Gut Bacterial and Fungal Communities of the Wild and Laboratory-Reared Thitarodes Larvae, Host of the Chinese Medicinal Fungus Ophiocordyceps sinensis on Tibetan Plateau
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ghost Moth Collection, Identification and Gut Preparation
2.2. Culture-Dependent Microbial Communities
2.3. Culture-Independent Microbial Communities
3. Results
3.1. Molecular Identification of the Ghost Moth Populations
3.2. Culture-Dependent Communities
3.3. Culture-Independent Communities
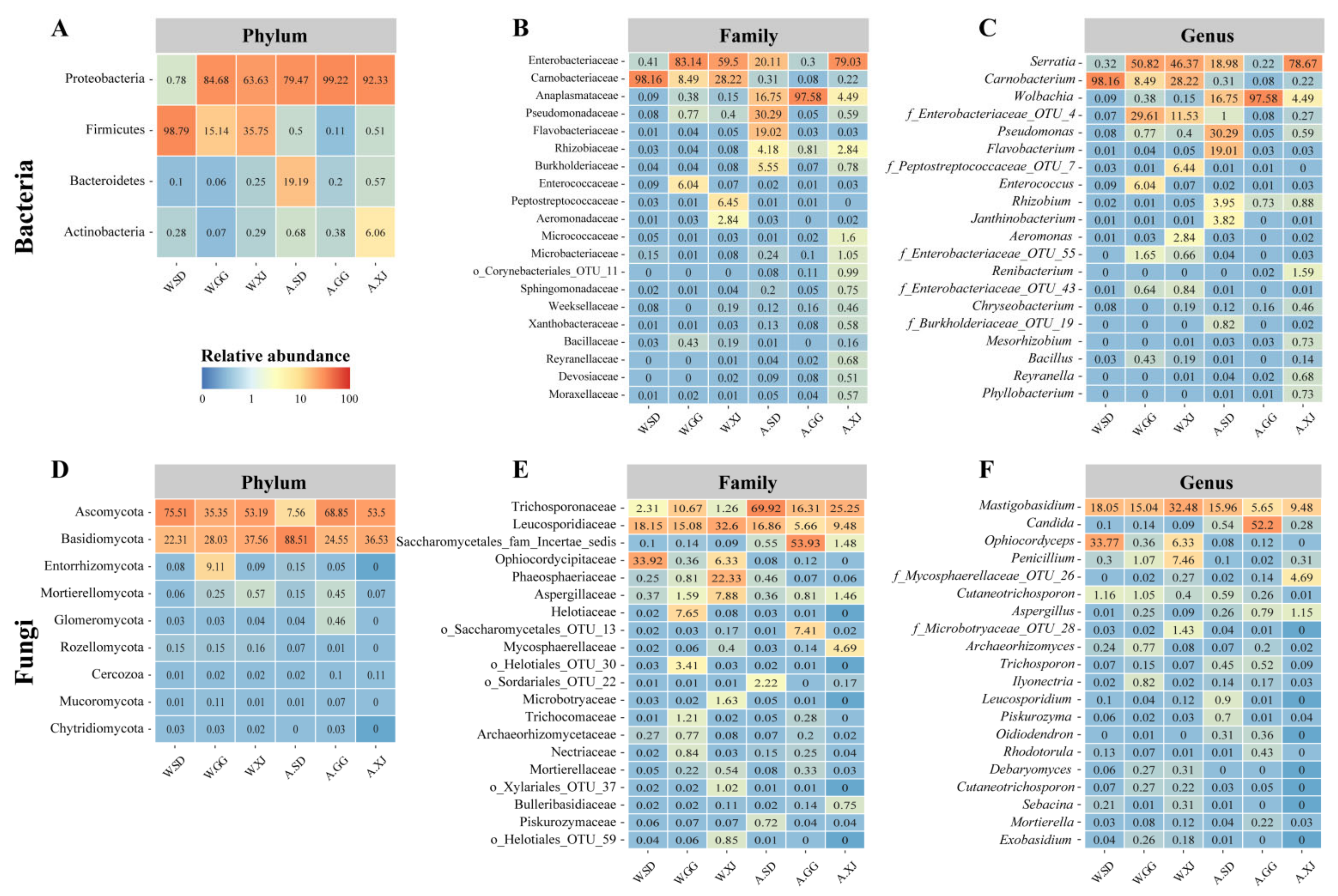
3.3.1. General Pattern of the Gut Microbiota of the Wild Ghost Moth Populations
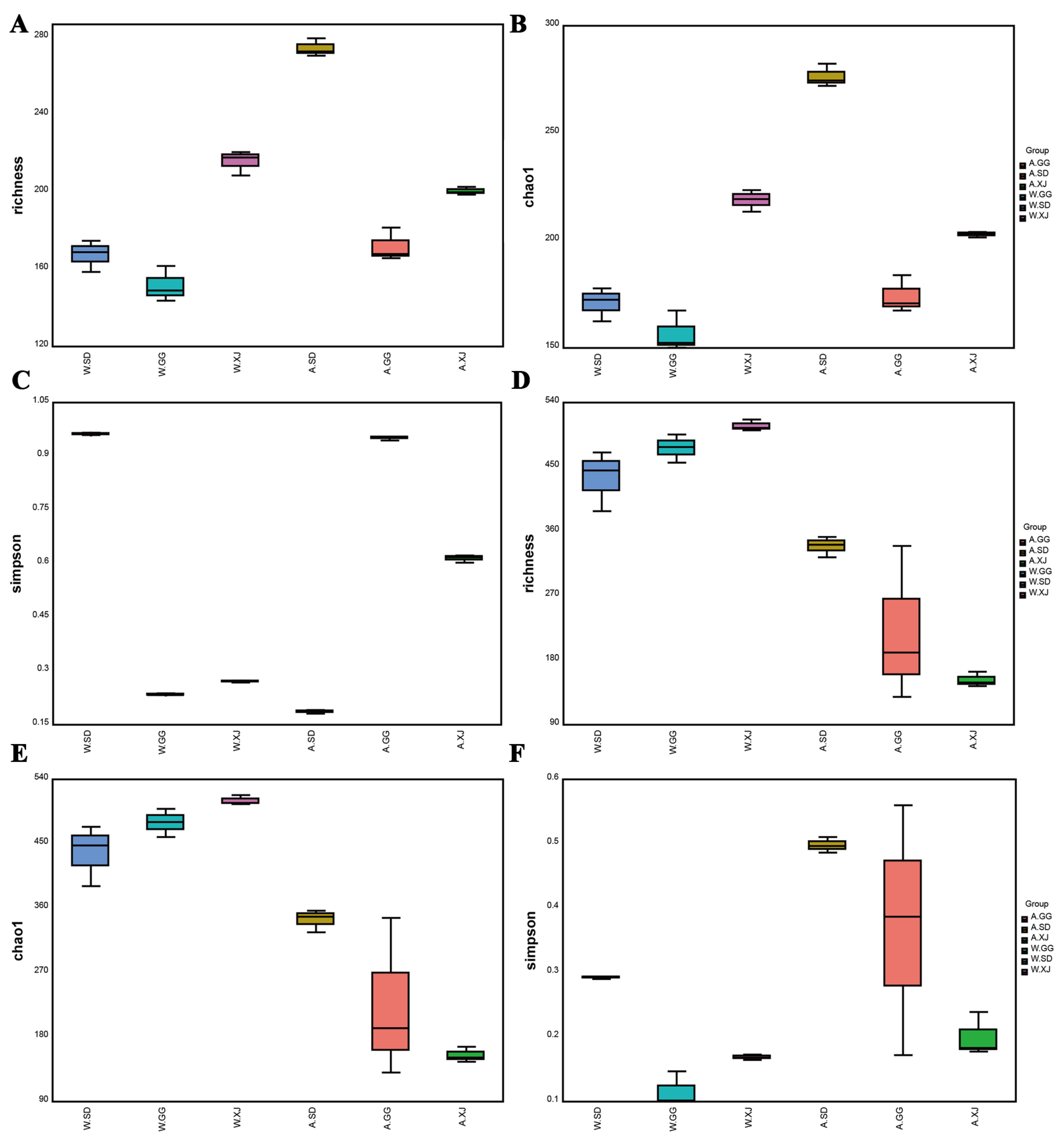
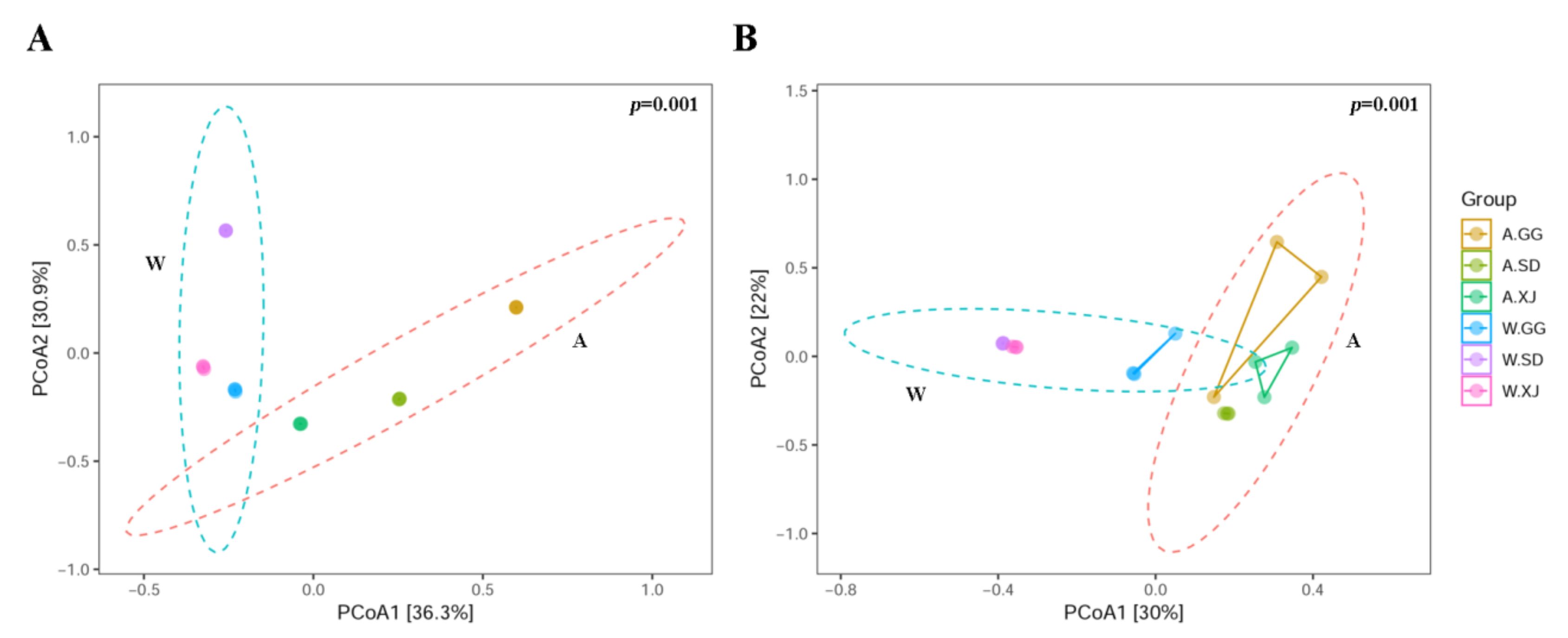
3.3.2. Comparison of the Gut Microbial Community Diversity between Insect Populations
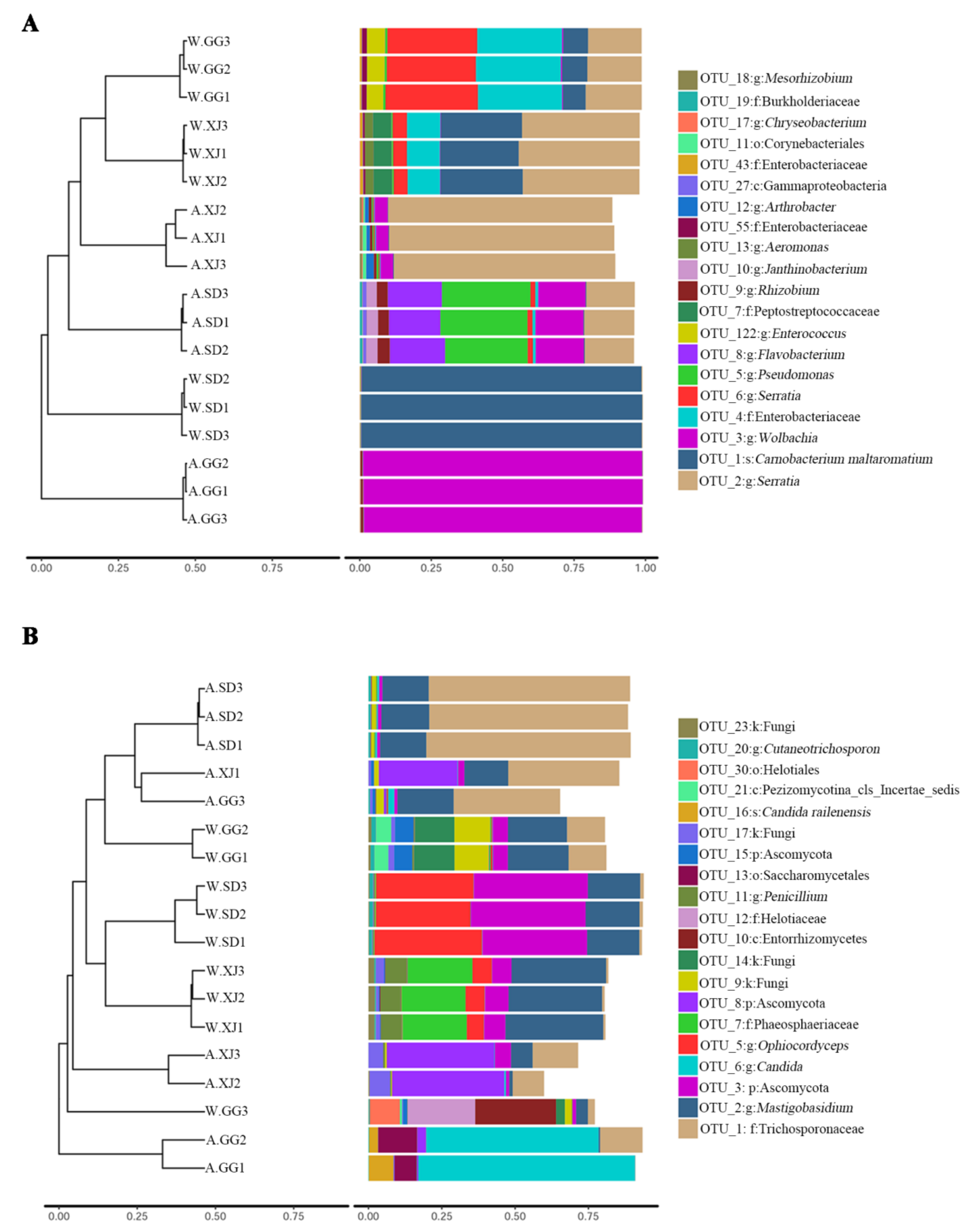
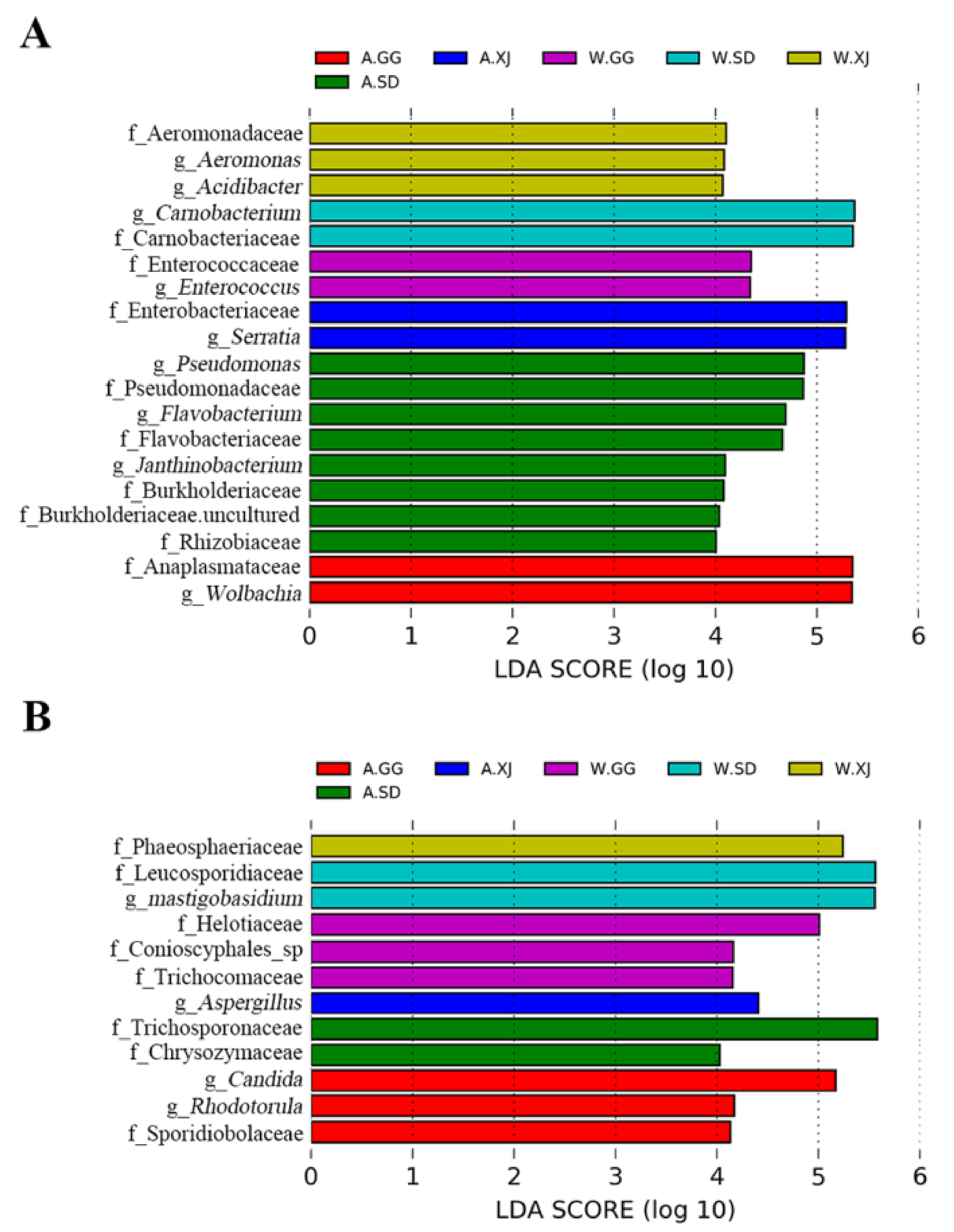
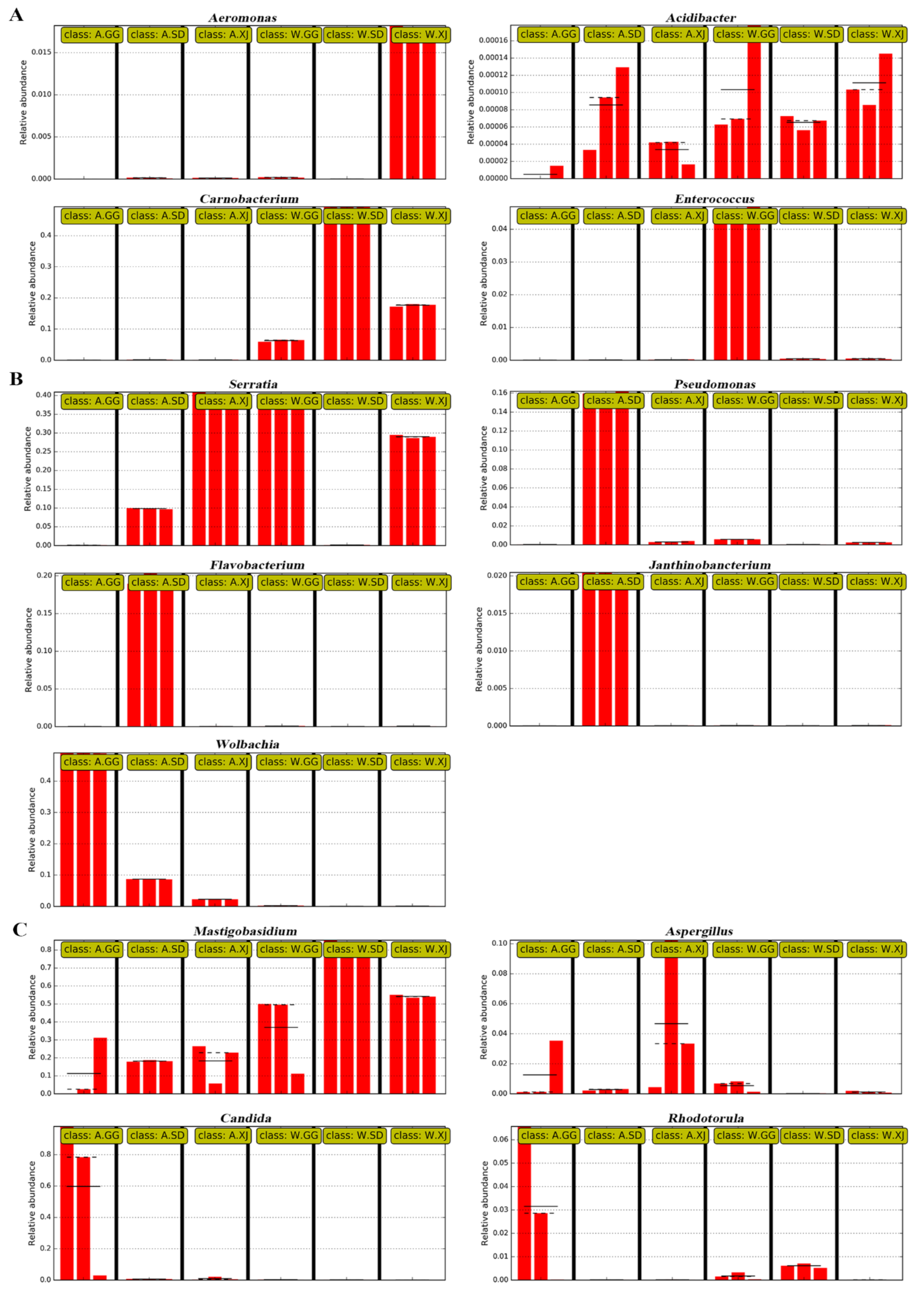
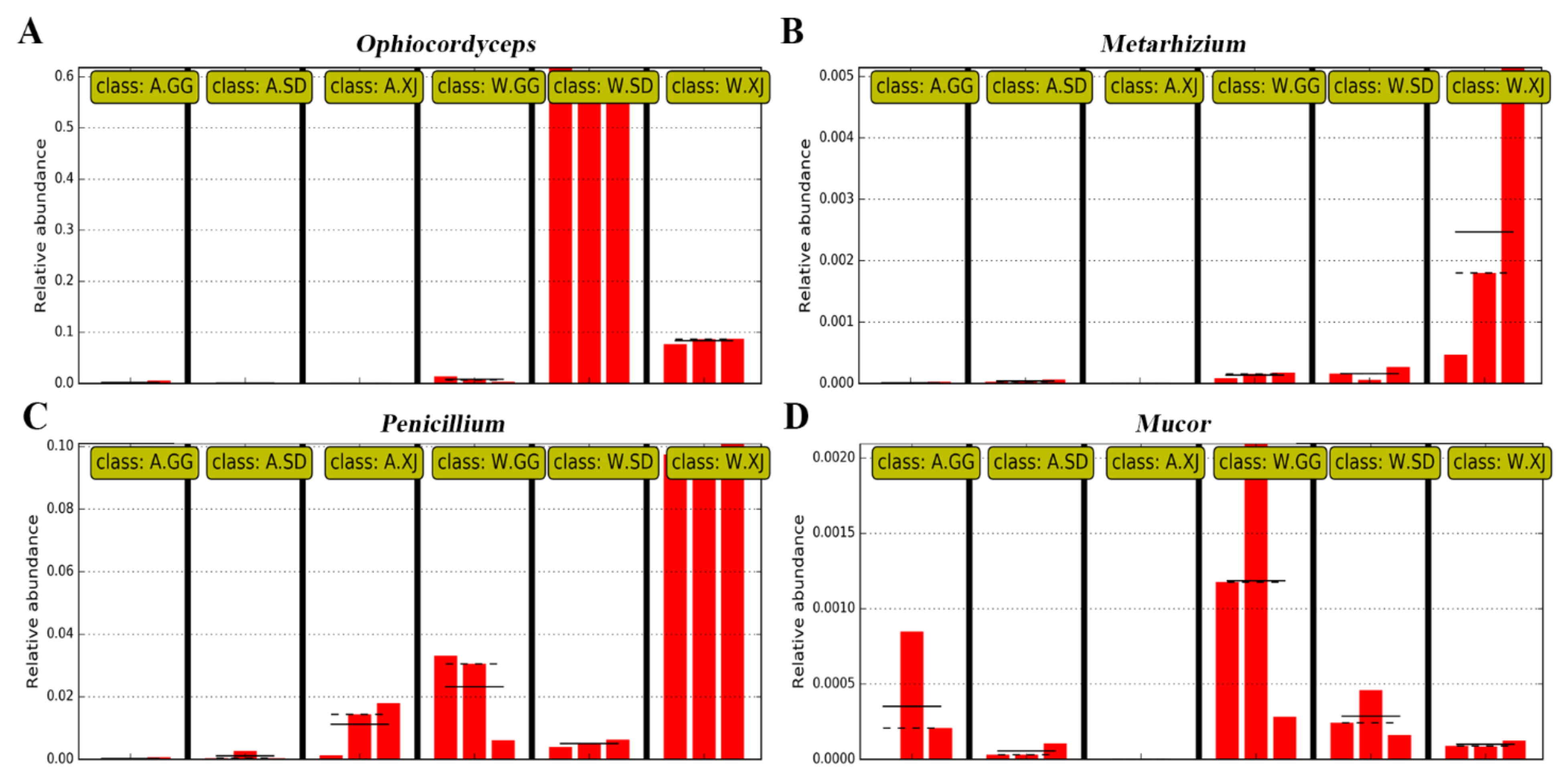
3.3.3. Differential Microbes among Insect Populations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sung, G.H.; Hywel-Jones, N.L.; Sung, J.M.; Luangsa-ard, J.J.; Shrestha, B.; Spatafora, J.W. Phylogenetic classification of Cordyceps and the clavicipitaceous fungi. Stud. Mycol. 2007, 57, 5–59. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.W.; Li, L.J.; Tian, E.W. Advances in research of the artificial cultivation of Ophiocordyceps sinensis in China. Crit. Rev. Biotechnol. 2014, 34, 233–243. [Google Scholar] [CrossRef]
- Han, R.C.; Wu, H.; Tao, H.P.; Qiu, X.H.; Liu, G.Q.; Rao, Z.C.; Cao, L. Research on Chinese cordyceps during the past 70 years in China. Chin. J. Appl. Entomol. 2019, 56, 849–883. [Google Scholar]
- Wiegmann, B.; Regier, J.; Mitter, C. Combined molecular and morphological evidence on the phylogeny of the lepidopteran lineages. Zool. Scr. 2002, 31, 67–81. [Google Scholar] [CrossRef]
- Wang, D.P.; Li, H.G.; Li, Y.J.; Guo, S.C.; Yang, J.; Qi, D.L.; Jin, C.; Zhao, X.Q. Hypoxia-inducible factor 1α cDNA cloning and its mRNA and protein tissue specific expression in domestic yak (Bos grunniens) from Qinghai-Tibetan plateau. Biochem. Biophys. Res. Commun. 2006, 348, 310–319. [Google Scholar] [CrossRef]
- Cui, X.; Gu, S.; Zhao, X.; Wu, J.; Kato, T.; Tang, Y. Diurnal and seasonal variations of UV radiation on the northern edge of the Qinghai-Tibetan Plateau. Agric. For. Meteorol. 2008, 148, 144–151. [Google Scholar] [CrossRef]
- Wang, X.L.; Yao, Y.J. Host insect species of Ophiocordyceps sinensis: A review. ZooKeys 2011, 43–59. [Google Scholar]
