Morphology of the Antennal Sensilla of Notonectoidea and Comparison of Evolutionary Changes in Sensilla Types and Distribution in Infraorder Nepomorpha (Insecta: Heteroptera)
Abstract
:Simple Summary
Abstract
1. Introduction
- The sensilla type and their arrangement on the antennae have a phylogenetic value (synapomorphies) resulting from the relationships of the taxa.
- The sensilla type and their arrangement on the antennae are the result of their evolution in the aquatic environment.
2. Materials and Methods
2.1. Taxon Samples
| Notonectidae: | Anisopinae: | Anisops debilis Gerstaecker, 1873 | |
| Anisops jaczewski Hutchinson, 1928 | |||
| Anisops sardea Herrich-Schaeffer, 1850 | |||
| Buenoa nitida Truxal, 1953 | |||
| Notonectinae: | Notonectini: | Enithares metallica Brooks, 1948 | |
| Enithares stridulata Brooks, 1948 | |||
| Notonecta ceres ceres Kirkaldy, 1897 | |||
| Notonecta disturbata Hungerford, 1926 | |||
| Notonecta glauca Linnaeus, 1758 | |||
| Notonecta maculata Fabricius, 1794 | |||
| Nychiini: | Martarega gonostyla Truxal, 1949 | ||
| Martarega uruguayensis Berg, 1883 | |||
| Pleidae: | Plea minutissima Leach, 1818 | ||
| Paraplea sp. | |||
| Helotrephidae: | Helotrephinae: | Hydrotrephes visayasensis Zettel, 2003 | |
| Neotrephinae: | Neotrephes lanemeloi Nieser and Chen, 2002 |
2.2. Programs Used for Cladistic Analysis
2.3. Description of the Character States
- Number of antennomeres: (0) 4, 5 (1) 3, (2) 2, (3) 1,
