Thermal Preferences of Cowpea Seed Beetles (Callosobruchus maculatus): Effects of Sex and Nuptial Gift Transfers
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Angilletta, M.J. Thermal Adaptation: A Theoretical and Empirical Synthesis; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Pörtner, H.O.; Van Dijk, P.L.M.; Hardewig, I.; Sommer, A. Levels of Metabolic Cold Adaptation: Tradeoffs in Eurythermal and Stenothermal Ectotherms; Davison, W., Howard-Williams, C., Broady, P., Eds.; Antarctic Ecosystems: Models for Wider Ecological Understanding; Caxton Press: Christchurch, New Zealand, 2000; pp. 109–122. [Google Scholar]
- Pörtner, H.O.; Bennett, A.F.; Bozinovic, F.; Clarke, A.; Lardies, M.A.; Lucassen, M.; Pelster, B.; Schiemer, F.; Stillman, J.H. Trade-offs in thermal adaptation: The need for a molecular to ecological integration. Physiol. Biochem. Zool. 2006, 79, 295–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huey, R.B. Behavioral thermoregulation in lizards: Importance of associated costs. Science 1974, 184, 1001–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huey, R. Temperature, Physiology, and the Ecology of Reptiles; Gans, C., Pough, F.H., Eds.; Biology of the Reptilia; Academic Press: New York, NY, USA, 1982; pp. 25–91. [Google Scholar]
- Huey, R.B.; Slatkin, M. Cost and benefits of lizard thermoregulation. Q. Rev. Biol. 1976, 51, 363–384. [Google Scholar] [CrossRef] [PubMed]
- Harrington, R.; Woiwod, I.; Sparks, T. Climate change and trophic interactions. Trends Ecol. Evol. 1999, 14, 146–150. [Google Scholar] [CrossRef]
- Huey, R.B.; Stevenson, R.D. Integrating thermal physiology and ecology of ectotherms: Discussion of approaches. Am. Zool. 1979, 19, 357–366. [Google Scholar] [CrossRef] [Green Version]
- Huey, R.B.; Berrigan, D. Temperature, demography, and ectotherm fitness. Am. Nat. 2001, 158, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, R.D. The relative importance of behavioral and physiological adjustments controlling body temperature in terrestrial ectotherms. Am. Nat. 1985, 126, 362–386. [Google Scholar] [CrossRef]
- Cowles, R.B.; Bogert, C.M. A preliminary study of the thermal requirements of desert reptiles. Bull. Am. Mus. Nat. 1944, 83, 261–296. [Google Scholar]
- Huey, R.B.; Hertz, P.E.; Sinervo, B. Behavioral drive versus behavioral inertia in evolution: A null model approach. Am. Nat. 2003, 161, 357–366. [Google Scholar] [CrossRef] [Green Version]