- Wang, H.S.; Zeng, H.; Xu, H.F. Study on change regulation of environment factors at Cordyceps sinensis growth area. Chin. Qinghai J. Anim. Vet. Sci. 2006, 36. [Google Scholar]
- Nielsen, E.S.; Robinson, G.S.; Wagner, D.L. Ghost-moths of the world: A global inventory and bibliography of the Exoporia (Mnesarchaeoidea and Hepialoidea) (Lepidoptera). J. Nat. Hist. 2000, 34, 823–878. [Google Scholar] [CrossRef]
- Tu, Y.Q.; Zhang, D.R.; Zeng, W.; Chen, S.J. Study on biological characteristic of Hepialus xiaojinensis in sichuan. Chin. J. Appl. Entomol. 2011, 48, 990–996. [Google Scholar]
- Guo, X.; Liu, B.; Ma, S.B.; Gui, M.Y. The ecological environmental investigation and biological characteristic analysis of Cordyceps sinensis in Yunnan province. Edible Fungi China 2008, 27, 8–11. [Google Scholar]
- Lu, Z.H.; Shi, P.; He, Y.C.; Zhang, D.L.; He, Z.Y.; Chen, S.J.; Tu, Y.Q.; Li, L.; Liu, F.; Zeng, W. Review on natural enemies and diseases in the artificial cultivation of Chinese caterpillar mushroom, Ophiocordyceps sinensis (Ascomycetes). Int. J. Med. Mushrooms 2015, 17, 693–700. [Google Scholar] [CrossRef]
- Yang, R.H.; Wang, X.L.; Su, J.H.; Li, Y.; Jiang, S.P.; Gu, F.; Yao, Y.J. Bacterial diversity in native habitats of the medicinal fungus Ophiocordyceps sinensis on Tibetan plateau as determined using illumina sequencing data. FEMS Microbiol. Lett. 2015, 362. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, H.; Li, X.; Meng, Q.; Shu, R.; Wang, M.; Zhou, G.; Wang, H.; Miao, L.; Zhang, J.; et al. Cold adaptation mechanisms in the ghost moth Hepialus xiaojinensis: Metabolic regulation and thermal compensation. J. Insect Physiol. 2016, 85, 76–85. [Google Scholar] [CrossRef]
- Wu, W.; Sun, H.; Guo, J.; Jiang, F.; Liu, X.; Zhang, G. De novo transcriptome characterization of the ghost moth, Thitarodes pui, and elevation-based differences in the gene expression of its larvae. Gene 2015, 574, 95–105. [Google Scholar] [CrossRef]
- Rao, Z.; Cao, L.; Qiu, X.; Han, R. Comparative transcriptome analysis reveals molecular strategies of ghost moth Thitarodes armoricanus in response to hypoxia and anoxia. J. Insect Physiol. 2019, 112, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.X.; Xu, X.M.; Liang, F.R.; Yuan, J.P.; Peng, J.; Wu, C.F.; Wang, J.H. Morphological observations and fatty acid composition of indoor-cultivated Cordyceps sinensis at a high-altitude laboratory on Sejila mountain, Tibet. PLoS ONE 2015, 10, e0126095. [Google Scholar]
- Liu, G.; Han, R.; Cao, L. Artificial cultivation of the Chinese cordyceps from injected ghost moth larvae. Environ. Entomol. 2019, 48, 1088–1094. [Google Scholar] [CrossRef]
