From Global to Local—New Insights into Features of Pyrethroid Detoxification in Vector Mosquitoes
Abstract
Simple Summary
Abstract
1. Introduction
Multiple Pathways Contribute to Resistance Phenotypes
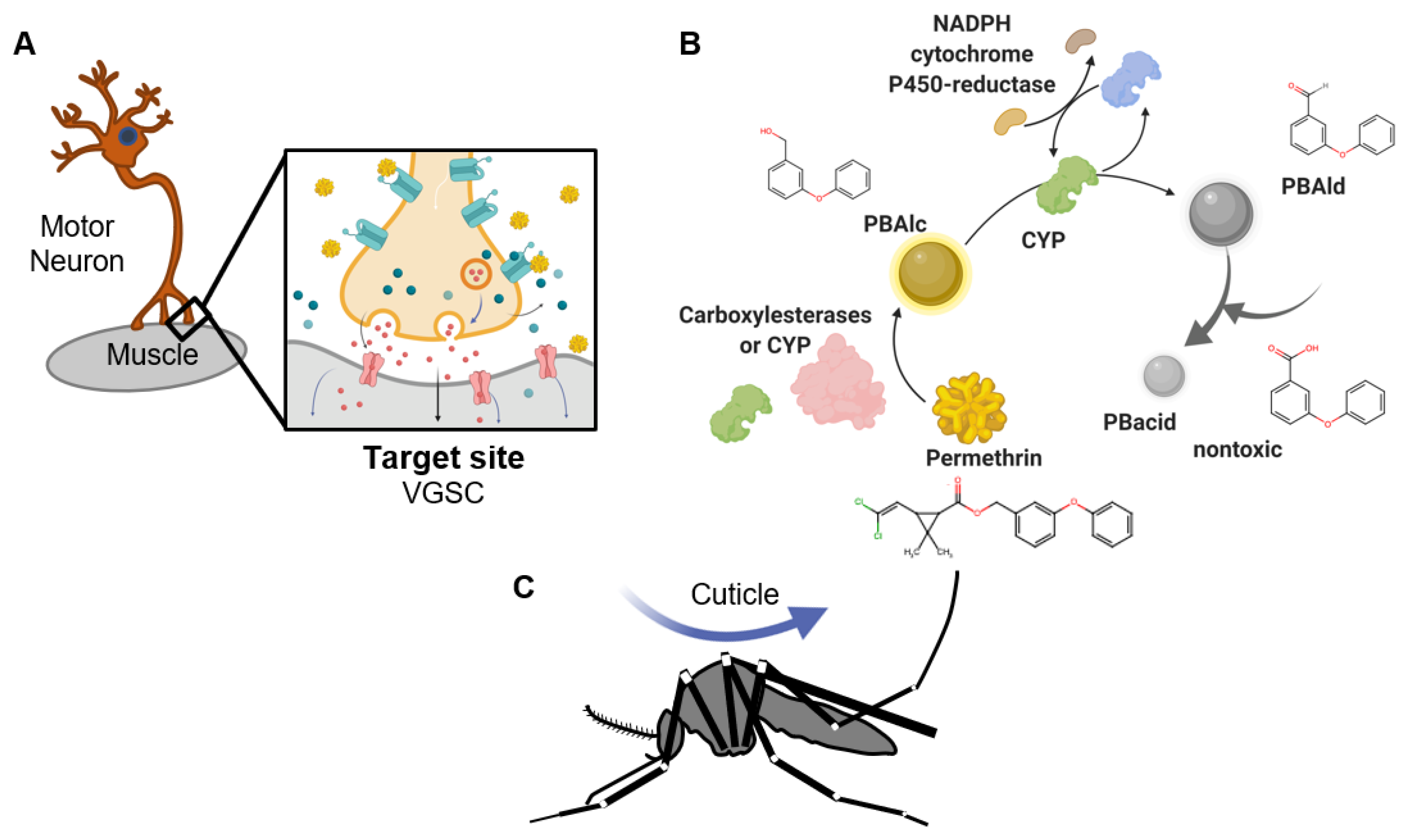
2. Biochemical Resistance
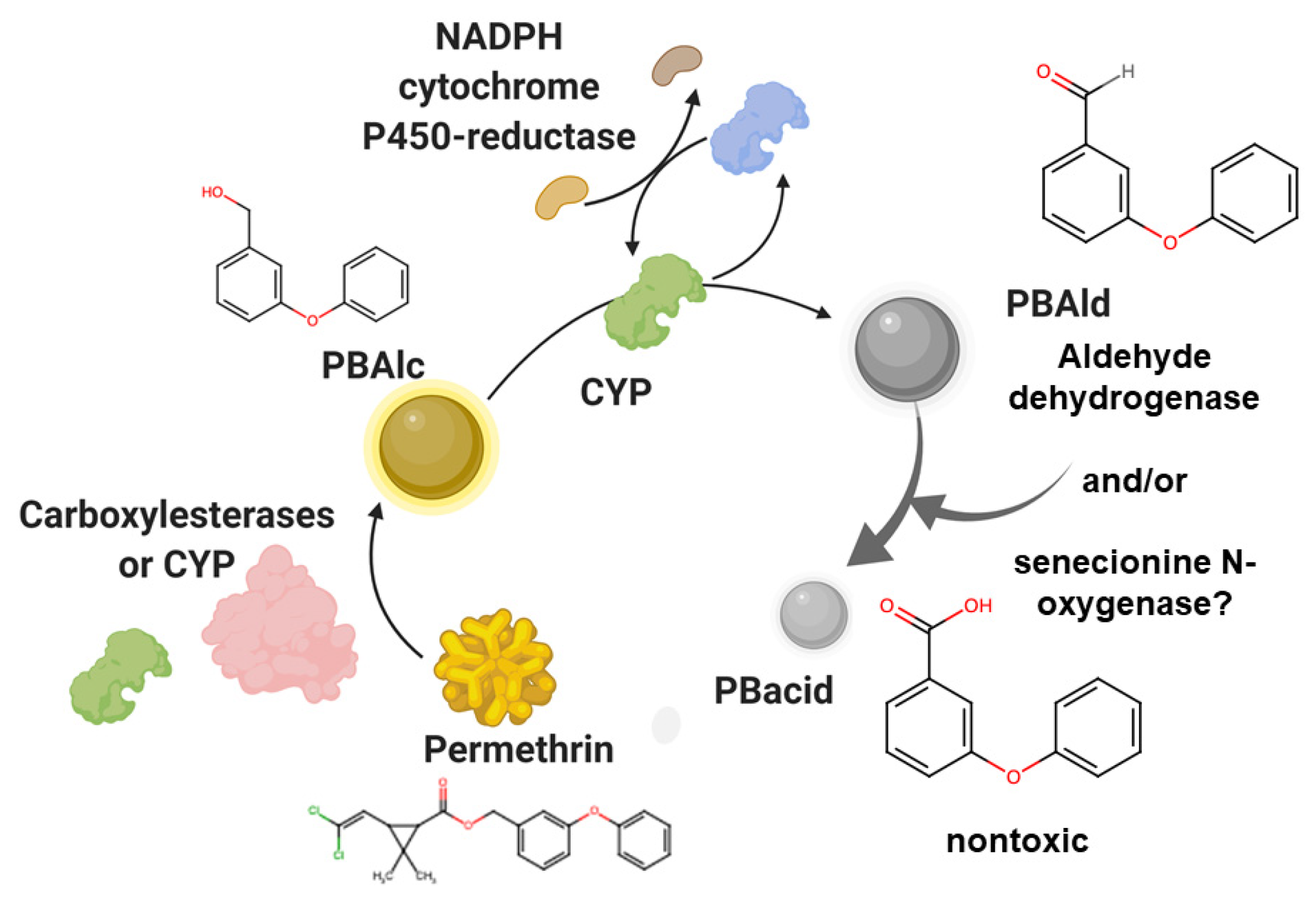
2.1. Detoxification
2.2. Cuticular Modifications
2.3. Synergisms
2.4. Excretion of Metabolic Intermediates
3. Newly-Described Features of Resistance
3.1. Post-Transcriptional Regulation
3.1.1. Small RNA Profiling
3.1.2. Nuanced Post-Transcriptional Regulation of VGSC
3.2. New Potential Detoxification Effectors
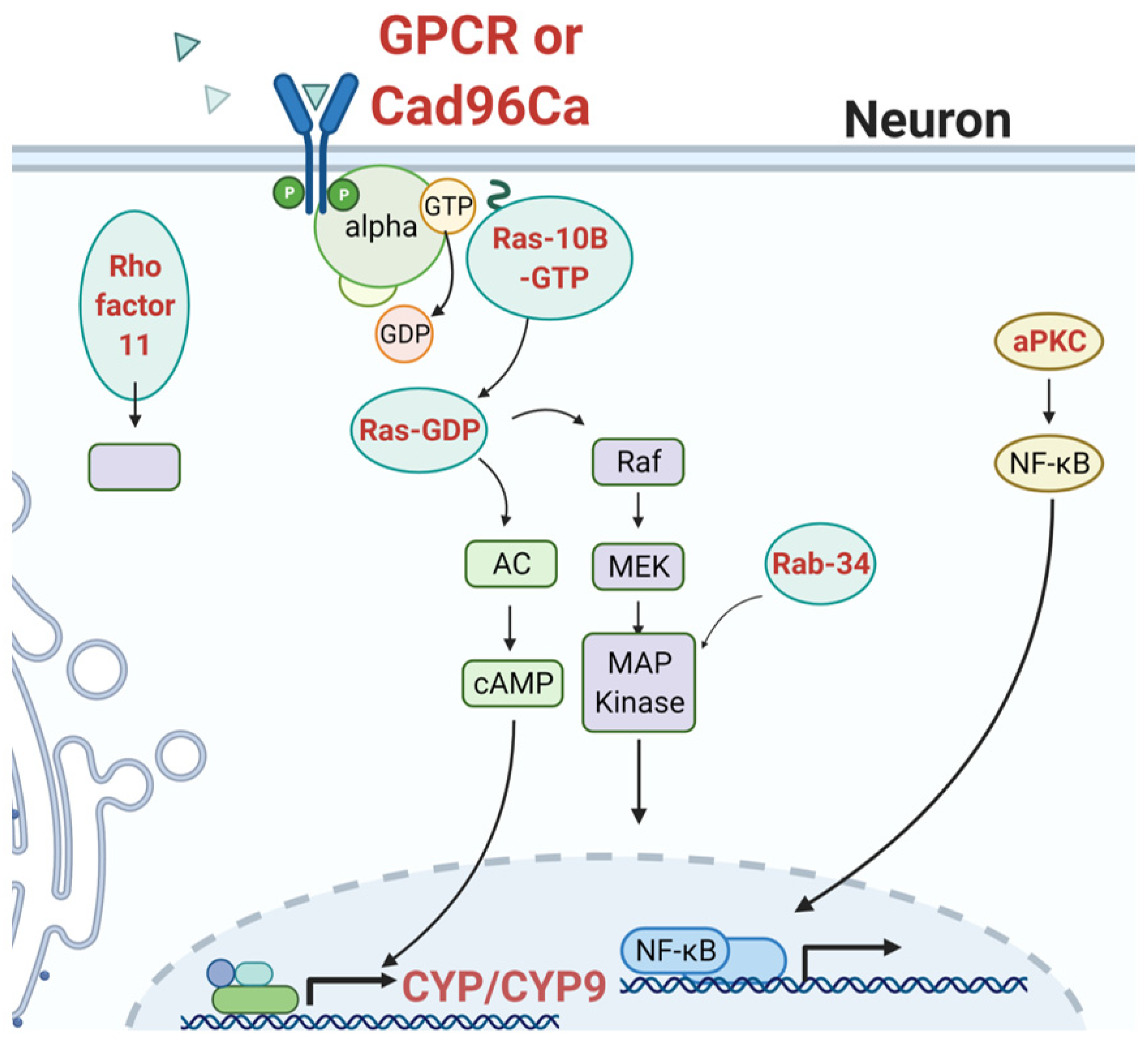
3.3. Signaling Mechanisms
4. Convergent Evolution of Insecticide Resistance
5. Conclusions
6. Criteria for Review
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Franklinos, L.H.V.; Jones, K.E.; Redding, D.W.; Abubakar, I. The effect of global change on mosquito-borne disease. Lancet Infect. Dis. 2019, 19, e302–e312. [Google Scholar] [CrossRef]
- Matiya, D.J.; Philbert, A.B.; Kidima, W.; Matowo, J.J. Dynamics and monitoring of insecticide resistance in malaria vectors across mainland Tanzania from 1997 to 2017: A systematic review. Malar. J. 2019, 18, 102. [Google Scholar] [CrossRef]
- Da-Cunha, M.P.; Lima, J.B.P.; Brogdon, W.G.; Moya, G.E.; Valle, D. Monitoring of resistance to the pyrethroid cypermethrin in Brazilian Aedes aegypti (Diptera: Culicidae) populations collected between 2001 and 2003. Mem. Instig. Oswaldo Cruz 2005, 100, 441–444. [Google Scholar] [CrossRef] [PubMed]
- Flores, A.E.; Ponce, G.; Silva, B.G.; Gutierrez, S.M.; Bobadilla, C.; Lopez, B.; Mercado, R.; Black, W.C. Wide Spread Cross Resistance to Pyrethroids in Aedes aegypti (Diptera: Culicidae) From Veracruz State Mexico. J. Econ. Èntomol. 2013, 106, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.F.; Ranson, H.; Rajatileka, S. Pyrethroid Resistance in Aedes aegypti from Grand Cayman. Am. J. Trop. Med. Hyg. 2010, 83, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Marcombe, S.; Poupardin, R.; Darriet, F.; Reynaud, S.; Bonnet, J.; Strode, C.; Brengues, C.; Yébakima, A.; Ranson, H.; Corbel, V.; et al. Exploring the molecular basis of insecticide resistance in the dengue vector Aedes aegypti: A case study in Martinique Island (French West Indies). BMC Genom. 2009, 10, 494. [Google Scholar] [CrossRef]
- García, G.P.; Flores, A.E.; Fernández-Salas, I.; Saavedra-Rodríguez, K.; Reyes-Solis, G.; Lozano-Fuentes, S.; Bond, J.G.; Casas-Martínez, M.; Ramsey, J.M.; García-Rejón, J.; et al. Recent Rapid Rise of a Permethrin Knock Down Resistance Allele in Aedes aegypti in México. PLoS Negl. Trop. Dis. 2009, 3, e531. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, L.C.; Ponce, G.; Saavedra-Rodriguez, K.; Lopez, B.; Flores, A.E. Frequency of V1016I and F1534C mutations in the voltage-gated sodium channel gene in Aedes aegypti in Venezuela. Pest Manag. Sci. 2014, 71, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Busvine, J.R. Mechanism of Resistance to Insecticide in Houseflies. Nat. Cell Biol. 1951, 168, 193–195. [Google Scholar] [CrossRef]
- Busvine, J.R. Forms of Insecticide-Resistance in Houseflies and Body Lice. Nat. Cell Biol. 1953, 171, 118–119. [Google Scholar] [CrossRef]
- Du, Y.; Nomura, Y.; Satar, G.; Hu, Z.; Nauen, R.; He, S.Y.; Zhorov, B.S.; Dong, K. Molecular evidence for dual pyrethroid-receptor sites on a mosquito sodium channel. Proc. Natl. Acad. Sci. USA 2013, 110, 11785–11790. [Google Scholar] [CrossRef] [PubMed]
- Flores, A.E.; Grajales, J.S.; Salas, I.F.; Garcia, G.P.; Becerra, M.H.; Lozano, S.; Brogdon, W.G.; Black, W.C.; Beaty, B. Mechanisms of insecticide resistance in field populations of Aedes aegypti (L.) from Quintana Roo, Southern Mexico. J. Am. Mosq. Control. Assoc. 2006, 22, 672–677. [Google Scholar] [CrossRef]
- Bingham, G.; Strode, C.; Tran, L.; Khoa, P.T.; Jamet, H.P. Can piperonyl butoxide enhance the efficacy of pyrethroids against pyrethroid-resistant Aedes aegypti? Trop. Med. Int. Health 2011, 16, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Itokawa, K.; Komagata, O.; Kasai, S.; Masada, M.; Tomita, T. Cis-acting mutation and duplication: History of molecular evolution in a P450 haplotype responsible for insecticide resistance in Culex quinquefasciatus. Insect Biochem. Mol. Biol. 2011, 41, 503–512. [Google Scholar] [CrossRef]
- Abdalla, H.; Wilding, C.S.; Nardini, L.; Pignatelli, P.; Koekemoer, L.L.; Ranson, H.; Coetzee, M. Insecticide resistance in Anopheles arabiensis in Sudan: Temporal trends and underlying mechanisms. Parasites Vectors 2014, 7, 213. [Google Scholar] [CrossRef]
- Riveron, J.M.; Osae, M.; Egyir-Yawson, A.; Irving, H.; Ibrahim, S.S.; Wondji, C.S. Multiple insecticide resistance in the major malaria vector Anopheles funestus in southern Ghana: Implications for malaria control. Parasites Vectors 2016, 9, 504. [Google Scholar] [CrossRef]
- Dusfour, I.; Zorrilla, P.; Guidez, A.; Issaly, J.; Girod, R.; Guillaumot, L.; Robello, C.; Strode, C. Deltamethrin Resistance Mechanisms in Aedes aegypti Populations from Three French Overseas Territories Worldwide. PLoS Negl. Trop. Dis. 2015, 9, e0004226. [Google Scholar] [CrossRef] [PubMed]
- Balabanidou, V.; Kampouraki, A.; MacLean, M.; Blomquist, G.J.; Tittiger, C.; Juárez, M.P.; Mijailovsky, S.J.; Chalepakis, G.; Anthousi, A.; Lynd, A.; et al. Cytochrome P450 associated with insecticide resistance catalyzes cuticular hydrocarbon production in Anopheles gambiae. Proc. Natl. Acad. Sci. USA 2016, 113, 9268–9273. [Google Scholar] [CrossRef]
- Kasai, S.; Komagata, O.; Itokawa, K.; Shono, T.; Ng, L.C.; Kobayashi, M.; Tomita, T. Mechanisms of Pyrethroid Resistance in the Dengue Mosquito Vector, Aedes aegypti: Target Site Insensitivity, Penetration, and Metabolism. PLoS Negl. Trop. Dis. 2014, 8, e2948. [Google Scholar] [CrossRef] [PubMed]
- Simma, E.A.; Dermauw, W.; Balabanidou, V.; Snoeck, S.; Bryon, A.; Clark, R.M.; Yewhalaw, D.; Vontas, J.; Duchateau, L.; Van Leeuwen, T. Genome-wide gene expression profiling reveals that cuticle alterations and P450 detoxification are associated with deltamethrin and DDT resistance in Anopheles arabiensis populations from Ethiopia. Pest Manag. Sci. 2019, 75, 1808–1818. [Google Scholar] [CrossRef] [PubMed]
- Wood, O.R.; Hanrahan, S.; Coetzee, M.; Koekemoer, L.L.; Brooke, B.D. Cuticle thickening associated with pyrethroid resistance in the major malaria vector Anopheles funestus. Parasites Vectors 2010, 3, 67. [Google Scholar] [CrossRef]
- Yahouédo, G.A.; Chandre, F.; Rossignol, M.; Ginibre, C.; Balabanidou, V.; Mendez, N.G.A.; Pigeon, O.; Vontas, J.; Cornelie, S. Contributions of cuticle permeability and enzyme detoxification to pyrethroid resistance in the major malaria vector Anopheles gambiae. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Assogba, B.S.; Djogbénou, L.S.; Saizonou, J.; Milesi, P.; Djossou, L.; Djégbe, I.; A Oumbouke, W.; Chandre, F.; Baba-Moussa, L.; Weill, M.; et al. Phenotypic effects of concomitant insensitive acetylcholinesterase (ace-1 R ) and knockdown resistance (kdr R ) in Anopheles gambiae: A hindrance for insecticide resistance management for malaria vector control. Parasites Vectors 2014, 7, 548. [Google Scholar] [CrossRef]