- Kellermann, V.; Chown, S.L.; Schou, M.F.; Aitkenhead, I.; Janion-Scheepers, C.; Clemson, A.; Scott, M.T.; Sgrò, C.M. Comparing thermal performance curves across traits: How consistent are they? J. Exp. Biol. 2019, 222, 193433. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Dong, S.; Ji, T. Effect of different thermal regimes on growth and physiological performance of the sea cucumber Apostichopus japonicus Selenka. Aquaculture 2008, 275, 329–334. [Google Scholar] [CrossRef]
- Stearns, S.C. The Evolution of Life Histories; Oxford University Press: Oxford, UK, 1992; (No. 575 S81). [Google Scholar]
- Kozłowski, J.; Czarnoleski, M.; Dańko, M. Can optimal resource allocation models explain why ectotherms grow larger in cold? Integr. Comp. Biol. 2004, 44, 480–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antoł, A.; Rojek, W.; Singh, S.; Piekarski, D.; Czarnoleski, M. Hypoxia causes woodlice (Porcellio scaber) to select lower temperatures and impairs their thermal performance and heat tolerance. PLoS ONE 2019, 14, e0220647. [Google Scholar] [CrossRef] [Green Version]
- Morita, K.; Fukuwaka, M.A.; Tanimata, N.; Yamamura, O. Size-dependent thermal preferences in a pelagic fish. Oikos 2010, 119, 1265–1272. [Google Scholar] [CrossRef]
- Macnab, V.; Barber, I. Some (worms) like it hot: Fish parasites grow faster in warmer water, and alter host thermal preferences. Glob. Change Biol. 2012, 18, 1540–1548. [Google Scholar] [CrossRef]
- Kinzner, M.T.; Kinzner, M.C.; Kaufmann, R.; Hoffmann, A.A.; Arthofer, W.; Schlick-Steiner, B.C.; Steiner, F.M. Is temperature preference in the laboratory ecologically relevant for the field? The case of Drosophila nigrosparsa. Glob. Ecol. Conserv. 2019, 18, e00638. [Google Scholar] [CrossRef]
- Jury, S.H.; Watson III, W.H. Seasonal and sexual differences in the thermal preferences and movements of American lobsters. Can. J. Fish Aquat. Sci. 2013, 70, 1650–1657. [Google Scholar] [CrossRef]
- Lailvaux, S.P.; Alexander, G.J.; Whiting, M.J. Sex-based differences and similarities in locomotor performance, thermal preferences, and escape behaviour in the lizard Platysaurus intermedius wilhelmi. Physiol. Biochem. Zool. 2003, 76, 511–521. [Google Scholar] [CrossRef] [Green Version]
- Le Galliard, J.F.; Le Bris, M.; Clobert, J. Timing of locomotor impairment and shift in thermal preferences during gravidity in a viviparous lizard. Funct. Ecol. 2003, 17, 877–885. [Google Scholar] [CrossRef] [Green Version]
- Fox, C.W.; Hickman, D.L.; Raleigh, E.L.; Mousseau, T.A. Paternal investment in a seed beetle (Coleoptera: Bruchidae): Influence of male size, age, and mating history. Ann. Entomol. Soc. Am. 1995, 88, 100–103. [Google Scholar] [CrossRef]