- Meng, Q.; Yu, H.Y.; Zhang, H.; Zhu, W.; Wang, M.L.; Zhang, J.H.; Zhou, G.L.; Li, X.; Qin, Q.L.; Hu, S.N.; et al. Transcriptomic insight into the immune defenses in the ghost moth, Hepialus xiaojinensis, during an Ophiocordyceps sinensis fungal infection. Insect Biochem. Mol. Biol. 2015, 64, 1–15. [Google Scholar] [CrossRef]
- Rao, Z.C.; Cao, L.; Wu, H.; Qiu, X.H.; Liu, G.Q.; Han, R.C. Comparative transcriptome analysis of Thitarodes armoricanus in response to the entomopathogenic fungi Paecilomyces hepiali and Ophiocordyceps sinensis. Insects 2020, 11, 4. [Google Scholar] [CrossRef]
- Wu, H.; Rao, Z.C.; Cao, L.; De Clercq, P.; Han, R.C. Infection of Ophiocordyceps sinensis fungus causes dramatic changes in the microbiota of its thitarodes host. Front. Microbiol. 2020, 11, 577268. [Google Scholar] [CrossRef] [PubMed]
- Janson, E.M.; Stirema, J.O., 3rd; Singer, M.S.; Abbot, P. Phytophagous insect-microbe mutualisms and adaptive evolutionary diversification. Evolution 2008, 62, 997–1012. [Google Scholar] [CrossRef] [PubMed]
- Sommer, F.; Bäckhed, F. The gut microbiota-masters of host development and physiology. Nat. Rev. Microbiol. 2013, 11, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Coon, K.L.; Valzania, L.; McKinney, D.A.; Vogel, K.J.; Brown, M.R.; Strand, M.R. Bacteria-mediated hypoxia functions as a signal for mosquito development. Proc. Natl. Acad. Sci. USA 2017, 114, E5362–E5369. [Google Scholar] [CrossRef]
- Hu, Y.; Sanders, J.G.; Lukasik, P.; D’Amelio, C.L.; Millar, J.S.; Vann, D.R.; Lan, Y.; Newton, J.A.; Schotanus, M.; Kronauer, D.J.C.; et al. Herbivorous turtle ants obtain essential nutrients from a conserved nitrogen-recycling gut microbiome. Nat. Commun. 2018, 9, 964. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Zmora, N.; Levy, M.; Elinav, E. The microbiome and innate immunity. Nature 2016, 535, 65–74. [Google Scholar] [CrossRef]
- Martinson, V.G.; Danforth, B.N.; Minckley, R.L.; Rueppell, O.; Tingek, S.; Moran, N.A. A simple and distinctive microbiota associated with honey bees and bumble bees. Mol. Ecol. 2011, 20, 619–628. [Google Scholar] [CrossRef]
- Ceja-Navarro, J.A.; Vega, F.E.; Karaoz, U.; Hao, Z.; Jenkins, S.; Lim, H.C.; Kosina, P.; Infante, F.; Northen, T.R.; Brodie, E.L. Gut microbiota mediate caffeine detoxification in the primary insect pest of coffee. Nat. Commun. 2015, 6, 7618. [Google Scholar] [CrossRef]
- Francoeur, C.B.; Khadempour, L.; Moreira-Soto, R.D.; Gotting, K.; Book, A.J.; Pinto-Tomás, A.A.; Keefover-Ring, K.; Currie, C.R. Bacteria contribute to plant secondary compound degradation in a generalist herbivore system. mBio 2020, 11, e02146-20. [Google Scholar] [CrossRef]
