Simple Summary
The brown planthopper (BPH), Nilaparvata lugens, is one of the most harmful rice crop pest insects. The use of RNAi is a feasible strategy for controlling this pest. In this study, we evaluated the importance of PCE3 in the development and reproduction of male BPH. We found that PCE3 could regulate the development of the male internal genitalia and reduce the oviposition level of the females that mated with males treated with dsRNA targeting the N. lugens PCE3 gene, causing eggs not to hatch. Our findings indicate that PCE3 is an important gene in regulating male fecundity and a promising target for controlling BPH.
Abstract
Nilaparvata lugens proclotting enzymes (NlPCEs) belong to the clip domain serine protease (clip-SP) family, which is a characteristic protease family in arthropods. NlPCE3 was previously reported to regulate egg production and development in female N. lugens, but its role in male N. lugens is unclear. In the present study, qPCR analysis showed that NlPCE3 was expressed in three different tissues (gut, testis and fat body). RNAi revealed that dsNlPCE3 injection made the male vas deferens thinner and reduced the oviposition level of the females that mated with dsNlPCE3-treated males, causing eggs not to hatch. Furthermore, immunofluorescence staining showed that NlPCE3 was widely expressed in the male internal genitalia. However, after dsNlPCE3 injection, expression of NlPCE3 was diffuse in the male internal genitalia, whose peripheral cells seemed degraded. Overall, these results indicate that NlPCE3 is important for reproduction in male N. lugens.
1. Introduction
The serine protease (SP) and serine protease homolog (SPH) family is one of the most characteristic enzyme families, playing vital roles in the process of digestion, blood clotting and immunity [1,2]. Certain SPs and SPHs containing one or more disulfide-bridged structures named clip domains are called clip serine proteases (clip-SPs) [3]. In contrast to other SPs, clip-SPs are non-digestive. In addition to one or more N-terminal clip domains, clip-SPs contain a C-terminal S1A family SP domain and a linker sequence [3]. The clip domain may regulate protein–protein interaction similarly to other motifs that are found in SPs, such as low-density lipoprotein receptor class A repeat, scavenger receptor cysteine-rich domain and thrombospondin 2 domain. The clip domain may also anchor enzymes to the surface of invaded organisms by participating in a cascade pathway involving SP-related proteins. The C-terminal S1A family SP domain usually adopts a chymotrypsin-like fold, which consists of two adjacent β-barrel-like structures arranged perpendicularly to each other [4]. This C-terminal domain contains a His-Asp-Ser catalytic triad that is responsible for the acyl transfer [5]. Clip-SPs are divided by phylogenetic analysis into four groups named clip-A, clip-B, clip-C and clip-D [6]. According to sequence similarity and the distances between six highly conserved cysteines, clip-SPs can be further divided into group 1a, group 1b, group 2 and group 3 [7], and they are related to clip-C, clip-D, clip-B and clip-A, respectively [8].
To date, clip-SPs have been found only in arthropods such as Acyrthosiphon pisum, Drosophila melanogaster, Manduca sexta, Scylla paramamosain, Eriocheir sinensis and Apis mellifera. Clip-SPs in arthropods have been reported functioning in the control of dorsoventral patterning during fertilization or embryonic development [1,9,10,11] and participating in various aspects of innate immune regulation, including the regulation of specific activation of prophenoloxidase required for melanization response [12,13,14], hemolymph blood clotting function [15], defense responses to microbial infection mediated by activation of the Toll pathway [1] and antibacterial activity [16,17,18].
The brown planthopper (BPH), Nilaparvata lugens (Hemiptera: Delphacidae), is one of the most destructive rice plant pests in China and Southeast Asia. BPH is an r-selected species with high fertility and is difficult to effectively control. PCE3 is one clip-SP gene found in N. lugens (NlPCE3). In a previous study, we demonstrated that NlPCE3 could be a target for controlling BPH as it has an important role in female reproduction, including reduced egg production, hatching rate and ovarian development [19]. In addition, the experimental data showed that NlPCE3 has a substantially high expression level in male adults, suggesting that it may also play important roles in males. In the present study, we further examine the function of NlPCE3 in male BPH to better elucidate the functions of this enzyme.