- Sensilla trichodea ST1: (0) present on the 3rd and 4th antennomeres, (1) present on all antennomeres or absent only on the first, (2) absent on all antennomeres (3) absent only on the 3rd antennomere (4) present only on the 3rd antennomere, (5) present on the 2nd antennomere or on the 2nd and 3nd antennomeres, (6) absent only on the 4th antennomere, (7) absent only on the 2nd antennomere, (8) present only on the 4th antennomere (9) present only on the 1st and 2nd antennomeres,
- Sensilla trichodea ST2: (0) present on all antennomeres, (1) absent on all antennomeres, (2) absent only on the 1st antennomere, (3) present only on the 3rd antennomere, (4) present only on the 1st antennomere, (5) present only on the 2nd antennomere, (6) present only on the 1st and 2nd antennomeres, (7) absent only on the 2nd antennomere, (8) present only on the 2nd and 3rd antennomeres, (9) present only on the 4th antennomere,
- Sensilla trichodea ST3: (0) present on the 1st and 2nd antennomeres (1) present only on the 3rd and 4th antennomeres, (2) absent, (3) present only on the 3rd antennomere, (4) present only on the 1st antennomere (5) present only on the 2nd antennomere, (6) present only on the 2nd and 4th antennomeres,
- Sensilla trichodea ST4a: (0) absent, (1) present only on the 3rd antennomere,
- Sensilla trichodea ST4b: (0) absent, (1) absent only on the 1st antennomere, (2) present only on the 3rd and 4th antennomeres, (3) present only on the 3rd antennomere,
- Sensilla trichodea ST4c: (0) absent, (1) present only on the 3rd antennomere, (2) present only on the 3rd and 4th antennomeres, (3) present only on the 2nd antennomere,
- Sensilla trichodea ST5a: (0) absent, (1) present only on the 3rd antennomere, (2) present only on the 3rd and 4th antennomeres,