- Denham, S.; Eisen, L.; Beaty, M.; Beaty, B.J.; Black, W.C.; Saavedra-Rodriguez, K. Two Novel Bioassays to Assess the Effects of Pyrethroid-Treated Netting on Knockdown-Susceptible Versus Resistant Strains of Aedes aegypti. J. Am. Mosq. Control. Assoc. 2015, 31, 52–62. [Google Scholar] [CrossRef]
- Maestre-Serrano, R.; Gomez-Camargo, D.; Ponce-Garcia, G.; Flores, A.E. Susceptibility to insecticides and resistance mechanisms in Aedes aegypti from the Colombian Caribbean Region. Pestic. Biochem. Physiol. 2014, 116, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Field, L.M.; Davies, T.G.E.; O’Reilly, A.O.; Williamson, M.S.; Wallace, B.A. Voltage-gated sodium channels as targets for pyrethroid insecticides. Eur. Biophys. J. 2017, 46, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Saavedra-Rodriguez, K.; Maloof, F.V.; Campbell, C.L.; Garcia-Rejon, J.; Lenhart, A.; Penilla, P.; Rodriguez, A.; Sandoval, A.A.; Flores, A.E.; Ponce, G.; et al. Parallel evolution of vgsc mutations at domains IS6, IIS6 and IIIS6 in pyrethroid resistant Aedes aegypti from Mexico. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.P.B.; Santos, J.M.M.; Martins, A.J. Mutations in the voltage-gated sodium channel gene of anophelines and their association with resistance to pyrethroids–a review. Parasites Vectors 2014, 7, 450. [Google Scholar] [CrossRef]
- Zhorov, B.S.; Dong, K. Elucidation of pyrethroid and DDT receptor sites in the voltage-gated sodium channel. NeuroToxicology 2017, 60, 171–177. [Google Scholar] [CrossRef]
- Du, Y.; Nomura, Y.; Zhorov, B.S.; Dong, K. Sodium Channel Mutations and Pyrethroid Resistance in Aedes aegypti. Insects 2016, 7, 60. [Google Scholar] [CrossRef]
- Campbell, C.L.; Saavedra-Rodriguez, K.; Kubik, T.D.; Lenhart, A.; Lozano-Fuentes, S.; Black, W.C. Vgsc-interacting proteins are genetically associated with pyrethroid resistance in Aedes aegypti. PLoS ONE 2019, 14, e0211497. [Google Scholar] [CrossRef]
- David, J.-P.; Faucon, F.; Chandor-Proust, A.; Poupardin, R.; Riaz, M.A.; Bonin, A.; Navratil, V.; Reynaud, S. Comparative analysis of response to selection with three insecticides in the dengue mosquito Aedes aegypti using mRNA sequencing. BMC Genom. 2014, 15, 174. [Google Scholar] [CrossRef] [PubMed]
- Faucon, F.; Dusfour, I.; Gaude, T.; Navratil, V.; Boyer, F.; Chandre, F.; Sirisopa, P.; Thanispong, K.; Juntarajumnong, W.; Poupardin, R.; et al. Identifying genomic changes associated with insecticide resistance in the dengue mosquito Aedes aegypti by deep targeted sequencing. Genome Res. 2015, 25, 1347–1359. [Google Scholar] [CrossRef]
- Li, T.; Cao, C.; Yang, T.; Zhang, L.; He, L.; Xi, Z.; Bian, G.; Liu, N. A G-protein-coupled receptor regulation pathway in cytochrome P450-mediated permethrin-resistance in mosquitoes, Culex quinquefasciatus. Sci. Rep. 2015, 5, 17772. [Google Scholar] [CrossRef]
- Bonizzoni, M.; Ochomo, E.; Dunn, W.A.; Britton, M.; Afrane, Y.; Zhou, G.; Hartsel, J.; Lee, M.-C.; Xu, J.; Githeko, A.; et al. RNA-seq analyses of changes in the Anopheles gambiae transcriptome associated with resistance to pyrethroids in Kenya: Identification of candidate-resistance genes and candidate-resistance SNPs. Parasites Vectors 2015, 8, 474. [Google Scholar] [CrossRef] [PubMed]
- Strode, C.; Wondji, C.S.; David, J.-P.; Hawkes, N.J.; Lumjuan, N.; Nelson, D.R.; Drane, D.R.; Karunaratne, S.P.; Hemingway, J.; Black, W.C.; et al. Genomic analysis of detoxification genes in the mosquito Aedes aegypti. Insect Biochem. Mol. Biol. 2008, 38, 113–123. [Google Scholar] [CrossRef]
- Aldridge, R.L.; Bloomquist, J.R.; A Gezan, S.; Linthicum, K.J.; E Kaufman, P. Application Site and Mosquito Age Influences Malathion- and Permethrin-Induced Mortality in Culex quinquefasciatus (Diptera: Culicidae). J. Med. Èntomol. 2017, 54, 1692–1698. [Google Scholar] [CrossRef]
- Aldridge, R.L.; Kaufman, P.E.; Bloomquist, J.R.; Gezan, S.A.; Linthicum, K.J. Permethrin and malathion LD 90 values for Culex quinquefasciatus vary with topical application site. Med. Veter Èntomol. 2017, 57, 306–311. [Google Scholar] [CrossRef]
- Vais, H.; Williamson, M.S.; Goodson, S.J.; Devonshire, A.L.; Warmke, J.W.; Usherwood, P.N.; Cohen, C.J. Activation of Drosophila Sodium Channels Promotes Modification by Deltamethrin. J. Gen. Physiol. 2000, 115, 305–318. [Google Scholar] [CrossRef]
- Behura, S.K.; Sarro, J.; Li, P.; Mysore, K.; Severson, D.W.; Emrich, S.J.; Duman-Scheel, M. High-throughput cis-regulatory element discovery in the vector mosquito Aedes aegypti. BMC Genom. 2016, 17, 341. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.L.; Yerbanga, R.S.; Lefèvre, T.; Ouedraogo, J.B.; Corces, V.G.; Gómez-Díaz, E. Chromatin changes in Anopheles gambiae induced by Plasmodium falciparum infection. Epigenet. Chromatin 2019, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- Seong, K.M.; Coates, B.S.; Kim, D.-H.; Hansen, A.K.; Pittendrigh, B.R. Differentially expressed microRNAs associated with changes of transcript levels in detoxification pathways and DDT-resistance in the Drosophila melanogaster strain 91-R. PLoS ONE 2018, 13, e0196518. [Google Scholar] [CrossRef] [PubMed]
- Epis, S.; Porretta, D.; Mastrantonio, V.; Comandatore, F.; Sassera, D.; Rossi, P.; Cafarchia, C.; Otranto, D.; Favia, G.; Genchi, C.; et al. ABC transporters are involved in defense against permethrin insecticide in the malaria vector Anopheles stephensi. Parasites Vectors 2014, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Terhzaz, S.; Cabrero, P.; Brinzer, R.A.; Halberg, K.A.; Dow, J.A.; Davies, S.-A. A novel role of Drosophila cytochrome P450-4e3 in permethrin insecticide tolerance. Insect Biochem. Mol. Biol. 2015, 67, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Li, T.; Feng, Y.; Liu, N. The function of two P450s, CYP9M10 and CYP6AA7, in the permethrin resistance of Culex quinquefasciatus. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Stevenson, B.J.; Bibby, J.; Pignatelli, P.; Muangnoicharoen, S.; O’Neill, P.M.; Lian, L.-Y.; Müller, P.; Nikou, D.; Steven, A.; Hemingway, J.; et al. Cytochrome P450 6M2 from the malaria vector Anopheles gambiae metabolizes pyrethroids: Sequential metabolism of deltamethrin revealed. Insect Biochem. Mol. Biol. 2011, 41, 492–502. [Google Scholar] [CrossRef]
- Chandor-Proust, A.; Bibby, J.; Régent-Kloeckner, M.; Roux, J.; Guittard-Crilat, E.; Poupardin, R.; Riaz, M.A.; Paine, M.; Dauphin-Villemant, C.; Reynaud, S.; et al. The central role of mosquito cytochrome P450 CYP6Zs in insecticide detoxification revealed by functional expression and structural modelling. Biochem. J. 2013, 455, 75–85. [Google Scholar] [CrossRef]
- Brogdon, W.G.; Barber, A.M. Microplate assay of glutathione s-transferase activity for resistance detection in single-mosquito triturates. Comp. Biochem. Physiol. Part B Comp. Biochem. 1990, 96, 339–342. [Google Scholar] [CrossRef]
- Brogdon, W.G.; McAllister, J.C. Simplification of adult mosquito bioassays through use of time-mortality determinations in glass bottles. J. Am. Mosq. Control. Assoc. 1998, 14, 159–164. [Google Scholar] [PubMed]
