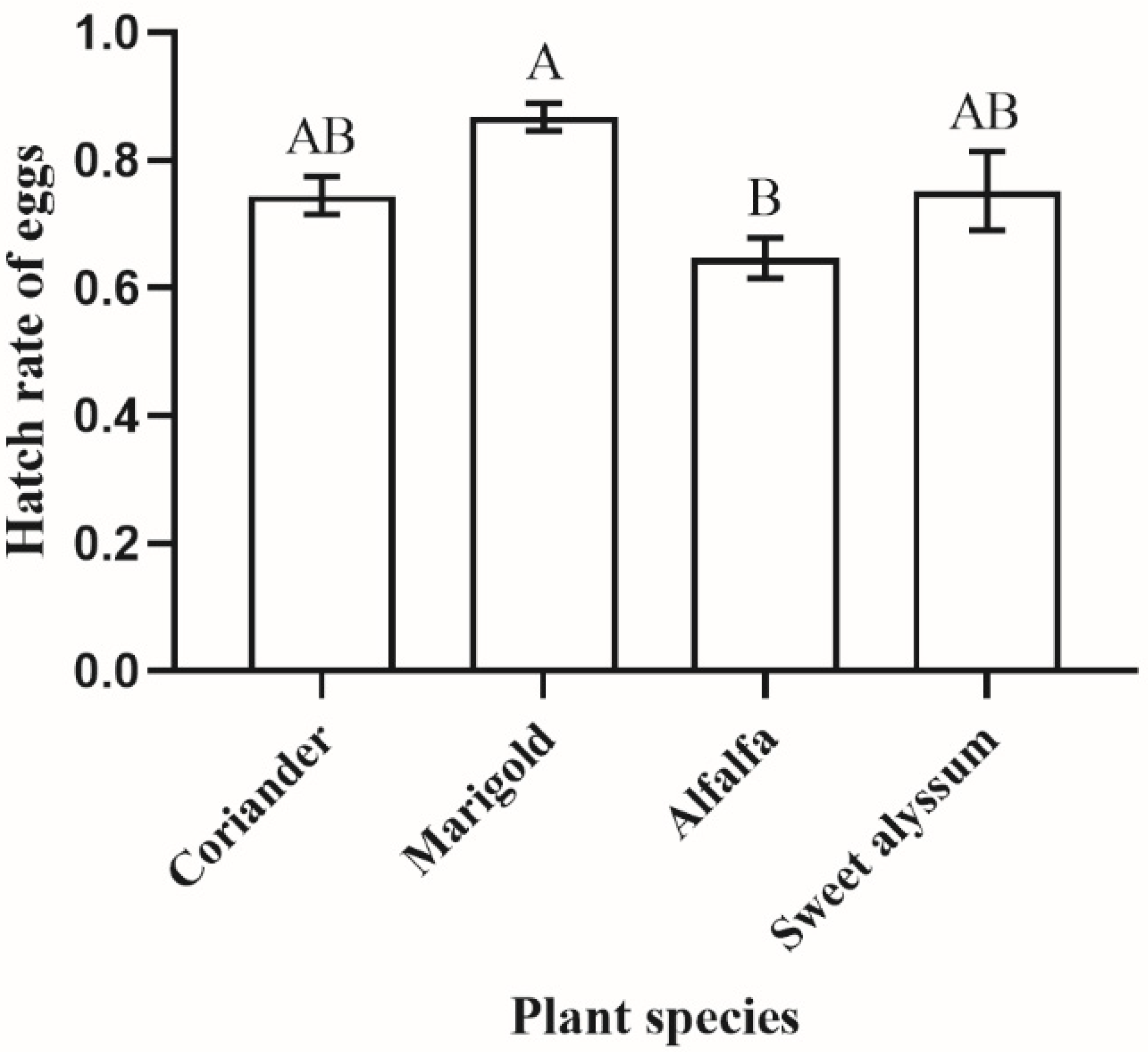
2.5. Egg Hatch on Different Plant Species
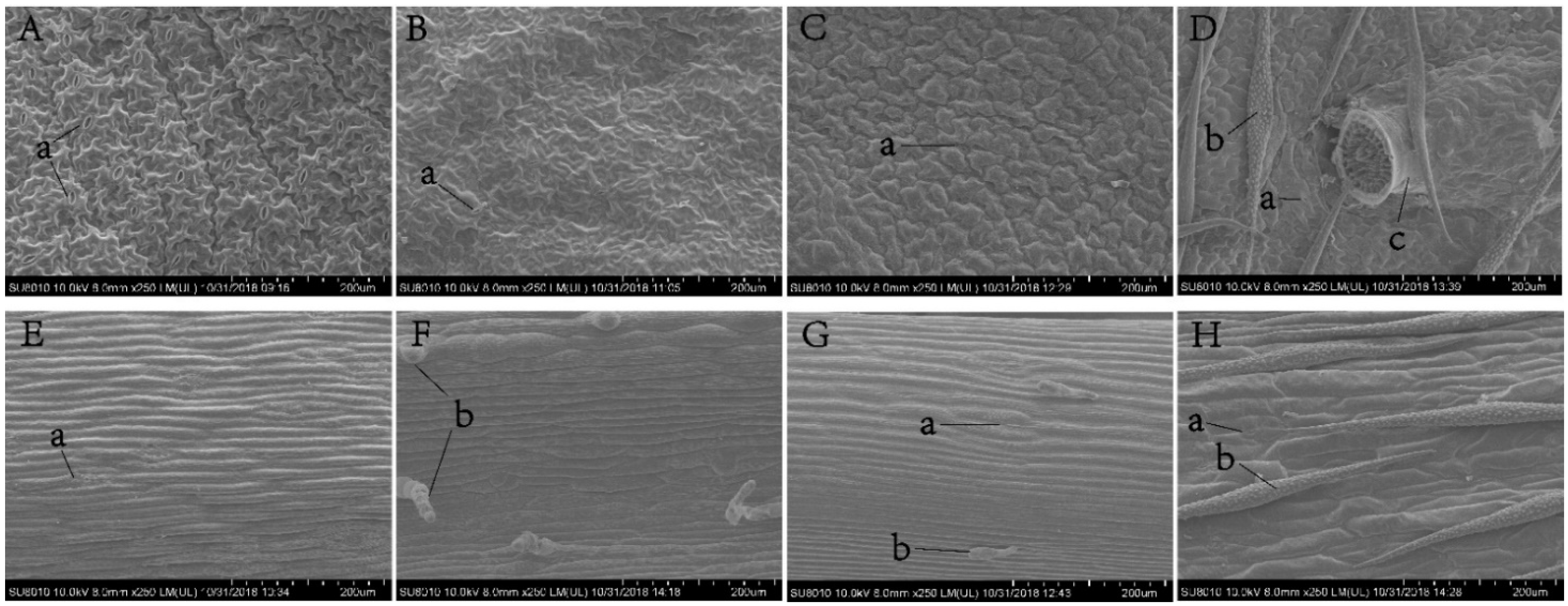
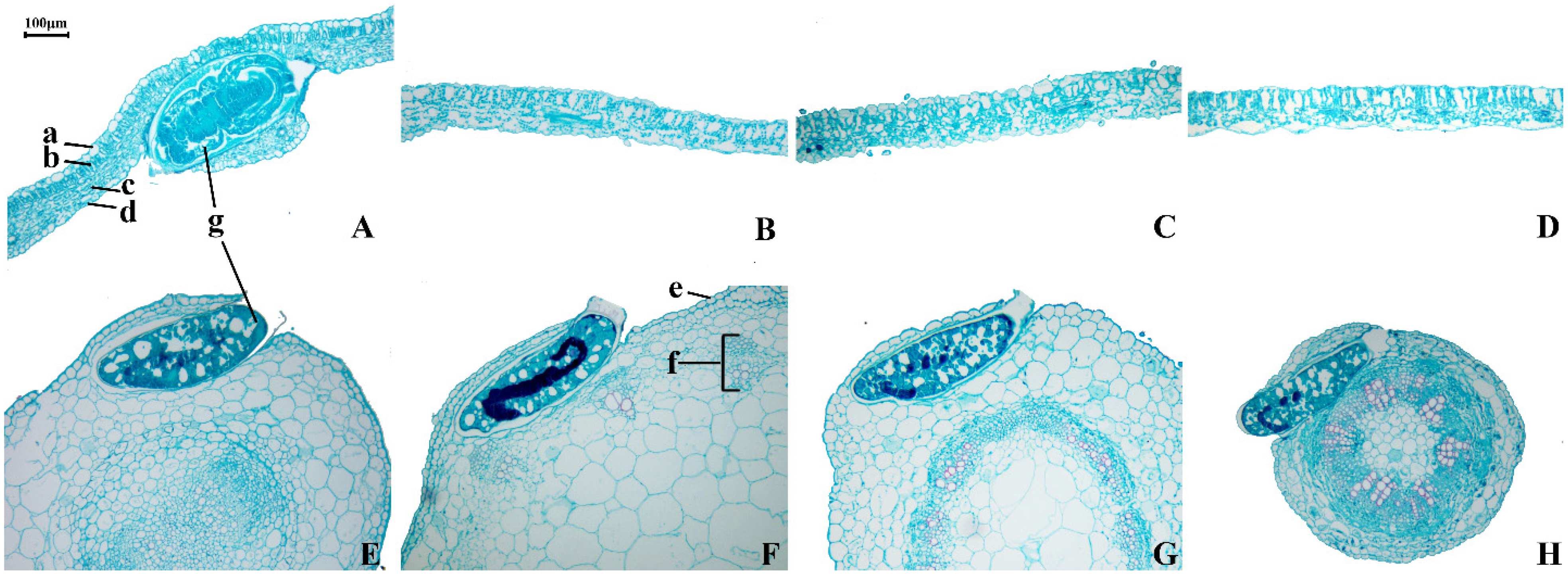
2.6. Plant Morphological Characteristics
2.7. Statistical Analysis
3. Results
3.1. Behavioral Preferences
3.2. Plant Acceptability and Ovipositional Preferences for Different Kinds of Plants
3.3. Egg Hatch on Different Plant Species
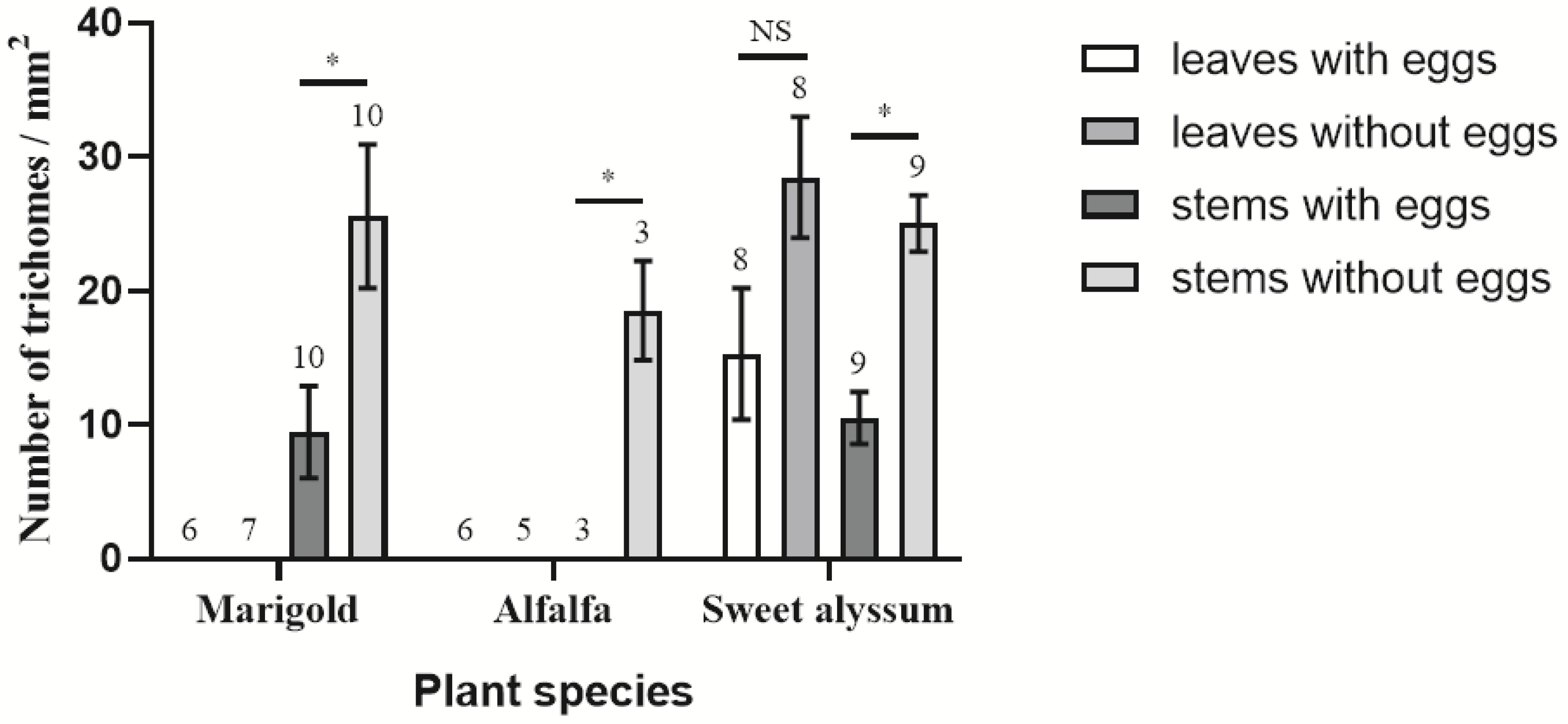
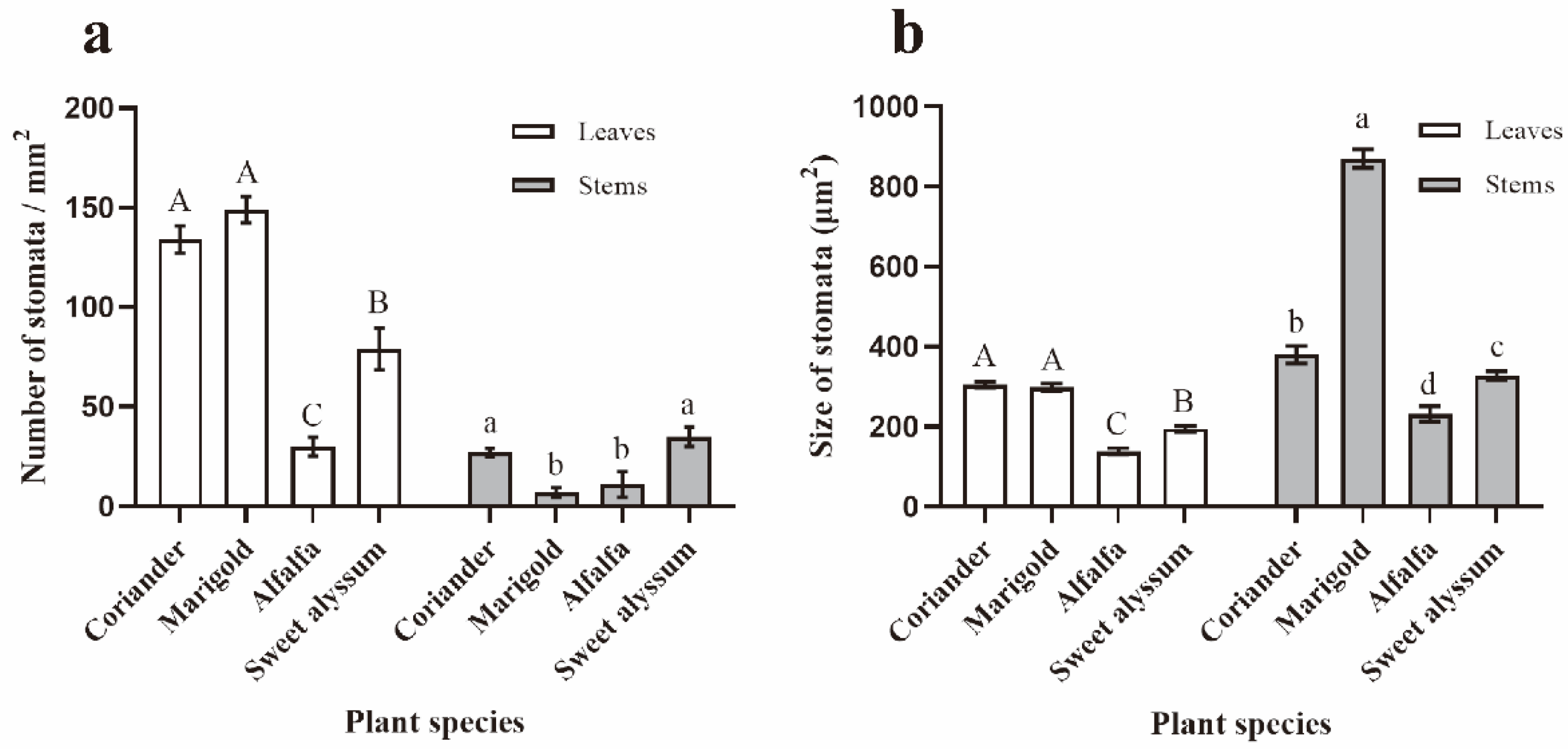
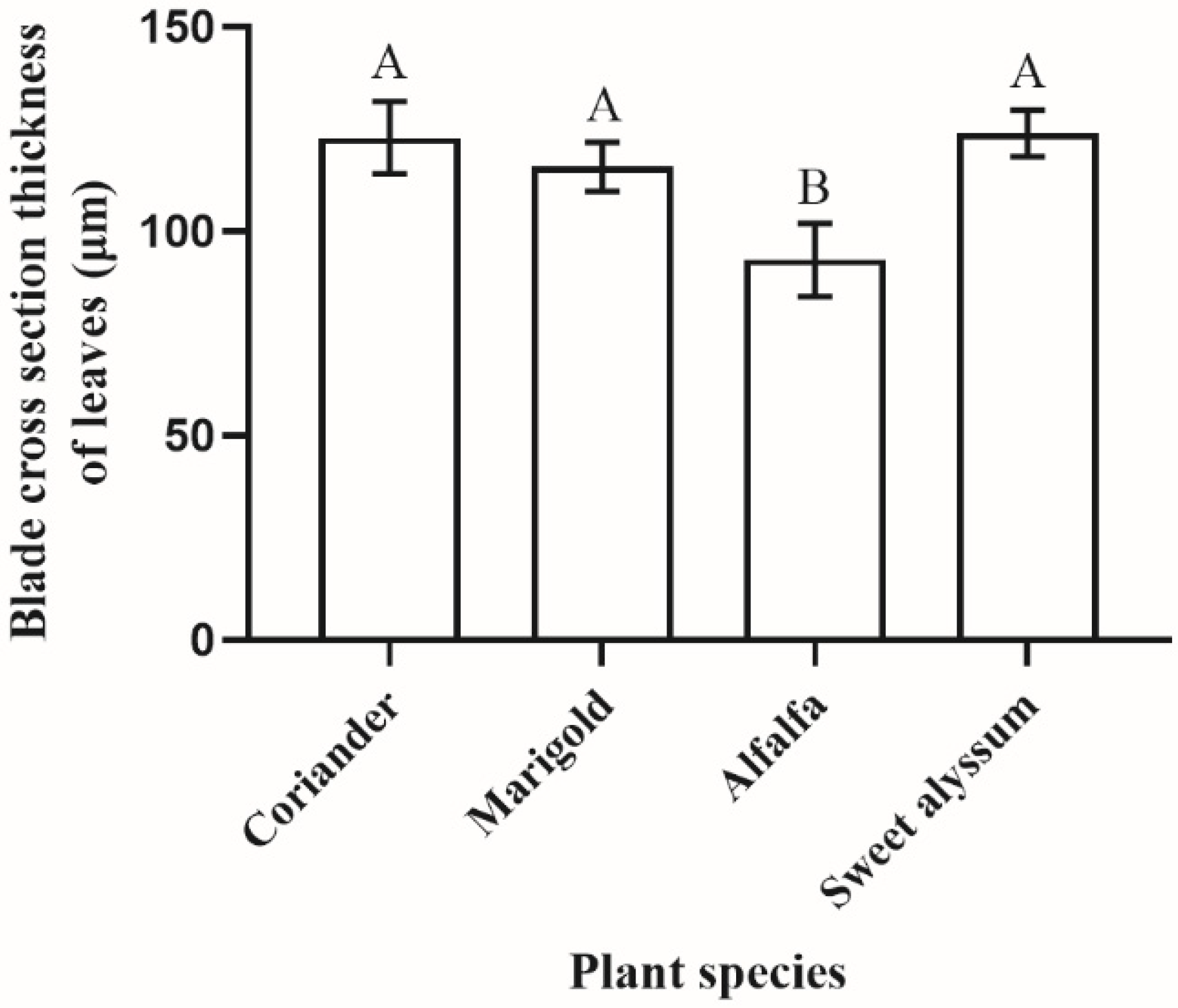
3.4. Effects of Plant Morphological Characteristics
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lattin, J.D. Bionomics of the Anthocoridae. Annu. Rev. Entomol. 1999, 44, 207–231. [Google Scholar] [CrossRef] [PubMed]
- Blaeser, P.; Sengonca, C.; Zegula, T. The potential use of different predatory bug species in the biological control of Frankliniella occidentalis (Pergande) (Thysanoptera: Thripidae). J. Pest. Sci. 2004, 77, 211–219. [Google Scholar] [CrossRef]
- Xu, X.; Borgemeister, C.; Poehling, H. Interactions in the biological control of western flower thrips, Frankliniella occidentalis (Pergande) and two-spotted spider mite, Tetranychus urticae Koch by the predatory bug Orius insidiosus Say on beans. Biol. Control. 2006, 36, 57–64. [Google Scholar] [CrossRef]
- Obrycki, J.J.; Harwood, J.D.; Kring, T.J.; Neil, R.J.O. Aphidophagy by Coccinellidae: Application of biological control in agroecosystems. Biol. Control. 2009, 51, 244–254. [Google Scholar] [CrossRef]
- Zhao, J.; Guo, X.; Tan, X.; Desneux, N.; Zappala, L.; Zhang, F.; Wang, S. Using Calendula officinalis as a floral resource to enhance aphid and thrips suppression by the flower bug Orius sauteri (Hemiptera: Anthocoridae). Pest. Manag. Sci. 2017, 73, 515–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rebek, E.J.; Sadof, C.S.; Hanks, L.M. Manipulating the abundance of natural enemies in ornamental landscapes with floral resource plants. Biol. Control. 2005, 33, 203–216. [Google Scholar] [CrossRef]