2. Materials and Methods
2.1. Culture of Insects
N. lugens used in this study was maintained in a climatron at China Jiliang University, Hangzhou, China. The insects were reared on rice seedlings (Taichung Native 1) at 27 ± 1 °C with 60% humidity under a 16 h light: 8 h dark photoperiod.
2.2. Real-Time Quantitative PCR Analysis
The expression level of NlPCE3 in male BPH samples was analyzed using real-time quantitative PCR (qPCR). Total RNA from males was extracted with a TaKaRa MiniBEST Universal RNA Extraction Kit (TaKaRa, Dalian, China), and the cDNA template for qPCR was synthesized with a Perfect Real Time PrimeScript™ RT reagent Kit with a gDNA Eraser (TaKaRa). The quality of extracted RNA was determined using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) and confirmed with agarose gel electrophoresis. The qPCR reagent was SYBR® Premix ExTaq™ II (Tli RNaseH Plus) (TaKaRa). The special primers for NlPCE3 qPCR were qNlPCE3-F and qNlPCE3-R, and 18S rRNA was used as the qPCR internal reference, with primers qNl18s-F and qNl18s-R (Supplementary Table S1) [19]. Reaction system in the amount of 20 µL was used for qPCR with a StepOnePlus™ Real-Time PCR System (Applied Biosystems, Carlsbad, CA, USA), and the program was as follows: 94 °C for 30 s, followed by 40 cycles at 94 °C for 5 s, and 60 °C for 30 s. Relative transcript level of NlPCE3 in different samples was evaluated using the 2−∆∆Ct method.
2.3. dsRNA Synthesis and Injection
As with our previous methods [19], the DNA template of NlPCE3 for dsRNA synthesis was 630 bp with primers dsNlPCE3-F and dsNlPCE3-R (Supplementary Table S1). dsRNA of the GFP gene (dsGFP) was taken as the negative control. The GFP gene sequence was synthesized in vitro based on the sequence of the binary vector pCAMBIA-1302 (GenBank: AF234298.1). The DNA template of GFP for dsRNA synthesis was 350 bp with primers dsGFP-F and dsGFP-R (Supplementary Table S1). dsNlPCE3 and dsGFP were synthesized according to the instructions for a MEGAscriptTM T7 High Yield Transcription Kit (Thermo Fisher Scientific, Waltham, MA, USA). The concentration of synthesized dsRNA was determined using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific). RNA interference was performed by injecting approximately 50 nL of dsRNA (5000 ng µL−1) into the abdomen of each 5th instar nymph using a manual microinjector. Injection of dsNlPCE3 was made to 100 nymphs, and 100 nymphs received dsGFP as the negative control.
2.4. Observation of the Male Internal Genitalia and Fertility Analysis
After injection, once the 5th instar male nymphs emerged, one male was placed in a 50 mL centrifuge tube with two untreated virgin female adults and fed fresh rice seedlings. The seedlings were changed every day and the tube orifice was covered with gauze. The survival rate and the number of eggs laid by the untreated females were recorded and statistically analyzed. Subsequently, three-day-old male adults were dissected under a Nikon SMZ1500 stereozoom microscope (Nikon, Tokyo, Japan), and morphological changes were observed and photographed with the NIS Elements software (Nikon, Tokyo, Japan).
2.5. Immunofluorescence Staining
Immunofluorescence was used to analyze localization and distribution of target proteins in male internal genitalia under different treatments. All samples were washed three times in phosphate-buffered saline (PBS), then fixed with 4% paraformaldehyde in PBS for 2 h at room temperature. Immunofluorescence staining was performed as previously described [19,20]. The primary antibody was a rabbit polyclonal antibody against a NlPCE3 polypeptide (VEGKSRHRRSIGDQ), and the secondary antibody was a goat anti-rabbit IgG antibody conjugated with Dylight 488 fluorescent dye (Abbkine, Wuhan, China). The samples were observed with laser scanning confocal microscopy (Leica SP8, Mannheim, Germany).