- Brogdon, W.G.; McAllister, J.C.; Vulule, J. Heme peroxidase activity measured in single mosquitoes identifies individuals expressing an elevated oxidase for insecticide resistance. J. Am. Mosq. Control. Assoc. 1997, 13, 233–237. [Google Scholar]
- Martins, A.J.; Linss, J.G.B.; Valle, D.; Peixoto, A.A.; Lins, R.M.M.D.A. Voltage-Gated Sodium Channel Polymorphism and Metabolic Resistance in Pyrethroid-Resistant Aedes aegypti from Brazil. Am. J. Trop. Med. Hyg. 2009, 81, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Heidari, R.; Devonshire, A.L.; Campbell, B.E.; Dorrian, S.J.; Oakeshott, J.G.; Russell, R.J. Hydrolysis of pyrethroids by carboxylesterases from Lucilia cuprina and Drosophila melanogaster with active sites modified by in vitro mutagenesis. Insect Biochem. Mol. Biol. 2005, 35, 597–609. [Google Scholar] [CrossRef]
- Nakamura, Y.; Sugihara, K.; Sone, T.; Isobe, M.; Ohta, S.; Kitamura, S. The in vitro metabolism of a pyrethroid insecticide, permethrin, and its hydrolysis products in rats. Toxicology 2007, 235, 176–184. [Google Scholar] [CrossRef]
- Lumjuan, N.; Wicheer, J.; Leelapat, P.; Choochote, W.; Somboon, P. Identification and Characterisation of Aedes aegypti Aldehyde Dehydrogenases Involved in Pyrethroid Metabolism. PLoS ONE 2014, 9, e102746. [Google Scholar] [CrossRef]
- Nwane, P.; Etang, J.; Chouaïbou, M.; Toto, J.C.; Koffi, A.; Mimpfoundi, R.; Simard, F. Multiple insecticide resistance mechanisms in Anopheles gambiae s.l. populations from Cameroon, Central Africa. Parasites Vectors 2013, 6, 41. [Google Scholar] [CrossRef]
- Vontas, J.; Grigoraki, L.; Morgan, J.; Tsakireli, D.; Fuseini, G.; Segura, L.; de Carvalho, J.N.; Nguema, R.; Weetman, D.; Slotman, M.A.; et al. Rapid selection of a pyrethroid metabolic enzyme CYP9K1 by operational malaria control activities. Proc. Natl. Acad. Sci. USA 2018, 115, 4619–4624. [Google Scholar] [CrossRef] [PubMed]
- Barnes, K.G.; Weedall, G.D.; Ndula, M.; Irving, H.; Mzihalowa, T.; Hemingway, J.; Wondji, C.S. Genomic Footprints of Selective Sweeps from Metabolic Resistance to Pyrethroids in African Malaria Vectors Are Driven by Scale up of Insecticide-Based Vector Control. PLoS Genet. 2017, 13, e1006539. [Google Scholar] [CrossRef]
- David, J.-P.; Ismail, H.M.; Chandor-Proust, A.; Paine, M.J.I. Role of cytochrome P450s in insecticide resistance: Impact on the control of mosquito-borne diseases and use of insecticides on Earth. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20120429. [Google Scholar] [CrossRef] [PubMed]
- Bamou, R.; Sonhafouo-Chiana, N.; Mavridis, K.; Tchuinkam, T.; Wondji, C.S.; Vontas, J.; Antonio-Nkondjio, C. Status of Insecticide Resistance and Its Mechanisms in Anopheles gambiae and Anopheles coluzzii Populations from Forest Settings in South Cameroon. Genes 2019, 10, 741. [Google Scholar] [CrossRef] [PubMed]
- Weedall, G.D.; Mugenzi, L.M.J.; Menze, B.D.; Tchouakui, M.; Ibrahim, S.S.; Amvongo-Adjia, N.; Irving, H.; Wondji, M.J.; Tchoupo, M.; Djouaka, R.; et al. A cytochrome P450 allele confers pyrethroid resistance on a major African malaria vector, reducing insecticide-treated bednet efficacy. Sci. Transl. Med. 2019, 11, eaat7386. [Google Scholar] [CrossRef]
- Ingham, V.A.; Wagstaff, S.; Ranson, H. Transcriptomic meta-signatures identified in Anopheles gambiae populations reveal previously undetected insecticide resistance mechanisms. Nat. Commun. 2018, 9, 1–11. [Google Scholar] [CrossRef]
- Riveron, J.M.; Ibrahim, S.S.; Chanda, E.; Mzilahowa, T.; Cuamba, N.; Irving, H.; Barnes, K.G.; Ndula, M.; Wondji, C.S. The highly polymorphic CYP6M7 cytochrome P450 gene partners with the directionally selected CYP6P9a and CYP6P9b genes to expand the pyrethroid resistance front in the malaria vector Anopheles funestus in Africa. BMC Genom. 2014, 15, 1–19. [Google Scholar] [CrossRef]
- Cattel, J.; Faucon, F.; Le Péron, B.; Sherpa, S.; Monchal, M.; Grillet, L.; Gaude, T.; Laporte, F.; Dusfour, I.; Reynaud, S.; et al. Combining genetic crosses and pool targeted DNA-seq for untangling genomic variations associated with resistance to multiple insecticides in the mosquito Aedes aegypti. Evol. Appl. 2019, 13, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Riveron, J.M.; Irving, H.; Ndula, M.; Barnes, K.G.; Ibrahim, S.S.; Paine, M.J.; Wondji, C.S. Directionally selected cytochrome P450 alleles are driving the spread of pyrethroid resistance in the major malaria vector Anopheles funestus. Proc. Natl. Acad. Sci. USA 2013, 110, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.M.; Good, R.T.; Appleton, B.; Sherrard, J.; Raymant, G.C.; Bogwitz, M.R.; Martin, J.; Daborn, P.J.; Goddard, M.E.; Batterham, P.; et al. Copy Number Variation and Transposable Elements Feature in Recent, Ongoing Adaptation at the Cyp6g1 Locus. PLoS Genet. 2010, 6, e1000998. [Google Scholar] [CrossRef] [PubMed]
- Soper, F.L. The Elimination of Urban Yellow Fever in the Americas Through the Eradication of Aedes aegypti. Am. J. Public Health Nations Health 1963, 53, 7–16. [Google Scholar] [CrossRef]
- Symes, C.M.; Thompson, R.C.M.; Busvine, J.R. Insect Control in Public Health; Elsevier: New York, NY, USA, 1962. [Google Scholar]
- Faucon, F.; Gaude, T.; Dusfour, I.; Navratil, V.; Corbel, V.; Juntarajumnong, W.; Girod, R.; Poupardin, R.; Boyer, F.; Reynaud, S.; et al. In the hunt for genomic markers of metabolic resistance to pyrethroids in the mosquito Aedes aegypti: An integrated next-generation sequencing approach. PLoS Negl. Trop. Dis. 2017, 11, e0005526. [Google Scholar] [CrossRef]
- Menze, B.D.; Kouamo, M.F.; Wondji, M.J.; Tchapga, W.; Tchoupo, M.; Kusimo, M.O.; Mouhamadou, C.S.; Riveron, J.M.; Wondji, C.S. An Experimental Hut Evaluation of PBO-Based and Pyrethroid-Only Nets against the Malaria Vector Anopheles funestus Reveals a Loss of Bed Nets Efficacy Associated with GSTe2 Metabolic Resistance. Genes 2020, 11, 143. [Google Scholar] [CrossRef]
- Mugenzi, L.M.J.; Menze, B.D.; Tchouakui, M.; Wondji, M.J.; Irving, H.; Tchoupo, M.; Hearn, J.; Weedall, G.D.; Riveron, J.M.; Wondji, C.S. Cis-regulatory CYP6P9b P450 variants associated with loss of insecticide-treated bed net efficacy against Anopheles funestus. Nat. Commun. 2019, 10, 4652. [Google Scholar] [CrossRef]
- Smith, L.B.; Tyagi, R.; Kasai, S.; Scott, J.G. CYP-mediated permethrin resistance in Aedes aegypti and evidence for trans-regulation. PLoS Negl. Trop. Dis. 2018, 12, e0006933. [Google Scholar] [CrossRef]
- Djouaka, R.F.; Bakare, A.A.; Coulibaly, O.N.; Akogbeto, M.C.; Ranson, H.; Hemingway, J.; Strode, C. Expression of the cytochrome P450s, CYP6P3 and CYP6M2 are significantly elevated in multiple pyrethroid resistant populations of Anopheles gambiae s.s. from Southern Benin and Nigeria. BMC Genom. 2008, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, K.; Højland, D.H.; Asp, T.; Kristensen, M. Transcriptome Analysis of an Insecticide Resistant Housefly Strain: Insights about SNPs and Regulatory Elements in Cytochrome P450 Genes. PLoS ONE 2016, 11, e0151434. [Google Scholar] [CrossRef][Green Version]
- Saavedra-Rodriguez, K.; Campbell, C.L.; Lenhart, A.; Penilla, P.; Lozano-Fuentes, S.; Black, W.C. Exome-wide association of deltamethrin resistance in Aedes aegypti from Mexico. Insect Mol. Biol. 2019, 28, 591–604. [Google Scholar] [CrossRef]
