Diversity, Co-Occurrence, and Nestedness Patterns of Sand Fly Species (Diptera: Psychodidae) in Two Rural Areas of Western Panamá
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Sand Fly Sampling
2.3. Sand Fly Species’ Identification
2.4. Statistical Analysis
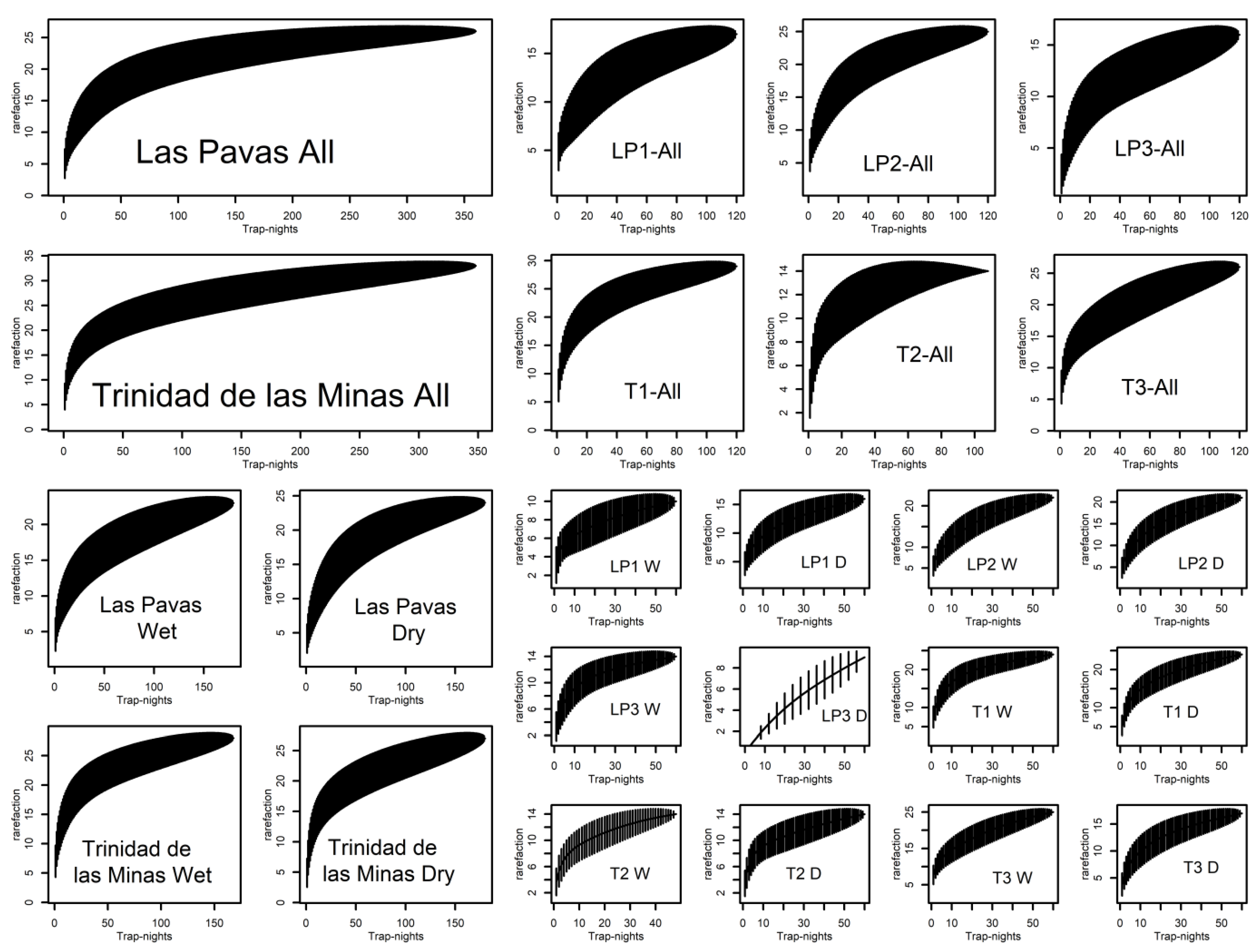
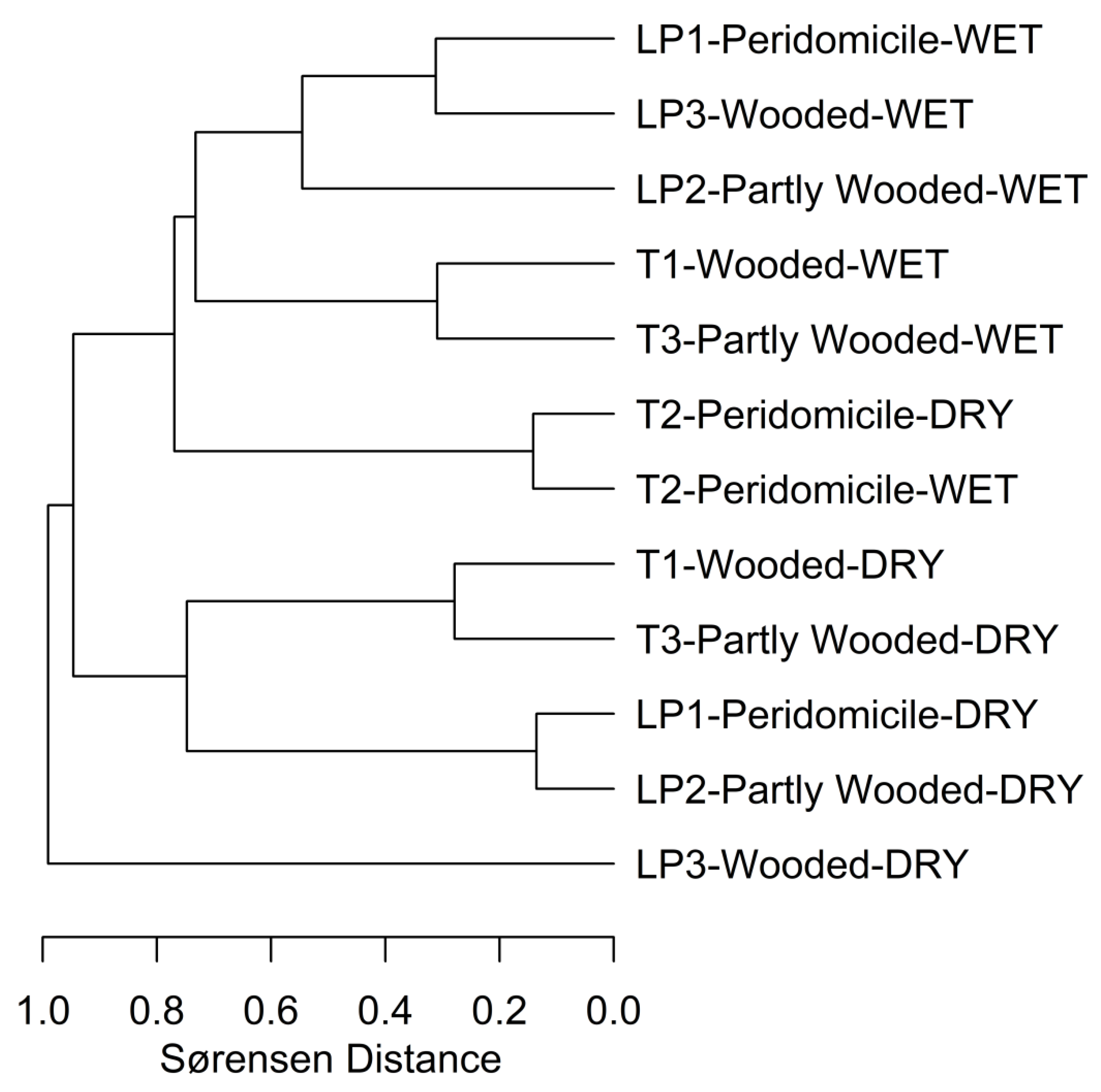
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; den Boer, M.; WHO Leishmaniasis Control Team. Leishmaniasis Worldwide and Global Estimates of Its Incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef] [PubMed]
- PAHO. Leishmaniases: Epidemiological Report of the Americas; PAHO: Washington, DC, USA, 2019; p. 4. [Google Scholar]
- Rigg, C.A.; Calzada, J.E.; Saldaña, A.; Perea, M.; Chaves, L.F.; Valderrama, A. Leishmania spp. Infection Rate and Feeding Patterns of Sand Flies (Diptera: Psychodidae) from a Hyperendemic Cutaneous Leishmaniasis Community in Panamá. Am. J. Trop. Med. Hyg. 2019, 100, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Miranda, A.; Carrasco, R.; Paz, H.; Pascale, J.M.; Samudio, F.; Saldaña, A.; Santamaría, G.; Mendoza, Y.; Calzada, J.E. Molecular Epidemiology of American Tegumentary Leishmaniasis in Panama. Am. J. Trop. Med. Hyg. 2009, 81, 565–571. [Google Scholar] [CrossRef] [PubMed]
- González, K.; Calzada, J.E.; Saldaña, A.; Rigg, C.; Alvarado, G.; Rodríguez-Herrera, B.; Kitron, U.D.; Adler, G.H.; Gottdenker, N.L.; Chaves, L.F.; et al. Survey of Wild Mammal Hosts of Cutaneous Leishmaniasis Parasites in Panamá and Costa Rica. Trop. Med. Health 2015, 43, 75–78. [Google Scholar] [CrossRef]
- Fitzpatrick, C.; Engels, D. Leaving no one behind: A neglected tropical disease indicator and tracers for the Sustainable Development Goals. Int. Health 2016, 8, i15–i18. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Valderrama, A.; Gottdenker, N.; Cerezo, L.; Minakawa, N.; Saldaña, A.; Calzada, J.E.; Chaves, L.F. Macroecological patterns of American Cutaneous Leishmaniasis transmission across the health areas of Panamá (1980–2012). Parasite Epidemiol. Control 2016, 1, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Quintana, M.G.; Salomon, O.D.; De Grosso, M.; Lizarralde, S. Distribution of Phlebotomine Sand Flies (Diptera: Psychodidae) in a Primary Forest-Crop Interface, Salta, Argentina. J. Med. Entomol. 2010, 47, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Chaniotis, B.N.; Neely, J.M.; Correa, M.A.; Tesh, R.B.; Johnson, K.M. Natural population dynamics of Phlebotomine Sand flies in Panama. J. Med. Entomol. 1971, 8, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Salomon, O.D.; Wilson, M.L.; Munstermann, L.E.; Travi, B.L. Spatial and Temporal Patterns of Phlebotomine Sand Flies (Diptera: Psychodidae) in a Cutaneous Leishmaniasis Focus in Northern Argentina. J. Med. Entomol. 2004, 41, 33–39. [Google Scholar] [CrossRef]
- Chaves, L.F.; Calzada, J.E.; Valderama, A.; Saldaña, A. Cutaneous Leishmaniasis and Sand Fly fluctuations are associated with El Niño in Panamá. PLoS Negl. Trop. Dis. 2014, 8, e3210. [Google Scholar] [CrossRef]