- Lewis, S.M.; Vahed, K.; Koene, J.M.; Engqvist, L.; Bussiere, L.F.; Perry, J.C.; Gwynne, D.T.; Lehmann, G.U. Emerging issues in the evolution of animal nuptial gifts. Biol. Lett. 2014, 10, 20140336. [Google Scholar] [CrossRef] [PubMed]
- Arnqvist, G.; Jones, T.M.; Elgar, M.A. Reversal of sex roles in nuptial feeding. Nature 2003, 424, 387. [Google Scholar] [CrossRef]
- Gwynne, D.T. Sexual conflict over nuptial gifts in insects. Annu. Rev. Entomol. 2008, 53, 83–101. [Google Scholar] [CrossRef] [Green Version]
- Fox, C.W. Multiple mating, lifetime fecundity and female mortality of the bruchid beetle, Callosobruchus maculatus (Coleoptera: Bruchidae). Funct Ecol. 1993, 7, 203–208. [Google Scholar] [CrossRef]
- Savalli, U.M.; Fox, C.W. The effect of male mating history on paternal investment, fecundity and female remating in the seed beetle Callosobruchus maculatus. Func. Ecol. 1999, 13, 169–177. [Google Scholar] [CrossRef]
- Eady, P.E.; Wilson, N.; Jackson, M. Copulating with multiple mates enhances female fecundity but not egg-to-adult survival in the bruchid beetle Callosobruchus maculatus. Evolution 2000, 54, 2161–2165. [Google Scholar] [CrossRef]
- Eady, P.E.; Hamilton, L.; Lyons, R.E. Copulation, genital damage and early death in Callosobruchus maculatus. Proc. R. Soc. Lond. B 2007, 274, 247–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paukku, S.; Kotiaho, J.S. Cost of reproduction in Callosobruchus maculatus: Effects of mating on male longevity and the effect of male mating status on female longevity. J. Insect Physiol. 2005, 51, 1220–1226. [Google Scholar] [CrossRef] [PubMed]
- Małek, D.K.; Dańko, M.J.; Czarnoleski, M. Does seed size mediate sex-specific reproduction costs in the Callosobruchus maculatus bean beetle? PLoS ONE 2019, 14, e0225967. [Google Scholar] [CrossRef]
- Ursprung, C.; Den Hollander, M.; Gwynne, D.T. Female seed beetles, Callosobruchus maculatus, remate for male-supplied water rather than ejaculate nutrition. Behav. Ecol. Sociobiol. 2009, 63, 781–788. [Google Scholar] [CrossRef]
- Edvardsson, M. Female Callosobruchus maculatus mate when they are thirsty: Resource-rich ejaculates as mating effort in a beetle. Anim. Behav. 2007, 74, 183–188. [Google Scholar] [CrossRef]
- Edney, E.B. Metabolic Water. In Water Balance in Land Arthropods. Zoophysiology and Ecology; Springer: Berlin/Heidelberg, Germany, 1977; Volume 9. [Google Scholar] [CrossRef]