- Itokawa, K.; Komagata, O.; Kasai, S.; Kawada, H.; Mwatele, C.; O Dida, G.; Njenga, S.M.; Mwandawiro, C.; Tomita, T. Global spread and genetic variants of the two CYP9M10 haplotype forms associated with insecticide resistance in Culex quinquefasciatus Say. Hered 2013, 111, 216–226. [Google Scholar] [CrossRef]
- Liu, N.; Li, T.; Reid, W.R.; Yang, T.; Zhang, L. Multiple Cytochrome P450 Genes: Their Constitutive Overexpression and Permethrin Induction in Insecticide Resistant Mosquitoes, Culex quinquefasciatus. PLoS ONE 2011, 6, e23403. [Google Scholar] [CrossRef] [PubMed]
- Tchouakui, M.; Miranda, J.R.; Mugenzi, L.M.J.; Djonabaye, D.; Wondji, M.J.; Tchoupo, M.; Tchapga, W.; Njiokou, F.; Wondji, C.S. Cytochrome P450 metabolic resistance (CYP6P9a) to pyrethroids imposes a fitness cost in the major African malaria vector Anopheles funestus. Hered 2020, 124, 621–632. [Google Scholar] [CrossRef]
- Tchouakui, M.; Riveron, J.M.; Djonabaye, D.; Tchapga, W.; Irving, H.; Takam, P.S.; Njiokou, F.; Wondji, C.S. Fitness Costs of the Glutathione S-Transferase Epsilon 2 (L119F-GSTe2) Mediated Metabolic Resistance to Insecticides in the Major African Malaria Vector Anopheles Funestus. Genes 2018, 9, 645. [Google Scholar] [CrossRef] [PubMed]
- Amichot, M.; Tares, S.; Brun-Barale, A.; Arthaud, L.; Bride, J.-M.; Berge, J.-B. Point mutations associated with insecticide resistance in the Drosophila cytochrome P450 Cyp6a2 enable DDT metabolism. JBIC J. Biol. Inorg. Chem. 2004, 271, 1250–1257. [Google Scholar] [CrossRef]
- Battlay, P.; Leblanc, P.B.; Green, L.; Garud, N.R.; Schmidt, J.M.; Fournier-Level, A.; Robin, C. Structural Variants and Selective Sweep Foci Contribute to Insecticide Resistance in theDrosophilaGenetic Reference Panel. Genes Genomes Genet. 2018, 8, 3489–3497. [Google Scholar] [CrossRef]
- Ibrahim, S.S.; Mukhtar, M.M.; Irving, H.; Riveron, J.M.; Fadel, A.N.; Tchapga, W.; Hearn, J.; Muhammad, A.; Sarkinfada, F.; Wondji, C.S. Exploring the Mechanisms of Multiple Insecticide Resistance in a Highly Plasmodium-Infected Malaria Vector Anopheles funestus Sensu Stricto from Sahel of Northern Nigeria. Genes 2020, 11, 454. [Google Scholar] [CrossRef]
- Riveron, J.M.; Yunta, C.; Ibrahim, S.S.; Djouaka, R.; Irving, H.; Menze, B.D.; Ismail, H.M.; Hemingway, J.; Ranson, H.; Albert, A.; et al. A single mutation in the GSTe2 gene allows tracking of metabolically based insecticide resistance in a major malaria vector. Genome Biol. 2014, 15, R27. [Google Scholar] [CrossRef] [PubMed]
- Tchigossou, G.; Djouaka, R.; Akoton, R.; Riveron, J.M.; Irving, H.; Atoyebi, S.; Moutairou, K.; Yessoufou, A.; Wondji, C.S. Molecular basis of permethrin and DDT resistance in an Anopheles funestus population from Benin. Parasites Vectors 2018, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wilding, C.S.; Weetman, D.; Rippon, E.J.; Steen, K.; Mawejje, H.D.; Barsukov, I.; Donnelly, M.J. Parallel evolution or purifying selection, not introgression, explains similarity in the pyrethroid detoxification linked GSTE4 of Anopheles gambiae and An. arabiensis. Mol. Genet. Genom. 2014, 290, 201–215. [Google Scholar] [CrossRef]
- Wongtrakul, J.; Pongjaroenkit, S.; Leelapat, P.; Nachaiwieng, W.; Prapanthadara, L.-A.; Ketterman, A.J. Expression and Characterization of Three New Glutathione Transferases, an Epsilon (AcGSTE2- 2), Omega (AcGSTO1- 1), and Theta (AcGSTT1- 1) From Anopheles cracens (Diptera: Culicidae), a Major Thai Malaria Vector. J. Med. Èntomol. 2010, 47, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Lumjuan, N.; McCarroll, L.; Prapanthadara, L.-A.; Hemingway, J.; Ranson, H. Elevated activity of an Epsilon class glutathione transferase confers DDT resistance in the dengue vector, Aedes aegypti. Insect Biochem. Mol. Biol. 2005, 35, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Vontas, J.G.; Small, G.J.; Hemingway, J. Glutathione S-transferases as antioxidant defence agents confer pyrethroid resistance in Nilaparvata lugens. Biochem. J. 2001, 357, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Kostaropoulos, I.; Papadopoulos, A.I.; Metaxakis, A.; Boukouvala, E.; Papadopoulou-Mourkidou, E. Glutathione S-transferase in the defence against pyrethroids in insects. Insect Biochem. Mol. Biol. 2001, 31, 313–319. [Google Scholar] [CrossRef]
- Chenprakhon, P.; Wongnate, T.; Chaiyen, P. Monooxygenation of aromatic compounds by flavin-dependent monooxygenases. Protein Sci. 2018, 28, 8–29. [Google Scholar] [CrossRef]
- Lilly, D.G.; Latham, S.L.; Webb, C.E.; Doggett, S.L. Cuticle Thickening in a Pyrethroid-Resistant Strain of the Common Bed Bug, Cimex lectularius L. (Hemiptera: Cimicidae). PLoS ONE 2016, 11, e0153302. [Google Scholar] [CrossRef]
- Mamidala, P.; Wijeratne, A.J.; Wijeratne, S.; Kornacker, K.; Sudhamalla, B.; Rivera-Vega, L.J.; Hoelmer, A.; Meulia, T.; Jones, S.C.; Mittapalli, O. RNA-Seq and molecular docking reveal multi-level pesticide resistance in the bed bug. BMC Genom. 2012, 13, 6. [Google Scholar] [CrossRef]
- Samantsidis, G.-R.; Panteleri, R.; Denecke, S.; Kounadi, S.; Christou, I.; Nauen, R.; Douris, V.; Vontas, J. ‘What I cannot create, I do not understand’: Functionally validated synergism of metabolic and target site insecticide resistance. Proc. R. Soc. B Boil. Sci. 2020, 287, 20200838. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.A.A. Resistance to insecticides and synergism by enzyme inhibitors in Aedes albopictus from Punjab, Pakistan. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Vijayan, V.A.; Kumar, B.Y.S.; Ganesh, K.N.; Urmila, J.; Fakoorziba, M.R.; Makkapati, A.K. Efficacy of piperonyl butoxide (PBO) as a synergist with deltamethrin on five species of mosquitoes. J. Commun. Dis. 2007, 39, 159–163. [Google Scholar] [PubMed]
- Queiroz, M.C.V.; Sato, M.E. Pyrethroid resistance in Phytoseiulus macropilis (Acari: Phytoseiidae): Cross-resistance, stability and effect of synergists. Exp. Appl. Acarol. 2015, 68, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Pasay, C.; Arlian, L.; Morgan, M.; Gunning, R.; Rossiter, L.; Holt, D.; Walton, S.; Beckham, S.; McCarthy, J. The Effect of Insecticide Synergists on the Response of Scabies Mites to Pyrethroid Acaricides. PLoS Negl. Trop. Dis. 2009, 3, e354. [Google Scholar] [CrossRef] [PubMed]
- Thiaw, O.; Doucouré, S.; Sougoufara, S.; Bouganali, C.; Konaté, L.; Diagne, N.; Faye, O.; Sokhna, C. Investigating insecticide resistance and knock-down resistance (kdr) mutation in Dielmo, Senegal, an area under long lasting insecticidal-treated nets universal coverage for 10 years. Malar. J. 2018, 17, 1–11. [Google Scholar] [CrossRef]
- Kona, M.P.; Kamaraju, R.; Donnelly, M.J.; Bhatt, R.M.; Nanda, N.; Chourasia, M.K.; Swain, D.K.; Suman, S.; Uragayala, S.; Kleinschmidt, I.; et al. Characterization and monitoring of deltamethrin-resistance in Anopheles culicifacies in the presence of a long-lasting insecticide-treated net intervention. Malar. J. 2018, 17, 414. [Google Scholar] [CrossRef] [PubMed]