- Sensilla trichodea ST5b: (0) absent, (1) present only on the 1st antennomere, (2) present on the 2nd antennomere, (3) present only on the 3rd antennomere, (4) present only on the 2nd and 3rd antennomeres, (6) absent only on the 1st antennomere, (6) present only on the 3rd and 4th antennomeres, (7) present only on the 2nd and 4th antennomeres,
- Sensilla chaetica SCh: (0) present on all antennomeres, (1) absent, (2) present only on the 3rd and 4th antennomeres, (3) present only on the 4th antennomere, (4) present only on the 2nd antennomere, (5) present only on the 1st antennomere,
- Sensilla cone-like SCoL: (0) absent, (1) present,
- Sensilla brush-like SBL: (0) absent, (1) present,
- Sensilla club-like SClL: (0) absent, (1) present only on the 1st antennomere, (2) present only on the 1st and 2nd antennomeres,
- Sensilla paddle-like SPL1: (0) absent, (1) present only on the 1st antennomere, (2) present only on the 3rd antennomere,
- Sensilla paddle-like SPL2: (0) absent, (1) present,
- Sensilla squamiformia SSq: (0) absent, (1) present,
- Sensilla campaniformia SCa: (0) present, (1) absent,
- Sensilla basiconica SB1: (0) present, (1) absent,
- Sensilla basiconica SB2: (0) present only on the 4th antennomere, (1) present only on the 3rd and 4th antennomeres, (2) present only on the 2nd and 3rd antennomeres (3) absent, (4) present on the 2nd, 3rd and 4th antennomeres, (5) present only on the 1st antennomere, (6) present only on the 3rd antennomere, (7) absent only on the 1st antennomere,
- Sensilla basiconica SB3a: (0) absent, (1) present only on the 3rd and 4th antennomeres, (2) present only on the 4th antennomere, (3) present only on the 3rd antennomere, (4) present only on the 2nd and 3rd antennomere, (5) present only on the 2nd antennomere,
