Recent Advancements in Studies on Chemosensory Mechanisms Underlying Detection of Semiochemicals in Dacini Fruit Flies of Economic Importance (Diptera: Tephritidae)
Abstract
Simple Summary
Abstract
1. Introduction
2. Semiochemicals Involved in the Life Cycle of Dacini Fruit Flies
2.1. Male Attractants and Sex Pheromones
2.2. Common Plant Volatiles Other Than Phenylpropanoids and Phenylbutanoids as Attractants
3. Detection of Male Attractants by the Peripheral Sensory Organs
4. Chemosensory-Related Behaviors Modulated by Physiological Status
5. Major Molecular Components in Insect Chemoreception
5.1. OBPs
5.2. ORs
5.3. Other Chemosensory Receptors
6. Future Research
6.1. Characterization of Chemosensory Receptors Responding to Male Attractants and Sex Pheromones
6.2. Characterization of Chemosensory Receptors Responding to Common Plant Volatiles
6.3. Information Processing of Chemosensory Inputs
6.4. Mechanisms of Physiological Change Associated with Mating Behavior
6.5. New Approaches Such as Promoter Analysis and Ectopic Expression Using New Genetic Techniques
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Krosch, M.N.; Schutze, M.K.; Armstrong, K.F.; Graham, G.C.; Yeates, D.K.; Clarke, A.R. A molecular phylogeny for the Tribe Dacini (Diptera: Tephritidae): Systematic and biogeographic implications. Mol. Phylogenet. Evol. 2012, 64, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Bernays, E.A.; Chapman, R.F. Host-Plant Selection by Phytophagous Insects; Chapman & Hall: New York, NY, USA, 1994; ISBN 9780585304557. [Google Scholar]
- Tan, K.H.; Nishida, R. Methyl eugenol: Its occurrence, distribution, and role in nature, especially in relation to insect behavior and pollination. J. Insect Sci. 2012, 12, 56. [Google Scholar] [CrossRef] [PubMed]
- Steiner, L.F.; Mitchell, W.C.; Harris, E.J.; Kozuma, T.T.; Fujimoto, M.S. Oriental fruit fly eradication by male annihilation. J. Econ. Entomol. 1965, 58, 961–964. [Google Scholar] [CrossRef]
- Steiner, L.F.; Hart, W.G.; Harris, E.J.; Cunningham, R.T.; Ohinata, K.; Kamakahi, D.C. Eradication of the oriental fruit fly from the Mariana Islands by the methods of male annihilation and sterile insect release. J. Econ. Entomol. 1970, 63, 131–135. [Google Scholar] [CrossRef]
- Koyama, J.; Teruya, T.; Tanaka, K. Eradication of the oriental fruit fly (Diptera: Tephritidae) from the Okinawa Islands by a male annihilation method. J. Econ. Entomol. 1984, 77, 468–472. [Google Scholar] [CrossRef]
- Siderhurst, M.; Jang, E. Female-biased attraction of oriental fruit fly, Bactrocera dorsalis (Hendel), to a blend of host fruit volatiles from Terminalia catappa L. J. Chem. Ecol. 2006, 32, 2513–2524. [Google Scholar] [CrossRef]
- Siderhurst, M.; Jang, E. Attraction of female oriental fruit fly, Bactrocera dorsalis, to Terminalia catappa fruit extracts in wind tunnel and olfactometer tests. Formos. Entomol. 2006, 26, 45–55. [Google Scholar]
- Kamala Jayanthi, P.D.; Woodcock, C.M.; Caulfield, J.; Birkett, M.A.; Bruce, T.J.A. Isolation and identification of host cues from mango, Mangifera indica, that attract gravid female oriental fruit fly, Bactrocera dorsalis. J. Chem. Ecol. 2012, 38, 361–369. [Google Scholar] [CrossRef]
- Kamala Jayanthi, P.D.; Kempraj, V.; Aurade, R.M.; Venkataramanappa, R.K.; Nandagopal, B.; Verghese, A.; Bruce, T.J.A. Specific volatile compounds from mango elicit oviposition in gravid Bactrocera dorsalis females. J. Chem. Ecol. 2014, 40, 259–266. [Google Scholar] [CrossRef]
- Biasazin, T.D.; Karlsson, M.F.; Hillbur, Y.; Seyoum, E.; Dekker, T. Identification of host blends that attract the african invasive fruit fly, Bactrocera invadens. J. Chem. Ecol. 2014, 40, 966–976. [Google Scholar] [CrossRef]
- Nishida, R.; Tan, K.H. Search for new fruit fly attractants from plants: A review. In Proceedings of the 9th International Symposium on Fruit Flies of Economic Importance, Bangkok, Thailand, 12 May 2014; pp. 249–262. [Google Scholar]
- Tan, K.H.; Nishida, R.; Jang, E.B.; Shelly, T.E. Pheromones, Male Lures, and Trapping of Tephritid Fruit Flies; Springer: Dordrecht, The Netherlands, 2014; ISBN 9789401791922. [Google Scholar]
- Shelly, T. Effects of methyl eugenol and raspberry ketone/cue lure on the sexual behavior of Bactrocera species (Diptera: Tephritidae). Appl. Entomol. Zool. 2010, 45, 349–361. [Google Scholar] [CrossRef]
- Howlett, F.M. VII. The effect of oil of Citronella on two species of Dacus. Trans. R. Entomol. Soc. Lond. 1912, 60, 412–418. [Google Scholar] [CrossRef]
- Howlett, F. Chemical reactions of fruit-flies. Bull. Entomol. Res. 1915, 6, 297–305. [Google Scholar] [CrossRef]
- Royer, J.E.; Khan, M.; Mayer, D.G. Methyl-isoeugenol, a highly attractive male lure for the cucurbit flower pest Zeugodacus diversus (coquillett) (syn. Bactrocera diversa) (Diptera: Tephritidae: Dacinae). J. Econ. Entomol. 2018, 111, 1197–1201. [Google Scholar] [CrossRef]
- Drew, R.A.I.; Hooper, G.H.S. The responses of fruit fly species (Diptera: Tephritidae) in Australia to various attractants. Aust. J. Entomol. 1981, 20, 201–205. [Google Scholar] [CrossRef]
- Drew, R.A.I. The responses of fruit fly species (Diptera: Tephritidae) in the South Pacific area to male attractants. Aust. J. Entomol. 1974, 13, 267–270. [Google Scholar] [CrossRef]
- Tan, K.H.; Nishida, R. Synomone or kairomone?—Bulbophyllum apertum flower releases raspberry ketone to attract Bactrocera fruit flies. J. Chem. Ecol. 2005, 31, 497–507. [Google Scholar] [CrossRef]
- Tan, K.; Nishida, R.; Toong, Y. Floral synomone of a wild orchid, Bulbophyllum cheiri, lures Bactrocera fruit flies for pollination. J. Chem. Ecol. Ecol. 2002, 28, 1161–1172. [Google Scholar] [CrossRef]