2.6. Data Analysis
Statistical analyses were conducted using one-way ANOVA followed by Tukey’s test for multiple comparisons and unpaired two-tailed Student’s t-tests using GraphPad Prism Software 8.0.2 (GraphPad Software, San Diego, CA, USA). All data are presented as the means ± standard error.
3. Results
3.1. Tissue-Specific Expression of NlPCE3
Our previous experimental data showed that NlPCE3 had a higher expression level in male adults than in female adults [19]. To further study the tissue-specific expression of NlPCE3 in male adults, 50 three-day-old male adults were dissected and their guts, testes and fat bodies were collected for qPCR analysis. The results showed that NlPCE3 had a higher expression level in the gut than in the fat body and testis (Figure 1).
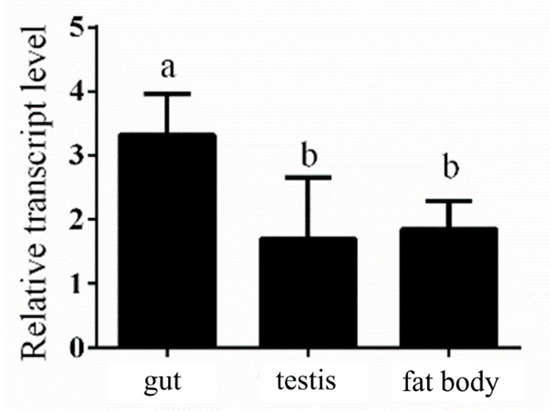
Figure 1.
Expression of NlPCE3 in different tissues of three-day-old male adults. The different tissues were dissected from 50 male individuals and divided into three pools for RNA extraction and qPCR analysis. Data are presented as the means ± standard error. Statistical analyses were conducted using one-way ANOVA and unpaired two-tailed Student’s t-test using GraphPad Prism Software 8.0.2 (GraphPad Software, San Diego, CA, USA). Lowercase letters indicate significant differences (p < 0.05).
3.2. Effect of RNAi on BPH Fecundity
Following dsRNA injection, one- and three-day-old male adults were selected for qPCR detection of the RNAi effect on the NlPCE3 expression level. We found that the transcriptional expression level of NlPCE3 was prominently inhibited, especially in one-day-old males, indicating RNAi by dsRNA injection was effective (Figure 2A). The survival curve illustrated that the dsNlPCE3 treatment group had a relatively higher mortality than the dsGFP treatment group, although this difference was not statistically significant (Figure 2B).
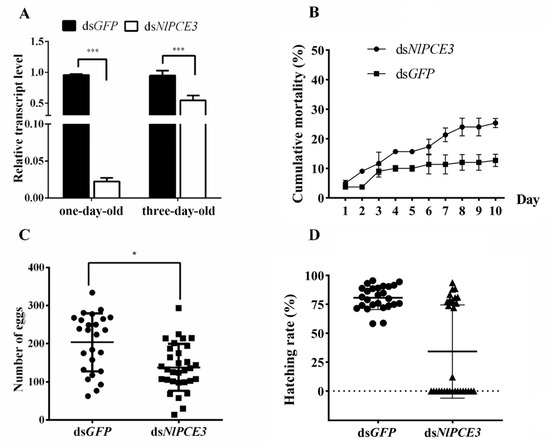
Figure 2.
Effect of RNAi on BPH fecundity. (A) Downregulation of NlPCE3 expression in one- and three-day-old male adults by dsRNA injection (three repetitions, five adults in one repetition). qPCR data of one- and three-day-old male subjects were analyzed independently. (B) Cumulative mortality of male BPH after dsNlPCE3 and dsGFP injection (three repetitions, 20 individuals in one repetition). (C) The number of eggs laid by untreated females that mated with dsNlPCE3- (n = 31) and dsGFP-treated (n = 25) males. Each point on the graph represents the number of eggs laid by each female. (D) The hatching rate of eggs laid by untreated females that mated with dsNlPCE3-(n = 31) and dsGFP-treated (n = 23) males. Each point on the graph represents the hatching rate of eggs laid by each female. Data are presented as the means ± standard error. Statistical analyses were conducted using one-way ANOVA and unpaired two-tailed Student’s t-tests using GraphPad Prism Software 8.0.2 (GraphPad Software). In (A) and (C), * and *** represent significant differences of p < 0.05 and p < 0.001, respectively.