- Rozen-Rechels, D.; Dupoué, A.; Lourdais, O.; Chamaillé-Jammes, S.; Meylan, S.; Clobert, J.; Le Galliard, J.F. When water interacts with temperature: Ecological and evolutionary implications of thermo-hydroregulation in terrestrial ectotherms. Ecol. Evol. 2019, 9, 10029–10043. [Google Scholar] [CrossRef] [Green Version]
- Tuda, M.; Kagoshima, K.; Toquenaga, Y.; Arnqvist, G. Global genetic differentiation in a cosmopolitan pest of stored beans: Effects of geography, host-plant usage and anthropogenic factors. PLoS ONE 2014, 9, e106268. [Google Scholar] [CrossRef] [Green Version]
- Kébé, K.; Alvarez, N.; Tuda, M.; Arnqvist, G.; Fox, C.W.; Sembène, M.; Espíndola, A. Global phylogeography of the insect pest Callosobruchus maculatus (Coleoptera: Bruchinae) relates to the history of its main host, Vigna unguiculata. J. Biogeogr. 2017, 44, 2515–2526. [Google Scholar] [CrossRef]
- Asare, A.T.; Gowda, B.S.; Galyuon, I.K.A.; Aboagye, L.L.; Takrama, J.F.; Timko, M.P. Assessment of the genetic diversity in cowpea (Vigna unguiculata L. Walp.) germplasm from Ghana using simple sequence repeat markers. Plant Genet. Resour. 2010, 8, 142. [Google Scholar] [CrossRef]
- Zannou, E.T.; Glitho, I.A.; Huignard, J.; Monge, J.P. Life history of flight morph females of Callosobruchus maculatus F.: Evidence of a reproductive diapause. J. Insect Physiol. 2003, 49, 575–582. [Google Scholar] [CrossRef]
- Prevett, P.F. Field infestation of cowpea (Vigna unguiculata) pods by beetles of the families Bruchidae and Curculionidae in Northern Nigeria. Bull. Entomol. Res. 1961, 52, 635–645. [Google Scholar] [CrossRef]
- Sano, I. Density effect and environmental temperature as the factors producing the active form of Callosobruchus maculatus. J. Stored Prod. Res. 1967, 2, 187–195. [Google Scholar] [CrossRef]
- Sano Fuji, I. Effect of parental age and developmental rate on the production of the active form of Callosobruchus maculatus. Mech. Ageing Dev. 1980, 10, 283–293. [Google Scholar] [CrossRef]
- Sano Fuji, I. Effect of bean water content on the production of the active form of Callosobruchus maculatus. J. Stored Prod. Res. 1984, 22, 115–123. [Google Scholar] [CrossRef]
- Ouedraogo, A.P.; Monge, J.P.; Huignard, J. Importance of temperature and seed water on induction of imaginal polymorphism in Callosobruchus maculatus. Entomol. Exp. Appl. 1991, 59, 59–66. [Google Scholar] [CrossRef]
- Monge, J.P.; Huignard, J. Population fluctuations of two bruchids species Callosobruchus maculatus and Bruchidius atrolineatus and their parasitoids Dinarmus basalis and Eupelmus vuilleti in a storage situation in Niger. J. Afr. Zool. 1991, 105, 187–196. [Google Scholar]