- Beroza, M.; Alexanderl, B.H.; Steiner, F.; Mitchell, W.C.; Miyashita, D.H. New synthetic lures for the male melon fly. Science 1960, 131, 1044–1045. [Google Scholar] [CrossRef]
- Tan, K.H.; Nishida, R. Mutual reproductive benefits between a wild orchid, Bulbophyllum patens, and Bactrocera fruit flies via a floral synomone. J. Chem. Ecol. 2000, 26, 533–546. [Google Scholar] [CrossRef]
- Nishida, R.; Tan, K.H.; Serit, M.; Lajis, N.H.; Sukari, A.M.; Takahashi, S.; Fukami, H. Accumulation of phenylpropanoids in the rectal glands of males of the oriental fruit fly, Dacus dorsalis. Experientia 1988, 44, 534–536. [Google Scholar] [CrossRef]
- Fletcher, B.S. Storage and release of a sex pheromone by the Queensland fruit fly, Dacus tryoni (Diptera: Trypetidae). Nature 1968, 219, 631–632. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, B.S. The structure and function of the sex pheromone glands of the male Queensland fruit fly, Dacus tryoni. J. Insect Physiol. 1969, 15, 1309–1322. [Google Scholar] [CrossRef]
- Nishida, R.; Iwahashi, O.; Tan, K.H. Accumulation of Dendrobium superbum (Orchidaceae) fragrance in the rectal glands by males of the melon fly, Dacus cucurbitae. J. Chem. Ecol. 1993, 19, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.H.; Nishida, R. Incorporation of raspberry ketone in the rectal glands of males of the Queensland fruit fly, Bactrocera tryoni Froggatt (Diptera: Tephritidae). Appl. Entomol. Zool. 1995, 30, 494–497. [Google Scholar] [CrossRef][Green Version]
- Kobayashi, R.M.; Ohinata, K.; Chambers, D.L.; Fujimoto, M.S. Sex pheromones of the oriental fruit fly and the melon fly: Mating behavior, bioassay method, and attraction of females by live males and by suspected pheromone glands of males. Environ. Entomol. 1978, 7, 107–112. [Google Scholar] [CrossRef]
- Khoo, C.C.H.; Yuen, K.H.; Tan, K.H. Attraction of female Bactrocera papayae to sex pheromone components with two different release devices. J. Chem. Ecol. 2000, 26, 2487–2496. [Google Scholar] [CrossRef]
- Hee, A.K.W.; Tan, K.H. Attraction of female and male Bactrocera papayae to conspecific males fed with methyl eugenol and attraction of females to male sex pheromone components. J. Chem. Ecol. 1998, 24, 753–764. [Google Scholar] [CrossRef]
- Ohinata, K.; Jacobson, M.; Kobayashi, R.M.; Chambers, D.L.; Fujimoto, M.S.; Higa, H.H. Oriental fruit fly and melon fly; biological and chemical studies of smoke produced by males. J. Environ. Sci. Health Part A Environ. Sci. Eng. 1982, 17, 197–216. [Google Scholar] [CrossRef]
- Khoo, C.C.H.; Tan, K.H. Attraction of both sexes of melon fly, Bactrocera cucurbitae to conspecific males—A comparison after pharmacophagy of cue-lure and a new attractant—Zingerone. Entomol. Exp. Appl. 2000, 97, 317–320. [Google Scholar] [CrossRef]
- Haniotakis, G.E.; Mazomenos, B.E.; Tumlinson, J.H. A sex attractant of the olive fruit fly, Dacus oleae and its biological activity under laboratory and field conditions. Entomol. Exp. Appl. 1977, 21, 81–87. [Google Scholar] [CrossRef]
- Baker, R.; Herbert, R.; Howse, P.E.; Jones, O.T.; Francke, W.; Reith, W. Identification and synthesis of the major sex pheromone of the olive fly (Dacus oleae). J. Chem. Soc. Chem. Commun. 1980, 2, 52–53. [Google Scholar] [CrossRef]
- Mazomenos, B.E.; Haniotakis, G.E. Male olive fruit fly attraction to synthetic sex pheromone components in laboratory and field tests. J. Chem. Ecol. 1985, 11, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Haniotakis, G.; Francke, W.; Mori, K.; Redlich, H.; Schurig, V. Sex-specific activity of (R)-(-)- and (S)- (+)-1,7-dioxaspiro[5.5]undecane, the major pheromone of Dacus oleae. J. Chem. Ecol. 1986, 12, 1559–1568. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, M.T.; Kitching, W. Chemistry of fruit flies. Chem. Rev. 1995, 95, 789–828. [Google Scholar] [CrossRef]
- Quilici, S.; Atiama-Nurbel, T.; Brévault, T. Plant odors as fruit fly attractants. In Trapping and the Detection, Control, and Regulation of Tephritid Fruit Flies; Shelly, T.E., Epsky, N., Jang, E.B., Reyes-Flores, J., Vargas, R.I., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 119–144. ISBN 978-94-017-9192-2. [Google Scholar]
- Light, D.M.; Jang, E.B. Electroantennogram responses of the oriental fruit fly, Dacus dorsalis, to a spectrum of alcohol and aldehyde plant volatiles. Entomol. Exp. Appl. 1987, 45, 55–64. [Google Scholar] [CrossRef]
- Damodaram, K.J.P.; Kempraj, V.; Aurade, R.M.; Venkataramanappa, R.K.; Nandagopal, B.; Verghese, A.; Bruce, T. Oviposition site-selection by Bactrocera dorsalis is mediated through an innate recognition template tuned to γ-octalactone. PLoS ONE 2014, 9, e85764. [Google Scholar] [CrossRef]
- Liu, Z.; Smagghe, G.; Lei, Z.; Wang, J.J. Identification of male- and female-specific olfaction genes in antennae of the oriental fruit fly (Bactrocera dorsalis). PLoS ONE 2016, 11, e0147783. [Google Scholar] [CrossRef]
- Tokushima, I.; Orankanok, W.; Tan, K.H.; Ono, H.; Nishida, R. Accumulation of phenylpropanoid and sesquiterpenoid volatiles in male rectal pheromonal glands of the guava fruit fly, Bactrocera correcta. J. Chem. Ecol. 2010, 36, 1327–1334. [Google Scholar] [CrossRef]
- Wee, S.L.; Chinvinijkul, S.; Tan, K.H.; Nishida, R. A new and highly effective male lure for the guava fruit fly Bactrocera correcta. J. Pest. Sci. 2018, 91, 691–698. [Google Scholar] [CrossRef]
- Kamiji, T.; Kaneda, M.; Sasaki, M.; Ohto, K. Sexual maturation of male Bactrocera correcta (Diptera: Tephritidae) and age-related responses to β-caryophyllene and methyl eugenol. Appl. Entomol. Zool. 2018, 53, 41–46. [Google Scholar] [CrossRef]