- Cisse, M.B.M.; Sangare, D.; Oxborough, R.M.; Dicko, A.; Dengela, D.; Sadou, A.; Mihigo, J.; George, K.; Norris, L.; Fornadel, C. A village level cluster-randomized entomological evaluation of combination long-lasting insecticidal nets containing pyrethroid plus PBO synergist in Southern Mali. Malar. J. 2017, 16, 477. [Google Scholar] [CrossRef]
- Staedke, S.G.; Gonahasa, S.; Dorsey, G.; Kamya, M.R.; Maiteki-Sebuguzi, C.; Lynd, A.; Katureebe, A.; Kyohere, M.; Mutungi, P.; Kigozi, S.P.; et al. Effect of long-lasting insecticidal nets with and without piperonyl butoxide on malaria indicators in Uganda (LLINEUP): A pragmatic, cluster-randomised trial embedded in a national LLIN distribution campaign. Lancet 2020, 395, 1292–1303. [Google Scholar] [CrossRef]
- Groves, R.L.; A Dame, D.; Meek, C.L.; Meisch, M.V. Efficacy of three synthetic pyrethroids against three mosquito species in Arkansas and Louisiana. J. Am. Mosq. Control. Assoc. 1997, 13, 184–188. [Google Scholar] [PubMed]
- Bariami, V.; Jones, C.M.; Poupardin, R.; Vontas, J.; Ranson, H. Gene Amplification, ABC Transporters and Cytochrome P450s: Unraveling the Molecular Basis of Pyrethroid Resistance in the Dengue Vector, Aedes aegypti. PLoS Negl. Trop. Dis. 2012, 6, e1692. [Google Scholar] [CrossRef]
- Epis, S.; Porretta, D.; Mastrantonio, V.; Urbanelli, S.; Sassera, D.; De Marco, L.; Mereghetti, V.; Montagna, M.; Ricci, I.; Favia, G.; et al. Temporal dynamics of the ABC transporter response to insecticide treatment: Insights from the malaria vector Anopheles stephensi. Sci. Rep. 2015, 4, 7435. [Google Scholar] [CrossRef] [PubMed]
- Mangia, C.; Vismarra, A.; Genchi, M.; Epis, S.; Bandi, C.; Grandi, G.; Bell-Sakyi, L.; Otranto, D.; Passeri, B.; Kramer, L. Exposure to amitraz, fipronil and permethrin affects cell viability and ABC transporter gene expression in an Ixodes ricinus cell line. Parasites Vectors 2018, 11, 1–9. [Google Scholar] [CrossRef]
- Mastrantonio, V.; Ferrari, M.; Negri, A.; Sturmo, T.; Favia, G.; Porretta, D.; Epis, S.; Urbanelli, S. Insecticide Exposure Triggers a Modulated Expression of ABC Transporter Genes in Larvae of Anopheles gambiae s.s. Insects 2019, 10, 66. [Google Scholar] [CrossRef] [PubMed]
- Negri, A.; Ferrari, M.; Nodari, R.; Coppa, E.; Mastrantonio, V.; Zanzani, S.; Porretta, D.; Bandi, C.; Urbanelli, S.; Epis, S. Gene silencing through RNAi and antisense Vivo-Morpholino increases the efficacy of pyrethroids on larvae of Anopheles stephensi. Malar. J. 2019, 18, 1–12. [Google Scholar] [CrossRef]
- Pignatelli, P.; Ingham, V.A.; Balabanidou, V.; Vontas, J.; Lycett, G.; Ranson, H. The Anopheles gambiae ATP-binding cassette transporter family: Phylogenetic analysis and tissue localization provide clues on function and role in insecticide resistance. Insect Mol. Biol. 2017, 27, 110–122. [Google Scholar] [CrossRef]
- Zuber, R.; Norum, M.; Wang, Y.; Oehl, K.; Gehring, N.; Accardi, D.; Bartozsewski, S.; Berger, J.; Flötenmeyer, M.; Moussian, B. The ABC transporter Snu and the extracellular protein Snsl cooperate in the formation of the lipid-based inward and outward barrier in the skin of Drosophila. Eur. J. Cell Biol. 2018, 97, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Fabian, M.R.; Sonenberg, N. The mechanics of miRNA-mediated gene silencing: A look under the hood of miRISC. Nat. Struct. Mol. Biol. 2012, 19, 586–593. [Google Scholar] [CrossRef]
- Hong, S.; Guo, Q.; Wang, W.; Hu, S.; Fang, F.; Lv, Y.; Yu, J.; Zou, F.; Lei, Z.; Ma, K.; et al. Identification of differentially expressed microRNAs in Culex pipiens and their potential roles in pyrethroid resistance. Insect Biochem. Mol. Biol. 2014, 55, 39–50. [Google Scholar] [CrossRef]
- Sun, X.H.; Xu, N.; Xu, Y.; Zhou, D.; Sun, Y.; Wang, W.J.; Ma, L.; Zhu, C.L.; Shen, B. A novel miRNA, miR-13664, targetsCpCYP314A1to regulate deltamethrin resistance inCulex pipiens pallens. Parasitology 2019, 146, 197–205. [Google Scholar] [CrossRef]
- Tian, M.; Liu, B.; Hu, H.; Li, X.; Guo, Q.; Zou, F.; Liu, X.; Hu, M.; Guo, J.; Ma, L.; et al. MiR-285 targets P450 (CYP6N23) to regulate pyrethroid resistance in Culex pipiens pallens. Parasitol. Res. 2016, 115, 4511–4517. [Google Scholar] [CrossRef]
- Okamura, K.; Ladewig, E.; Zhou, L.; Lai, E.C. Functional small RNAs are generated from select miRNA hairpin loops in flies and mammals. Genes Dev. 2013, 27, 778–792. [Google Scholar] [CrossRef] [PubMed]
- Kubik, T.D.; Snell, T.K.; Saavedra-Rodriguez, K.; Wilusz, J.; Anderson, J.R.; Lozano-Fuentes, S.; Black, W.C.; Campbell, C.L. Aedes aegypti miRNA-33 Modulates Permethrin-Induced Toxicity by Regulating VGSC Transcripts Scientific Reports. 2020; in press. [Google Scholar]
- Muraro, N.I.; Weston, A.J.; Gerber, A.P.; Luschnig, S.; Moffat, K.G.; Baines, R.A. Pumilio Binds para mRNA and Requires Nanos and Brat to Regulate Sodium Current in Drosophila Motoneurons. J. Neurosci. 2008, 28, 2099–2109. [Google Scholar] [CrossRef]
- Sehlmeyer, S.; Wang, L.; Langel, D.; Heckel, D.G.; Mohagheghi, H.; Petschenka, G.; Ober, D. Flavin-Dependent Monooxygenases as a Detoxification Mechanism in Insects: New Insights from the Arctiids (Lepidoptera). PLoS ONE 2010, 5, e10435. [Google Scholar] [CrossRef]
- Li, T.; Liu, L.; Zhang, L.; Liu, N. Role of G-protein-coupled Receptor-related Genes in Insecticide Resistance of the Mosquito, Culex quinquefasciatus. Sci. Rep. 2015, 4, 6474. [Google Scholar] [CrossRef]
- Fung, S.; Wang, F.; Chase, M.; Godt, R.; Hartenstein, V. Expression profile of the cadherin family in the developingDrosophila brain. J. Comp. Neurol. 2007, 506, 469–488. [Google Scholar] [CrossRef]
- Tsarouhas, V.; Yao, L.; Samakovlis, C. Src kinases and ERK activate distinct responses to Stitcher receptor tyrosine kinase signaling during wound healing in Drosophila. J. Cell Sci. 2014, 127, 1829–1839. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Tsarouhas, V.; Xylourgidis, N.; Sabri, N.; Tiklová, K.; Nautiyal, N.; Gallio, M.; Samakovlis, C. The tyrosine kinase Stitcher activates Grainy head and epidermal wound healing in Drosophila. Nat. Cell Biol. 2009, 11, 890–895. [Google Scholar] [CrossRef] [PubMed]
- Nevil, M.; Bondra, E.R.; Schulz, K.N.; Kaplan, T.; Harrison, M.M. Stable Binding of the Conserved Transcription Factor Grainy Head to its Target Genes Throughout Drosophila melanogaster Development. Genetics 2017, 205, 605–620. [Google Scholar] [CrossRef]
- Shieh, B.-H.; Parker, L.; Popescu, D. Protein kinase C (PKC) isoforms in Drosophila. J. Biochem. 2002, 132, 523–527. [Google Scholar] [CrossRef]
- Wang, S.; Samakovlis, C. Grainy Head and Its Target Genes in Epithelial Morphogenesis and Wound Healing. Curr. Top. Dev. Biol. 2012, 98, 35–63. [Google Scholar] [CrossRef] [PubMed]
- Riveron, J.M.; Ibrahim, S.S.; Mulamba, C.; Djouaka, R.; Irving, H.; Wondji, M.J.; Ishak, I.H.; Wondji, C.S. Genome-Wide Transcription and Functional Analyses Reveal Heterogeneous Molecular Mechanisms Driving Pyrethroids Resistance in the Major Malaria Vector Anopheles funestus Across Africa. Genes Genomes Genet. 2017, 7, 1819–1832. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.J.; Mundis, S.J.; Aguirre, A.; Lippi, C.A.; Beltrán, E.; Heras, F.; Sanchez, V.; Borbor-Cordova, M.J.; Sippy, R.; Stewart-Ibarra, A.M.; et al. Seasonal and geographic variation in insecticide resistance in Aedes aegypti in southern Ecuador. PLoS Negl. Trop. Dis. 2019, 13, e0007448. [Google Scholar] [CrossRef]