- Christensen, H.A.; Fairchild, G.B.; Herrer, A.; Johnson, C.M.; Young, D.G.; Vasquez, A.M.d. The ecology of cutaneous leishmaniasis in the republic of Panama. J. Med. Entomol. 1983, 20, 463–484. [Google Scholar] [CrossRef] [PubMed]
- Saldaña, A.; Chaves, L.F.; Rigg, C.A.; Wald, C.; Smucker, J.E.; Calzada, J.E. Clinical Cutaneous Leishmaniasis Rates Are Associated with Household Lutzomyia gomezi, Lu. panamensis, and Lu. trapidoi Abundance in Trinidad de Las Minas, Western Panama. Am. J. Trop. Med. Hyg. 2013, 88, 572–574. [Google Scholar] [CrossRef] [PubMed]
- Chaves, L.F.; Hernandez, M.-J.; Dobson, A.P.; Pascual, M. Sources and sinks: Revisiting the criteria for identifying reservoirs for American cutaneous leishmaniasis. Trends Parasitol. 2007, 23, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.O.K.M.; de Oliveira, T.M.; de Assis Aguiar, A.N.; Pinto, P.E.M.; Barbosa, D.S.; de Araújo Diniz, S.; Silva, M.X. Profile of American tegumentary leishmaniasis in transmission areas in the state of Minas Gerais, Brazil, from 2007 to 2017. BMC Infect. Dis. 2020, 20, 163. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, D.T.; de Souza, D.C.; de Castro, V.N.; Geiger, S.M.; Barbosa, D.S. Identification of priority areas for surveillance of cutaneous leishmaniasis using spatial analysis approaches in Southeastern Brazil. BMC Infect. Dis. 2019, 19, 318. [Google Scholar] [CrossRef] [PubMed]
- Añez, N.; Nieves, E.; Cazorla, D.; Oviedo, M.; Lugo de Yarbuh, A.; Valera, M. Epidemiology of cutaneous leishmaniasis in Merida, Venezuela. III. Altitudinal distribution, age structure, natural infection and feeding behaviour of sand flies and their relation to the risk of transmission. Ann. Trop. Med. Parasitol. 1994, 88, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Chaves, L.F.; Añez, N. Species co-occurrence and feeding behavior in sand fly transmission of American cutaneous leishmaniasis in western Venezuela. Acta Trop. 2004, 92, 219–224. [Google Scholar] [CrossRef]
- Chaves, L.F.; Añez, N. Nestedness patterns of sand fly (Diptera: Psychodidae) species in a neotropical semi-arid environment. Acta Trop. 2016, 153, 7–13. [Google Scholar] [CrossRef][Green Version]
- Feliciangeli, M.D. Ecology of sand flies (Diptera: Psychodidae) in a restricted focus of cutaneous leishmaniasis en Northern Venezuela: I. Description of the study area, catching methods and species composition. Mem. Inst. Oswaldo Cruz 1987, 82, 119–124. [Google Scholar] [CrossRef]
- Feliciangeli, M.D. Ecology of sand flies (Diptera: Psychodidae) in a restricted focus of cutaneous leishmaniasis in Northern Venezuela: II. Species composition in relation to habitat, catching method and hour of catching. Mem. Inst. Oswaldo Cruz 1987, 82, 125–131. [Google Scholar] [CrossRef]