Further analysis revealed that the mean number of eggs laid by untreated female adults that mated with dsNlPCE3-treated male adults was less than that of the eggs laid by the females that mated with dsGFP-treated male adults (Figure 2C). In addition, the hatching rate of eggs in the dsNlPCE3 treatment group was reduced. Among the 31 females that mated with dsNlPCE3-treated males, 17 females laid eggs that could not hatch (Figure 2D), and no eye-spot (as described by Fan et al. (2020) [21]) was found on these non-viable eggs (Figure 3).

Figure 3.
Five-day-old eggs. (A) Normal eggs laid by an untreated female that mated with a dsGFP-treated male. (B) Abnormal eggs laid by an untreated female that mated with a dsNlPCE3-treated male. The abnormal eggs laid by untreated females that mated with dsNlPCE3-treated males are unable to hatch and lack eye-spots.
3.3. Effect of RNAi on Male Internal Genitalia and Sperm
Observation of male internal genitalia revealed that the vas deferens of dsNlPCE3-treated males was markedly thinner than that of dsGFP-treated males (Figure 4 and Figure 5). Immunofluorescence staining showed that NlPCE3 was widely expressed in male internal genitalia (Figure 5A,D), but after dsNlPCE3 treatment, expression of NlPCE3 was diffuse in the vas deferens of dsNlPCE3-treated males (Figure 5B,E). In addition, the peripheral cells of dsNlPCE3-treated males’ internal genitalia seemed degraded (Figure 5Bi,Ei). However, the size of the testes appeared not affected by dsNlPCE3 treatment.
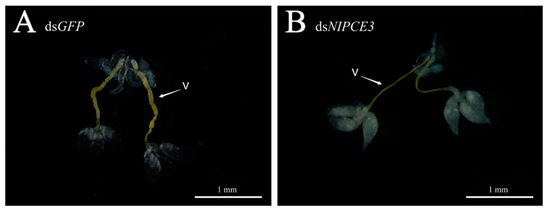
Figure 4.
Effect of RNA interference on the internal genitalia development of a three-day-old male adult. (A) The internal genitalia of a dsGFP-treated male. (B) The internal genitalia of a dsNlPCE3-treated male. V: vas deferens.
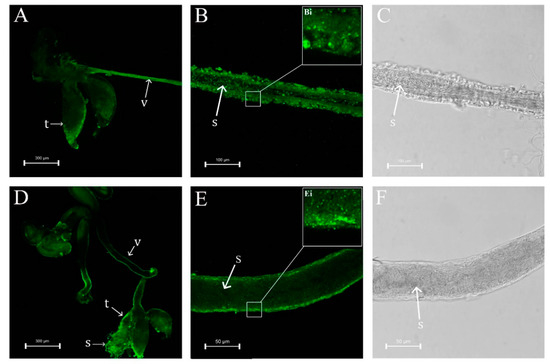
Figure 5.
Effect of RNAi on NlPCE3 expression in the internal genitalia of a three-day-old male adult. (A) The internal genitalia of a male treated with dsNlPCE3. (B,C) The vas deferens of a male treated with dsNlPCE3. (D) The internal genitalia of a male treated with dsGFP. (E,F) The vas deferens of a male treated with dsGFP. t: testis, v: vas deferens, s: sperm.
Furthermore, we investigated the effect of dsNlPCE3 on sperm. We found sperm in the vas deferens was active, and there was a large number of sperm in the testes of dsNlPCE3-treated males (Figure 5C,F) and in the spermathecae and bursae copulatrix of untreated female adults that mated with dsNlPCE3-treated males (Figure 6).