- Cheng, D.; Guo, Z.; Riegler, M.; Xi, Z.; Liang, G.; Xu, Y. Gut symbiont enhances insecticide resistance in a significant pest, the oriental fruit fly Bactrocera dorsalis (Hendel). Microbiome 2017, 5, 13. [Google Scholar] [CrossRef]
- Engel, P.; Moran, N.A. The gut microbiota of insects-diversity in structure and function. FEMS Microbiol. Rev. 2013, 37, 699–735. [Google Scholar] [CrossRef]
- Chouaia, B.; Goda, N.; Mazza, G.; Alali, S.; Florian, F.; Gionechetti, F.; Callegari, M.; Gonella, E.; Magoga, G.; Fusi, M.; et al. Developmental stages and gut microenvironments influence gut microbiota dynamics in the invasive beetle Popillia japonica Newman (Coleoptera: Scarabaeidae). Environ. Microbiol. 2019, 21, 4343–4359. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, D.; Augustinos, A.; Doudoumis, V.; Bel Mokhtar, N.; Maiga, H.; Tsiamis, G.; Bourtzis, K. Multiple factors determine the structure of bacterial communities associated with Aedes albopictus under artificial rearing conditions. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef]
- Mikaelyan, A.; Dietrich, C.; Kohler, T.; Poulsen, M.; Sillam-Dusses, D.; Brune, A. Diet is the primary determinant of bacterial community structure in the guts of higher termites. Mol. Ecol. 2015, 24, 5284–5295. [Google Scholar] [CrossRef]
- Martinez-Solis, M.; Collado, M.C.; Herrero, S. Influence of diet, sex, and viral infections on the gut microbiota composition of Spodoptera exigua caterpillars. Front. Microbiol. 2020, 11, 753. [Google Scholar] [CrossRef]
- Li, W.J.; Dong, C.H.; Liu, X.Z.; Li, Q.P.; Xia, J.M.; Liang, F. Research advances in artificial cultivation of Chinese cordyceps. Mycosystema 2016, 35, 375–387. [Google Scholar]
- Qin, Q.L.; Zhou, G.L.; Zhang, H.; Meng, Q.; Zhang, J.H.; Wang, H.T.; Miao, L.; Li, X. Obstacles and approaches in artificial cultivation of Chinese cordyceps. Mycology 2018, 9, 7–9. [Google Scholar] [CrossRef]
- Liu, G.H.; Cao, L.; Qiu, X.H.; Han, R.C. Quorum sensing activity and hyphal growth by external stimuli in the entomopathogenic fungus Ophiocordyceps Sinensis. Insects 2020, 11, 205. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, Z.K.; Yu, H.W.; Chen, S.J.; Yan, G.F.; Xia, Y.X. Analysis of the bacterial diversity in intestines of Hepialus gonggaensis larvae. Acta Mycol. Sin. 2008, 48, 616–622. [Google Scholar]
- Yu, H.; Wang, Z.; Liu, L.; Xia, Y.; Yin, Y.; Yuan, Q.; Cao, Y.; Guoxiong, P. Analysis of fungal diversity in intestines of Hepialus gonggaensis larvae. Acta Microbiol. Sin. 2008, 48, 439–445. [Google Scholar]