- Sensilla basiconica SB3b: (0) absent, (1) present only on the 2nd antennomere, (2) present only on the 2nd and 3rd antennomeres, (3) present only on the 3rd antennomere, (4) present only on the 3rd and 4th antennomeres, (5) present only on the 4th antennomere,
- Sensilla basiconica SB3c: (0) absent, (1) present only on the 3rd and 4th antennomeres, (2) present on the 2nd and 3rd antennomeres,
- Sensilla basiconica SB4: (0) present only on the 4th antennomere, (1) absent, (2) present only on the 3rd antennomere, (3) present only on the 2nd, 3rd and 4th antennomeres, (4) present only on the 2nd and 3rd antennomeres, (5) present only on the 2nd and 4th antennomeres, (6) present only on the 2nd antennomere, (7) present only on the 3rd and 4th antennomeres,
- Sensilla coeloconica SCo1a: (0) absent, (1) present,
- Sensilla coeloconica SCo2a: (0) absent, (1) present only on the 4th antennomere, (2) present on the 3rd antennomere,
- Sensilla coeloconica SCo3: (0) absent, (1) present only on the 3rd and 4th antennomeres, (2) present only on the 2nd and 3rd antennomeres,
- Sensilla coeloconica SCo1b: (0) sparsely distributed all over the antenna, (1) absent, (2) present only on the 3rd antennomere, (3) present only on the 3rd and 4th antennomeres, (4) present only on the 2nd antennomere, (5) present only on the 4th antennomere (6) present only on the 2nd and 3rd antennomeres,
- Sensilla coeloconica SCo2b: (0) absent, (1) present on the 3rd antennomere, (2) present on the 4th antennomere,
- Sensilla ampullacea SA: (0) present, (1) absent,
- Sensilla plate-like SPl: (0) absent, (1) present,
- Sensilla placodea multilobated SPM: (0) absent, (1) present,