- García-Franco, F.; Black, W.C.; Beaty, B.J.; Garcia-Rejon, J.; Fernandez-Salas, I.; Lozano-Fuentes, S.; Muńoz, M.D.L. Large genetic distances among Aedes aegypti populations along the South Pacific coast of Mexico. Am. J. Trop. Med. Hyg. 2002, 66, 594–598. [Google Scholar] [CrossRef][Green Version]
- Gorrochotegui-Escalante, N.; Lozano-Fuentes, S.; Black, W.C.; Fernandez-Salas, L.; Garcia-Rejon, J.; Gomez-Machorro, C.; Beaty, B.J.; A Farfan-Ale, J.; Munoz, M.D.L. Breeding structure of Aedes aegypti populations in Mexico varies by region. Am. J. Trop. Med. Hyg. 2002, 66, 213–222. [Google Scholar] [CrossRef]
- Lozano-Fuentes, S.; Fernandez-Salas, I.; Muñoz, M.D.L.; García-Rejón, J.E.; Olson, K.E.; Beaty, B.J.; Black, W.C. The Neovolcanic Axis Is a Barrier to Gene Flow among Aedes aegypti Populations in Mexico That Differ in Vector Competence for Dengue 2 Virus. PLoS Negl. Trop. Dis. 2009, 3, e468. [Google Scholar] [CrossRef] [PubMed]
- Saavedra-Rodriguez, K.; Ponce-Garcia, G.; Loroño-Pino, M.A.; García-Rejón, J.E.; Beaty, M.; Eisen, L.; Flores-Suarez, A.; Reyes-Solis, G.; Lozano-Fuentes, S.; Black, W.C.; et al. Local Evolution of Pyrethroid Resistance Offsets Gene Flow Among Aedes aegypti Collections in Yucatan State, Mexico. Am. J. Trop. Med. Hyg. 2015, 92, 201–209. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fine, B.C. Pattern of Pyrethrin-Resistance in Houseflies. Nat. Cell Biol. 1961, 191, 884–885. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie-Impoinvil, L.; Weedall, G.D.; Lol, J.C.; Pinto, J.; Vizcaino, L.; Dzuris, N.; Riveron, J.; Padilla, N.; Wondji, C.; Lenhart, A. Contrasting patterns of gene expression indicate differing pyrethroid resistance mechanisms across the range of the New World malaria vector Anopheles albimanus. PLoS ONE 2019, 14, e0210586. [Google Scholar] [CrossRef]
- Jones, C.M.; A Haji, K.; O Khatib, B.; Bagi, J.; Mcha, J.; Devine, G.J.; Daley, M.; Kabula, B.; Ali, A.S.; Majambere, S.; et al. The dynamics of pyrethroid resistance in Anopheles arabiensis from Zanzibar and an assessment of the underlying genetic basis. Parasites Vectors 2013, 6, 343. [Google Scholar] [CrossRef]
- Witzig, C.; Parry, M.A.J.; Morgan, J.C.; Irving, H.R.; Steven, A.; Cuamba, N.; Kerahhinzoumbe, C.; Ranson, H.; Wondji, C.S. Genetic mapping identifies a major locus spanning P450 clusters associated with pyrethroid resistance in kdr-free Anopheles arabiensis from Chad. Hered 2013, 110, 389–397. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nardini, L.; Christian, R.N.; Coetzer, N.; Ranson, H.; Coetzee, M.; Koekemoer, L.L. Detoxification enzymes associated with insecticide resistance in laboratory strains of Anopheles arabiensis of different geographic origin. Parasites Vectors 2012, 5, 113. [Google Scholar] [CrossRef]
- Ibrahim, S.S.; Fadel, A.N.; Tchouakui, M.; Terence, E.; Wondji, M.J.; Tchoupo, M.; Kérah-Hinzoumbé, C.; Wanji, S.; Wondji, C.S. High insecticide resistance in the major malaria vector Anopheles coluzzii in Chad Republic. Infect. Dis. Poverty 2019, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.S.; Mukhtar, M.M.; Irving, H.; Labbo, R.; Kusimo, M.O.; Mahamadou, I.; Wondji, C.S. High Plasmodium infection and multiple insecticide resistance in a major malaria vector Anopheles coluzzii from Sahel of Niger Republic. Malar. J. 2019, 18, 181. [Google Scholar] [CrossRef] [PubMed]
- Main, B.J.; Everitt, A.; Cornel, A.J.; Hormozdiari, F.; Lanzaro, G.C. Genetic variation associated with increased insecticide resistance in the malaria mosquito, Anopheles coluzzii. Parasites Vectors 2018, 11, 225. [Google Scholar] [CrossRef]
- Djouaka, R.; Riveron, J.M.; Yessoufou, A.; Tchigossou, G.; Akoton, R.; Irving, H.; Djegbe, I.; Moutairou, K.; Adeoti, R.; Tamò, M.; et al. Multiple insecticide resistance in an infected population of the malaria vector Anopheles funestus in Benin. Parasites Vectors 2016, 9, 1–12. [Google Scholar] [CrossRef]
- Mulamba, C.; Riveron, J.M.; Ibrahim, S.S.; Irving, H.; Barnes, K.G.; Mukwaya, L.G.; Birungi, J.; Wondji, C.S. Widespread Pyrethroid and DDT Resistance in the Major Malaria Vector Anopheles funestus in East Africa Is Driven by Metabolic Resistance Mechanisms. PLoS ONE 2014, 9, e110058. [Google Scholar] [CrossRef]
- Rakotondranaivo, T.; Randriamanarivo, S.F.; Tanjona, M.R.; Vigan-Womas, I.; Randrianarivelojosia, M.; Ndiath, M.O. Evidence of Insecticide Resistance to Pyrethroids and Bendiocarb in Anopheles funestus from Tsararano, Marovoay District, Madagascar. BioMed. Res. Int. 2018, 2018, 1–9. [Google Scholar] [CrossRef]
- Barnes, K.G.; Irving, H.; Chiumia, M.; Mzilahowa, T.; Coleman, M.; Hemingway, J.; Wondji, C.S. Restriction to gene flow is associated with changes in the molecular basis of pyrethroid resistance in the malaria vector Anopheles funestus. Proc. Natl. Acad. Sci. USA 2017, 114, 286–291. [Google Scholar] [CrossRef]
- Irving, H.R.; Riveron, J.M.; Ibrahim, S.S.; Lobo, N.F.; Wondji, C.S. Positional cloning of rp2 QTL associates the P450 genes CYP6Z1, CYP6Z3 and CYP6M7 with pyrethroid resistance in the malaria vector Anopheles funestus. Hered 2012, 109, 383–392. [Google Scholar] [CrossRef]
- Nardini, L.; Hunt, R.H.; Dahan-Moss, Y.L.; Christie, N.; Christian, R.N.; Coetzee, M.; Koekemoer, L.L. Malaria vectors in the Democratic Republic of the Congo: The mechanisms that confer insecticide resistance in Anopheles gambiae and Anopheles funestus. Malar. J. 2017, 16, 1–15. [Google Scholar] [CrossRef]
- Samb, B.; Konate, L.; Irving, H.; Riveron, J.M.; Dia, I.; Faye, O.; Wondji, C.S. Investigating molecular basis of lambda-cyhalothrin resistance in an Anopheles funestus population from Senegal. Parasites Vectors 2016, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Djouaka, R.; Irving, H.; Tukur, Z.; Wondji, C.S. Exploring Mechanisms of Multiple Insecticide Resistance in a Population of the Malaria Vector Anopheles funestus in Benin. PLoS ONE 2011, 6, e27760. [Google Scholar] [CrossRef]
- Ibrahim, S.S.; Ndula, M.; Riveron, J.M.; Irving, H.; Wondji, C.S. The P450 CYP6Z1 confers carbamate/pyrethroid cross-resistance in a major African malaria vector beside a novel carbamate-insensitive N485I acetylcholinesterase-1 mutation. Mol. Ecol. 2016, 25, 3436–3452. [Google Scholar] [CrossRef]
- Morgan, J.C.; Irving, H.; Okedi, L.M.; Steven, A.; Wondji, C.S. Pyrethroid Resistance in an Anopheles funestus Population from Uganda. PLoS ONE 2010, 5, e11872. [Google Scholar] [CrossRef] [PubMed]
- Riveron, J.M.; Chiumia, M.; Menze, B.D.; Barnes, K.G.; Irving, H.; Ibrahim, S.S.; Weedall, G.D.; Mzilahowa, T.; Wondji, C.S. Rise of multiple insecticide resistance in Anopheles funestus in Malawi: A major concern for malaria vector control. Malar. J. 2015, 14, 1–9. [Google Scholar] [CrossRef]