- Feliciangeli, M.D. Ecology of sand flies Diptera: Psychodidae) in a restricted focus of cutaneous leishmaniasis in Northern Venezuela: IV. Sand fly monthly fluctuation and leishmaniasis incidence relationship. Mem. Inst. Oswaldo Cruz 1987, 82, 177–179. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Feliciangeli, M.D. Ecology of sand flies (Diptera: Psychodidae) in a restricted focus of cutaneous leishmaniasis in Northern Venezuela: III. Seasonal fluctuation. Mem. Inst. Oswaldo Cruz 1987, 82, 167–176. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ferro, C.; Morrison, A.C.; Torres, M.; Pardo, R.; Wilson, M.L.; Tesh, R.B. Species Composition and Relative Abundance of Sand Flies of the Genus Lutzomyia (Diptera: Psychodidae) at an Endemic Focus of Visceral Leishmaniasis in Colombia. J. Med. Entomol. 1995, 32, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, A.E.; Rojas, J.C.; Vargas, F.; Herrero, M.V. Temporal and Spatial Variation of Phlebotomine (Diptera: Psychodidae) Community Diversity in a Cutaneous Leishmaniasis Endemic Area of Costa Rica. J. Med. Entomol. 2000, 37, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Baselga, A. Partitioning the turnover and nestedness components of beta diversity. Glob. Ecol. Biogeogr. 2010, 19, 134–143. [Google Scholar] [CrossRef]
- Levins, R.; Awerbuch, T.; Brinkmann, U.; Eckardt, I.; Epstein, P.; Makhoul, N.; Depossas, C.A.; Puccia, C.; Spielman, A.; Wilson, M.E. The emergence of new diseases. Am. Sci. 1994, 82, 52–60. [Google Scholar]
- Chaves, L.F. Sand fly species co-occurrence at the local scale: Differences between agricultural and forested areas. Bol. Malariol. Salud Ambient. 2011, 51, 35–39. [Google Scholar]
- Laporta, G.Z.; de Prado, P.I.K.L.; Kraenkel, R.A.; Coutinho, R.M.; Sallum, M.A.M. Biodiversity can help prevent malaria outbreaks in tropical forests. PLoS Negl. Trop. Dis. 2013, 7, e2139. [Google Scholar] [CrossRef]
- Gotelli, N.J. Null model analysis of species co-occurrence patterns. Ecology 2000, 81, 2606–2621. [Google Scholar] [CrossRef]
- Ulrich, W.; Gotelli, N.J. Null model analysis of species nestedness patterns. Ecology 2007, 88, 1824–1831. [Google Scholar] [CrossRef]
- Ulrich, W.; Gotelli, N.J. Disentangling community patterns of nestedness and species co-occurrence. Oikos 2007, 116, 2053–2061. [Google Scholar] [CrossRef]
- Holdridge, L. Ecología Basada en Zonas de Vida; IICA: San José, Costa Rica, 1987. [Google Scholar]
- Gottdenker, N.L.; Calzada, J.E.; Saldaña, A.; Carroll, C.R. Association of Anthropogenic Land Use Change and Increased Abundance of the Chagas Disease Vector Rhodnius pallescens in a Rural Landscape of Panama. Am. J. Trop. Med. Hyg. 2011, 84, 70–77. [Google Scholar] [CrossRef] [PubMed]
- PMCC. Informe Final: Proyecto Monitoreo de la Cuenca del Canal de Panamá; USAIDANAM-STRI: Balboa, Panamá, 1999. [Google Scholar]
- Chaves, L.F.; Calzada, J.E.; Rigg, C.; Valderrama, A.; Gottdenker, N.L.; Saldaña, A. Leishmaniasis sand fly vector density reduction is less marked in destitute housing after insecticide thermal fogging. Parasites Vectors 2013, 6, 164. [Google Scholar] [CrossRef] [PubMed]