- Sensilla trichodea curved: (0) present, (1) absent.
3. Results
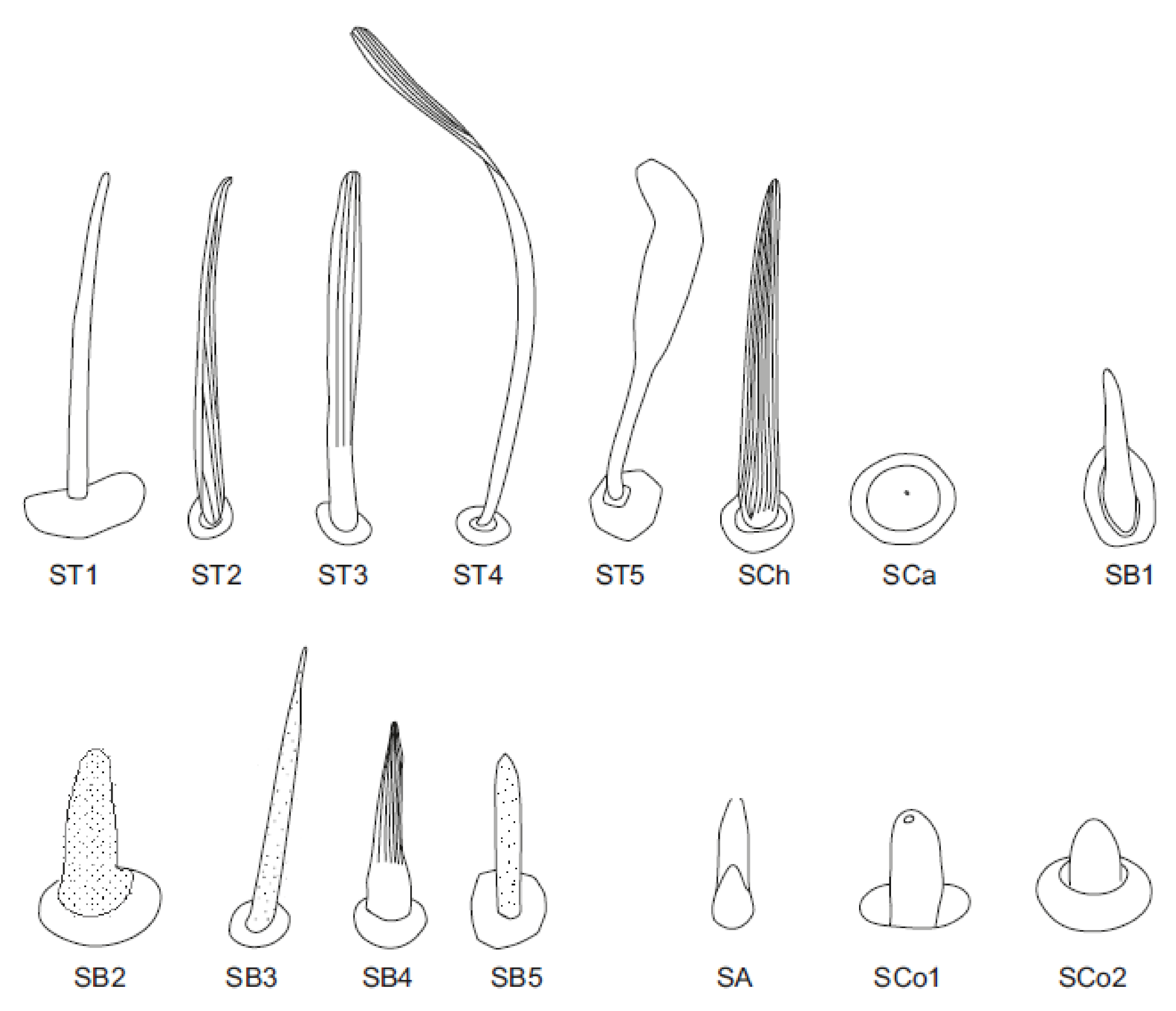
3.1. Morphology and Categories of Antennal Sensilla
- Sensilla trichodea—sensilla responsible for mechanoreception. Straight, long and hair like structures. Usually, they cover a large surface on the antennae. They arise from flexible sockets, which is common for mechanoreceptive sensilla. A flexible socket is a socket with a thin cuticular membrane that is continuous with the cuticle and hair of the general body. A flexible socket provides greater mobility at the base of the sensillum. The following sensilla trichodea subtypes were observed:
- -
- -
- -
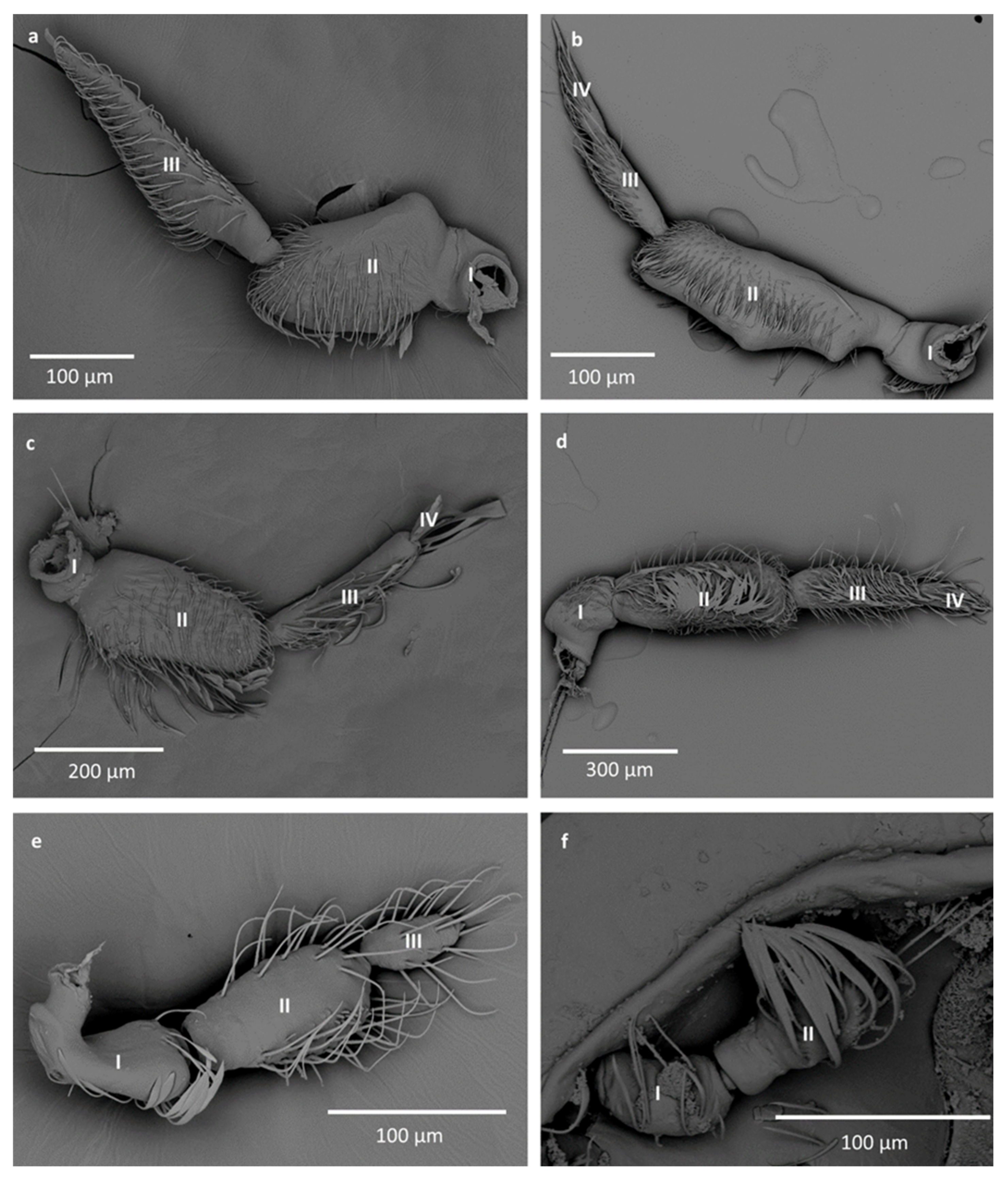
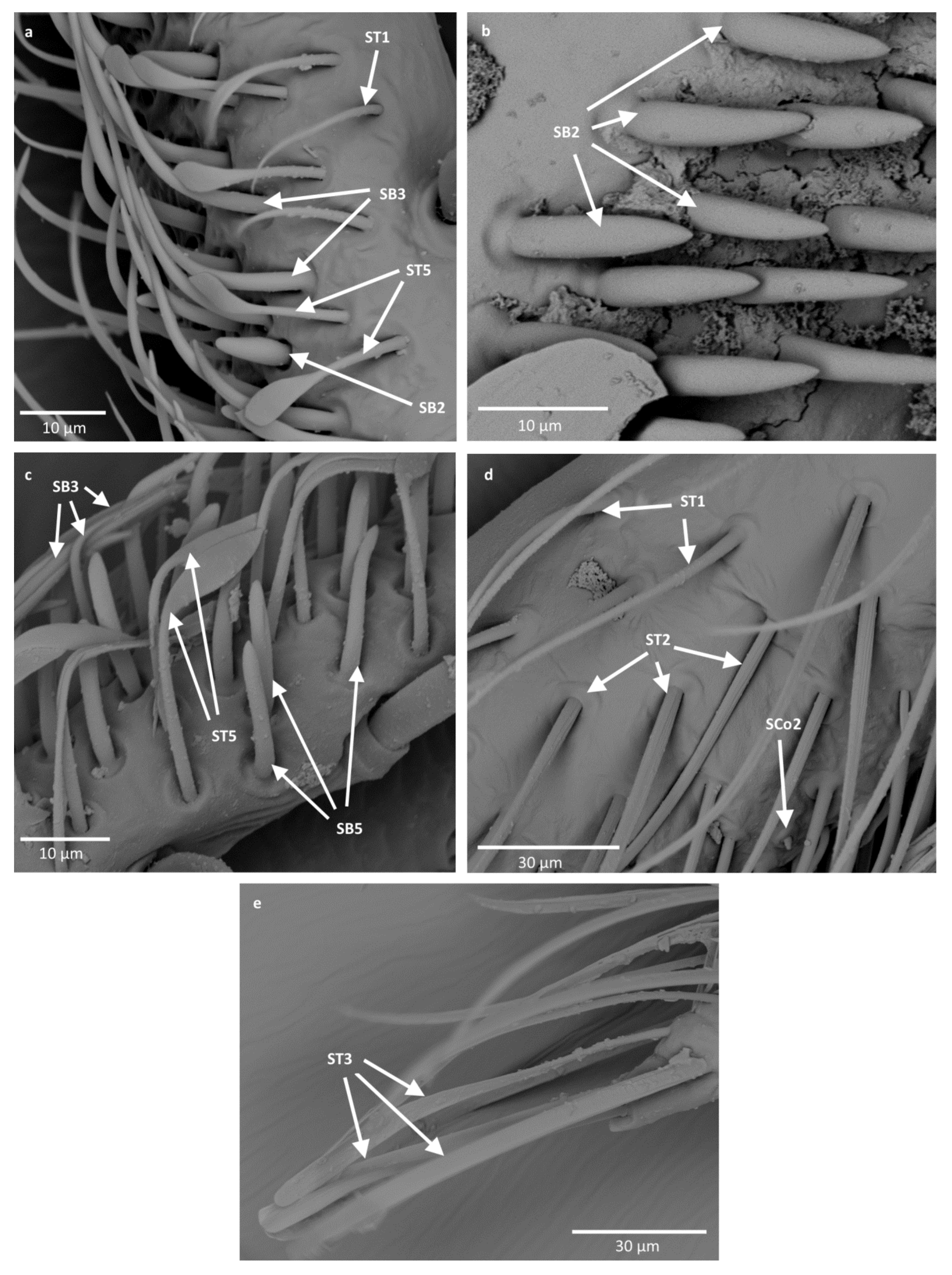
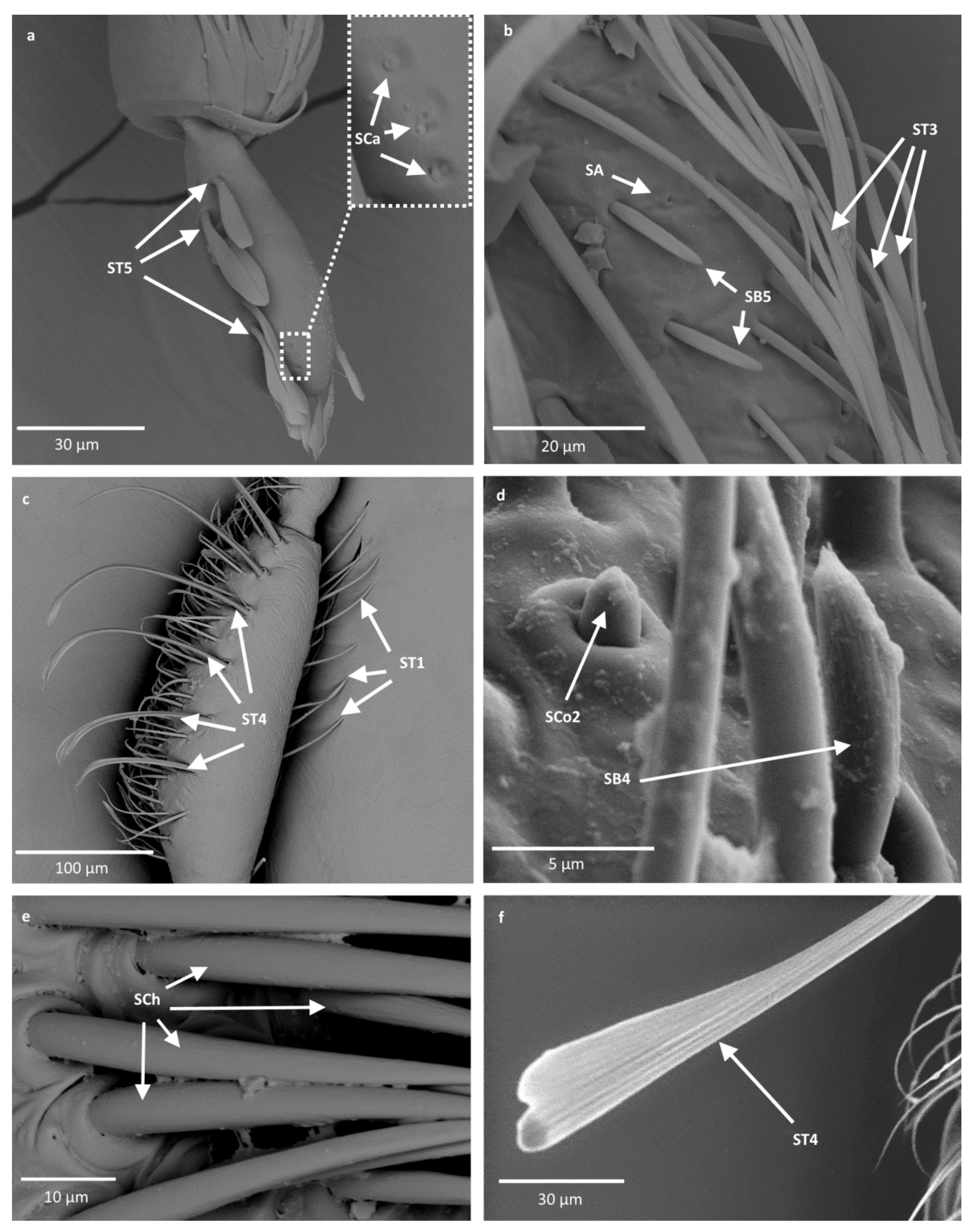
- Sensilla trichodea (ST3)—long structures with a ribbed surface on one side, widening more less in the middle. These sensilla were usually found on the second last and last antennomeres. They usually cover a large part of the antennomere, protecting smaller sensilla underneath. They were found in Anisops debilis, Anisops sardea, Buenoa nitida, Enithares, Martarega uruguayensis and Hydrotrephes visayasensis, on different antennomeres (Figure 3, Figure 4e and Figure 5b).
- -
- Sensilla trichodea (ST4)—long structures protruding above all other sensilla. They are ribbed and resemble an oar. These sensilla were present in all studied species, except Enithares and the species of Pleidae and Helotrephidae. They were found on the second last and last antennomeres, usually organized in a row through the length of the antennomere (Figure 3 and Figure 5c,f).