- Tao, Z.; Cao, L.; Zhang, Y.; Ye, Y.S.; Han, R.C. Laboratory rearing of Thitarodes armoricanus and Thitarodes jianchuanensis (Lepidoptera: Hepialidae), hosts of the Chinese medicinal fungus Ophiocordyceps sinensis (Hypocreales: Ophiocordycipitaceae). J. Econ. Eentomol. 2016, 109, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.W.; Liu, X.; Zhang, G.R. Revision of taxonomic system of the genus Hepialus (Lepidoptera, Hepialidae) currently adopted in China. J. Hunan Univ. Sci. Technol. 2010, 25, 114–120. [Google Scholar]
- Cao, L.; Ye, Y.S.; Han, R.C. Fruiting body production of the medicinal Chinese caterpillar mushroom, Ophiocordyceps sinensis (Ascomycetes), in artificial medium. Int. J. Med. Mushrooms 2015, 17, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Andersen, K.; Kirkegaard, R.; Karst, S.; Albertsen, M. Ampvis2: An r package to analyse and visualise 16s rrna amplicon data. bioRxiv 2018. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.R.; Yu, J.F.; Wu, G.G.; Liu, X. Factors influencing the occurrence of Ophiocordyceps sinensis. Acta Ecol. Sin. 2011, 31, 4117–4125. [Google Scholar]
- Chu, H.F.; Wang, L.Y.; Han, H.X. Lepidoptera, Hepialidae and Epiplemidae in Fauna Sinica, Insecta; Beijing Science Press: Beijing, China, 2004; Volume 38, pp. 1–194. [Google Scholar]
- Chao, A. Nonparametric estimation of the number of classes in a population. Scand. J. Stat. 1984, 11, 265–270. [Google Scholar]
- Lemos, L.N.; Fulthorpe, R.R.; Triplett, E.W.; Roesch, L.F. Rethinking microbial diversity analysis in the high throughput sequencing era. J. Microbiol. Methods 2011, 86, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.Q.; Han, R.C. Detection primer set, detection kit and detection method of Isaria farinosa in culture medium of Cordyceps sinensis. Chin. Pat. 2017, 201711340871.8. [Google Scholar]
- Zhuo, F.P.; Chen, S.J.; Yin, Y.P.; Wang, Z.K.; Xia, Y.X. Analysis on the Hepialus gonggaensis’s intestinal bacterial flora. J. Chongqing Univ. 2004, 27, 26–29. [Google Scholar]
- Liu, Y.; Shen, Z.; Yu, J.; Li, Z.; Liu, X.; Xu, H. Comparison of gut bacterial communities and their associations with host diets in four fruit borers. Pest Manag. Sci. 2020, 76, 1353–1362. [Google Scholar] [CrossRef]
- Chen, B.; Du, K.; Sun, C.; Vimalanathan, A.; Liang, X.; Li, Y.; Wang, B.; Lu, X.; Li, L.; Shao, Y. Gut bacterial and fungal communities of the domesticated silkworm (Bombyx mori) and wild mulberry-feeding relatives. ISME J. 2018, 12, 2252–2262. [Google Scholar] [CrossRef]