- Gontijo, L.M.; Beers, E.H.; Snyder, W.E. Flowers promote aphid suppression in apple orchards. Biol. Control. 2013, 66, 8–15. [Google Scholar] [CrossRef]
- Minarro, M.; Prida, E. Hedgerows surrounding organic apple orchards in north-west Spain: Potential to conserve beneficial insects. Agr. Forest Entomol. 2013, 15, 382–390. [Google Scholar] [CrossRef]
- Wong, S.K.; Frank, S.D. Pollen increases fitness and abundance of Orius insidiosus Say (Heteroptera: Anthocoridae) on banker plants. Biol. Control. 2013, 64, 45–50. [Google Scholar] [CrossRef]
- James, D.G.; Seymour, L.; Lauby, G.; Buckley, K. Beneficial insects attracted to native flowering buckwheats (Eriogonum Michx) in central Washington. Environ. Entomol. 2014, 43, 942–948. [Google Scholar] [CrossRef]
- Zhou, H.; Yi, Y.; Tan, X.; Chen, A.; Feng, J. Biological control of insect pests in apple orchards in China. Biol. Control. 2014, 68, 47–56. [Google Scholar] [CrossRef]
- Blaauw, B.R.; Isaacs, R. Larger wildflower plantings increase natural enemy density, diversity, and biological control of sentinel prey, without increasing herbivore density. Ecol. Entomol. 2012, 37, 386–394. [Google Scholar] [CrossRef]
- Balmer, O.; Pfiffner, L.; Schied, J.; Willareth, M.; Leimgruber, A.; Luka, H.; Traugott, M. Noncrop flowering plants restore top-down herbivore control in agricultural fields. Ecol. Evol. 2013, 3, 2634–2646. [Google Scholar] [CrossRef] [Green Version]
- Belz, E.; Kolliker, M.; Balmer, O. Olfactory attractiveness of flowering plants to the parasitoid Microplitis mediator: Potential implications for biological control. BioControl 2013, 58, 163–173. [Google Scholar] [CrossRef]
- Balmer, O.; Géneau, C.E.; Belz, E.; Weishaupt, B.; Förderer, G.; Moos, S.; Ditner, N.; Juric, I.; Luka, H. Wildflower companion plants increase pest parasitation and yield in cabbage fields: Experimental demonstration and call for caution. Biol. Control. 2014, 76, 19–27. [Google Scholar] [CrossRef]
