The European Map Butterfly Araschnia levana as a Model to Study the Molecular Basis and Evolutionary Ecology of Seasonal Polyphenism
Abstract
Simple Summary
Abstract
1. Introduction
2. Environment and Phenotype
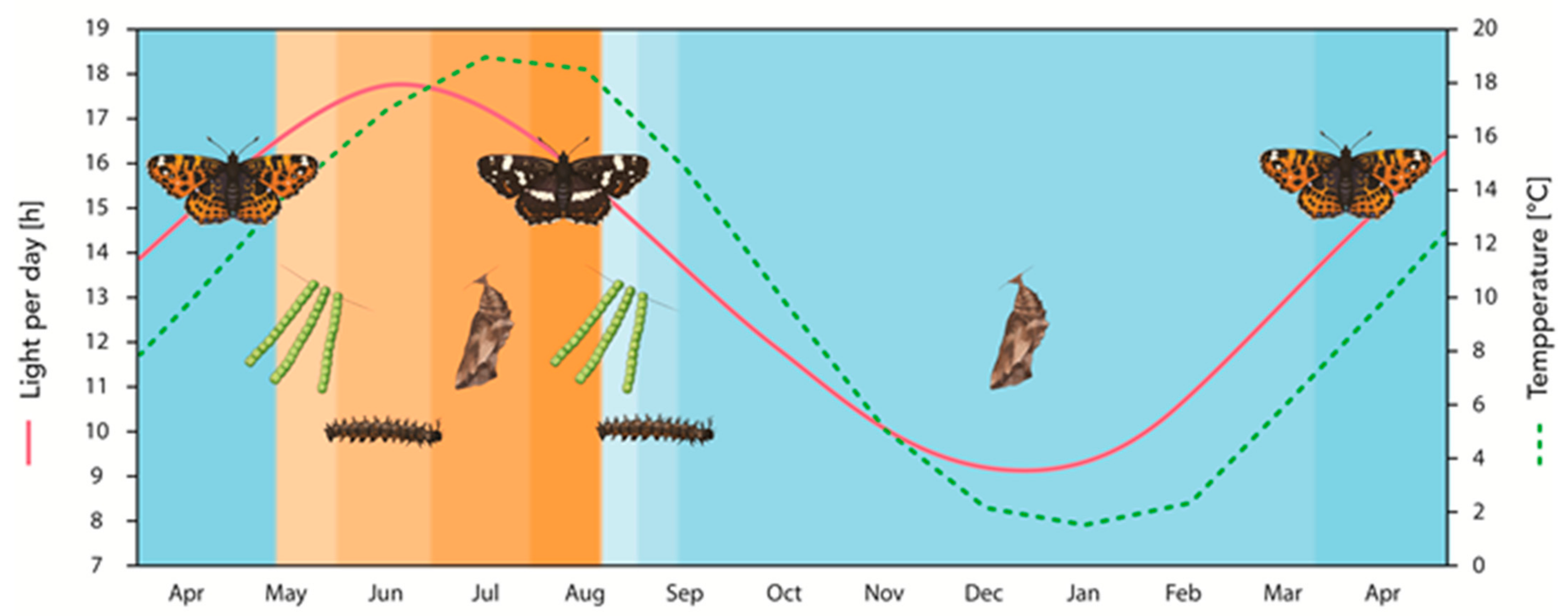
2.1. Photoperiodism and Temperature
2.2. Food Quality
3. Hormonal and Epigenetic Control of the Phenotype
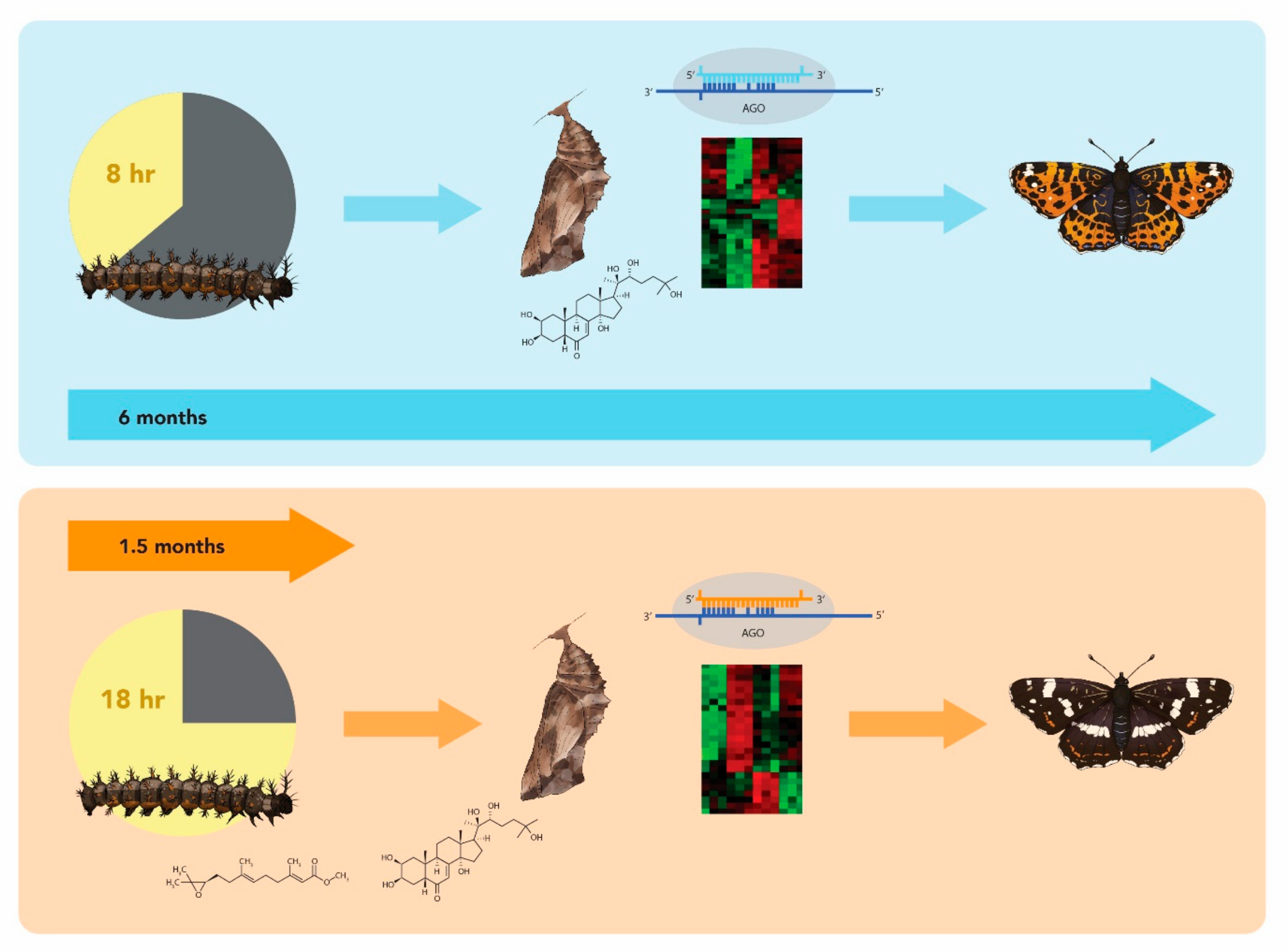
3.1. Hormones
3.2. Circadian Clocks and Epigenetics
4. Photoperiod-Specific Polyphenic Responses
4.1. Morphology
4.2. Immunity
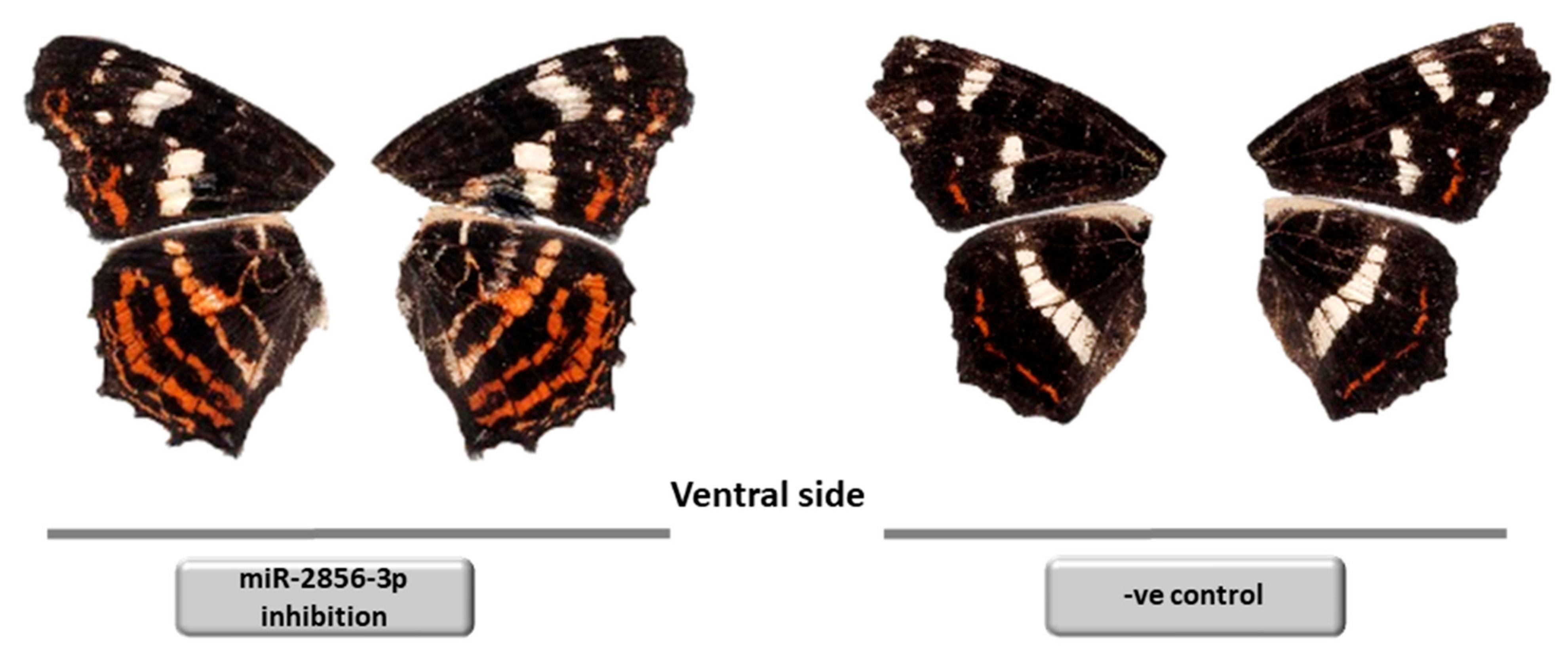
4.3. Wing Pattern and Color
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Panzeri, I.; Pospisilik, J.A. Epigenetic control of variation and stochasticity in metabolic disease. Mol. Metab. 2018, 14, 26–38. [Google Scholar] [CrossRef]
- Geng, Y.; Gao, L.; Yang, J. Epigenetic Flexibility Underlying Phenotypic Plasticity. In Progress in Botany; Springer: Berlin/Heidelberg, Germany, 2013; pp. 153–163. [Google Scholar] [CrossRef]
- Yang, C.H.; Pospisilik, J.A. Polyphenism—A window into gene-environment interactions and phenotypic plasticity. Front. Genet. 2019, 10, 132. [Google Scholar] [CrossRef] [PubMed]
- Janzen, F.J.; Phillips, P.C. Exploring the evolution of environmental sex determination, especially in reptiles. J. Evol. Biol. 2006, 19, 1775–1784. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Todd, E.V.; Lokman, P.M.; Lamm, M.S.; Godwin, J.R.; Gemmell, N.J. Sexual plasticity: A fishy tale. Mol. Reprod. Dev. 2017, 84, 171–194. [Google Scholar] [CrossRef]
- Miyakawa, H.; Gotoh, H.; Sugimoto, N.; Miura, T. Effect of juvenoids on predator-induced polyphenism in the water flea, Daphnia pulex. J. Exp. Zool. Part A Ecol. Genet. Physiol. 2013, 319, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, K.; Baudach, A. Epigenetic Control of Polyphenism in Aphids. In Biology and Ecology of Aphids; Vilcinskas, A., Ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 89–99. [Google Scholar]
- Brakefield, P.M.; Frankino, W.A. Polyphenisms in Lepidoptera: Multidisciplinary approaches to studies of evolution. In Phenotypic Plasticity in Insects. Mechanisms and Consequences; Whitman, D.W., Ananthakrishnan, N.T., Eds.; Science Publishers: Plymouth, UK, 2007; pp. 121–151. [Google Scholar]