- -
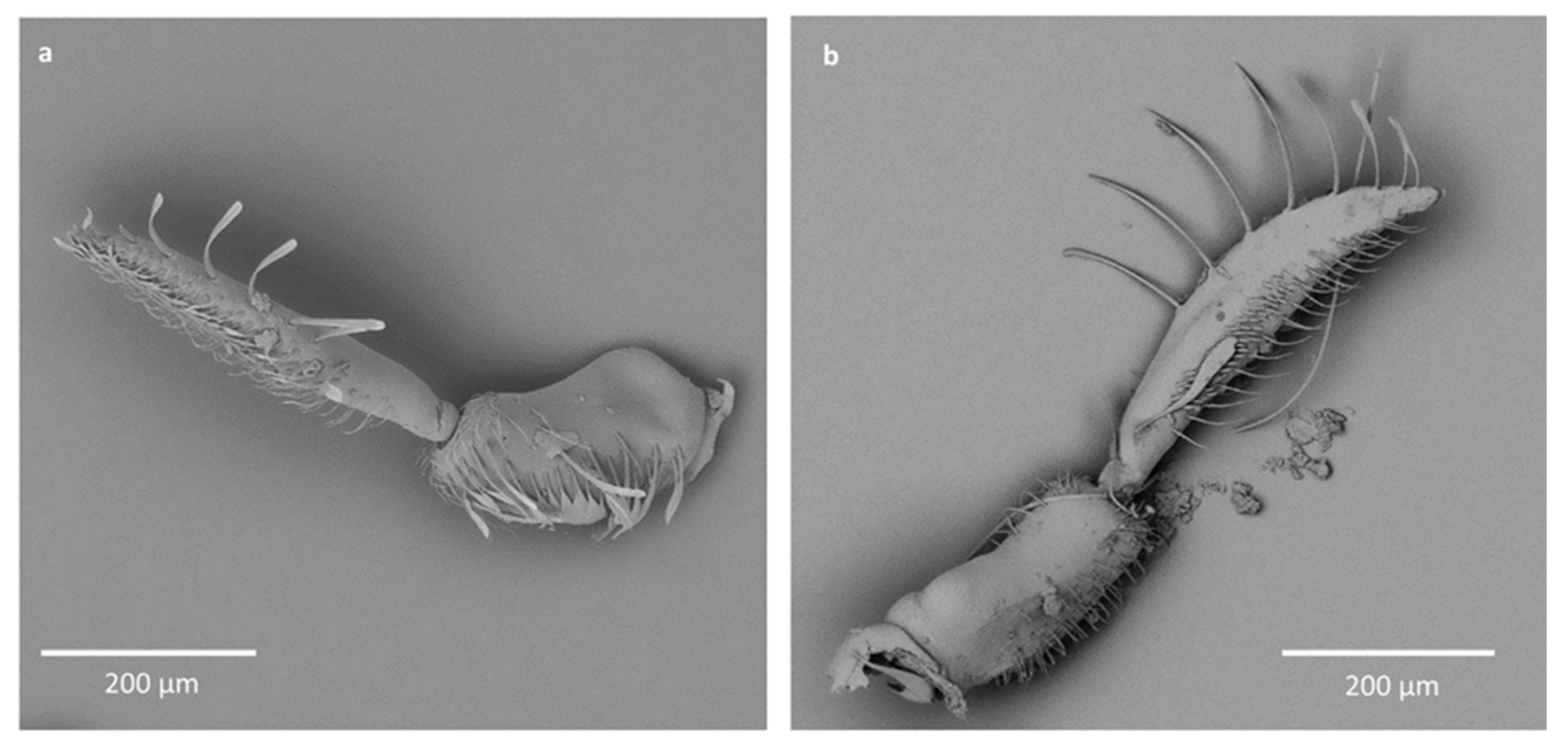
- Sensilla trichodea (ST5)—long structures, cylindrical at the base with the rest of sensillum flattened and resembling a leaf. Overall, they were found on every antennomere. Depending on the species, however, they occur on one, two or three antennomeres, but never in all four of them at once. As with sensilla trichodea ST3, they cover a large part of the antennomere, protecting the smaller sensilla underneath. They were found on different antennomeres in all species except Enithares and Neotrephes lanemeloi (Figure 3, Figure 4a,c, Figure 5a and Figure 6a).
- Sensilla chaetica (SCh)—long structures, much more rigid than trichoid sensilla. They are sharpened at the tip, ribbed, and arise from flexible sockets. Sensilla chaetica are much less common than sensilla trichodea. They were only found in Notonecta, grouped on the second antennomere, except in Notonecta disturbata (we expect that sensilla chaetica are also present there; however, the antennae of this species were on a slightly different angle and therefore it is possible that the sensilla were occluded from view) (Figure 3 and Figure 5e).
- Sensilla campaniformia (SCa)—sensilla also responsible for mechanoreception, therefore arising from flexible sockets. These are flat structures, resembling round or oval discs, with a single pore in the middle. This type was found on different antennomeres in Buenoa nitida, Enithares stridulata, Notonecta ceres ceres, Martega, Paraplea sp. and in the species of Helotrephidae (Figure 3, Figure 5a and Figure 6b).