- Medeiros, M.A.; Sujii, E.R.; Morais, H.C. Effect of plant diversification on abundance of South American tomato pinworm and predators in two cropping systems. Hortic. Bras. 2009, 27, 300–306. [Google Scholar] [CrossRef] [Green Version]
- Resende, A.L.S.; Haro, M.M.D.; Da Silva, V.F.; Souza, B.; Silveira, L.C.P. Diversity of predators in coriander, dill and fennel under organic management. Arq. Inst. Biol. Sao Paulo 2012, 79, 193–199. [Google Scholar] [CrossRef] [Green Version]
- Paterno Silveira, L.C.; Berti Filho, E.; Rosa Pierre, L.S.; Cunha Peres, F.S.; Cassa Louzada, J.N. Marigold (Tagetes erecta L.) as an attractive crop to natural enemies in onion fields. Sci. Agric. 2009, 66, 780–787. [Google Scholar] [CrossRef]
- Sampaio, M.V.; Bueno, V.H.P.; Silveira, L.C.P.; Auad, A.M. Biological Control of Insect Pests in the Tropics. In Encyclopedia of Life Support Systems; Delclaro, K., Ed.; EOLSS Publishers: Oxford, UK, 2008. [Google Scholar]
- Yang, F.; Wang, S.; Zhang, J. Olfactory influences and filed attractions of enhancing plant and herbivore induced defense volatiles to predacious flower bug Orius sauteri (Hemoptera: Anthocoridae) and parasitoid wasp Encarsia sophia (Hymenoptera: Aphelinidae). J. Environ. Entomol. 2017, 39, 1250–1257. [Google Scholar]
- Pico, F.X.; Retana, J. Temporal variation in the female components of reproductive success over the extended flowering season of a Mediterranean perennial herb. OIKOS 2000, 89, 485–492. [Google Scholar] [CrossRef]
- Pease, C.G.; Zalom, F.G. Influence of non-crop plants on stink bug (Hemiptera: Pentatomidae) and natural enemy abundance in tomatoes. J. Appl. Entomol. 2010, 134, 626–636. [Google Scholar] [CrossRef]
- Bennison, J.; Pope, T.; Maulden, K. The potential use of flowering alyssum as a ‘banker’ plant to support the establishment of Orius laevigatus in everbearer strawberry for improved biological control of western flower thrips. IOBC/WPRS Bull. 2011, 68, 15–18. [Google Scholar]
- Pumariño, L.; Alomar, O. The role of omnivory in the conservation of predators: Orius majusculus (Heteroptera: Anthocoridae) on sweet alyssum. Biol. Control. 2012, 62, 24–28. [Google Scholar] [CrossRef]