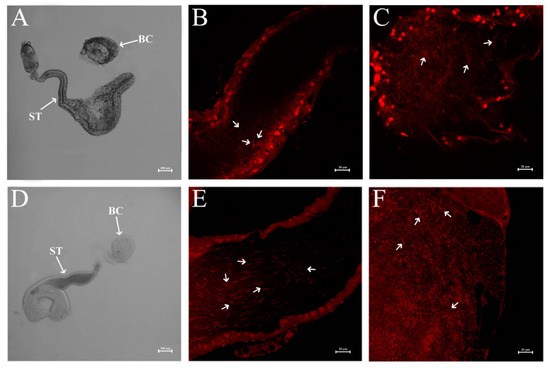
Figure 6.
Sperm in the spermatheca and bursa copulatrix. (A) Spermatheca and bursa copulatrix of an untreated female that mated with dsGFP-treated males. (B) Sperm in the spermatheca of an untreated female that mated with dsGFP-treated males. (C) Sperm in the bursa copulatrix of an untreated female that mated with dsGFP-treated males. (D) Spermatheca and bursa copulatrix of an untreated female that mated with dsNlPCE3-treated males. (E) Sperm in the spermatheca of an untreated female that mated with dsNlPCE3-treated males. (F) Sperm in the bursa copulatrix of an untreated female that mated with dsNlPCE3-treated males. Nuclei were stained with propidium iodide. ST: spermatheca, BC: bursa copulatrix. White arrows indicate sperm in (B,C,E,F).
4. Discussion
In internally fertilizing species, seminal fluid proteins (SFPs) are transferred with sperm from males to their mates during copulation. These proteins play significant roles in the process of reproduction, including sperm capacitation, sperm storage and competition and fertilization; they can also influence female behavior and physiology in some cases [22,23,24]. Proteolysis regulators (proteases and their inhibitors) are a component of the seminal fluid of many animals regulating functions of the proteins necessary for male or female reproduction by degradation or removal of inhibitory peptides or through processing of prohormones [25]. The predicted proteolysis regulators in humans and insects mainly include SPs, cysteine proteases, threonine proteases and SP inhibitors [26]. The SPs and SP inhibitors are the most common types of proteases and protease inhibitors, respectively [25].
In insects, SFPs have been proven to be one of the seminal fluid substances involved in the regulation of reproduction. Females have higher levels of oogenesis and oviposition when they receive SFPs [27]. SFPs of the SP family have been detected in male gonads of D. melanogaster, and these proteins affect female oviposition through proteolysis cascade regulation [28]. SPs in males were demonstrated to induce oviposition by females, with SPs and SP inhibitors playing a key role in the phenotypic changes between pre-fertilization and post-mating eggs [29]. D. melanogaster females that mated with males whose seminase (a predicted SP) was knocked down by RNAi failed to maintain a high level of oviposition [25]. In Allonemobius socius, RNAi knockdown of the ejaculate serine protease in males weakened their ability to induce female oviposition [30]. SPs are also an energy source of sperm motility, inducing sperm movement [31]. In M. sexta, sperm motility depends on the proteases secreted from the male reproductive tract [32]. In Aquarius remigis, a trypsase plays important roles in activating the signal pathway of sperm motility [33].