- Jaleel, W.; He, Y.; Lü, L. The response of two Bactrocera species (Diptera: Tephritidae) to fruit volatiles. J. Asia. Pac. Entomol. 2019, 22, 758–765. [Google Scholar] [CrossRef]
- Flath, R.A.; Cunningham, R.T.; Liquido, N.; McGovern, T.P. Alpha-ionol as attractant for trapping Bactrocera latifrons (Diptera: Tephritidae). J. Econ. Entomol. 1994, 87, 1470–1476. [Google Scholar] [CrossRef]
- Ishida, T.; Enomoto, H.; Nishida, R. New attractants for males of the solanaceous fruit fly Bactrocera latifrons. J. Chem. Ecol. 2008, 34, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Nishida, R.; Enomoto, H.; Shelly, T.E.; Ishida, T. Sequestration of 3-oxygenated α-ionone derivatives in the male rectal gland of the solanaceous fruit fly, Bactrocera latifrons. Entomol. Exp. Appl. 2009, 131, 85–92. [Google Scholar] [CrossRef]
- Enomoto, H.; Ishida, T.; Hamagami, A.; Nishida, R. 3-Oxygenated α-ionone derivatives as potent male attractants for the solanaceous fruit fly, Bactrocera latifrons (Diptera: Tephritidae), and sequestered metabolites in the rectal gland. Appl. Entomol. Zool. 2010, 45, 551–556. [Google Scholar] [CrossRef]
- Vosshall, L.B.; Stocker, R.F. Molecular architecture of smell and taste in Drosophila. Annu. Rev. Neurosci. 2007, 30, 505–533. [Google Scholar] [CrossRef]
- Chieng, A.C.T.; Hee, A.K.W.; Wee, S.L. Involvement of the antennal and maxillary palp structures in detection and response to methyl eugenol by male Bactrocera dorsalis (Diptera: Tephritidae). J. Insect Sci. 2018, 18, 19. [Google Scholar] [CrossRef]
- Verschut, T.A.; Farnier, K.; Cunningham, J.P.; Carlsson, M.A. Behavioral and physiological evidence for palp detection of the male-specific attractant cuelure in the Queensland fruit fly (Bactrocera tryoni). Front. Physiol. 2018, 9, 990. [Google Scholar] [CrossRef]
- Park, K.C.; Jeong, S.A.; Kwon, G.; Oh, H.W. Olfactory attraction mediated by the maxillary palps in the striped fruit fly, Bactrocera scutellata: Electrophysiological and behavioral study. Arch. Insect Biochem. Physiol. 2018, 99, 1–13. [Google Scholar] [CrossRef]
- Shiraiwa, T. Multimodal chemosensory integration through the maxillary palp in Drosophila. PLoS ONE 2008, 3, e2191. [Google Scholar] [CrossRef] [PubMed]
- Maeda, T.; Tamotsu, S.; Iwasaki, M.; Nisimura, T.; Shimohigashi, M.; Hojo, M.K.; Ozaki, M. Neuronal projections and putative interaction of multimodal inputs in the subesophageal ganglion in the blowfly, Phormia regina. Chem. Senses 2014, 39, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, E.S.; Lettvin, J.Y.; Roeder, K.D. Physiology of a primary chemoreceptor unit. Science 1955, 122, 417–418. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Fleischer, F.; Piñero, J.; Shelly, T. Interactions between tephritid fruit fly physiological state and stimuli from baits and traps: Looking for the pied piper of hamelin to lure pestiferous fruit flies. In Trapping and the Detection, Control, and Regulation of Tephritid Fruit Flies; Shelly, T., Epsky, N., Jang, E., Reyes-Flores, J., Vargas, R., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 145–172. [Google Scholar]
- Gadenne, C.; Barrozo, R.B.; Anton, S. Plasticity in insect olfaction: To smell or not to smell? Annu. Rev. Entomol. 2016, 61, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Manoukis, N.C.; Jang, E.B. The diurnal rhythmicity of Bactrocera cucurbitae (Diptera: Tephritidae) attraction to cuelure: Insights from an interruptable lure and computer vision. Ann. Entomol. Soc. Am. 2013, 106, 136–142. [Google Scholar] [CrossRef]
- Raghu, S.; Clarke, A.R. Spatial and temporal partitioning of behaviour by adult dacines: Direct evidence for methyl eugenol as a mate rendezvous cue for Bactrocera cacuminata. Physiol. Entomol. 2003, 28, 175–184. [Google Scholar] [CrossRef]
- Fitt, G.P. The influence of age, nutrition and time of day on the responsiveness of male Dacus opiliae to the synthetic lure, methyl eugenol. Entomol. Exp. Appl. 1981, 30, 83–90. [Google Scholar] [CrossRef]
- Wee, S.L.; Hee, A.K.W. Diurnal attraction of fruit flies (Diptera: Tephritidae) to methyl eugenol in a village ecosystem in Tanjung Bungah, Penang, Malaysia. Serangga 2018, 23, 83–91. [Google Scholar]
- Hiap, W.W.; Wee, S.L.; Tan, K.H.; Hee, A.K.W. Phenylpropanoid sex pheromone component in hemolymph of male Carambola fruit fly, Bactrocera carambolae (Diptera: Tephritidae). Chemoecology 2019, 29, 25–34. [Google Scholar] [CrossRef]
- Hee, A.K.; Tan, K.H. Transport of methyl eugenol-derived sex pheromonal components in the male fruit fly, Bactrocera dorsalis. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2006, 143, 422–428. [Google Scholar] [CrossRef]
- Hee, A.K.W.; Tan, K.H. Bioactive fractions containing methyl eugenol-derived sex pheromonal components in haemolymph of the male fruit fly Bactrocera dorsalis (Diptera: Tephritidae). Bull. Entomol. Res. 2005, 95, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Hee, A.K.; Tan, K. Male sex pheromonal components derived from methyl eugenol in the hemolymph of the fruit fly Bactrocera papayae. J. Chem. Ecol. 2004, 30, 2127–2138. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.; Kirton, L.; Serit, M. Age response of Dacus dorsalis (Hendel) to methyl eugenol in A) a wind tunnel and B) traps set in a village, and its implication in population estimation. In Fruit Flies: Proceedings of the Second International Symposium; Economopoulos, A.P., Ed.; Elsevier: New York, NY, USA, 1987; pp. 425–432. ISBN 978-0444989468. [Google Scholar]