- Chen, M.; Zhou, Z.; Luo, J. Niche and temporal pattern of arthropod community in cotton-alfalfa intercrop fields. Acta Prataculturae Sin. 2008, 17, 132–140. [Google Scholar]
- McCaffrey, J.P.; Horsburgh, R.L. Biology of Orius insidiosus (Heteroptera, Anthocoridae)—A predator in Virginia apple orchards. Environ. Entomol. 1986, 15, 984–988. [Google Scholar] [CrossRef]
- Li, S.; Tan, X.; Desneux, N.; Benelli, G.; Zhao, J.; Li, X.; Zhang, F.; Gao, X.; Wang, S. Innate positive chemotaxis to pollen from crops and banker plants in predaceous biological control agents: Towards new field lures? Sci. Rep. 2015, 5, 12729. [Google Scholar] [CrossRef] [Green Version]
- Reddy, G.V.P.; Guerrero, A. Interactions of insect pheromones and plant semiochemicals. Trends Plant. Sci. 2004, 9, 253–261. [Google Scholar] [CrossRef]
- Clavijo McCormick, A.; Unsicker, S.B.; Gershenzon, J. The specificity of herbivore-induced plant volatiles in attracting herbivore enemies. Trends Plant. Sci. 2012, 17, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Xiu, C.; Xu, B.; Pan, H.; Zhang, W.; Yang, Y.; Lu, Y. Volatiles from Sophora japonica flowers attract Harmonia axyridis adults (Coleoptera: Coccinellidae). J. Integr. Agr. 2019, 18, 873–883. [Google Scholar] [CrossRef] [Green Version]
- Kugimiya, S.; Uefune, M.; Shimoda, T.; Takabayashi, J. Orientation of the parasitic wasp, Cotesia vestalis (Haliday) (Hymenoptera: Braconidae), to visual and olfactory cues of field mustard flowers, Brassica rapa L. (Brassicaceae), to exploit food sources. Appl. Entomol. Zool. 2010, 45, 369–375. [Google Scholar] [CrossRef] [Green Version]
- Bolter, C.J.; Dicke, M.; VanLoon, J.J.A.; Visser, J.H.; Posthumus, M.A. Attraction of Colorado potato beetles to herbivore-damaged plants during herbivory and after its termination. J. Chem. Ecol. 1997, 23, 1003–1023. [Google Scholar] [CrossRef]
- Pichersky, E.; Gershenzon, J. The formation and function of plant volatiles: Perfumes for pollinator attraction and defense. Curr. Opin. Plant. Biol. 2002, 5, 237–243. [Google Scholar] [CrossRef]