- Gong, Q.; Cao, L.J.; Sun, L.N.; Chen, J.C.; Gong, Y.J.; Pu, D.Q.; Huang, Q.; Hoffmann, A.A.; Wei, S.J. Similar gut bacterial microbiota in two fruit-feeding moth pests collected from different host species and locations. Insects 2020, 11, 840. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Hong, Y.; Mai, Z.; Zhu, Q.; Guo, L. Internal and external microbial community of the Thitarodes moth, the host of Ophiocordyceps sinensis. Microorganisms 2019, 7, 517. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.I.; Jacquet, T.; Delaunay, S.; Borges, F.; Millière, J.B.; Revol-Junelles, A.M.; Cailliez-Grimal, C. Carnobacterium maltaromaticum: Identification, isolation tools, ecology and technological aspects in dairy products. Food Microbiol. 2010, 27, 573–579. [Google Scholar] [CrossRef]
- Leisner, J.J.; Groth, L.B.; Prévost, H.; Djamel, D.; Paw, D. Carnobacterium: Positive and negative effects in the environment and in foods. FEMS Microbiol. Rev. 2007, 31, 592–613. [Google Scholar] [CrossRef]
- Martinsen, L.; Salma, W.; Myklebust, R.; Mayhew, T.; Ringoe, E. Carnobacterium maltaromaticum vs. Vibrio (Listonella) anguillarum in the midgut of Atlantic cod (Gadus morhua L.): An ex vivo study. Aquac. Res. 2011, 42, 1830–1839. [Google Scholar]
- Ahmed, M.Z.; Araujo-Jnr, E.V.; Welch, J.J.; Kawahara, A.Y. Wolbachia in butterflies and moths: Geographic structure in infection frequency. Front. Zool. 2015, 12, 16. [Google Scholar] [CrossRef]
- Narita, S.; Nomura, M.; Kageyama, D. Naturally occurring single and double infection with Wolbachia strains in the butterfly Eurema hecabe: Transmission efficiencies and population density dynamics of each Wolbachia strain. FEMS Microbiol. Ecol. 2007, 61, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Werren, J.H.; Baldo, L.; Clark, M.E. Wolbachia: Master manipulators of invertebrate biology. Nat. Rev. Microbiol. 2008, 6, 741–751. [Google Scholar] [CrossRef]
- Teixeira, L.; Ferreira, A.; Ashburner, M. The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol. 2008, 6, 2753–2763. [Google Scholar] [CrossRef]
- Ford, S.A.; Allen, S.L.; Ohm, J.R.; Sigle, L.T.; Sebastian, A.; Albert, I.; Chenoweth, S.F.; McGraw, E.A. Selection on Aedes aegypti alters Wolbachia-mediated dengue virus blocking and fitness. Nat. Microbiol. 2019, 4, 1832–1839. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Sun, P.; Nie, K.X.; Zhu, Y.B.; Shi, M.Y.; Xiao, C.G.; Liu, H.; Liu, Q.Y.; Zhao, T.Y.; Chen, X.G.; et al. A gut commensal bacterium promotes mosquito permissiveness to arboviruses. Cell Host Microbe 2019, 25, 110–112. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Lai, Y.L.; Wang, G.D.; Chen, H.; Li, F.; Wang, S.B. Insect pathogenic fungus interacts with the gut microbiota to accelerate mosquito mortality. Proc. Natl. Acad. Sci. USA 2017, 114, 5994–5999. [Google Scholar] [CrossRef]