- Von Linnaeus, C. Systema Naturae; Laurentii Salvii: Stockholm, Sweden, 1758. [Google Scholar]
- Reinhardt, R. Der Landkärtchenfalter Araschnia levana—Einfluß der Umwelt auf den Gestaltswechsel, 2nd ed.; Die Neue Brehm-Bücherei: Karl-Marx-Stadt, Germany, 1984. [Google Scholar]
- Fric, Z.; Konvicka, M. Adult population structure and behaviour of two seasonal generations of the European Map Butterfly, Araschnia levana, species with seasonal polyphenism (Nymphalidae). Nota Lepidopterol. 2000, 23, 2–25. [Google Scholar]
- Dorfmeister, G. Über die Einwirkung verschiedener, während der Entwicklungsperioden angewendeter Wärmegrade auf die Färbung und Zeichnung der Schmetterlinge. Mitt. Naturw. Ver. Steiermark 1864, 99–108. [Google Scholar]
- Dorfmeister, G. Über den Einfluß der Temperatur bei der Erzeugung von Schmetterlingsvaritäten. Mitt. Naturw. Ver. Steiermark 1879, 1–8. [Google Scholar]
- Standfuß, M. Experimentelle zoologische Studien mit Lepidopteren. A Temperaturexperimente. N. Denkschr. Schweiz. Ges. 1891, 36, 1–82. [Google Scholar]
- Fischer, E. Zur Physiologie der Aberrationen- und Varietätenbildung der Schmetterlinge. Arch. Rassen Ges. 1907, 761–792. [Google Scholar]
- Weismann, A. Studien zur Descendenz-Theorie. I. Über den Saison-Dimorphismus der Schmetterlinge; Verlag von Wilhelm Engelmann: Leipzig, Germany, 1875; ISBN 9788578110796. [Google Scholar]
- Marcovitch, S. The migration of the aphididae and the appearance of the sexual forms as affected by the relative length of daily light exposure. J. Agric. Res. 1924, XXVII, 513–522. [Google Scholar]
- Müller, H.J. Die Saisonformenbildung von Arachnia levana, ein photoperiodisch gesteuerter Diapauseeffekt. Naturwissenschaften 1955, 42, 134–135. [Google Scholar] [CrossRef]
- Koch, P.B.; Bückmann, D. Hormonal control of seasonal morphs by the timing of ecdysteroid release in Araschnia levana L. (Nymphalidae: Lepidoptera). J. Insect Physiol. 1987, 33, 823–829. [Google Scholar] [CrossRef]
- Brakefield, P.M.; Reitsma, N. Phenotypic plasticity, seasonal climate and the population biology of Bicyclus butterflies (Satyridae) in Malawi. Ecol. Entomol. 1991, 16, 291–303. [Google Scholar] [CrossRef]