- Wong, T.T.Y.; McInnis, D.O.; Nishimoto, J.I. Relationship of sexual maturation rate to response of oriental fruit fly strains (Diptera: Tephritidae) to methyl eugenol. J. Chem. Ecol. 1989, 15, 1399–1405. [Google Scholar] [CrossRef] [PubMed]
- Shelly, T.E.; Edu, J.; Pahio, E.; Wee, S.L.; Nishida, R. Re-examining the relationship between sexual maturation and age of response to methyl eugenol in males of the oriental fruit fly. Entomol. Exp. Appl. 2008, 128, 380–388. [Google Scholar] [CrossRef]
- Wee, S.L.; Tan, K.H. Sexual maturity and intraspecific mating success of two sibling species of the Bactrocera dorsalis complex. Entomol. Exp. Appl. 2000, 94, 133–139. [Google Scholar] [CrossRef]
- Wong, T.T.Y.; McInnis, D.O.; Ramadan, M.M.; Nishimoto, J.I. Age-related response of male melon flies Dacus cucurbitae (Diptera: Tephritidae) to cue-lure. J. Chem. Ecol. 1991, 17, 2481–2487. [Google Scholar] [CrossRef] [PubMed]
- Wee, S.L.; Abdul Munir, M.Z.; Hee, A.K.W. Attraction and consumption of methyl eugenol by male Bactrocera umbrosa Fabricius (Diptera: Tephritidae) promotes conspecific sexual communication and mating performance. Bull. Entomol. Res. 2018, 108, 116–124. [Google Scholar] [CrossRef]
- Wee, S.L.; Peek, T.; Clarke, A.R. The responsiveness of Bactrocera jarvisi (Diptera: Tephritidae) to two naturally occurring phenylbutaonids, zingerone and raspberry ketone. J. Insect Physiol. 2018, 109, 41–46. [Google Scholar] [CrossRef]
- Roan, C.C.; Flitters, N.E.; Davis, C.J. Light intensity and temperature as factors limiting the mating of the oriental fruit fly. Ann. Entomol. Soc. Am. 1954, 47, 593–594. [Google Scholar] [CrossRef]
- Arakaki, N.; Kuba, H.; Soemori, H. Mating behavior of the oriental fruit fly, Dacus dorsalis Hendel (Diptera: Tephritidae). Appl. Entomol. Zool. 1984, 19, 42–51. [Google Scholar] [CrossRef]
- Kokkari, A.I.; Pliakou, O.D.; Floros, G.D.; Kouloussis, N.A.; Koveos, D.S. Effect of fruit volatiles and light intensity on the reproduction of Bactrocera (Dacus) oleae. J. Appl. Entomol. 2017, 141, 841–847. [Google Scholar] [CrossRef]
- Jang, E.B. Effects of mating and accessory gland injections on olfactory-mediated behavior in the female Mediterranean fruit fly, Ceratitis capitata. J. Insect Physiol. 1995, 41, 705–710. [Google Scholar] [CrossRef]
- Cornelius, M.L.; Nergel, L.; Duan, J.J.; Messing, R.H. Responses of female oriental fruit flies (Diptera: Tephritidae) to protein and host fruit odors in field cage and open field tests. Environ. Entomol. 2000, 29, 14–19. [Google Scholar] [CrossRef][Green Version]
- Hagen, K.S.; Finney, G.L. A food supplement for effectively increasing the fecundity of certain Tephritid species. J. Econ. Entomol. 1950, 43, 735. [Google Scholar] [CrossRef]
- Perez-Staples, D.; Prabhu, V.; Taylor, P.W. Post-teneral protein feeding enhances sexual performance of Queensland fruit flies. Physiol. Entomol. 2007, 32, 225–232. [Google Scholar] [CrossRef]
- Shelly, T.E.; Edu, J.; Pahio, E. Influence of diet and methyl eugenol on the mating success of males of the oriental fruit fly, Bactrocera dorsalis (Diptera: Tephritidae). Fla. Entomol. 2005, 88, 307–313. [Google Scholar] [CrossRef]
- Shelly, T.E.; Edu, J.; Pahio, E. Condition-dependent mating success in male fruit flies: Ingestion of a pheromone precursor compensates for a low-quality diet. J. Insect Behav. 2007, 20, 347–365. [Google Scholar] [CrossRef]
- Weldon, C.W.; Perez-Staples, D.; Taylor, P.W. Feeding on yeast hydrolysate enhances attraction to cue-lure in Queensland fruit flies, Bactrocera tryoni. Entomol. Exp. Appl. 2008, 129, 200–209. [Google Scholar] [CrossRef]
- Shelly, T.E.; Dewire, A.L.M. Chemically mediated mating success in male oriental fruit flies (Diptera: Tephritidae). Ann. Entomol. Soc. Am. 1994, 87, 375–382. [Google Scholar] [CrossRef]
- Khan, M.A.M.; Shuttleworth, L.A.; Osborne, T.; Collins, D.; Gurr, G.M.; Reynolds, O.L. Raspberry ketone accelerates sexual maturation and improves mating performance of sterile male Queensland fruit fly, Bactrocera tryoni (Froggatt). Pest Manag. Sci. 2019, 75, 1942–1950. [Google Scholar] [CrossRef]
- Akter, H.; Mendez, V.; Morelli, R.; Pérez, J.; Taylor, P.W. Raspberry ketone supplement promotes early sexual maturation in male Queensland fruit fly, Bactrocera tryoni (Diptera: Tephritidae). Pest Manag. Sci. 2017, 73, 1764–1770. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.M.; Manoukis, N.C.; Osborne, T.; Barchia, I.M.; Gurr, G.M.; Reynolds, O.L. Semiochemical mediated enhancement of males to complement sterile insect technique in management of the tephritid pest Bactrocera tryoni (Froggatt). Sci. Rep. 2017, 7, 13366. [Google Scholar] [CrossRef] [PubMed]
- Akter, H.; Adnan, S.; Morelli, R.; Rempoulakis, P.; Taylor, P.W. Suppression of cuelure attraction in male Queensland fruit flies provided raspberry ketone supplements as immature adults. PLoS ONE 2017, 12, e0184086. [Google Scholar] [CrossRef] [PubMed]
- Shelly, T.E.; Villalobos, E.M. Cue lure and the mating behavior of male melon flies (Diptera: Tephritidae). Fla. Entomol. 1995, 78, 473–482. [Google Scholar] [CrossRef]
- Fezza, T.J.; Shelly, T.E. Raspberry ketone-supplemented diet has no effect on fitness parameters or lure responsiveness in male melon flies, Zeugodacus cucurbitae (Diptera: Tephritidae). J. Asia Pac. Entomol. 2018, 21, 1384–1388. [Google Scholar] [CrossRef]
- Wolfson, J.L. Bioassay techniques—An ecological perspective. J. Chem. Ecol. 1988, 14, 1951–1963. [Google Scholar] [CrossRef]
- Miyatake, T. Genetic changes of life history and behavioral traits during mass-rearing in the melon fly, Bactrocera cucurbitae (Diptera: Tephritidae). Res. Popul. Ecol. 1998, 40, 301–310. [Google Scholar] [CrossRef]