- Ouedraogo, A.P.; Sou, S.; Sanon, A.; Monge, J.P.; Huignard, J.; Tran, M.D.; Credland, P.F. Influence of temperature and humidity on populations of Callosobruchus maculatus (Coleoptera: Bruchidae) and its parasitoid Dinarmus basalis (Pteromalidae) in two zones of Burkina Faso. Bull. Entomol. Res. 1996, 86, 695–702. [Google Scholar] [CrossRef]
- Tatar, M.; Carey, J.R. Nutrition mediates reproductive trade-offs with age-specific mortality in the beetle Callosobruchus maculatus. Ecology 1995, 76, 2066–2073. [Google Scholar] [CrossRef]
- Romeis, J.; Städler, E.; Wäckers, F. Nectar- and pollen-feeding by adult herbivorous insects. In Plant-Provided Food for Carnivorous Insects; Wäckers, F.L., van Rijn, P.C.J., Bruin, J., Eds.; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar] [CrossRef]
- Alzouma, I.; Huignard, J. Donne’es pre´liminaires sur la biologie et le comportement de ponte dans la nature de Bruchidius atrolineatus (Pic) (Coleoptere Bruchidae) dans une zone sud-sahe´lienne au Niger Acta Oecologica. Oecol. Appl. 1981, 2, 391–400. [Google Scholar]
- Rönn, J.L.; Katvala, M.; Arnqvist, G. Interspecific variation in ejaculate allocation and associated effects on female fitness in seed beetles. J. Evol. Biol. 2008, 21, 461–470. [Google Scholar] [CrossRef] [Green Version]
- Dillon, M.E.; Wang, G.; Garrity, P.A.; Huey, R.B. Thermal preference in Drosophila. J. Therm. Biol. 2009, 34, 109–119. [Google Scholar] [CrossRef] [Green Version]
- Ahrens, W.H.; Cox, D.J.; Budhwar, G. Use of the arcsine and square root transformations for subjectively determined percentage data. Weed Sci. 1990, 38, 452–458. [Google Scholar] [CrossRef]
- Zari, T.A. Effects of sexual condition on food consumption and temperature selection in the herbivorous desert lizard, Uromastyx philbyi. J. Arid Environ. 1998, 38, 371–377. [Google Scholar] [CrossRef]
- Singh, S.; Smyth, A.K.; Blomberg, S.P. Thermal ecology and structural habitat use of two sympatric lizards (Carlia vivax and Lygisaurus foliorum) in subtropical Australia. Austral. Ecol. 2002, 27, 616–623. [Google Scholar] [CrossRef]
- Chen, T.H.; Lue, K.Y. Thermal preference of the yellow-margined box turtle (Cuora flavomarginata) (Testudines: Geoemydidae) inhabiting a mesic lowland forest, northern Taiwan. Amphib. Reptil. 2008, 29, 513–522. [Google Scholar] [CrossRef]
- Beal, M.S.; Lattanzio, M.S.; Miles, D.B. Differences in the thermal physiology of adult Yarrow’s spiny lizards (Sceloporus jarrovii) in relation to sex and body size. Ecol. Evol. 2014, 4, 4220–4229. [Google Scholar] [CrossRef] [Green Version]