- Riveron, J.M.; Huijben, S.; Tchapga, W.; Tchouakui, M.; Wondji, M.J.; Tchoupo, M.; Irving, H.; Cuamba, N.; Maquina, M.; Paaijmans, K.; et al. Escalation of Pyrethroid Resistance in the Malaria Vector Anopheles funestus Induces a Loss of Efficacy of Piperonyl Butoxide–Based Insecticide-Treated Nets in Mozambique. J. Infect. Dis. 2019, 220, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Kamgang, B.; Tchapga, W.; Ngoagouni, C.; Sangbakembi-Ngounou, C.; Wondji, M.; Riveron, J.M.; Wondji, C.S. Exploring insecticide resistance mechanisms in three major malaria vectors from Bangui in Central African Republic. Pathog. Glob. Health 2018, 112, 349–359. [Google Scholar] [CrossRef]
- Namountougou, M.; Simard, F.; Baldet, T.; Diabaté, A.; Ouédraogo, J.B.; Martin, T.; Dabiré, R.K. Multiple Insecticide Resistance in Anopheles gambiae s.l. Populations from Burkina Faso, West Africa. PLoS ONE 2012, 7, e48412. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.; Mavridis, K.; Vontas, J.; Rodrigues, A.; Osório, H.C. Monitoring and molecular profiling of contemporary insecticide resistance status of malaria vectors in Guinea–Bissau. Acta Trop. 2020, 206, 105440. [Google Scholar] [CrossRef] [PubMed]
- Stica, C.; Jeffries, C.L.; Irish, S.R.; Barry, Y.; Camara, D.; Yansane, I.; Kristan, M.; Walker, T.; Messenger, L.A. Characterizing the molecular and metabolic mechanisms of insecticide resistance in Anopheles gambiae in Faranah, Guinea. Malar. J. 2019, 18, 1–15. [Google Scholar] [CrossRef]
- Tene, B.F.; Poupardin, R.; Costantini, C.; Awono-Ambene, P.; Wondji, C.S.; Ranson, H.; Antonio-Nkondjio, C. Resistance to DDT in an Urban Setting: Common Mechanisms Implicated in Both M and S Forms of Anopheles gambiae in the City of Yaoundé Cameroon. PLoS ONE 2013, 8, e61408. [Google Scholar] [CrossRef]
- Isaacs, A.T.; Mawejje, H.D.; Tomlinson, S.; Rigden, D.J.; Donnelly, M.J. Genome-wide transcriptional analyses in Anopheles mosquitoes reveal an unexpected association between salivary gland gene expression and insecticide resistance. BMC Genom. 2018, 19, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Edi, C.V.; Djogbénou, L.; Jenkins, A.M.; Regna, K.; Muskavitch, M.A.T.; Poupardin, R.; Jones, C.M.; Essandoh, J.; Kétoh, G.K.; Paine, M.J.I.; et al. CYP6 P450 Enzymes and ACE-1 Duplication Produce Extreme and Multiple Insecticide Resistance in the Malaria Mosquito Anopheles gambiae. PLoS Genet. 2014, 10, e1004236. [Google Scholar] [CrossRef]
- Kwiatkowska, R.M.; Platt, N.; Poupardin, R.; Irving, H.; Dabire, R.K.; Mitchell, S.; Jones, C.M.; Diabaté, A.; Ranson, H.; Wondji, C.S. Dissecting the mechanisms responsible for the multiple insecticide resistance phenotype in Anopheles gambiae s.s., M form, from Vallée du Kou, Burkina Faso. Gene 2013, 519, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Koffi, A.A.; Alou, L.P.A.; Kabran, J.-P.K.; N’Guessan, R.; Pennetier, C. Re-Visiting Insecticide Resistance Status in Anopheles gambiae from Côte d’Ivoire: A Nation-Wide Informative Survey. PLoS ONE 2013, 8, e82387. [Google Scholar] [CrossRef]
- Koffi, A.A.; Alou, L.P.A.; A Adja, M.; Chandre, F.; Pennetier, C. Insecticide resistance status of Anopheles gambiae s.s population from M’Bé: A WHOPES-labelled experimental hut station, 10 years after the political crisis in Côte d’Ivoire. Malar. J. 2013, 12, 151. [Google Scholar] [CrossRef] [PubMed]
- Koffi, A.A.; Alou, L.P.A.; Adja, M.A.; Koné, M.; Chandre, F.; N’Guessan, R. Update on resistance status of Anopheles gambiae s.s. to conventional insecticides at a previous WHOPES field site, "Yaokoffikro", 6 years after the political crisis in Côte d’Ivoire. Parasites Vectors 2012, 5, 68. [Google Scholar] [CrossRef]
- Gorouhi, M.A.; Oshaghi, M.A.; Vatandoost, H.; Enayati, A.A.; Raeisi, A.; Abai, M.R.; Salim-Abadie, Y.; Hanafi-Bojd, A.A.; Paksa, A.; Nikpoor, F. Biochemical Basis of Cyfluthrin and DDT Resistance in Anopheles stephensi (Diptera: Culicidae) in Malarious Area of Iran. J. Arthropod Borne Dis. 2018, 12, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Sanil, D.; Shetty, V.; Shetty, N.J. Differential expression of glutathione s-transferase enzyme in different life stages of various insecticide-resistant strains of Anopheles stephensi: A malaria vector. J. Vector Borne Dis. 2014, 51, 97–105. [Google Scholar] [PubMed]
- Zhu, G.; Zhou, H.; Li, J.; Tang, J.; Bai, L.; Wang, W.; Gu, Y.; Liu, Y.; Lu, F.; Cao, Y.; et al. The colonization of pyrethroid resistant strain from wild Anopheles sinensis, the major Asian malaria vector. Parasites Vectors 2014, 7, 582. [Google Scholar] [CrossRef]
- Zhong, D.; Chang, X.; Zhou, G.; He, Z.; Fu, F.; Yan, Z.; Zhu, G.; Xu, T.; Bonizzoni, M.; Wang, M.-H.; et al. Relationship between Knockdown Resistance, Metabolic Detoxification and Organismal Resistance to Pyrethroids in Anopheles sinensis. PLoS ONE 2013, 8, e55475. [Google Scholar] [CrossRef]
- Yan, Z.-W.; He, Z.-B.; Yan, Z.-T.; Si, F.-L.; Zhou, Y.; Chen, B. Genome-wide and expression-profiling analyses suggest the main cytochrome P450 genes related to pyrethroid resistance in the malaria vector, Anopheles sinensis (Diptera Culicidae). Pest Manag. Sci. 2018, 74, 1810–1820. [Google Scholar] [CrossRef]
- Salako, A.S.; Ahogni, I.; Aïkpon, R.; Sidick, A.; Dagnon, F.; Sovi, A.; Sominahouin, A.A.; Agossa, F.; Iyikirenga, L.; Akogbeto, M.C. Insecticide resistance status, frequency of L1014F Kdr and G119S Ace-1 mutations, and expression of detoxification enzymes in Anopheles gambiae (s.l.) in two regions of northern Benin in preparation for indoor residual spraying. Parasites Vectors 2018, 11, 618. [Google Scholar] [CrossRef] [PubMed]
- Camara, S.; Koffi, A.A.; Alou, L.P.A.; Koffi, K.; Kabran, J.-P.K.; Koné, A.; Koffi, M.F.; N’Guessan, R.; Pennetier, C. Mapping insecticide resistance in Anopheles gambiae (s.l.) from Côte d’Ivoire. Parasites Vectors 2018, 11, 1–11. [Google Scholar] [CrossRef]
- Chabi, J.; Baidoo, P.K.; Datsomor, A.K.; Okyere, D.; Ablorde, A.; Iddrisu, A.; Wilson, M.D.; Dadzie, S.K.; Jamet, H.P.; Ii, J.W.D. Insecticide susceptibility of natural populations of Anopheles coluzzii and Anopheles gambiae (sensu stricto) from Okyereko irrigation site, Ghana, West Africa. Parasites Vectors 2016, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cisse, M.B.M.; Keita, C.; Dicko, A.; Dengela, D.; Coleman, J.; Lucas, B.; Mihigo, J.; Sadou, A.; Belemvire, A.; George, K.; et al. Characterizing the insecticide resistance of Anopheles gambiae in Mali. Malar. J. 2015, 14, 327. [Google Scholar] [CrossRef] [PubMed]
- Yahouédo, G.A.; Cornelie, S.; Djègbè, I.; Ahlonsou, J.; Aboubakar, S.; Soares, C.; Akogbéto, M.; Corbel, V. Dynamics of pyrethroid resistance in malaria vectors in southern Benin following a large scale implementation of vector control interventions. Parasites Vectors 2016, 9, 385. [Google Scholar] [CrossRef]
- Scott, J.G.; Yoshimizu, M.H.; Kasai, S. Pyrethroid resistance in Culex pipiens mosquitoes. Pestic. Biochem. Physiol. 2015, 120, 68–76. [Google Scholar] [CrossRef]
- Tabbabi, A.; Daaboub, J.; Ben Cheikh, R.; Laamari, A.; Feriani, M.; Boubaker, C.; Ben Jha, I.; Ben Cheikh, H. Resistance status to deltamethrin pyrethroid of Culex pipiens pipiens (Diptera: Culicidae) collected from three districts of Tunisia. Afr. Health Sci. 2018, 18, 1182–1188. [Google Scholar] [CrossRef]
- Lv, Y.; Wang, W.; Hong, S.; Lei, Z.; Fang, F.; Guo, Q.; Hu, S.; Tian, M.; Liu, B.; Zhang, D.; et al. Comparative transcriptome analyses of deltamethrin-susceptible and -resistant Culex pipiens pallens by RNA-seq. Mol. Genet. Genom. 2016, 291, 309–321. [Google Scholar] [CrossRef]