In the present study, we found that NlPCE3 plays an important role in the reproduction of male BPH. First, we found that female adults that mated with dsNlPCE3-treated male adults laid fewer eggs than those that mated with dsGFP-treated males, and more than half of these eggs could not hatch and lacked eye-spots. However, we also found a large amount of sperm in the spermathecae and bursae copulatrix of the mating females. These results demonstrate that although sperm could still be transmitted from a male to a female after dsNlPCE3 treatment, male reproductive capability was affected. NlPCE3 belongs to the clip-SP family, which has only been identified in arthropods [1]. The catalytically active structure of NlPCE3 is the C-terminal SP domain, and the clip domain’s function may be to regulate the activity of SP in the last step of the insect SP pathway [3,7]. NlPCE3 may participate in sperm capacitation, increasing the odds of successful fertilization. Alternatively, NlPCE3 may participate in the functional regulation of SFPs of male BPH as a proteolysis regulator, enhancing the ability of male-to-female oviposition induction. Second, we also observed that the male vas deferens became thinner after RNAi, and the peripheral cells seemed degraded. Even though the size of the testes appeared not affected by dsNlPCE3 treatment, it cannot be excluded that the dsNIPCE3-treated males had delayed sexual maturation or development, since the vas deferens was thinner. It is questioned whether this weakness of the vas deferens might affect transmission or ejaculation efficiency of the sperm. We tried to compare the amount of sperm produced by dsGFP-treated and dsNIPCE3-treated males, but, regrettably, there is no effective method to quantify the amount of sperm. In female BPH, we also found that the follicular cells outside the terminal follicle were destroyed when the expression of NlPCE3 in follicular cells was disrupted by RNAi, and ovarian development was prominently inhibited [19]. Taken together, these results indicate that NlPCE3 affects development of both male and female internal genitalia. Since NlPCE3 is so important for BPH development, it is a possible target for controlling BPH.
5. Conclusions
In general, although the main functions of clip-SPs in insects have been studied, a full understanding of the role of clip-SPs remains incomplete. In a previous study, we found NlPCE3 was involved in egg production of female BPH [19]. In the present study, we show that NlPCE3 also plays an important role in the reproduction of male BPH. These results provide important evidence for revealing more functions of clip-SPs in insects. However, the tissue-specific expression of NlPCE3 differs between adult female and male BPH. In adult females, NlPCE3 expression is higher in the gut and fat body than in the ovaries, but in adult males, it is higher in the gut than in the fat body and testis. The reason for this difference between the sexes requires further study. Furthermore, as NlPCE3’s specific molecular interaction mechanism in BPH remains unknown, future work will focus on identifying the interacting proteins of NlPCE3 to reveal their interaction mechanism during the PCE3 functioning in BPH reproduction.
Supplementary Materials
The following are available online at https://www.mdpi.com/2075-4450/12/2/114/s1, Table S1: The primers used in this study.
Author Contributions
Conceptualization, R.-e.Z., J.W. and Y.X.; data curation, R.-e.Z. and J.W.; investigation, R.-e.Z., J.J., J.W., R.Z. and Y.L.; supervision, X.Y. and Y.X.; validation, R.-e.Z., J.J. and J.W.; writing—original draft, R.-e.Z.; writing—review and editing, Y.X. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (31871961 and 31501632) and a grant from the Zhejiang Provincial Key R&D Project (2019C02015).