- Koyama, J.; Kakinohana, H.; Miyatake, T. Eradication of the melon fly, Bactrocera cucurbitae, in Japan: Importance of behavior, ecology, genetics, and evolution. Annu. Rev. Entomol. 2004, 49, 331–349. [Google Scholar] [CrossRef]
- Fleischer, J.; Pregitzer, P.; Breer, H.; Krieger, J. Access to the odor world: Olfactory receptors and their role for signal transduction in insects. Cell. Mol. Life Sci. 2018, 75, 485–508. [Google Scholar] [CrossRef]
- Montell, C. A taste of the Drosophila gustatory receptors. Curr. Opin. Neurobiol. 2009, 19, 345–353. [Google Scholar] [CrossRef]
- Rytz, R.; Croset, V.; Benton, R. Ionotropic Receptors (IRs): Chemosensory ionotropic glutamate receptors in Drosophila and beyond. Insect Biochem. Mol. Biol. 2013, 43, 888–897. [Google Scholar] [CrossRef] [PubMed]
- Touhara, K.; Vosshall, L.B. Sensing odorants and pheromones with chemosensory receptors. Annu. Rev. Physiol. 2009, 71, 307–332. [Google Scholar] [CrossRef] [PubMed]
- Vogt, R.G.; Riddiford, L.M. Pheromone binding and inactivation by moth antennae. Nature 1981, 293, 161–163. [Google Scholar] [CrossRef] [PubMed]
- Brito, N.F.; Moreira, M.F.; Melo, A.C.A. A look inside odorant-binding proteins in insect chemoreception. J. Insect Physiol. 2016, 95, 51–65. [Google Scholar] [CrossRef]
- Butterwick, J.A.; del Mármol, J.; Kim, K.H.; Kahlson, M.A.; Rogow, J.A.; Walz, T.; Ruta, V. Cryo-EM structure of the insect olfactory receptor Orco. Nature 2018, 560, 447–452. [Google Scholar] [CrossRef]
- Abuin, L.; Bargeton, B.; Ulbrich, M.H.; Isacoff, E.Y.; Kellenberger, S.; Benton, R. Functional architecture of olfactory ionotropic glutamate receptors. Neuron 2011, 69, 44–60. [Google Scholar] [CrossRef]
- Benton, R.; Vannice, K.S.; Gomez-Diaz, C.; Vosshall, L.B. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell 2009, 136, 149–162. [Google Scholar] [CrossRef]
- Sato, K.; Pellegrino, M.; Nakagawa, T.; Nakagawa, T.; Vosshall, L.B.; Touhara, K. Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature 2008, 452, 1002–1006. [Google Scholar] [CrossRef]
- Wicher, D.; Schäfer, R.; Bauernfeind, R.; Stensmyr, M.C.; Heller, R.; Heinemann, S.H.; Hansson, B.S. Drosophila odorant receptors are both ligand-gated and cyclic-nucleotide- activated cation channels. Nature 2008, 452, 1007–1011. [Google Scholar] [CrossRef]
- Sato, K.; Tanaka, K.; Touhara, K. Sugar-regulated cation channel formed by an insect gustatory receptor. Proc. Natl. Acad. Sci. USA 2011, 108, 11680–11685. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, H.; Wang, Z.; Bin, S.; He, H.; Lin, J. Discovery of chemosensory genes in the oriental fruit fly, Bactrocera dorsalis. PLoS ONE 2015, 10, e0129794. [Google Scholar] [CrossRef] [PubMed]
- Elfekih, S.; Chen, C.; Hsu, J.; Belcaid, M.; Haymer, D. Identification and preliminary characterization of chemosensory perception-associated proteins in the melon fly Bactrocera cucurbitae using RNA-seq. Sci. Rep. 2016, 6, 19112. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.-F.; Yu, T.; Chen, Z.-J.; Chen, Y.-P.; Gao, L.; Zhang, W.-H.; Jiang, B.; Bai, X.; Liu, J.; Lu, Y.-Y. Identification and expression analysis of chemosensory genes in the citrus fruit fly Bactrocera (Tetradacus) minax. PeerJ Prepr. 2018, 6, e27297v1. [Google Scholar] [CrossRef]
- Wu, Z.; Kang, C.; Qu, M.; Chen, J.; Chen, M.; Bin, S.; Lin, J. Candidates for chemosensory genes identified in the Chinese citrus fly, Bactrocera minax, through a transcriptomic analysis. BMC Genom. 2019, 20, 646. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Cui, Y.; Ma, J.; Qu, M.; Lin, J. Analyses of chemosensory genes provide insight into the evolution of behavioral differences to phytochemicals in Bactrocera species. Mol. Phylogenet. Evol. 2020, 151, 106858. [Google Scholar] [CrossRef] [PubMed]
- Kaissling, K.E. Kinetics of olfactory responses might largely depend on the odorant-receptor interaction and the odorant deactivation postulated for flux detectors. J. Comp. Physiol. A 2013, 199, 879–896. [Google Scholar] [CrossRef] [PubMed]
- Larter, N.K.; Sun, J.S.; Carlson, J.R. Organization and function of Drosophila odorant binding proteins. eLife 2016, 5, e20242. [Google Scholar] [CrossRef]
- Zhu, G.H.; Zheng, M.Y.; Sun, J.B.; Khuhro, S.A.; Yan, Q.; Huang, Y.; Syed, Z.; Dong, S.L. CRISPR/Cas9 mediated gene knockout reveals a more important role of PBP1 than PBP2 in the perception of female sex pheromone components in Spodoptera litura. Insect Biochem. Mol. Biol. 2019, 115, 103244. [Google Scholar] [CrossRef]
- Chen, X.F.; Xu, L.; Zhang, Y.X.; Wei, D.; Wang, J.J.; Jiang, H.B. Genome-wide identification and expression profiling of odorant-binding proteins in the oriental fruit fly, Bactrocera dorsalis. Comp. Biochem. Physiol. Part D Genom. Proteom. 2019, 31, 100605. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, X.F.; Fu, L.; Han, Y.Y.; Chen, J.; Lu, Y.Y. BdorOBP2 plays an indispensable role in the perception of methyl eugenol by mature males of Bactrocera dorsalis (Hendel). Sci. Rep. 2017, 7, 15894. [Google Scholar] [CrossRef]
- Wu, Z.; Lin, J.; Zhang, H.; Zeng, X. BdorOBP83a-2 mediates responses of the oriental fruit fly to semiochemicals. Front. Physiol. 2016, 7, 452. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.B.; Light, D.M.; Flath, R.A.; Nagata, J.T.; Mon, T.R. Electroantennogram responses of the Mediterranean fruit fly, Ceratitis capitata, to the volatile constituents of nectarines. Entomol. Exp. Appl. 1989, 50, 7–19. [Google Scholar] [CrossRef]