- Sensilla basiconica (SB)—sensilla responsible for chemoreception in general, usually olfaction. Their surface is covered with wall pores which allow chemical particles to enter. Unlike mechanoreceptive sensilla, these structures arise from inflexible sockets. One subtype was found which we believe to be responsible for proprioception. The following subtypes were observed:
- -
- -
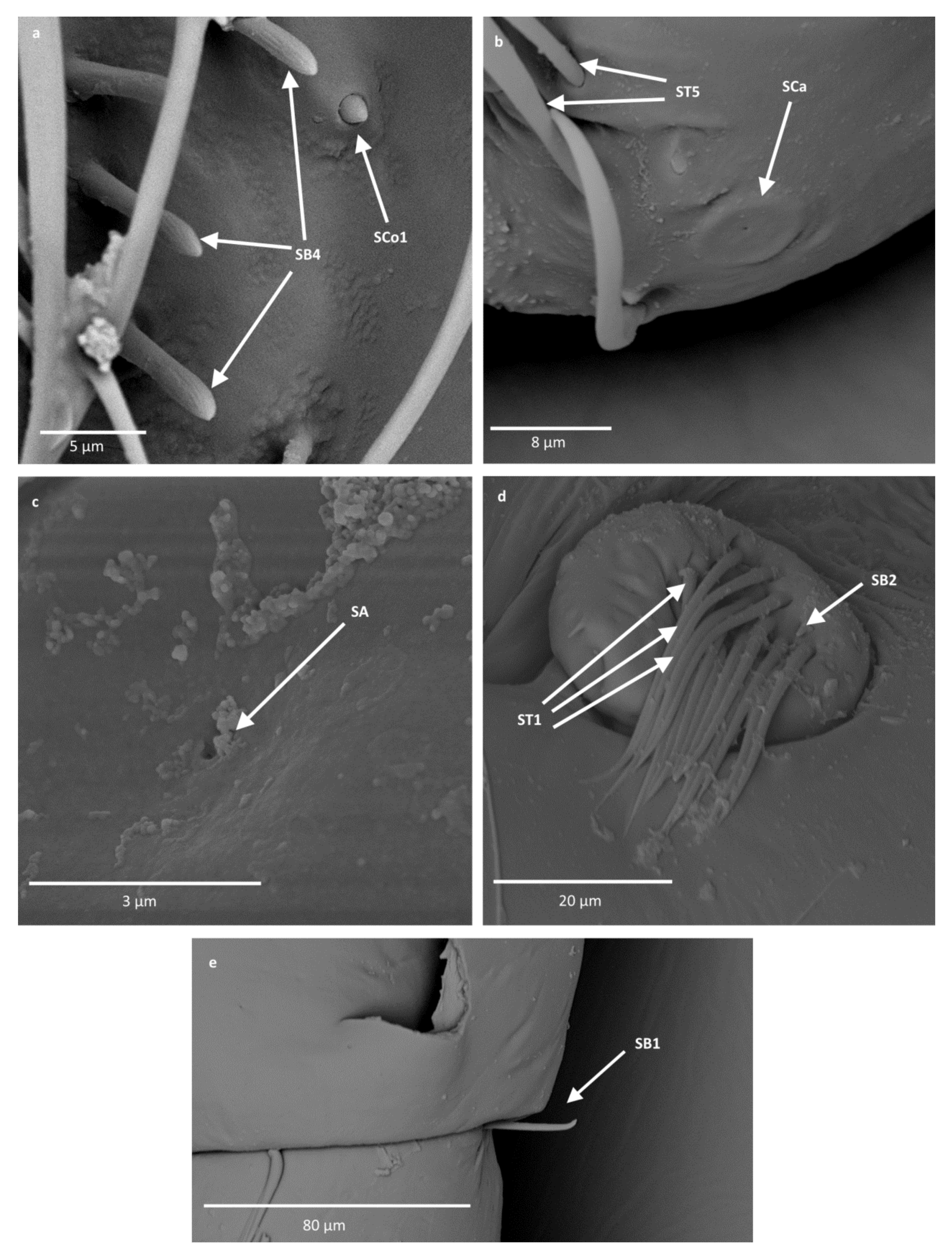
- Sensilla basiconica (SB2)—cone-like structures with a porous surface and slightly pointed tip. They were found in all studied species, except Buenoa nitida, Enithares metallica, Notonecta disturbata, Martarega, Plea minutissima, Hydrotrephes visayasensis and Neotrephes lanemeloi. This type was found on different antennomeres, both singularly and in groups (Figure 3, Figure 4a,b and Figure 6d).
- -
- Sensilla basiconica (SB3)—long, porous structures with a thick base and a thinner, slightly pointed tip. They were found in all studied species except Martarega and in the species of Helotrephidae. This type generally occurred on the second last and last antennomeres, in a group among sensilla trichodea and other sensilla basiconica. It is unusual for sensilla basiconica to be this long, but their external structure suggests a chemosensory function (Figure 3 and Figure 4a,c).
- -
- Sensilla basiconica (SB4)—cone-like structures, smooth at the base and ribbed along the length of sensillum. They were found in Anisops debilis, Anisops jaczewski, Enithares, Notonecta ceres ceres and Plea minutissima. These sensilla were found among sensilla trichodea ST3 and ST5, which covered and protected them (Figure 3, Figure 5d and Figure 6a).