- Hidrometeorología, D. Estadística Panameña. Situación Física; Etesa: Panamá, Panamá, 2014; p. 38. [Google Scholar]
- Lezcano, H.; Finegan, B.; Condit, R.; Delgado, D. Variación de las Características de la Comunidad Vegetal en Relación al Efecto de Borde en Fragmentos de bosque; Las Pavas, Cuenca del Canal de Panamá, Recursos Naturales y Ambiente: Panamá, Panamá, 2002. [Google Scholar]
- Navas, N.; Eyda, V.; Cedeño, B.H. Estadística de Panamá. Dirección de Estadística y Meteorológica, 1998–1999; Estadística de Panamá, Censo: Panamá, Panamá, 2001. [Google Scholar]
- Calzada, J.E.; Saldaña, A.; Rigg, C.; Valderrama, A.; Romero, L.; Chaves, L.F. Changes in phlebotomine sand fly species composition following insecticide thermal fogging in a rural setting of western Panamá. PLoS ONE 2013, 8, e53289. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, B.E. Plan Estratégico de Desarrollo Distrital en Capira 2018–2022. In Distrito Capira; Finanzas, M.E., Ed.; Dirección de Programación de Inversiones: Alcaldía de Capira, Panama, 2017; pp. 13–18. [Google Scholar]
- Chaniotis, B.N.; Tesh, R.B.; Correa, M.A.; Johnson, K.M. Diurnal resting sites of phlebotomine sand flies in a Panamanian tropical forest. J. Med. Entomol. 1972, 9, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Chaniotis, B.N.; Correa, M.A. Comparative flying and biting activity of Panamanian phlebotomine sand flies in a mature forest and adjacent open space. J. Med. Entomol. 1974, 11, 115–116. [Google Scholar] [CrossRef] [PubMed]
- Young, D.G.; Duncan, M.A. Guide to the Identification and Geographic Distribution of Lutzomyia sand Flies in Mexico, the West Indies, Central and South America (Diptera: Psychodidae); Associated Publishers: Gainesville, FL, USA, 1994; p. 881. [Google Scholar]
- Galati, E.A.B. Morfologia e Taxonomia: Morfologia, terminologia de adultos e identificação dos táxons da América. In Flebotomíneos do Brasil; Rangel, E.F., Lainson, R., Eds.; Editora Fiocruz: Rio de Janeiro, Brazil, 2003; pp. 53–75. [Google Scholar]
- Chao, A.; Chazdon, R.L.; Colwell, R.K.; Shen, T.J. A new statistical approach for assessing similarity of species composition with incidence and abundance data. Ecol. Lett. 2005, 8, 148–159. [Google Scholar] [CrossRef]
- Colwell, R.K.; Coddington, J.A. Estimating Terrestrial Biodiversity through Extrapolation. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1994, 345, 101–118. [Google Scholar]
- Almeida-Neto, M.; Guimarães, P.; Guimarães, P.R.; Loyola, R.D.; Ulrich, W. A consistent metric for nestedness analysis in ecological systems: Reconciling concept and measurement. Oikos 2008, 117, 1227–1239. [Google Scholar] [CrossRef]
- Ulrich, W.; Almeida-Neto, M.; Gotelli, N.J. A consumer’s guide to nestedness analysis. Oikos 2009, 118, 3–17. [Google Scholar] [CrossRef]