- Siciliano, P.; He, X.L.; Woodcock, C.; Pickett, J.A.; Field, L.M.; Birkett, M.A.; Kalinova, B.; Gomulski, L.M.; Scolari, F.; Gasperi, G.; et al. Identification of pheromone components and their binding affinity to the odorant binding protein CcapOBP83a-2 of the Mediterranean fruit fly, Ceratitis capitata. Insect Biochem. Mol. Biol. 2014, 48, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Papanicolaou, A.; Schetelig, M.F.; Arensburger, P.; Atkinson, P.W.; Benoit, J.B.; Bourtzis, K.; Castañera, P.; Cavanaugh, J.P.; Chao, H.; Childers, C.; et al. The whole genome sequence of the Mediterranean fruit fly, Ceratitis capitata (Wiedemann), reveals insights into the biology and adaptive evolution of a highly invasive pest species. Genome Biol. 2016, 17, 192. [Google Scholar] [CrossRef] [PubMed]
- Falchetto, M.; Ciossani, G.; Scolari, F.; Di Cosimo, A.; Nenci, S.; Field, L.M.; Mattevi, A.; Zhou, J.J.; Gasperi, G.; Forneris, F. Structural and biochemical evaluation of Ceratitis capitata odorant-binding protein 22 affinity for odorants involved in intersex communication. Insect Mol. Biol. 2019, 28, 431–443. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, P.; Zhang, J.; Yang, D.; Li, Z.; Zhang, X.; Zhu, S.; Yu, Y.; Chen, N. Identification of odorant binding proteins in Carpomya vesuviana and their binding affinity to the male-borne semiochemicals and host plant volatiles. J. Insect Physiol. 2017, 100, 100–107. [Google Scholar] [CrossRef]
- Li, H.; Ren, L.; Xie, M.; Gao, Y.; He, M.; Hassan, B.; Lu, Y.; Cheng, D. Egg-surface bacteria are indirectly associated with oviposition aversion in Bactrocera dorsalis. Curr. Biol. 2020, 30, 4432–4440. [Google Scholar] [CrossRef]
- Yao, R.; Zhao, M.; Zhong, L.; Li, Y.; Li, D.; Deng, Z.; Ma, X. Characterization of the binding ability of the odorant binding protein BminOBP9 of Bactrocera minax to citrus volatiles. Pest Manag. Sci. 2020. [Google Scholar] [CrossRef]
- Nakagawa, T. Insect sex-pheromone signals mediated by specific combinations of olfactory receptors. Science 2005, 307, 1638–1642. [Google Scholar] [CrossRef]
- Miyazaki, H.; Otake, J.; Mitsuno, H.; Ozaki, K.; Kanzaki, R.; Chui-Ting Chieng, A.; Kah-Wei Hee, A.; Nishida, R.; Ono, H. Functional characterization of olfactory receptors in the oriental fruit fly Bactrocera dorsalis that respond to plant volatiles. Insect Biochem. Mol. Biol. 2018, 101, 32–46. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Jiang, X.; Wang, G. Cloning and functional characterization of three new pheromone receptors from the diamondback moth, Plutella xylostella. J. Insect Physiol. 2018, 107, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Miura, N.; Nakagawa, T.; Tatsuki, S.; Touhara, K.; Ishikawa, Y. A male-specific odorant receptor conserved through the evolution of sex pheromones in Ostrinia moth species. Int. J. Biol. Sci. 2009, 5, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Miura, N.; Nakagawa, T.; Touhara, K.; Ishikawa, Y. Broadly and narrowly tuned odorant receptors are involved in female sex pheromone reception in Ostrinia moths. Insect Biochem. Mol. Biol. 2010, 40, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Mitsuno, H.; Sakurai, T.; Murai, M.; Yasuda, T.; Kugimiya, S.; Ozawa, R.; Toyohara, H.; Takabayashi, J.; Miyoshi, H.; Nishioka, T. Identification of receptors of main sex-pheromone components of three Lepidopteran species. Eur. J. Neurosci. 2008, 28, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Forstner, M.; Breer, H.; Krieger, J. A receptor and binding protein interplay in the detection of a distinct pheromone component in the silkmoth Antheraea polyphemus. Int. J. Biol. Sci. 2009, 5, 745–757. [Google Scholar] [CrossRef]
- Sakurai, T.; Nakagawa, T.; Mitsuno, H.; Mori, H.; Endo, Y.; Tanoue, S.; Yasukochi, Y.; Touhara, K.; Nishioka, T. Identification and functional characterization of a sex pheromone receptor in the silkmoth Bombyx mori. Proc. Natl. Acad. Sci. USA 2004, 101, 16653–16658. [Google Scholar] [CrossRef]
- Wanner, K.W.; Nichols, A.S.; Allen, J.E.; Bunger, P.L.; Garczynski, S.F.; Linn, C.E.; Robertson, H.M.; Luetje, C.W. Sex pheromone receptor specificity in the european corn borer moth, Ostrinia nubilalis. PLoS ONE 2010, 5, e8685. [Google Scholar] [CrossRef]
- Zheng, W.; Zhu, C.; Peng, T.; Zhang, H. Odorant receptor co-receptor Orco is upregulated by methyl eugenol in male Bactrocera dorsalis (Diptera: Tephritidae). J. Insect Physiol. 2012, 58, 1122–1127. [Google Scholar] [CrossRef]
- Liu, H.; Chen, Z.S.; Zhang, D.J.; Lu, Y.Y. BdorOR88a modulates the responsiveness to methyl eugenol in mature males of Bactrocera dorsalis (Hendel). Front. Physiol. 2018, 9, 987. [Google Scholar] [CrossRef]
- Tan, K.H.; Nishida, R. Sex pheromone and mating competition after methyl eugenol consumption in the Bactrocera dorsalis complex. In Fruit Fly Pests: A World Assessment of Their Biology and Management; McPheron, B.A., Steck, G.J., Eds.; St. Lucie Press: Delray Beach, FL, USA, 1996; pp. 147–153. ISBN 978-1574440140. [Google Scholar]
- Tan, K.H.; Tokushima, I.; Ono, H.; Nishida, R. Comparison of phenylpropanoid volatiles in male rectal pheromone gland after methyl eugenol consumption, and molecular phylogenetic relationship of four global pest fruit fly species: Bactrocera invadens, B. dorsalis, B. correcta and B. zonata. Chemoecology 2011, 21, 25–33. [Google Scholar] [CrossRef]
- Wee, S.; Tan, K. Male endogenous pheromonal component of Bactrocera carambolae (Diptera: Tephritidae) deterred gecko predation. Chemoecology 2005, 203, 199–203. [Google Scholar] [CrossRef]