- Deal, J. The temperature preferendum of certain insects. J. Anim. Ecol. 1941, 10, 323–356. [Google Scholar] [CrossRef]
- Kaikaew, K.; Steenbergen, J.; Themmen, A.P.; Visser, J.A.; Grefhorst, A. Sex difference in thermal preference of adult mice does not depend on presence of the gonads. Biol. Sex Differ. 2017, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Wedell, N. Variation in nuptial gift quality in bush crickets (Orthoptera: Tettigoniidae). Behav. Ecol. 1994, 5, 418–425. [Google Scholar] [CrossRef]
- Kindle, T.K.; Johnson, K.M.; Ivy, T.M.; Weddle, C.B.; Sakaluk, S.K. Female mating frequency increases with temperature in two cricket species, Gryllodes sigillatus and Acheta domesticus (Orthoptera: Gryllidae). Can. J. Zool. 2009, 84, 1345–1350. [Google Scholar] [CrossRef]
- Castañeda, L.E.; Romero-Soriano, V.; Mesas, A.; Roff, D.A.; Santos, M. Evolutionary potential of thermal preference and heat tolerance in Drosophila subobscura. J. Evol. Biol. 2019, 32, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Poiani, A. Complexity of seminal fluid: A review. Behav. Ecol. Sociobiol. 2006, 60, 289–310. [Google Scholar] [CrossRef]
- Eisner, T.; Smedley, S.R.; Young, D.K.; Eisner, M.; Roach, B.; Meinwald, J. Chemical basis of courtship in a beetle (Neopyrochroa flabellata): Cantharidin as “nuptial gift”. Proc. Natl. Acad. Sci. USA 1996, 93, 6499–6503. [Google Scholar] [CrossRef] [Green Version]
- Chapman, T. The soup in my fly: Evolution, form and function of seminal fluid proteins. PLoS Biol. 2008, 6, e179. [Google Scholar] [CrossRef]
- Krstevska, B.; Hoffmann, A.A. The effects of acclimation and rearing conditions on the response of tropical and temperate populations of Drosophila melanogaster and D. simulans to a temperature gradient (Diptera: Drosophilidae). J. Insect Behav. 1994, 7, 279–288. [Google Scholar] [CrossRef]
- Taylor, M.L.; Wigmore, C.; Hodgson, D.J.; Wedell, N.; Hosken, D.J. Multiple mating increases female fitness in Drosophila simulans. Anim. Behav. 2008, 76, 963–970. [Google Scholar] [CrossRef]
- Bressac, C.; Fleury, A.; Lachaise, D. Another way of being anisogamous in Drosophila subgenus species: Giant sperm, one-to-one gamete ratio, and high zygote provisioning. Proc. Natl. Acad. Sci. USA 1994, 91, 10399–10402. [Google Scholar] [CrossRef] [Green Version]