Institutional Review Board Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Veillard, F.; Troxler, L.; Reichhart, J.M. Drosophila melanogaster clip-domain serine proteases: Structure, function and regulation. Biochimie 2016, 122, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Volz, J.; Müller, H.M.; Zdanowicz, A.; Kafatos, F.C.; Osta, M.A. A genetic module regulates the melanization response of Anopheles to Plasmodium. Cell Microbiol. 2006, 8, 1392–1405. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Kanost, M.R. The clip-domain family of serine proteinases in arthropods. Insect Biochem. Mol. Biol. 2000, 30, 95–105. [Google Scholar] [CrossRef]
- Kanost, M.R.; Jiang, H. Clip-domain serine proteases as immune factors in insect hemolymph. Curr. Opin. Insect Sci. 2015, 11, 47–55. [Google Scholar] [CrossRef]
- Hedstrom, L. Serine protease mechanism and specificity. Chem. Rev. 2002, 102, 4501–4524. [Google Scholar] [CrossRef]
- Cao, X.; He, Y.; Hu, Y.; Zhang, X.; Wang, Y.; Zou, Z.; Chen, Y.; Blissard, G.W.; Kanost, M.R.; Jiang, H. Sequence conservation, phylogenetic relationships, and expression profiles of nondigestive serine proteases and serine protease homologs in Manduca sexta. Insect Biochem. Mol. Biol. 2015, 62, 51–63. [Google Scholar] [CrossRef]
- Ross, J.; Jiang, H.; Kanost, M.R.; Wang, Y. Serine proteases and their homologs in the Drosophila melanogaster genome: An initial analysis of sequence conservation and phylogenetic relationships. Gene 2003, 304, 117–131. [Google Scholar] [CrossRef]
- Waterhouse, R.M.; Kriventseva, E.V.; Meister, S.; Xi, Z.; Alvarez, K.S.; Bartholomay, L.C.; Barillas-Mury, C.; Bian, G.; Blandin, S.; Christensen, B.M.; et al. Evolutionary dynamics of immune-related genes and pathways in disease-vector mosquitoes. Science 2007, 316, 1738–1743. [Google Scholar] [CrossRef]
- Moussian, B.; Roth, S. Dorsoventral axis formation in the Drosophila embryo-shaping and transducing a morphogen gradient. Curr. Biol. 2005, 15, R887–R899. [Google Scholar] [CrossRef]
- Zou, Z.; Lopez, D.L.; Kanost, M.R.; Evans, J.D.; Jiang, H. Comparative analysis of serine protease-related genes in the honey bee genome: Possible involvement in embryonic development and innate immunity. Insect Mol. Biol. 2006, 15, 603–614. [Google Scholar] [CrossRef]
- Krem, M.M.; Di Cera, E. Evolution of enzyme cascades from embryonic development to blood coagulation. Trends Biochem. Sci. 2002, 27, 67–74. [Google Scholar] [CrossRef]
- Tang, H. Regulation and function of the melanization reaction in Drosophila. Fly (Austin) 2009, 3, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Wang, Y.; Sumathipala, N.; Cao, X.; Kanost, M.R.; Jiang, H. Manduca sexta serpin-12 controls the prophenoloxidase activation system in larval hemolymph. Insect Biochem. Mol. Biol. 2018, 99, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Chen, F.; Wang, W.; Xu, L.; Lu, Z.Q. Identification of two clip domain serine proteases involved in the pea aphid’s defense against bacterial and fungal infection. Insect Sci. 2020, 27, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wan, W.; Kong, T.; Zhang, M.; Aweya, J.J.; Gong, Y.; Li, S. A clip domain serine protease regulates the expression of proPO and hemolymph clotting in mud crab, Scylla paramamosain. Fish Shellfish Immunol. 2018, 79, 52–64. [Google Scholar] [CrossRef]
- Gao, L.; Wang, H.; Liu, Z.; Liu, S.; Zhao, G.; Xu, B.; Guo, X. The initial analysis of a serine proteinase gene (AccSp10) from Apis cerana cerana: Possible involvement in pupal development, innate immunity and abiotic stress responses. Cell Stress Chaperones 2017, 22, 867–877. [Google Scholar] [CrossRef]