- Monteiro, A.; Tong, X.; Bear, A.; Liew, S.F.; Bhardwaj, S.; Wasik, B.R.; Dinwiddie, A.; Bastianelli, C.; Cheong, W.F.; Wenk, M.R.; et al. Differential Expression of Ecdysone Receptor Leads to Variation in Phenotypic Plasticity across Serial Homologs. PLoS Genet. 2015, 11, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Vilcinskas, A.; Vogel, H. Seasonal phenotype-specific transcriptional reprogramming during metamorphosis in the European map butterfly Araschnia levana. Ecol. Evol. 2016, 6, 3476–3485. [Google Scholar] [CrossRef]
- Mukherjee, K.; Baudach, A.; Vogel, H.; Vilcinskas, A. Seasonal phenotype-specific expression of microRNAs during metamorphosis in the European map butterfly Araschnia levana. Arch. Insect Biochem. Physiol. 2020, 104, e21657. [Google Scholar] [CrossRef]
- Müller, H.J. Die Wirkung verschiedener diurnaler Licht-Dunkel-Relationen auf die Saisonformenbildung von Araschnia levana. Naturwissenschaften 1956, 21, 503–504. [Google Scholar] [CrossRef]
- Kratochwil, A. Die Anpassung der Generationenfolge von Araschnia levana L. (Lepidoptera, Nymphalidae) an den jahreszeitlichen Witterungsverlauf. Verh. Ges. Okol. 1980, Vlll, 395–401. [Google Scholar]
- Müller, H.J.; Reinhardt, R. Die Bedeutung von Temperatur und Tageslänge für die Entwicklung der Saisonformen von Araschnia levana L. (Lep. Nymphalidae). Entomol. Berichte 1969, 93–100. [Google Scholar]
- Friberg, M.; Haugen, I.M.A.; Dahlerus, J.; Gotthard, K.; Wiklund, C. Asymmetric life-history decision-making in butterfly larvae. Oecologia 2011, 165, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Steiner, A. Extreme Flugzeiten von Schmetterlingen in den Jahren 1989 und 1990—Auswirkungen der weltweiten Klimaveränderung? Atalanta 1991, 22, 237–244. [Google Scholar]
- Müller, H.J. Die Bedeutung der Photoperiode im Lebenslauf der Insekten. Z. Entomol. 1960, 47, 7–24. [Google Scholar]
- Mevi-Schütz, J.; Erhardt, A. Amino acids in nectar enhance butterfly fecundity: A long-awaited link. Am. Nat. 2005, 165, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Pullin, A.S. Changes in Leaf Quality Following Clipping and Regrowth of Urtica dioica, and Consequences for a Specialist Insect Herbivore, Aglais urticae. Oikos 1987, 49, 39. [Google Scholar] [CrossRef]