- Aspi, J. Incidence and adaptive significance of multiple mating in females of two boreal Drosophila virilis-group species. Finnish Zoological Publishing Board, formed by the Finnish Academy of Sciences, Societas Biologica Fennica Vanamo, Societas pro Fauna et Flora Fennica, and Societas Scientiarum Fennica. Ann. Zool. Fenn. 1992, 29, 147–159. [Google Scholar]
- Yamamoto, A.H. Temperature preference of Drosophila immigrans and D. virilis: Intra-and inter-population genetic variation. Jpn. J. Genet. 1994, 69, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Schilman, P.E.; Lazzari, C.R. Temperature preference in Rhodnius prolixus, effects and possible consequences. Acta. Trop. 2004, 90, 115–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sievert, L.M.; Hutchison, V.H. Influences of season, time of day, light and sex on the thermoregulatory behaviour of Crotaphytus collaris. J. Therm. Biol. 1989, 14, 159–165. [Google Scholar] [CrossRef]
- Lelièvre, H.; Blouin-Demers, G.; Pinaud, D.; Lisse, H.; Bonnet, X.; Lourdais, O. Contrasted thermal preferences translate into divergences in habitat use and realized performance in two sympatric snakes. J. Zool. 2011, 284, 265–275. [Google Scholar] [CrossRef]
- Teder, T.; Tammaru, T. Sexual size dimorphism within species increases with body size in insects. Oikos 2005, 108, 321–334. [Google Scholar] [CrossRef]
- Stillwell, R.C.; Fox, C.W. Environmental effects on sexual size dimorphism of a seed-feeding beetle. Oecologia 2007, 153, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Cecchetto, N.R.; Naretto, S. Do sex, body size and reproductive condition influence the thermal preferences of a large lizard? A study in Tupinambis merianae. J. Therm. Biol. 2015, 53, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, G.W. The consequences of sexual dimorphism in body size for butterfly flight and thermoregulation. Funct. Ecol. 1990, 4, 475–487. [Google Scholar] [CrossRef]
- Fogleman, J.C. Oviposition site preference for substrate temperature in Drosophila melanogaster. Behav. Genet. 1979, 9, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Berger, D.; Walters, R.; Gotthard, K. What limits insect fecundity? Body size-and temperature-dependent egg maturation and oviposition in a butterfly. Funct. Ecol. 2008, 22, 523–529. [Google Scholar] [CrossRef] [Green Version]
- Giga, D.E.; Smith, R.H. Egg production and development of Callosobruchus rhodesianus (Pic) and Callosobruchus maculatus (F.) (Coleoptera: Bruchidae) on several commodities at two different temperatures. J. Stored. Prod. Res. 1987, 23, 9–15. [Google Scholar] [CrossRef]