- Tu, Y.Q.; Zhang, D.L.; Zeng, W.; Chen, S.J.; Yin, D.H. Study on the infection of Cordyceps sinensis on the host larva Hepialus sp. Edible Fungi 2010, 3, 16–17. [Google Scholar]
| Group Name 1 | Altitude (Masl) | Environmental Temperature 2 | Major Diet 3 | Sampling Location |
|---|---|---|---|---|
| W.SD (W) | ~4128 | Wild, −20~10 °C | plant roots of alpine meadow | Shade Town, Kangding City, Ganzi Tibetan Autonomous Prefecture, Sichuan Province, China |
| W.GG (W) | ~3958 | Wild, −20~10 °C | plant roots of alpine meadow | Hailuogou, Gongga mountain, Moxi Town, Luding County, Ganzi Tibetan Autonomous Prefecture, Sichuan Province, China |
| W.XJ (W) | ~3823 | Wild, −20~10 °C | plant roots of alpine meadow | Xiaojin County, Aba Tibetan and Qiang Autonomous Prefecture, Sichuan Province, China |
| A.SD (A) | ~43 | Artificial rearing at 8 °C | roots of P. anserina | Haizhu District, Guangzhou City, Guangdong Province, China |
| A.GG (A) | ~43 | Artificial rearing at 8 °C | roots of P. anserina | Haizhu District, Guangzhou City, Guangdong Province, China |
| A.XJ (A) | ~43 | Artificial rearing at 8 °C | roots of P. anserina | Haizhu District, Guangzhou City, Guangdong Province, China |
| Category | Microbe Species | Phylum | Population Sampled | |||||
|---|---|---|---|---|---|---|---|---|
| W.SD | W.GG | W.XJ | A.SD | A.GG | A.XJ | |||
| Bacteria | Acinetobacter lwoffii | Proteobacteria | + | + | ||||
| Aeromonas sp. | Proteobacteria | + | ++ | |||||
| Agromyces sp. | Actinobacteria | + | ||||||
| Arthrobacter sp. | Actinobacteria | + | + | + | + | |||
| Bacillus mycoides | Firmicutes | + | ||||||
| Bacillus sp. | Firmicutes | + | + | + | ||||
| Buttiauxella sp. | Proteobacteria | + | ||||||
| Carnobacterium maltaromaticum | Firmicutes | ++++ | ++++ | ++++ | +++ | +++ | +++ | |
| Chryseobacterium sp. | Bacteroidetes | + | + | + | + | + | ||
| Enterococcus sp. | Firmicutes | ++ | + | |||||
| Glutamicibacter sp. | Actinobacteria | + | + | |||||
| Microbacterium foliorum | Actinobacteria | + | ||||||
| Microbacterium sp. | Actinobacteria | + | + | + | + | ++ | ||
| Oerskovia sp. | Actinobacteria | + | ||||||
| Pantoea sp. | Proteobacteria | + | ||||||
| Pseudomonas sp. | Proteobacteria | + | + | + | ++ | + | + | |
| Pseudomonas fragi | Proteobacteria | + | + | + | + | |||