- Ament, K.; Kant, M.R.; Sabelis, M.W.; Haring, M.A.; Schuurink, R.C. Jasmonic acid is a key regulator of spider mite-induced volatile terpenoid and methyl salicylate emission in tomato. Plant. Physiol. 2004, 135, 2025–2037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.; Park, K. Methyl salicylate, a soybean aphid-induced plant volatile attractive to the predator Coccinella septempunctata. J. Chem. Ecol. 2005, 31, 1733–1746. [Google Scholar] [CrossRef]
- Rodriguez-Saona, C.; Kaplan, I.; Braasch, J.; Chinnasamy, D.; Williams, L. Field responses of predaceous arthropods to methyl salicylate: A meta-analysis and case study in cranberries. Biol. Control. 2011, 59, 294–303. [Google Scholar] [CrossRef]
- Togni, P.H.B.; Venzon, M.; Muniz, C.A.; Martins, E.F.; Pallini, A.; Sujii, E.R. Mechanisms underlying the innate attraction of an aphidophagous coccinellid to coriander plants: Implications for conservation biological control. Biol. Control. 2016, 92, 77–84. [Google Scholar] [CrossRef]
- Powell, J.E.; Lambert, L. Soybean genotype effects on bigeyed bug feeding on corn-earworm in the laboratory. Crop. Sci. 1993, 33, 556–559. [Google Scholar] [CrossRef]
- Lundgren, J.G.; Fergen, J.K.; Riedell, W.E. The influence of plant anatomy on oviposition and reproductive success of the omnivorous bug Orius insidiosus. Anim. Behav. 2008, 75, 1495–1502. [Google Scholar] [CrossRef]
- Lundgren, J.G.; Fergen, J.K. The oviposition behavior of the predator Orius insidiosus: Acceptability and preference for different plants. BioControl 2006, 51, 217–227. [Google Scholar] [CrossRef]
- Waite, M.O.; Scott-Dupree, C.D.; Brownbridge, M.; Buitenhuis, R.; Murphy, G. Evaluation of seven plant species/cultivars for their suitability as banker plants for Orius insidiosus Say. BioControl 2014, 59, 79–87. [Google Scholar] [CrossRef]
- Jensen, W.A. Botanical Histochemistry: Principles and Practice.; W.H. Freeman and Company: San Fransisco, CA, USA, 1962. [Google Scholar]