- Morehouse, N.I.; Mandon, N.; Christides, J.P.; Body, M.; Bimbard, G.; Casas, J. Seasonal selection and resource dynamics in a seasonally polyphenic butterfly. J. Evol. Biol. 2013, 26, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Mevi-Schütz, J.; Erhardt, A. Larval nutrition affects female nectar amino acid preference in the map butterfly (Araschnia levana). Ecology 2003, 84, 2788–2794. [Google Scholar] [CrossRef]
- Fric, Z.; Klimova, M.; Konvicka, M. Mechanical design indicates differences in mobility among butterfly generations. Evol. Ecol. Res. 2006, 8, 1511–1522. [Google Scholar]
- Fric, Z.; Konvička, M. Generations of the polyphenic butterfly Araschnia levana differ in body design. Evol. Ecol. Res. 2002, 4, 1017–1032. [Google Scholar]
- Esperk, T.; Tammaru, T. Ontogenetic Basis of Among-Generation Differences in Size-Related Traits in a Polyphenic Butterfly. Front. Ecol. Evol. 2021, 9, 1–11. [Google Scholar] [CrossRef]
- Denlinger, D.L. Hormonal control of diapause. In Comprehensive Insect Physiology Biochemistry and Pharmacology; Kerkut, A.G., Gilbert, L.I., Eds.; Pergamon Press: Oxford, UK, 1985; Volume 8. [Google Scholar]
- Koch, P.B.; Bückmann, D. The seasonal dimorphism of Araschnia levana L. (Nymphalidae) in relation to hormonal controlled development. Verh. Zool. Ges. 1985, 78, 260. [Google Scholar]
- Keino, H.; Endo, K. Studies of the determination of the seasonal forms in the butterfly Araschnia burejana Bermer. Zool. Mag. 1973, 82, 48–52. [Google Scholar]
- Koch, P.B. Preadult Changes of Ecdysteroid and Juvenile Hormone Titers in Relation to Diapause and Pigmental Variations in two Lepidopteran Species, Cerura vinula and Araschnia levana (Lepidoptera: Notodontidae/Nymphalidae). Entomol. Gen. 1996, 20, 143–155. [Google Scholar] [CrossRef]
- Gebert, L.F.R.; MacRae, I.J. Regulation of microRNA function in animals. Nat. Rev. Mol. Cell Biol. 2019, 20, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Koch, P.B. Die Steuerung der saisondimorphen Flügelfärbung von Araschnia levana L. (Nymphalidae, Lepidoptera). Mitt. Dtsch. Ges. Allg. Angew. Ent. 1987, 5, 195–197. [Google Scholar]
- Denlinger, D.L.; Hahn, D.A.; Merlin, C.; Holzapfel, C.M.; Bradshaw, W.E. Keeping time without a spine: What can the insect clock teach us about seasonal adaptation? Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160257. [Google Scholar] [CrossRef] [PubMed]