| Pseudoclavibacter sp. | Actinobacteria | + | ||||||
| Rahnella aquatilis | Proteobacteria | + | + | + | + | + | + | |
| Raoultella terrigena | Proteobacteria | + | + | + | ||||
| Rhodococcus sp. | Actinobacteria | + | + | + | ||||
| Serratia fonticola | Proteobacteria | + | + | ++ | ||||
| Serratia plymuthica | Proteobacteria | + | + | + | + | |||
| Serratia proteamaculans | Proteobacteria | + | + | |||||
| Staphylococcus sp. | Firmicutes | + | + | + | + | |||
| Streptomyces sp. | Actinobacteria | + | + | + | + | + | + | |
| Fungi | Apiotrichum sp. | Basidiomycota | + | |||||
| Aspergillus sp. | Ascomycota | + | + | + | ||||
| Candida sp. | Ascomycota | ++ | ++ | + | + | |||
| Chaetomium sp. | Ascomycota | + | + | |||||
| Cladosporium sp. | Ascomycota | + | + | + | + | |||
| Mucor hiemalis | Mucoromycota | + | ||||||
| Mucor racemosus | Mucoromycota | + | + | |||||
| Nectriaceae | Ascomycota | + | + | + | ||||
| Penicillium polonicum | Ascomycota | + | ||||||
| Penicillium sp. | Ascomycota | + | + | + | ||||
| Rhodotorula sp. | Basidiomycota | + | ||||||
| Trichoderma sp. | Ascomycota | + | ||||||
| Sporocadaceae | Ascomycota | + | ||||||
| Sterigmatomyces halophilus | Basidiomycota | + | + | |||||
| Bacterial species | 15 | 14 | 14 | 12 | 10 | 14 | ||
| Fungal species | 5 | 9 | 7 | 3 | 2 | 3 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, G.; Zheng, X.; Long, H.; Rao, Z.; Cao, L.; Han, R. Gut Bacterial and Fungal Communities of the Wild and Laboratory-Reared Thitarodes Larvae, Host of the Chinese Medicinal Fungus Ophiocordyceps sinensis on Tibetan Plateau. Insects 2021, 12, 327. https://doi.org/10.3390/insects12040327
Liu G, Zheng X, Long H, Rao Z, Cao L, Han R. Gut Bacterial and Fungal Communities of the Wild and Laboratory-Reared Thitarodes Larvae, Host of the Chinese Medicinal Fungus Ophiocordyceps sinensis on Tibetan Plateau. Insects. 2021; 12(4):327. https://doi.org/10.3390/insects12040327
Chicago/Turabian StyleLiu, Guiqing, Xuehong Zheng, Hailin Long, Zhongchen Rao, Li Cao, and Richou Han. 2021. "Gut Bacterial and Fungal Communities of the Wild and Laboratory-Reared Thitarodes Larvae, Host of the Chinese Medicinal Fungus Ophiocordyceps sinensis on Tibetan Plateau" Insects 12, no. 4: 327. https://doi.org/10.3390/insects12040327
APA StyleLiu, G., Zheng, X., Long, H., Rao, Z., Cao, L., & Han, R. (2021). Gut Bacterial and Fungal Communities of the Wild and Laboratory-Reared Thitarodes Larvae, Host of the Chinese Medicinal Fungus Ophiocordyceps sinensis on Tibetan Plateau. Insects, 12(4), 327. https://doi.org/10.3390/insects12040327