- Hoshi, T.; Imanishi, N.; Higa, Y.; Chaves, L.F. Mosquito Biodiversity Patterns Around Urban Environments in South-Central Okinawa Island, Japan. J. Am. Mosq. Control Assoc. 2014, 30, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Chaves, L.F.; Hamer, G.L.; Walker, E.D.; Brown, W.M.; Ruiz, M.O.; Kitron, U.D. Climatic variability and landscape heterogeneity impact urban mosquito diversity and vector abundance and infection. Ecosphere 2011, 2, art70. [Google Scholar] [CrossRef]
- Travi, B.L.; Adler, G.H.; Lozano, M.; Cadena, H.; Montoya-Lerma, J. Impact of Habitat Degradation on Phlebotominae (Diptera: Psychodidae) of Tropical Dry Forests in Northern Colombia. J. Med. Entomol. 2002, 39, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Nieves, E.; Oraá, L.; Rondón, Y.; Sánchez, M.; Sánchez, Y.; Rojas, M.; Rondón, M.; Rujano, M.; González, N.; Cazorla, D. Effect of Environmental Disturbance on the Population of Sand flies and Leishmania Transmission in an Endemic Area of Venezuela. J. Trop. Med. 2014, 2014, 280629. [Google Scholar] [CrossRef] [PubMed]
- Chaniotis, B.N.; Correa, M.A.; Tesh, R.B.; Johnson, K.M. Horizontal and vertical movements of phlebotomine sand flies in a Panamanian rain forest. J. Med. Entomol. 1974, 11, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Chaniotis, B.N. Improved trapping of phlebotomine sand flies (Diptera: Psychodidae) in light traps supplemented with dry ice in a neotropical rain forest. J. Med. Entomol. 1983, 20, 222–223. [Google Scholar] [CrossRef]
- Chagas, E.C.d.S.; Silva, A.S.; Fé, N.F.; Ferreira, L.S.; Sampaio, V.d.S.; Terrazas, W.C.M.; Guerra, J.A.O.; Souza, R.A.F.d.; Silveira, H.; Guerra, M.d.G.V.B. Composition of sand fly fauna (Diptera: Psychodidae) and detection of Leishmania DNA (Kinetoplastida: Trypanosomatidae) in different ecotopes from a rural settlement in the central Amazon, Brazil. Parasites Vectors 2018, 11, 180. [Google Scholar] [CrossRef]
- Valderrama, A.; Herrera, M.; Salazar, A. Relación entre la composición de especies del género de Lutzomyia Franca (Diptera: Psychodidae, Phlebotominae) y los diferentes tipos de bosques en Panamá. Acta Zool. Mex. 2008, 24, 67–68. [Google Scholar]
- Chaniotis, B.N.; Parsons, R.E.; Harlan, H.J.; Correa, M.A. A pilot study to control phlebotomine Sand Flies (Diptera: Psychodidae) in a Neotropical Rain Forest. J. Med. Entomol. 1982, 19, 1–5. [Google Scholar] [CrossRef]
- Christensen, H.A.; Herrer, A. Panamanian Lutzomyia (Diptera: Psychodidae) host attraction profiles. J. Med. Entomol. 1980, 17, 522–528. [Google Scholar] [CrossRef]
- Tesh, R.B.; Chaniotis, B.N.; Aronson, M.D.; Johnson, K.M. Natural Host Preferences of Panamanian Phlebotomine Sand flies as Determined by Precipitin Test. Am. J. Trop. Med. Hyg. 1971, 20, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Tesh, R.B.; Chaniotis, B.N.; Carrera, B.R.; Johnson, K.M. Further studies on the natural host preferences of Panamanian phlebotomine sand flies. Am. J. Epidemiol. 1972, 95, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Thatcher, V.E.; Hertig, M. Field Studies on the Feeding Habits and Diurnal Shelters of Some Phlebotomus Sand flies (Diptera: Psychodidae) in Panama. Ann. Entomol. Soc. Am. 1966, 59, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Santamaría, E.; Cabrera, O.L.; Marceló, C.; Goenaga-Olaya, S.; Maestre-Serrano, R. Behavioral Aspects of Phlebotomine Sand Flies Associated with a Case of Cutaneous Leishmaniasis in Atlántico, Northern Colombia. Am. J. Trop. Med. Hyg. 2020, 102, 768–776. [Google Scholar] [CrossRef]