- Zhou, W.; Wang, R.; Qiu, S. Use of soybean sprouts as the oviposition material in mass rearing of Orius sauteri (Hemiptera: Anthocoridae). Chin. J. Biol. Control. 1991, 7, 7–9. [Google Scholar]
- Tan, X.; Wang, S.; Liu, T. Acceptance and suitability of four plant substrates for rearing Orius sauteri (Hemiptera: Anthocoridae). Biocontrol Sci. Technol. 2014, 24, 291–302. [Google Scholar] [CrossRef]
- Armer, C.A.; Wiedenmann, R.N.; Irwin, M.E. Effects of soybean mosaic virus on the facultative phytophagous predator Orius insidiosus (Heteroptera: Anthocoridae). Environ. Entomol. 1999, 28, 1036–1043. [Google Scholar] [CrossRef]
- Mendel, Z.; Carmi-Gera, E.; Podoler, H.; Assael, F. Reproductive-behavior of the specialist predator Elatophilus hebraicus (Hemiptera, Anthocoridae). Ann. Entomol. Soc. Am. 1995, 88, 856–861. [Google Scholar] [CrossRef]
- Isenhour, D.J.; Yeargan, K.V. Effect of temperature on the development of Orius insidiosus, with notes on laboratory rearing. Ann. Entomol. Soc. Am. 1981, 74, 114–116. [Google Scholar] [CrossRef]
- Schmidt, J.; Richards, P.; Nadel, H.; Ferguson, G. A rearing method for the production of large numbers of the insidiosus flower bug, Orius insidiosus Say (Hemiptera: Anthocoridae). Can. Entomol. 1995, 127, 445–447. [Google Scholar] [CrossRef]
- Murai, T.; Narai, Y.; Sugiura, N. Utilization of germinated broad bean seeds as an oviposition substrate in mass rearing of the predatory bug, Orius sauteri Poppius (Heteroptera: Anthocoridae). Appl. Entomol. Zool. 2001, 36, 489–494. [Google Scholar] [CrossRef] [Green Version]
- Jaenike, J. On optimal oviposition behavior in phytophagous insects. Theor. Popul. Biol. 1978, 14, 350–356. [Google Scholar] [CrossRef]
- Heisswolf, A.; Obermaier, E.; Poethke, H.J. Selection of large host plants for oviposition by a monophagous leaf beetle: Nutritional quality or enemy-free space? Ecol. Entomol. 2005, 30, 299–306. [Google Scholar] [CrossRef]
- Tawfik, M.F.S.; Ata, A.M. Life history of Orius albidipennis Reut. (Hemiptera: Anthocoridae) on greenhouse sweet pepper. Bull. Soc. Entomol. Egypt 1973, 57, 117–126. [Google Scholar]