- Wee, S.; Tan, K.; Nishida, R. Pharmacophagy of methyl eugenol by males enhances sexual selection of Bactrocera carambolae. J. Chem. Ecol. 2007, 33, 1272–1282. [Google Scholar] [CrossRef] [PubMed]
- Wee, S.L.; Tan, K.H. Female sexual response to male rectal volatile constituents in the fruit fly, Bactrocera carambolae (Diptera: Tephritidae). Appl. Entomol. Zool. 2005, 40, 365–372. [Google Scholar] [CrossRef]
- Tan, K. Sex pheromone components in defense of melon fly, Bactrocera cucurbitae against Asian house gecko, Hemidactylus frenatus. J. Chem. Ecol. 2000, 26, 697–704. [Google Scholar] [CrossRef]
- Ono, H.; Miyazaki, H.; Mitsuno, H.; Ozaki, K.; Kanzaki, R.; Nishida, R. Functional characterization of olfactory receptors in three Dacini fruit flies (Diptera: Tephritidae) that respond to 1-nonanol analogs as components in the rectal glands. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2020, 239, 110346. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Qiu, Y.T.; Wang, G.; Kwon, J.Y.; Rutzler, M.; Kwon, H.W.; Pitts, R.J.; van Loon, J.J.A.; Takken, W.; Carlson, J.R.; et al. Odor coding in the maxillary palp of the malaria vector mosquito Anopheles gambiae. Curr. Biol. 2007, 17, 1533–1544. [Google Scholar] [CrossRef] [PubMed]
- Kreher, S.A.; Mathew, D.; Kim, J.; Carlson, J.R. Translation of sensory input into behavioral output via an olfactory system. Neuron 2008, 59, 110–124. [Google Scholar] [CrossRef]
- Bohbot, J.D.; Dickens, J.C. Characterization of an enantioselective odorant receptor in the yellow fever mosquito Aedes aegypti. PLoS ONE 2009, 4, e7032. [Google Scholar] [CrossRef]
- Hallem, E.A.; Nicole Fox, A.; Zwiebel, L.J.; Carlson, J.R. Mosquito receptor for human-sweat odorant. Nature 2004, 427, 212–213. [Google Scholar] [CrossRef]
- Wang, G.; Carey, A.F.; Carlson, J.R.; Zwiebel, L.J. Molecular basis of odor coding in the malaria vector mosquito Anopheles gambiae. Proc. Natl. Acad. Sci. USA 2010, 107, 4418–4423. [Google Scholar] [CrossRef]
- Liu, Y.; Cui, Z.; Si, P.; Liu, Y.; Zhou, Q.; Wang, G. Characterization of a specific odorant receptor for linalool in the Chinese citrus fly Bactrocera minax (Diptera: Tephritidae). Insect Biochem. Mol. Biol. 2020, 122, 103389. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cui, Z.; Wang, G.; Zhou, Q.; Liu, Y. Cloning and functional characterization of three odorant receptors from the Chinese citrus fly Bactrocera minax (Diptera: Tephritidae). Front. Physiol. 2020, 11, 246. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhou, Q. Olfactory response of female Bactrocera minax to chemical components of the preference host citrus volatile oils. J. Asia. Pac. Entomol. 2016, 19, 637–642. [Google Scholar] [CrossRef]
- Ai, M.; Blais, S.; Park, J.Y.; Min, S.; Neubert, T.A.; Suh, G.S.B. Ionotropic glutamate receptors IR64a and IR8a form a functional odorant receptor complex in vivo in Drosophila. J. Neurosci. 2013, 33, 10741–10749. [Google Scholar] [CrossRef] [PubMed]
- Choo, A.; Crisp, P.; Saint, R.; O’Keefe, L.V.; Baxter, S.W. CRISPR/Cas9-mediated mutagenesis of the white gene in the tephritid pest Bactrocera tryoni. J. Appl. Entomol. 2018, 142, 52–58. [Google Scholar] [CrossRef]
- Koh, T.W.; He, Z.; Gorur-Shandilya, S.; Menuz, K.; Larter, N.K.; Stewart, S.; Carlson, J.R. The Drosophila IR20a clade of ionotropic receptors are candidate taste and pheromone receptors. Neuron 2014, 83, 850–865. [Google Scholar] [CrossRef]
- Hanssen, B.L.; Park, S.J.; Royer, J.E.; Jamie, J.F.; Taylor, P.W.; Jamie, I.M. Systematic modification of zingerone reveals structural requirements for attraction of Jarvis’s Fruit Fly. Sci. Rep. 2019, 9, 19332. [Google Scholar] [CrossRef]
- Vosshall, L.B.; Laissue, P.P. The olfactory sensory map in Drosophila. Adv. Exp. Med. Biol. 2008, 628, 102–114. [Google Scholar] [CrossRef]
- Gao, Q.; Yuan, B.; Chess, A. Convergent projections of Drosophila olfactory neurons to specific glomeruli in the antennal lobe. Nat. Neurosci. 2000, 3, 780–785. [Google Scholar] [CrossRef]
- Couto, A.; Alenius, M.; Dickson, B.J. Molecular, anatomical, and functional organization of the Drosophila olfactory system. Curr. Biol. 2005, 15, 1535–1547. [Google Scholar] [CrossRef]
- Lin, T.; Li, C.; Liu, J.; Smith, B.H.; Lei, H.; Zeng, X. Glomerular organization in the antennal lobe of the oriental fruit fly Bactrocera dorsalis. Front. Neuroanat. 2018, 12, 71. [Google Scholar] [CrossRef] [PubMed]
- Riabinina, O.; Task, D.; Marr, E.; Lin, C.C.; Alford, R.; O’Brochta, D.A.; Potter, C.J. Organization of olfactory centres in the malaria mosquito Anopheles gambiae. Nat. Commun. 2016, 7, 13010. [Google Scholar] [CrossRef] [PubMed]
- Teal, P.E.A.; Gomez-Simuta, Y.; Proveaux, A.T. Mating experience and juvenile hormone enhance sexual signaling and mating in male Caribbean fruit flies. Proc. Natl. Acad. Sci. USA 2000, 97, 3708–3712. [Google Scholar] [CrossRef] [PubMed]
- Adnan, S.M.; Mendez, V.; Morelli, R.; Akter, H.; Farhana, I.; Taylor, P.W. Dietary methoprene supplement promotes early sexual maturation of male Queensland fruit fly Bactrocera tryoni. J. Pest Sci. 2018, 91, 1441–1454. [Google Scholar] [CrossRef]
- Haq, I.U.; Cáceres, C.; Hendrichs, J.; Teal, P.E.A.; Stauffer, C.; Robinson, A.S. Methoprene modulates the effect of diet on male melon fly, Bactrocera cucurbitae, performance at mating aggregations. Entomol. Exp. Appl. 2010, 136, 21–30. [Google Scholar] [CrossRef]
- Shelly, T.E.; Nishimoto, J.I.; Edu, J. Influence of methoprene on the age-related mating propensity of males of the oriental fruit fly and the Mediterranean fruit fly (Diptera: Tephritidae). Fla. Entomol. 2009, 92, 193–198. [Google Scholar] [CrossRef]