- Alexander, J.B. Dispersal of Phlebotomine Sand Flies (Diptera: Psychodidae) in a Colombian Coffee Plantation. J. Med. Entomol. 1987, 24, 552–558. [Google Scholar] [CrossRef]
- Teodoro, U.; La Salvia Filho, V.; Lima, E.M.d.; Misuta, N.M.; Verginassi, T.G.; Ferreira, M.E.M.C. Leishmaniose tegumentar americana: Flebotomíneos de área de transmissão no Norte do Paraná, Brasil. Rev. Saúde Pública 1991, 25, 129–133. [Google Scholar] [CrossRef][Green Version]
- Kelly, D.W.; Mustafa, Z.; Dye, C. Density-dependent feeding success in a field population of the sand fly, Lutzomyia longipalpis. J. Anim. Ecol. 1996, 65, 517–527. [Google Scholar] [CrossRef]
- Kelly, D.W. Why are some people bitten more than others? Trends Parasitol. 2001, 17, 578–581. [Google Scholar] [CrossRef]
- Feliciangeli, M.D.; Rodriguez, N.; Bravo, A.; Arias, F.; Guzman, B. Vectors of cutaneous leishmaniasis in north-central Venezuela. Med. Vet. Entomol. 1994, 8, 317–324. [Google Scholar] [CrossRef]
- MacArthur, R.H. Geographical Ecology: Patterns in the Distribution of Species; Princeton University Press: Princeton, NJ, USA, 1984. [Google Scholar]
- Chaves, L.F.; Cohen, J.M.; Pascual, M.; Wilson, M.L. Social Exclusion Modifies Climate and Deforestation Impacts on a Vector-Borne Disease. PLoS Negl. Trop. Dis. 2008, 2, e176. [Google Scholar] [CrossRef]
- Janzen, D.H. On Ecological Fitting. Oikos 1985, 45, 308–310. [Google Scholar] [CrossRef]
- Morrison, A.C.; Ferro, C.; Morales, A.; Tesh, R.B.; Wilson, M.L. Dispersal of the Sand Fly Lutzomyia longipalpis (Diptera: Psychodidae) at an Endemic Focus of Visceral Leishmaniasis in Colombia. J. Med. Entomol. 1993, 30, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Chaves, L.F.; Pascual, M. Climate cycles and forecasts of cutaneous leishmaniasis, a nonstationary vector-borne disease. PLoS Med. 2006, 3, e295. [Google Scholar] [CrossRef] [PubMed]
- Chaves, L.F.; Pascual, M. Comparing Models for Early Warning Systems of Neglected Tropical Diseases. PLoS Negl. Trop. Dis. 2007, 1, e33. [Google Scholar] [CrossRef] [PubMed]
- Alexander, B. Sampling methods for phlebotomine sand flies. Med. Vet. Entomol. 2000, 14, 109–122. [Google Scholar] [CrossRef]
- Moreno, M.; Guzmán-Rodríguez, L.; Valderrama-Ardila, C.; Alexander, N.; Ocampo, C.B. Land use in relation to composition and abundance of phlebotomines (Diptera: Psychodidae) in five foci of domiciliary transmission of cutaneous leishmaniasis in the Andean region of Colombia. Acta Trop. 2020, 203, 105315. [Google Scholar] [CrossRef]