- Wang, W.; Lv, Y.; Fang, F.; Hong, S.; Guo, Q.; Hu, S.; Zou, F.; Shi, L.; Lei, Z.; Ma, K.; et al. Identification of proteins associated with pyrethroid resistance by iTRAQ-based quantitative proteomic analysis in Culex pipiens pallens. Parasites Vectors 2015, 8, 95. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Sun, X.-H.; Liu, Z.-H.; Xu, Y.; Sun, Y.; Zhou, D.; Shen, B.; Zhu, C.-L. Identification and classification of differentially expressed genes in pyrethroid-resistant Culex pipiens pallens. Mol. Genet. Genom. 2019, 294, 861–873. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Liu, S.; Zhang, Y.; Gao, J.; Yang, M.; Liu, X.; Tao, L. Cypermethrin resistance conferred by increased target insensitivity and metabolic detoxification in Culex pipiens pallens Coq. Pestic. Biochem. Physiol. 2017, 142, 77–82. [Google Scholar] [CrossRef]
- Itokawa, K.; Komagata, O.; Kasai, S.; Ogawa, K.; Tomita, T. Testing the causality between CYP9M10 and pyrethroid resistance using the TALEN and CRISPR/Cas9 technologies. Sci. Rep. 2016, 6, 24652. [Google Scholar] [CrossRef]
- Gong, Y.; Li, T.; Zhang, L.; Gao, X.; Liu, N. Permethrin Induction of Multiple Cytochrome P450 Genes in Insecticide Resistant Mosquitoes, Culex quinquefasciatus. Int. J. Biol. Sci. 2013, 9, 863–871. [Google Scholar] [CrossRef]
- Polson, K.; Rawlins, S.; Brogdon, W.; Chadee, D. Characterisation of DDT and Pyrethroid Resistance in Trinidad and Tobago populations of Aedes aegypti. Bull. Èntomol. Res. 2011, 101, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-González, I.; Quiñones, M.L.; Lenhart, A.; Brogdon, W.G. Insecticide resistance status of Aedes aegypti (L.) from Colombia. Pest Manag. Sci. 2011, 67, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Aponte, A.; Penilla, R.P.; Rodríguez, A.D.; Ocampo, C.B. Mechanisms of pyrethroid resistance in Aedes (Stegomyia) aegypti from Colombia. Acta Trop. 2019, 191, 146–154. [Google Scholar] [CrossRef]
- Rocha, H.D.R.; Paiva, M.H.S.; Silva, N.M.; de Araújo, A.P.; Camacho, D.D.R.D.R.D.A.; da Moura, A.J.F.; Gómez, L.F.; Ayres, C.F.J.; Santos, M.A.V.D.M. Susceptibility profile of Aedes aegypti from Santiago Island, Cabo Verde, to insecticides. Acta Trop. 2015, 152, 66–73. [Google Scholar] [CrossRef]
- Lima, E.P.; Paiva, M.H.S.; De Araújo, A.P.; Da Silva, É.V.G.; Da Silva, U.M.; De Oliveira, L.N.; Santana, A.E.G.; Barbosa, C.N.; Neto, C.C.D.P.; Goulart, M.O.; et al. Insecticide resistance in Aedes aegypti populations from Ceará, Brazil. Parasites Vectors 2011, 4, 5. [Google Scholar] [CrossRef]
- Garcia, G.D.A.; David, M.R.; Martins, A.D.J.; Maciel-De-Freitas, R.; Linss, J.G.B.; Araújo, S.C.; Lima, J.B.P.; Valle, D. The impact of insecticide applications on the dynamics of resistance: The case of four Aedes aegypti populations from different Brazilian regions. PLoS Negl. Trop. Dis. 2018, 12, e0006227. [Google Scholar] [CrossRef]
- Ishak, I.H.; Kamgang, B.; Ibrahim, S.S.; Riveron, J.M.; Irving, H.; Wondji, C.S. Pyrethroid Resistance in Malaysian Populations of Dengue Vector Aedes aegypti Is Mediated by CYP9 Family of Cytochrome P450 Genes. PLoS Negl. Trop. Dis. 2017, 11, e0005302. [Google Scholar] [CrossRef] [PubMed]
- Goindin, D.; Delannay, C.; Gelasse, A.; Ramdini, C.; Gaude, T.; Faucon, F.; David, J.-P.; Gustave, J.; Vega-Rua, A.; Fouque, F. Levels of insecticide resistance to deltamethrin, malathion, and temephos, and associated mechanisms in Aedes aegypti mosquitoes from the Guadeloupe and Saint Martin islands (French West Indies). Infect. Dis. Poverty 2017, 6, 1–15. [Google Scholar] [CrossRef] [PubMed]
- De Araújo, A.P.; Paiva, M.H.S.; Cabral, A.M.; Cavalcanti, A.E.H.D.; Pessoa, L.F.F.; Diniz, D.F.A.; Helvecio, E.; Da Silva, E.V.G.; Da Silva, N.M.; Anastácio, D.B.; et al. Screening Aedes aegypti (Diptera: Culicidae) Populations From Pernambuco, Brazil for Resistance to Temephos, Diflubenzuron, and Cypermethrin and Characterization of Potential Resistance Mechanisms. J. Insect Sci. 2019, 19, 54. [Google Scholar] [CrossRef]
- Smith, L.B.; Sears, C.; Sun, H.; Mertz, R.W.; Kasai, S.; Scott, J.G. CYP-mediated resistance and cross-resistance to pyrethroids and organophosphates in Aedes aegypti in the presence and absence of kdr. Pestic. Biochem. Physiol. 2019, 160, 119–126. [Google Scholar] [CrossRef]
- Saavedra-Rodriguez, K.; Strode, C.; Flores, A.E.; Garcia-Luna, S.; Reyes-Solis, G.; Ranson, H.; Hemingway, J.; Black, W.C. Differential transcription profiles in Aedes aegypti detoxification genes after temephos selection. Insect Mol. Biol. 2013, 23, 199–215. [Google Scholar] [CrossRef]
- Rault, L.C.; O’Neal, S.T.; Johnson, E.J.; Anderson, T.D. Association of age, sex, and pyrethroid resistance status on survival and cytochrome P450 gene expression in Aedes aegypti (L.). Pestic. Biochem. Physiol. 2019, 156, 96–104. [Google Scholar] [CrossRef]
- Bisset, J.A.; Marín, R.; Rodríguez, M.M.; Severson, D.W.; Ricardo, Y.; French, L.; Díaz, M.; Pérez, O. Insecticide resistance in two Aedes aegypti (Diptera: Culicidae) strains from Costa Rica. J. Med. Èntomol. 2013, 50, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, J.; Zhong, D.; Zhang, H.; Yang, W.; Zhou, G.; Su, X.; Wu, Y.; Wu, K.; Cai, S.; et al. Evidence for multiple-insecticide resistance in urban Aedes albopictus populations in southern China. Parasites Vectors 2018, 11, 1–10. [Google Scholar] [CrossRef]
- Xu, J.; Su, X.; Bonizzoni, M.; Zhong, D.; Li, Y.; Zhou, G.; Nguyen, H.; Tong, S.; Yan, G.; Chen, X.-G. Comparative transcriptome analysis and RNA interference reveal CYP6A8 and SNPs related to pyrethroid resistance in Aedes albopictus. PLoS Negl. Trop. Dis. 2018, 12, e0006828. [Google Scholar] [CrossRef] [PubMed]
- Ishak, I.H.; Riveron, J.M.; Ibrahim, S.S.; Stott, R.; Longbottom, J.; Irving, H.; Wondji, C.S. The Cytochrome P450 gene CYP6P12 confers pyrethroid resistance in kdr-free Malaysian populations of the dengue vector Aedes albopictus. Sci. Rep. 2016, 6, 24707. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Black, W.C., IV; Snell, T.K.; Saavedra-Rodriguez, K.; Kading, R.C.; Campbell, C.L. From Global to Local—New Insights into Features of Pyrethroid Detoxification in Vector Mosquitoes. Insects 2021, 12, 276. https://doi.org/10.3390/insects12040276
Black WC IV, Snell TK, Saavedra-Rodriguez K, Kading RC, Campbell CL. From Global to Local—New Insights into Features of Pyrethroid Detoxification in Vector Mosquitoes. Insects. 2021; 12(4):276. https://doi.org/10.3390/insects12040276
Chicago/Turabian StyleBlack, William C., IV, Trey K. Snell, Karla Saavedra-Rodriguez, Rebekah C. Kading, and Corey L. Campbell. 2021. "From Global to Local—New Insights into Features of Pyrethroid Detoxification in Vector Mosquitoes" Insects 12, no. 4: 276. https://doi.org/10.3390/insects12040276
APA StyleBlack, W. C., IV, Snell, T. K., Saavedra-Rodriguez, K., Kading, R. C., & Campbell, C. L. (2021). From Global to Local—New Insights into Features of Pyrethroid Detoxification in Vector Mosquitoes. Insects, 12(4), 276. https://doi.org/10.3390/insects12040276