- Stillwell, R.C.; Wallin, W.G.; Hitchcock, L.J.; Fox, C.W. Phenotypic plasticity in a complex world: Interactive effects of food and temperature on fitness components of a seed beetle. Oecologia 2007, 153, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Vasudeva, R.; Deeming, D.C.; Eady, P.E. Developmental temperature affects the expression of ejaculatory traits and the outcome of sperm competition in Callosobruchus maculatus. J. Evol. Biol. 2014, 27, 1811–1818. [Google Scholar] [CrossRef]
- Feder, M.E.; Roberts, S.P.; Bordelon, A.C. Molecular thermal telemetry of free-ranging adult Drosoph melanogaster. Oecologia 2000, 123, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Stillwell, R.C.; Fox, C.W. Complex patterns of phenotypic plasticity: Interactive effects of temperature during rearing and oviposition. Ecology 2005, 86, 924–934. [Google Scholar] [CrossRef]
- Adhikary, P.; Barik, A. Effect of temperature on biology of Callosobruchus maculatus (F.). Indian. J. Entomol. 2012, 74, 261–266. [Google Scholar]
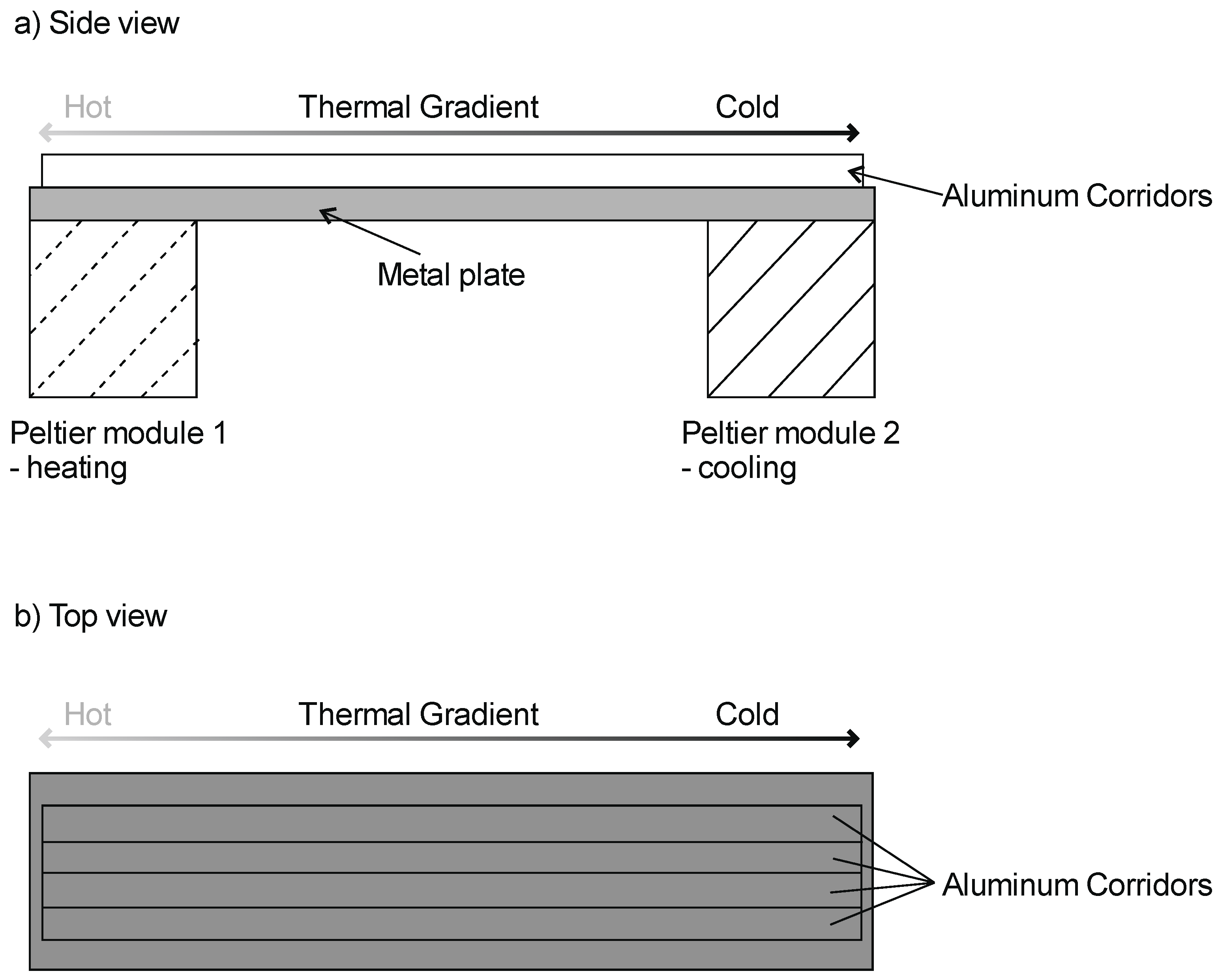
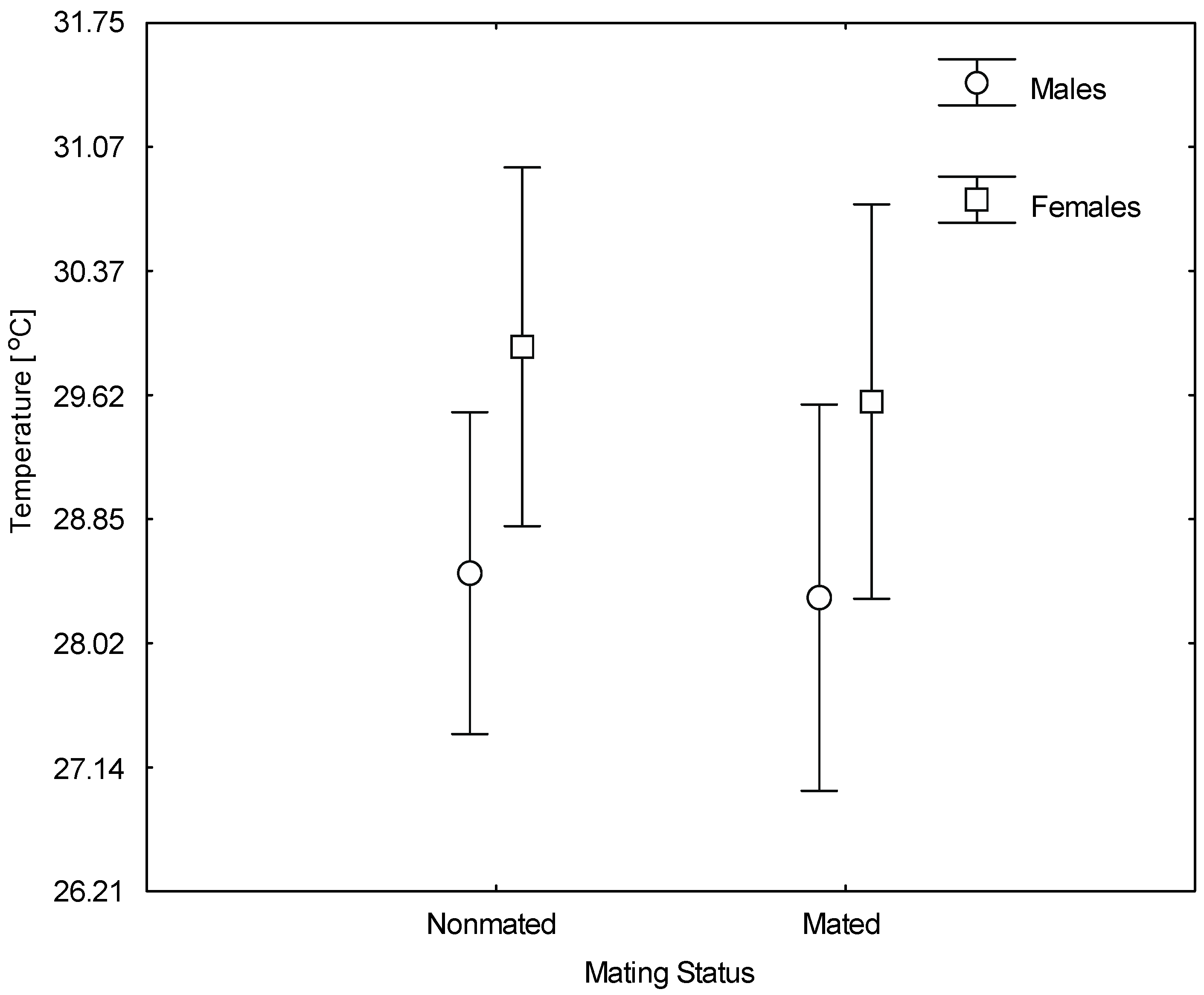
| Factor | Df | F | p | |
|---|---|---|---|---|
| (a) Body mass excluded | Sex | 1 | 5.218 | 0.024 |
| Mating Status | 1 | 0.184 | 0.669 | |
| Sex × Mating Status | 1 | 0.027 | 0.870 | |
| Error | 110 | |||
| (b) Body mass included | Sex | 1 | 0.038 | 0.845 |
| Mating Status | 1 | 0.107 | 0.744 | |
| Body mass | 1 | 0.528 | 0.469 | |
| Sex × Mating Status | 1 | 0.002 | 0.967 | |
| Error | 108 |
| Factor | Df | F | p | |
|---|---|---|---|---|
| (a) Males | Nuptial Gift Size | 1 | 0.179 | 0.676 |
| Error | 23 | |||
| (b) Females | Nuptial Gift Size | 1 | 2.013 | 0.171 |
| Error | 21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Małek, D.K.; Czarnoleski, M. Thermal Preferences of Cowpea Seed Beetles (Callosobruchus maculatus): Effects of Sex and Nuptial Gift Transfers. Insects 2021, 12, 310. https://doi.org/10.3390/insects12040310
Małek DK, Czarnoleski M. Thermal Preferences of Cowpea Seed Beetles (Callosobruchus maculatus): Effects of Sex and Nuptial Gift Transfers. Insects. 2021; 12(4):310. https://doi.org/10.3390/insects12040310
Chicago/Turabian StyleMałek, Dariusz Krzysztof, and Marcin Czarnoleski. 2021. "Thermal Preferences of Cowpea Seed Beetles (Callosobruchus maculatus): Effects of Sex and Nuptial Gift Transfers" Insects 12, no. 4: 310. https://doi.org/10.3390/insects12040310
APA StyleMałek, D. K., & Czarnoleski, M. (2021). Thermal Preferences of Cowpea Seed Beetles (Callosobruchus maculatus): Effects of Sex and Nuptial Gift Transfers. Insects, 12(4), 310. https://doi.org/10.3390/insects12040310