- Jindra, M.; Palli, S.R.; Riddiford, L.M. The Juvenile hormone signaling pathway in insect development. Annu. Rev. Entomol. 2013, 58, 181–204. [Google Scholar] [CrossRef]
- Li, K.; Jia, Q.Q.; Li, S. Juvenile hormone signaling—A mini review. Insect Sci. 2019, 26, 600–606. [Google Scholar] [CrossRef]
- Akami, M.; Andongma, A.A.; Zhengzhong, C.; Nan, J.; Khaeso, K.; Jurkevitch, E.; Niu, C.Y.; Yuval, B. Intestinal bacteria modulate the foraging behavior of the oriental fruit fly Bactrocera dorsalis (Diptera: Tephritidae). PLoS ONE 2019, 14, e0210109. [Google Scholar] [CrossRef]
- Akami, M.; Ren, X.M.; Qi, X.; Mansour, A.; Gao, B.; Cao, S.; Niu, C.Y. Symbiotic bacteria motivate the foraging decision and promote fecundity and survival of Bactrocera dorsalis (Diptera: Tephritidae). BMC Microbiol. 2019, 19, 229. [Google Scholar] [CrossRef]
- Jose, P.A.; Ben-Yosef, M.; Jurkevitch, E.; Yuval, B. Symbiotic bacteria affect oviposition behavior in the olive fruit fly Bactrocera oleae. J. Insect Physiol. 2019, 117, 103917. [Google Scholar] [CrossRef]
- Potter, C.J.; Tasic, B.; Russler, E.V.; Liang, L.; Luo, L. Resource the Q system: A repressible binary system for transgene expression, lineage tracing, and mosaic analysis. Cell 2010, 141, 536–548. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Betz, J.F.; Riabinina, O.; Lahondére, C.; Potter, C.J.; Afify, A.; Betz, J.F.; Riabinina, O.; Lahondère, C.; Potter, C.J. Commonly used insect repellents hide human odors from Anopheles mosquitoes. Curr. Biol. 2019, 29, 3669–3680. [Google Scholar] [CrossRef]
- Matthews, B.J.; Vosshall, L.B. How to turn an organism into a model organism in 10 “easy” steps. J. Exp. Biol. 2020, 223 (Suppl. 1). [Google Scholar] [CrossRef] [PubMed]
- Matthews, B.J.; Younger, M.A.; Vosshall, L.B. The ion channel ppk301 controls freshwater egg-laying in the mosquito Aedes aegypti. eLife 2019, 8, e43963. [Google Scholar] [CrossRef]
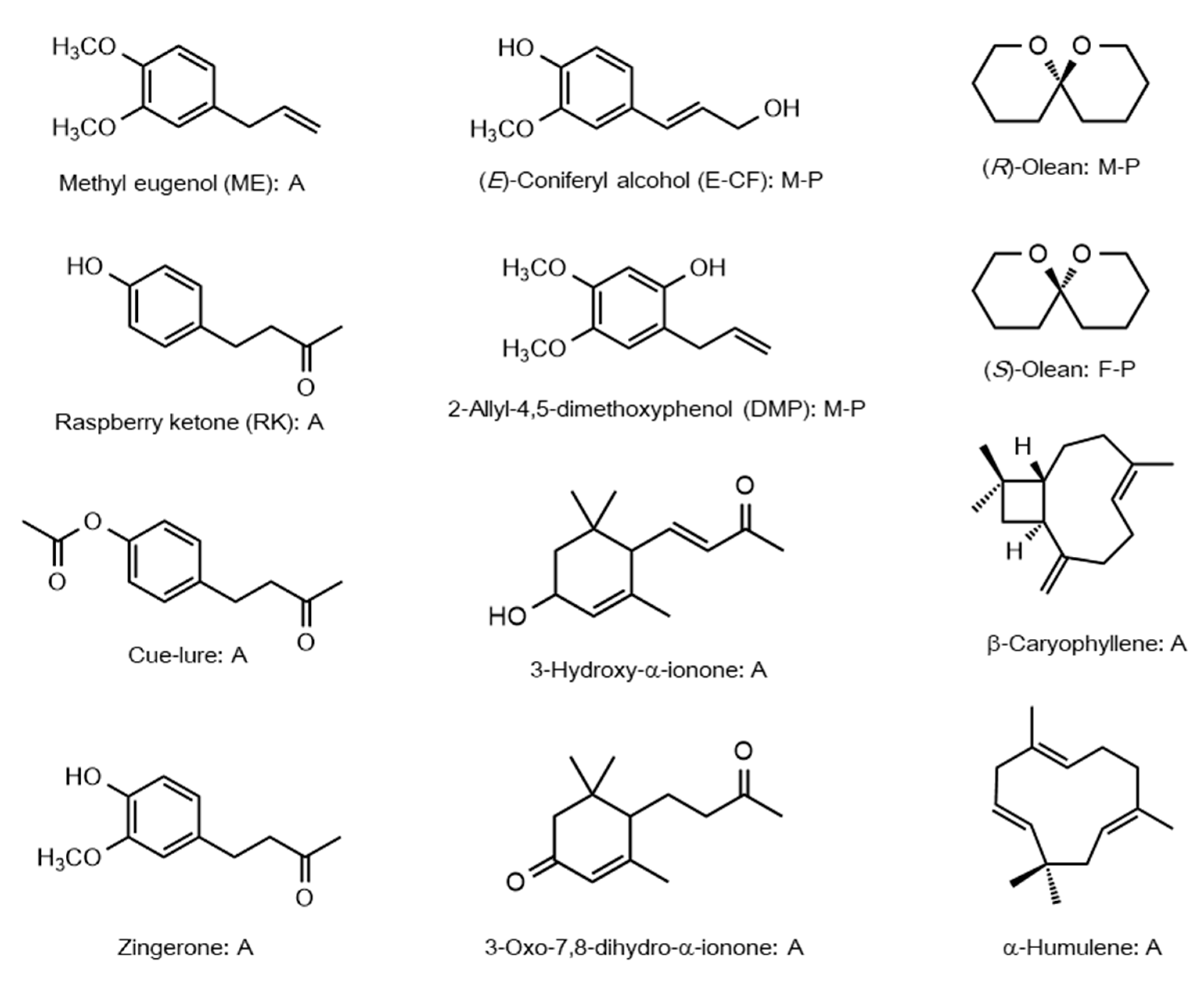
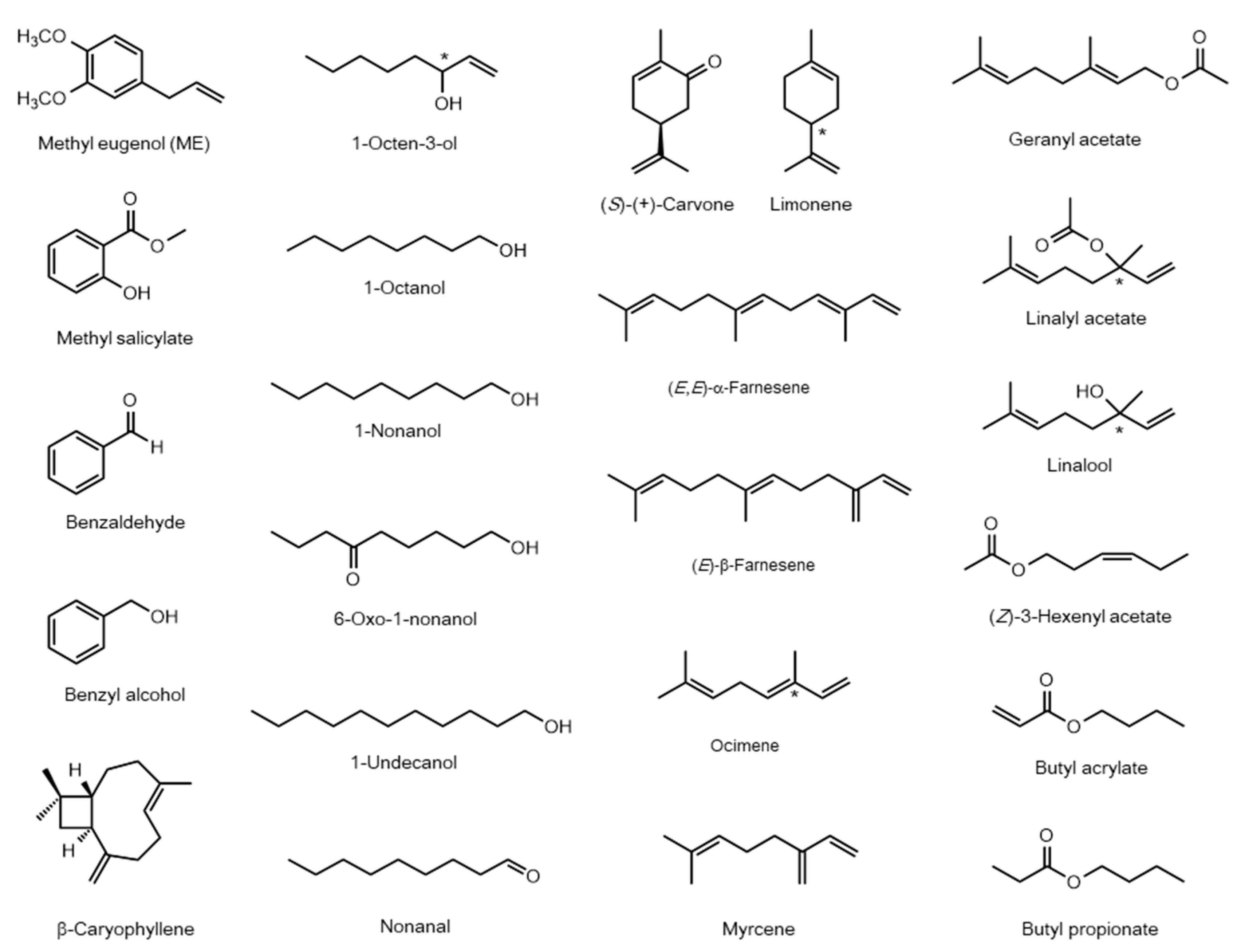
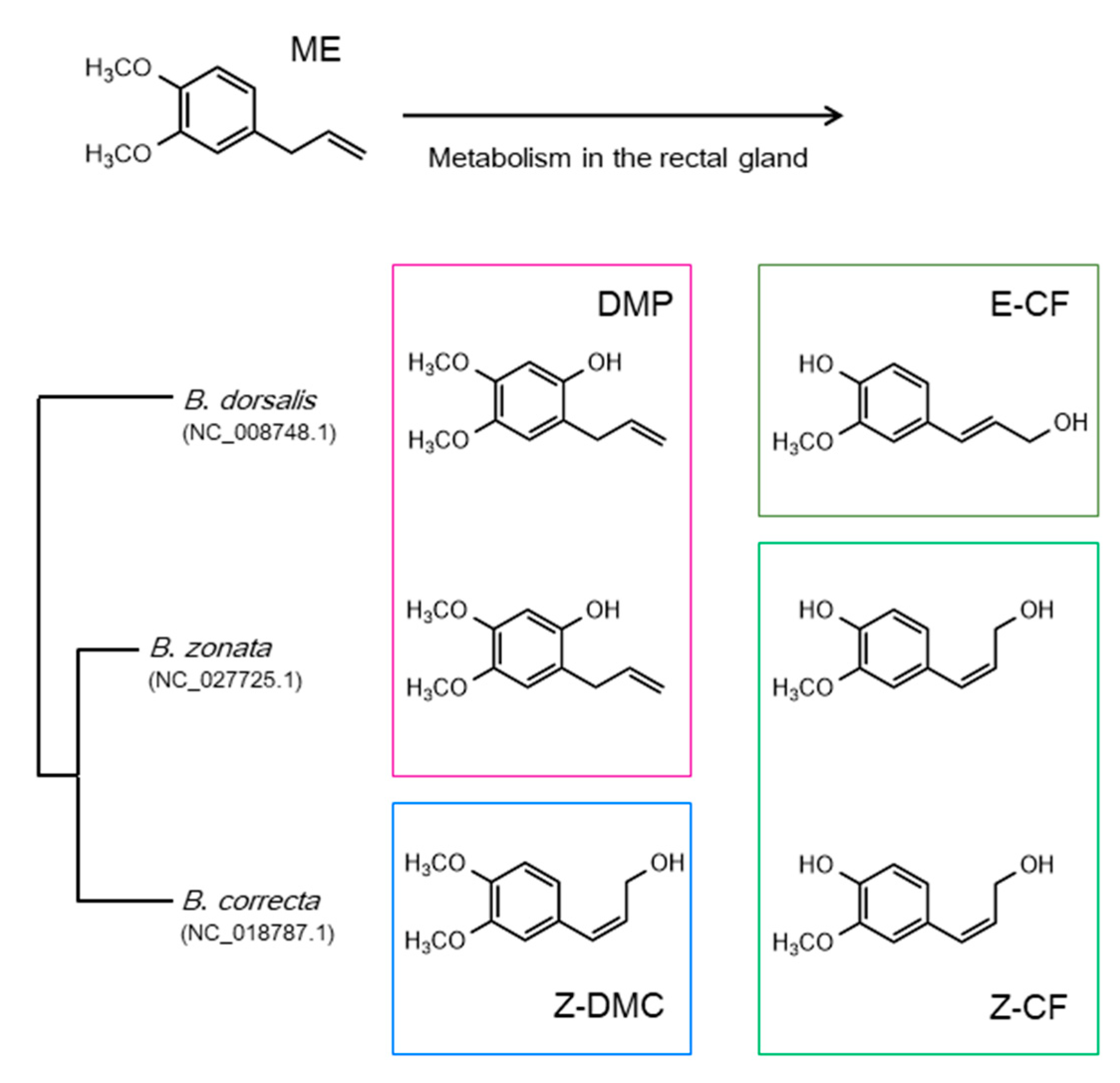
| Species | Name | Accession Number | Ligand | Ref. |
|---|---|---|---|---|
| Bactrocera dorsalis | BdorOBP56f-2 (BdorOBP2) | KC559113 | Methyl eugenol | [112] |
| BdorOBP83b (BdorOBP83a-2) | KP743700 | Methyl eugenol | [113] | |
| BdorOBP99b (BdorOBP99a) | KP743706 | Limonene | [120] | |
| Ocimene | ||||
| BdorOBP84a-1 | MT474623 | 1-Octen-3-ol | [113] | |
| Bactrocera minax | BminOBP9 (BminGOBP99a) | KY463449 | β-Caryophyllene | [120] |
| Ocimene | ||||
| Myrcene | ||||
| Ceratitis capitata | CcapOBP22 (CcapOBP69a) | NP_001295335.1 | (E,E)-α-Farnesene | [117] |
| CcapOBP83a-2 | XM_004523387.1 | (E,E)-α-Farnesene | [115] | |
| Carpomya vesuviana | CvesOBP1 | KU975053 | β-Caryophyllene | [118] |
| CvesOBP2 | KU975054 | (Z)-3-Hexenyl acetate | [118] | |
| CvesOBP3 | KU975055 | α-Farnesene | [118] | |
| CvesOBP4 | KX059394 | β-Caryophyllene | [118] | |
| CvesOBP5 | KU975056 | Nonanal | [118] | |
| CvesOBP6 | KX059395 | Nonanal | [118] |
| Species | Name | Accession Number | Drosophila Homolog (1) | E-Value | Ligand (2) | Ref. |
|---|---|---|---|---|---|---|
| Batrocera dorsalis | BdorOR13a | FX985890 | OR13a | 3e−122 | 1-Octen-3-ol (+++) | [122] |
| Batrocera dorsalis | BdorOR63a-1 | KP743726 | OR63a | 1e−95 | Methyl eugenol (+) | [131] |
| Batrocera dorsalis | BdorOR74a | FX985927 | OR74a | 5e−63 | 1-Nonanol (+++) | [138] |
| 6-Oxo-1-nonanol (++) | ||||||
| Batrocera dorsalis | BdorOR82a | FX985928 | OR82a | 2e−83 | Geranyl acetate (+++) | [122] |
| Farnesenes (3) (+) | ||||||
| Linalyl acetate (++) | ||||||
| Batrocera dorsalis | BdorOR88a | KP743732 | OR88a | 1e−35 | Methyl eugenol (+++) | [131] |
| Batrocera latifrons | BlatOR74a | FX986103 | OR74a | 2e−63 | 1-Nonanol (++) | [138] |
| Bactrocera minax | BminOR3 (4) | MN537976 | OR13a | 5e−119 | 1-Octen-3-ol (+++) | [145] |
| Bactrocera minax | BminOR12 | MN855530 | OR63a | 4e−109 | Methyl salicylate (+++) | [145] |
| Benzaldehyde (++) | ||||||
| (Z)-3-Hexenyl acetate (++) | ||||||
| Butyl acrylate (++) | ||||||
| Butyl propionate (++) | ||||||
| 1-Octanol (+) | ||||||
| (S)-(+)-Carvone (+) | ||||||
| Benzyl alcohol (+) | ||||||
| Bactrocera minax | BminOR16 | MN537977 | OR67c | 9e−33 | 1-Undecanol (++) | [145] |
| Bactrocera minax | BminOR24 | MN537978 | OR7a | 1e−50 | Linalool (+++) | [144] |
| Methyl eugenol (+) | ||||||
| Zeugodacus cucurbitae | ZcucOR74a | FX986226 | OR74a | 7e−64 | 1-Nonanol (+++) | [138] |
| 6-Oxo-1-nonanol (+) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ono, H.; Hee, A.K.-W.; Jiang, H. Recent Advancements in Studies on Chemosensory Mechanisms Underlying Detection of Semiochemicals in Dacini Fruit Flies of Economic Importance (Diptera: Tephritidae). Insects 2021, 12, 106. https://doi.org/10.3390/insects12020106
Ono H, Hee AK-W, Jiang H. Recent Advancements in Studies on Chemosensory Mechanisms Underlying Detection of Semiochemicals in Dacini Fruit Flies of Economic Importance (Diptera: Tephritidae). Insects. 2021; 12(2):106. https://doi.org/10.3390/insects12020106
Chicago/Turabian StyleOno, Hajime, Alvin Kah-Wei Hee, and Hongbo Jiang. 2021. "Recent Advancements in Studies on Chemosensory Mechanisms Underlying Detection of Semiochemicals in Dacini Fruit Flies of Economic Importance (Diptera: Tephritidae)" Insects 12, no. 2: 106. https://doi.org/10.3390/insects12020106
APA StyleOno, H., Hee, A. K.-W., & Jiang, H. (2021). Recent Advancements in Studies on Chemosensory Mechanisms Underlying Detection of Semiochemicals in Dacini Fruit Flies of Economic Importance (Diptera: Tephritidae). Insects, 12(2), 106. https://doi.org/10.3390/insects12020106