- Pascua, M.S.; Rocca, M.; De Clercq, P.; Greco, N.M. Host plant use for oviposition by the insidious flower bug (Hemiptera: Anthocoridae). J. Econ. Entomol. 2019, 112, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Naranjo, S.E.; Gibson, R.L. Phytophagy in Predaceous Heteroptera: Effects on life History and Population Dynamics. In Zoophytophagous Heteroptera: Implications for Life History and Integrated Pest Management; Alomar, O., Wiedenmann, R.N., Eds.; Entomological Society of America: Lanham, MD, USA, 1996; pp. 57–93. [Google Scholar]
- Armer, C.A.; Wiedenmann, R.N.; Bush, D.R. Plant feeding site selection on soybean by the facultatively phytophagous predator Orius insidiosus. Entomol. Exp. Appl. 1998, 86, 109–118. [Google Scholar] [CrossRef]
- Awmack, C.S.; Leather, S.R. Host plant quality and fecundity in herbivorous insects. Annu. Rev. Entomol. 2002, 47, 817–844. [Google Scholar] [CrossRef]
- Zhang, R.; Ji, D.; Zhang, Q.; Jin, L. Evaluation of eleven plant species as potential banker plants to support predatory Orius sauteri in tea plant systems. Insects 2021, 12, 162. [Google Scholar] [CrossRef] [PubMed]
- Messelink, G.J.; Bennison, J.; Alomar, O.; Ingegno, B.L.; Tavella, L.; Shipp, L.; Palevsky, E.; Wäckers, F.L. Approaches to conserving natural enemy populations in greenhouse crops: Current methods and future prospects. BioControl 2014, 59, 377–393. [Google Scholar] [CrossRef]
- Groenteman, R.; Guershon, M.; Coll, M. Effects of leaf nitrogen content on oviposition site selection, offspring performance, and intraspecific interactions in an omnivorous bug. Ecol. Entomol. 2006, 31, 155–161. [Google Scholar] [CrossRef]
- Mendel, Z.; Assael, F.; Dunkelblum, E. Kairomonal attraction of predatory bugs (Heteroptera: Anthocoridae) and brown lacewings (Neuroptera: Hemerobiidae) to sex pheromones of Matsucoccus species (Hemiptera: Matsucoccidae). Biol. Control. 2004, 30, 134–140. [Google Scholar] [CrossRef]