- Grimaldi, G.; Tesh, R.B.; McMahon-Pratt, D. A Review of the Geographic Distribution and Epidemiology of Leishmaniasis in the New World. Am. J. Trop. Med. Hyg. 1989, 41, 687–725. [Google Scholar] [CrossRef]
- Maroli, M.; Feliciangeli, M.D.; Bichaud, L.; Charrel, R.N.; Gradoni, L. Phlebotomine sand flies and the spreading of leishmaniases and other diseases of public health concern. Med. Vet. Entomol. 2013, 27, 123–147. [Google Scholar] [CrossRef]
- Feliciangeli, D.M.; Rabinovich, J. Abundance of Lutzomyia ovallesi but not Lu. gomezi (Diptera: Psychodidae) correlated with cutaneous leishmaniasis incidence in north-central Venezuela. Med. Vet. Entomol. 1998, 12, 121–131. [Google Scholar] [CrossRef]
- dos Reis, Y.V.; Alevi, K.C.C. Hybridization in Phlebotominae (Diptera: Psychodidae): A mini-review. Infect. Genet. Evol. 2020, 86, 104593. [Google Scholar] [CrossRef]
- Vamosi, S.M.; Heard, S.B.; Vamosi, J.C.; Webb, C.O. Emerging patterns in the comparative analysis of phylogenetic community structure. Mol. Ecol. 2009, 18, 572–592. [Google Scholar] [CrossRef] [PubMed]

| Location | LP | T | ||||
|---|---|---|---|---|---|---|
| Species | F | M | Total | F | M | Total |
| Brumptomyia hamata (Fairchild and Hertig, 1947) | 0 | 1 | 1 | 0 | 2 | 2 |
| Bichromomyia olmeca bicolor (Fairchild and Theodor, 1971) | 19 | 6 | 25 | 126 | 63 | 189 |
| Dampfomyia vesicifera (Fairchild and Hertig, 1947) | 4 | 0 | 4 | 25 | 2 | 27 |
| Dampfomyia vespertilionis (Fairchild and Hertig, 1947) | 7 | 2 | 9 | 8 | 9 | 17 |
| Evandromyia dubitans (Sherlock, 1962) | 0 | 0 | 0 | 1 | 0 | 1 |
| Evandromyia saulensis (Floch and Abonnenc, 1944) | 4 | 0 | 4 | 3 | 0 | 3 |
| Lutzomyia longipalpis (Lutz and Neiva, 1912) ** | 0 | 0 | 0 | 2 | 0 | 2 |
| Lutzomyia cruciata (Coquillett, 1907) | 0 | 0 | 0 | 1 | 0 | 1 |
| Lutzomyia gomezi (Nitzulescu, 1931) * | 369 | 213 | 582 | 134 | 88 | 222 |
| Lutzomyia sanguinaria (Fairchild and Hertig, 1957) * | 3 | 1 | 4 | 10 | 2 | 12 |
| Lutzomyia sp (França, 1924) | 1 | 10 | 11 | 22 | 5 | 27 |
| Micropygomyia micropyga (Mangabeira,1942) | 3 | 13 | 16 | 0 | 5 | 5 |
| Micropygomyia trinidadensis (Newstead, 1922) ** | 1 | 2 | 3 | 14 | 33 | 47 |
| Nyssomyia trapidoi (Fairchild and Hertig, 1952) * | 114 | 38 | 152 | 1684 | 594 | 2278 |
| Nyssomyia ylephiletor (Fairchild and Hertig, 1952) * | 7 | 9 | 16 | 260 | 208 | 468 |
| Pintomyia odax (Fairchild and Hertig, 1961) | 0 | 0 | 0 | 1 | 0 | 1 |
| Pintomyia serrana (Damasceno and Arouck, 1949) | 3 | 0 | 3 | 34 | 17 | 51 |
| Pintomyia ovallesi (Ortiz, 1952) ** | 15 | 7 | 22 | 215 | 59 | 274 |
| Pressatia camposi (Rodríguez, 1950) | 97 | 38 | 135 | 39 | 45 | 84 |
| Pressatia dysponeta (Fairchild and Hertig, 1952) | 293 | 195 | 488 | 80 | 45 | 125 |
| Psathyromyia abonnenci (Floch and Chassignet, 1947) | 0 | 0 | 0 | 1 | 0 | 1 |
| Psathyromyia dasymera (Fairchild and Hertig, 1961) | 0 | 0 | 0 | 2 | 0 | 2 |
| Psathyromyia punctigeniculata (Floch and Abonnenc, 1944) | 2 | 0 | 2 | 0 | 0 | 0 |
| Psathyromyia runoides (Faichild and Hertig, 1953) | 0 | 2 | 2 | 4 | 1 | 5 |