- Jia, Z.; Wang, M.; Zhang, H.; Wang, X.; Lv, Z.; Wang, L.; Song, L. Identification of a clip domain serine proteinase involved in immune defense in Chinese mitten crab Eriocheir sinensis. Fish Shellfish Immunol. 2018, 74, 332–340. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Y.; Song, C.; Ning, J.; Cui, Z. Functional characterization of two clip-domain serine proteases in the swimming crab Portunus trituberculatus. Fish Shellfish Immunol. 2019, 89, 98–107. [Google Scholar] [CrossRef]
- Wu, J.M.; Zheng, R.E.; Zhang, R.J.; Ji, J.L.; Yu, X.P.; Xu, Y.P. A clip domain serine protease involved in egg production in Nilaparvata lugens: Expression patterns and RNA interference. Insects 2019, 10, 378. [Google Scholar] [CrossRef]
- Nan, G.H.; Xu, Y.P.; Yu, Y.W.; Zhao, C.X.; Zhang, C.X.; Yu, X.P. Oocyte vitellogenesis triggers the entry of yeast-like symbionts into the oocyte of brown planthopper (Hemiptera: Delphacidae). Ann. Entomol. Soc. Am. 2016, 109, 753–758. [Google Scholar] [CrossRef]
- Fan, X.B.; Pang, R.; Li, W.X.; Ojha, A.; Li, D.; Zhang, W.Q. An Overview of Embryogenesis: External Morphology and Transcriptome Profiling in the Hemipteran Insect Nilaparvata lugens. Front. Physiol. 2020, 11, 106. [Google Scholar] [CrossRef] [PubMed]
- Poiani, A. Complexity of seminal fluid: A review. Behav. Ecol. Sociobiol. 2006, 60, 289–310. [Google Scholar] [CrossRef]
- Sirot, L.K.; LaFlamme, B.A.; Sitnik, J.L.; Rubinstein, C.D.; Avila, F.W.; Chow, C.Y.; Wolfner, M.F. Molecular social interactions: Drosophila melanogaster seminal fluid proteins as a case study. Adv. Genet. 2009, 68, 23–56. [Google Scholar] [PubMed]
- Zhao, Y.; Sun, W.; Zhang, P.; Chi, H.; Zhang, M.J.; Song, C.Q.; Ma, X.; Shang, Y.; Wang, B.; Hu, Y.; et al. Nematode sperm maturation triggered by protease involves sperm-secreted serine protease inhibitor (Serpin). Proc. Natl. Acad. Sci. USA 2012, 109, 1542–1547. [Google Scholar] [CrossRef]
- Laflamme, B.A.; Wolfner, M.F. Identification and function of proteolysis regulators in seminal fluid. Mol. Reprod. Dev. 2013, 80, 80–101. [Google Scholar] [CrossRef]
- Ravi Ram, K.; Sirot, L.K.; Wolfner, M.F. Predicted seminal astacin-like protease is required for processing of reproductive proteins in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2006, 103, 18674–18679. [Google Scholar] [CrossRef]
- Avila, F.W.; Sirot, L.K.; LaFlamme, B.A.; Rubinstein, C.D.; Wolfner, M.F. Insect seminal fluid proteins: Identification and function. Annu. Rev. Entomol. 2011, 56, 21–40. [Google Scholar] [CrossRef]
- LaFlamme, B.A.; Ram, K.R.; Wolfner, M.F. The Drosophila melanogaster seminal fluid protease "seminase" regulates proteolytic and post-mating reproductive processes. PLoS Genet. 2012, 8, e1002435. [Google Scholar] [CrossRef]
- Wong, A.; Turchin, M.C.; Wolfner, M.F.; Aquadro, C.F. Evidence for positive selection on Drosophila melanogaster seminal fluid protease homologs. Mol. Biol Evol. 2008, 25, 497–506. [Google Scholar] [CrossRef]
- Marshall, J.L.; Huestis, D.L.; Hiromasa, Y.; Wheeler, S.; Oppert, C.; Marshall, S.A.; Tomich, J.M.; Oppert, B. Identification, RNAi knockdown, and functional analysis of an ejaculate protein that mediates a postmating, prezygotic phenotype in a cricket. PLoS ONE 2009, 4, e7537. [Google Scholar] [CrossRef]
- Nagaoka, S.; Kato, K.; Takata, Y.; Kamei, K. Identification of the sperm-activating factor initiatorin, a prostatic endopeptidase of the silkworm, Bombyx mori. Insect Biochem. Mol. Biol. 2012, 42, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Friedländer, M.; Jeshtadi, A.; Reynolds, S.E. The structural mechanism of trypsin-induced intrinsic motility in Manduca sexta spermatozoa in vitro. J. Insect Physiol. 2001, 47, 245–255. [Google Scholar] [CrossRef]
- Miyata, H.; Thaler, C.D.; Haimo, L.T.; Cardullo, R.A. Protease activation and the signal transduction pathway regulating motility in sperm from the water strider Aquarius remigis. Cytoskeleton (Hoboken) 2012, 69, 207–220. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).