- Richard, G.; Le Trionnaire, G.; Danchin, E.; Sentis, A. Epigenetics and insect polyphenism: Mechanisms and climate change impacts. Curr. Opin. Insect Sci. 2019, 35, 138–145. [Google Scholar] [CrossRef]
- Dolezel, D. Photoperiodic time measurement in insects. Curr. Opin. Insect Sci. 2015, 7, 98–103. [Google Scholar] [CrossRef]
- Gegner, J.; Vogel, H.; Billion, A.; Förster, F.; Vilcinskas, A. Complete Metamorphosis in Manduca sexta Involves Specific Changes in DNA Methylation Patterns. Front. Ecol. Evol. 2021, 9, 6281. [Google Scholar] [CrossRef]
- Reynolds, J.A.; Peyton, J.T.; Denlinger, D.L. Changes in microRNA abundance may regulate diapause in the flesh fly, Sarcophaga bullata. Insect Biochem. Mol. Biol. 2017, 84, 1–14. [Google Scholar] [CrossRef]
- Isobe, M.; Kai, H.; Kurahashi, T.; Suwan, S.; Pitchayawasin-Thapphasaraphong, S.; Franz, T.; Tani, N.; Higashi, K.; Nishida, H. The molecular mechanism of the termination of insect diapause, part 1: A timer protein, TIME-EA4, in the diapause eggs of the silkworm Bombyx mori is a metallo-glycoprotein. ChemBioChem 2006, 7, 1590–1598. [Google Scholar] [CrossRef] [PubMed]
- Ylla, G.; Fromm, B.; Piulachs, M.D.; Belles, X. The microRNA toolkit of insects. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef]
- Collins, D.H.; Mohorianu, I.; Beckers, M.; Moulton, V.; Dalmay, T.; Bourke, A.F.G. MicroRNAs Associated with Caste Determination and Differentiation in a Primitively Eusocial Insect. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.K.; Nguyen, P.; Kim, A.Y.; Choi, A.Y.; Yu, Y.; Lee, G.S.; Koh, Y.H. Identification of microRNAs and their target transcripts in the migratory locust adult brain revealed their roles in the epigenetic regulation of polyphenisms. J. Asia Pac. Entomol. 2017, 20, 1396–1401. [Google Scholar] [CrossRef]
- Alexiuk, M.R.; Marcus, J.M.; Lalonde, M.M.L. The complete mitochondrial genome and phylogenetic analysis of the European map butterfly Araschnia levana (Insecta: Lepidoptera: Nymphalidae). Mitochondrial DNA Part B Resour. 2020, 5, 3264–3266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Reed, R.D. A Practical Guide to CRISPR/Cas9 Genome Editing in Lepidoptera. In Diversity and Evolution of Butterfly Wing Patterns; Sekimura, T., Nijhout, H., Eds.; Springer: Singapore, 2017. [Google Scholar]