- Teerling, C.R.; Gillespie, D.R.; Borden, J.H. Utilization of western flower thrips alarm pheromone as a prey-finding kairomone by predators. Can. Entomol. 1993, 125, 431–437. [Google Scholar] [CrossRef]
- Aldrich, J.R.; Oliver, J.E.; Shifflet, T.; Smith, C.L.; Dively, G.P. Semiochemical investigations of the insidious flower bug Orius insidiosus Say. J. Chem. Ecol. 2007, 33, 1477–1493. [Google Scholar] [CrossRef]
- Uehara, T.; Maeda, T.; Shimoda, M.; Fujiwara-Tsujii, N.; Yasui, H. Identification and characterization of the pheromones in the minute pirate bug Orius sauteri (Heteroptera: Anthocoridae). J. Chem. Ecol. 2019, 45, 811–817. [Google Scholar] [CrossRef]


| Effects | SS | Df | MS | F | p |
|---|---|---|---|---|---|
| Plants | 245.670 | 3 | 81.890 | 16.526 | <0.001 |
| Time | 0.384 | 3 | 0.128 | 0.026 | 0.994 |
| Plants*Time | 38.223 | 9 | 4.247 | 0.857 | 0.566 |
| Error | 475.714 | 96 | 4.955 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Qin, Z.; Liu, P.; Yin, Y.; Felton, G.W.; Shi, W. Influence of Plant Physical and Anatomical Characteristics on the Ovipositional Preference of Orius sauteri (Hemiptera: Anthocoridae). Insects 2021, 12, 326. https://doi.org/10.3390/insects12040326
Zhang L, Qin Z, Liu P, Yin Y, Felton GW, Shi W. Influence of Plant Physical and Anatomical Characteristics on the Ovipositional Preference of Orius sauteri (Hemiptera: Anthocoridae). Insects. 2021; 12(4):326. https://doi.org/10.3390/insects12040326
Chicago/Turabian StyleZhang, Liu, Zifang Qin, Pingping Liu, Yue Yin, Gary W. Felton, and Wangpeng Shi. 2021. "Influence of Plant Physical and Anatomical Characteristics on the Ovipositional Preference of Orius sauteri (Hemiptera: Anthocoridae)" Insects 12, no. 4: 326. https://doi.org/10.3390/insects12040326
APA StyleZhang, L., Qin, Z., Liu, P., Yin, Y., Felton, G. W., & Shi, W. (2021). Influence of Plant Physical and Anatomical Characteristics on the Ovipositional Preference of Orius sauteri (Hemiptera: Anthocoridae). Insects, 12(4), 326. https://doi.org/10.3390/insects12040326