| Psathyromyia aclydifera (Fairchild and Hertig, 1952) | 0 | 0 | 0 | 18 | 8 | 26 |
| Psathyromyia carpenteri (Fairchild and Hertig, 1953) | 12 | 12 | 24 | 10 | 5 | 15 |
| Psathyromyia shannoni (Dyar, 1929) ** | 6 | 1 | 7 | 2 | 4 | 6 |
| Psychodopygus geniculatus (Mangabeira,1941) | 1 | 0 | 1 | 0 | 0 | 0 |
| Psychodopygus panamensis (Shannon, 1926) * | 2104 | 1340 | 3444 | 653 | 459 | 1112 |
| Psychodopygus thula (Young, 1979) * | 61 | 107 | 168 | 113 | 90 | 203 |
| Sciopemyia sordellii (Shannon and Del Ponte, 1927) | 10 | 5 | 15 | 8 | 3 | 11 |
| Trichopygomyia triramula (Fairchild and Hertig, 1952) | 2874 | 1855 | 4729 | 611 | 452 | 1063 |
| Viannamyia caprina (Osorno-Mesa, Morales and Osorno, 1972) | 0 | 0 | 0 | 2 | 0 | 2 |
| Viannamyia furcata (Mangabeira, 1941) | 0 | 0 | 0 | 1 | 4 | 5 |
| Warileya rotundipennis (Fairchild and Hertig, 1951) | 0 | 1 | 1 | 1 | 0 | 1 |
| Total | 6010 | 3858 | 9868 | 4085 | 2203 | 6288 |
| Metric | Sampling | Estimated | Mean Simulation | 95% CI |
|---|---|---|---|---|
| C-score | C-score both | 0.23 * | 0.64 | (0.54, 0.71) |
| C-score wet | 0.13 * | 0.53 | (0.44, 0.59) | |
| C-score dry | 0.35 * | 0.64 | (0.54, 0.71) | |
| NODF-Global | NODF-both | 76.25 * | 67.71 | (65.85, 69.98) |
| NODF-wet | 57.55 * | 50.15 | (48.59, 52.17) | |
| NODF-dry | 52.48 * | 46.70 | (45.02, 48.83) | |
| NODF-Locations | NODF-both | 92.58 * | 71.18 | (49.51, 83.30) |
| NODF-wet | 88.89 * | 69.47 | (48.19, 82.21) | |
| NODF-dry | 86.38 * | 66.57 | (46.48, 78.25) | |
| NODF-Species | NODF-both | 75.84 * | 67.62 | (65.94, 69.75) |
| NODF-wet | 56.76 * | 49.67 | (48.29, 51.48) | |
| NODF-dry | 51.62 * | 46.20 | (44.57, 48.18) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rigg, C.A.; Perea, M.; González, K.; Saldaña, A.; Calzada, J.E.; Gao, Y.; Gottdenker, N.L.; Chaves, L.F. Diversity, Co-Occurrence, and Nestedness Patterns of Sand Fly Species (Diptera: Psychodidae) in Two Rural Areas of Western Panamá. Insects 2021, 12, 113. https://doi.org/10.3390/insects12020113
Rigg CA, Perea M, González K, Saldaña A, Calzada JE, Gao Y, Gottdenker NL, Chaves LF. Diversity, Co-Occurrence, and Nestedness Patterns of Sand Fly Species (Diptera: Psychodidae) in Two Rural Areas of Western Panamá. Insects. 2021; 12(2):113. https://doi.org/10.3390/insects12020113
Chicago/Turabian StyleRigg, Chystrie A., Milixa Perea, Kadir González, Azael Saldaña, José E. Calzada, Yan Gao, Nicole L. Gottdenker, and Luis Fernando Chaves. 2021. "Diversity, Co-Occurrence, and Nestedness Patterns of Sand Fly Species (Diptera: Psychodidae) in Two Rural Areas of Western Panamá" Insects 12, no. 2: 113. https://doi.org/10.3390/insects12020113
APA StyleRigg, C. A., Perea, M., González, K., Saldaña, A., Calzada, J. E., Gao, Y., Gottdenker, N. L., & Chaves, L. F. (2021). Diversity, Co-Occurrence, and Nestedness Patterns of Sand Fly Species (Diptera: Psychodidae) in Two Rural Areas of Western Panamá. Insects, 12(2), 113. https://doi.org/10.3390/insects12020113