- Baudach, A.; Lee, K.Z.; Vogel, H.; Vilcinskas, A. Immunological larval polyphenism in the map butterfly Araschnia levana reveals the photoperiodic modulation of immunity. Ecol. Evol. 2018, 8, 4891–4898. [Google Scholar] [CrossRef]
- Freitak, D.; Tammaru, T.; Sandre, S.L.; Meister, H.; Esperk, T. Longer life span is associated with elevated immune activity in a seasonally polyphenic butterfly. J. Evol. Biol. 2019, 32, 653–665. [Google Scholar] [CrossRef]
- Michie, L.J.; Masson, A.; Ware, R.L.; Jiggins, F.M. Seasonal phenotypic plasticity: Wild ladybirds are darker at cold temperatures. Evol. Ecol. 2011, 25, 1259–1268. [Google Scholar] [CrossRef]
- Knapp, M.; Nedvěd, O. Gender and Timing during Ontogeny Matter: Effects of a Temporary High Temperature on Survival, Body Size and Colouration in Harmonia axyridis. PLoS ONE 2013, 8, e74984. [Google Scholar] [CrossRef]
- Barber, N.A.; Wouk, J. Winter predation by insectivorous birds and consequences for arthropods and plants in summer. Oecologia 2012, 170, 999–1007. [Google Scholar] [CrossRef]
- Jervis, M.A.; Boggs, C.L. Linking nectar amino acids to fitness in female butterflies. Trends Ecol. Evol. 2005, 20, 585–587. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baudach, A.; Vilcinskas, A. The European Map Butterfly Araschnia levana as a Model to Study the Molecular Basis and Evolutionary Ecology of Seasonal Polyphenism. Insects 2021, 12, 325. https://doi.org/10.3390/insects12040325
Baudach A, Vilcinskas A. The European Map Butterfly Araschnia levana as a Model to Study the Molecular Basis and Evolutionary Ecology of Seasonal Polyphenism. Insects. 2021; 12(4):325. https://doi.org/10.3390/insects12040325
Chicago/Turabian StyleBaudach, Arne, and Andreas Vilcinskas. 2021. "The European Map Butterfly Araschnia levana as a Model to Study the Molecular Basis and Evolutionary Ecology of Seasonal Polyphenism" Insects 12, no. 4: 325. https://doi.org/10.3390/insects12040325
APA StyleBaudach, A., & Vilcinskas, A. (2021). The European Map Butterfly Araschnia levana as a Model to Study the Molecular Basis and Evolutionary Ecology of Seasonal Polyphenism. Insects, 12(4), 325. https://doi.org/10.3390/insects12040325