- -
- Sensilla basiconica (SB5)—structures resembling flattened cones. They are more or less the same length as sensilla basiconica, SB2 and SB3, with a round base that flattens along the rest of the sensillum. Their tip is rounded or slightly rounded. They were found in Buenoa nitida, Enithares, Notonecta ceres ceres, Notonecta disturbata, Martarega and Plea minutissima. These sensilla were found in groups; mostly they appeared as single structures on different antennomeres (Figure 3, Figure 4c and Figure 5b).
- Sensilla coeloconica (SCo)—sensilla responsible for thermo-hygroreception. They are peg-like structures embedded in a cavity of cuticle in inflexible sockets. The following subtypes were observed:
- -
- Sensilla coeloconica (SCo1)—pegs arising from the cuticle, sometimes on a protuberance of the cuticle. They were found on the top of the antennae in Notonecta ceres ceres, Notonecta glauca and Martarega. These sensilla were also present on the surface of the last two antennomeres in Plea minutissima and Paraplea (Figure 3 and Figure 6a).
- -
- Sensilla coeloconica (SCo2)—pegs arising from the cuticle, surrounded by a cuticular shaft. They were found on the surface of the second last and last antennomere, covered by sensilla trichodea, ST3 and ST5, in all studied species of Notonectidae, except in Anisops sardea, Buenoa nitida and Martarega gonostyla (Figure 3, Figure 4d and Figure 5d).
- Sensilla ampullacea (SA)—sensilla responsible for thermo-hygroreception. They are small pegs, hidden in a deep cavity and arising from inflexible sockets. They occurred singularly on different antennomeres in a few of the studied species. Only an opening to the cavity is visible from the outside, and therefore we are not able to confirm that those are, in fact, sensilla ampullacea. However, taking into account this morphological appearance and comparing it with other possibilities (such as glandular structures, which usually form a sieve structure rather than appear singularly), we believe these to be sensilla ampullacea. These structures were found in Anisops sardea, Notonecta ceres ceres, Martarega uruguayensis and Hydrotrephes visayasensis (Figure 3, Figure 5b and Figure 6c).
| Species Name | Sensilla Present on the I Antennomere | Sensilla Present on the I Antennomere | Sensilla Present on the III Antennomere | Sensilla Present on the III Antennomere |
|---|---|---|---|---|
| Anisops debilis | antennomere destroyed during dissection | ST1, ST2, ST3, ST4 | ST4, ST5, SB2, SB3, SB4, SCo2 | - |
| Anisops jaczewski | ST1, ST2 | ST1, ST2, ST5 | ST1, ST2, ST4, ST5, SCa, SB2, SB3, SB4, SCo2 | - |
| Anisops sardea | antennomere destroyed during dissection | ST1, ST2, ST3, ST5, SA | ST4, ST5, SB2, SB3 | - |
| Buenoa nitida | ST1, ST2, | ST1, ST2, ST3, SCa | ST4, ST5, SB3, SB5 | - |
| Enithares metallica | ST1, ST2, SB1 | ST1, ST2, ST3, SB5 | ST1, ST2, SB3, SB5 | ST2, ST3, SB3, SB4, SCo2 |
| Enithares stridulata | ST1, ST2, SCa, SB1 | ST3, SB5 | ST1, ST2, SB3, SB4, SCo2 | ST1, ST2, ST3, SB2 |
| Notonecta ceres ceres | SCa, SA, SB1 | ST1, ST2, ST5, SCh, SA | ST1, ST2, ST5, ST4, SB2, SB3, SB4, SCo2 | ST1, ST2, ST4, ST5, SB2, SB5, SCo1 |
| Notonecta disturbata | ST1, ST2, SB1 | ST1, ST2, ST5 | ST1, ST2, ST4, ST5, SCa, SB3, SCo2 | ST1, ST2, ST4, ST5, SB5 |
| Notonecta glauca | ST1, ST2 | ST1, ST2, ST5, SCh | ST1, ST2, ST4, SB2, SB3, SCo2 | ST1, ST2, ST4, ST5, SB2, SB3, SCo1 |
| Notonecta maculata | ST1, ST2 | ST1, ST2, ST5, SCh | ST1, ST2, ST4, ST5, SB2, SCo2 | ST1, ST2, ST5, SB2, SB3 |
| Martarega gonostyla | SCa | ST1, ST2, ST5 | ST1, ST2, ST4, ST5, SCa, SB5, SCo1 | antennomere destroyed during dissection |
| Martarega uruguayensis | SCa | ST1, ST2, ST4, ST5, SCa | ST1, ST2, ST3, ST4, SB5, SA, SCo2 | ST5, SCa, SCo1 |
| Plea minutissima | ST1, ST2, ST5 | ST1, SB3, SB4, SCo1 | ST1, SB5, SCo1 | - |
| Paraplea sp. | ST5, SCa, SB1 | ST1, SB2, SB3, SB4, SCo1 | ST1, SB2, SB3, SCo1 | - |
| Hydrotrephes visayasensis | ST1, ST3, SCa, SA | ST1, ST2, ST5, SCa, SB2 | - | - |
| Neotrephes lanemeloi | ST1, SCa, SB2 | - | - | - |
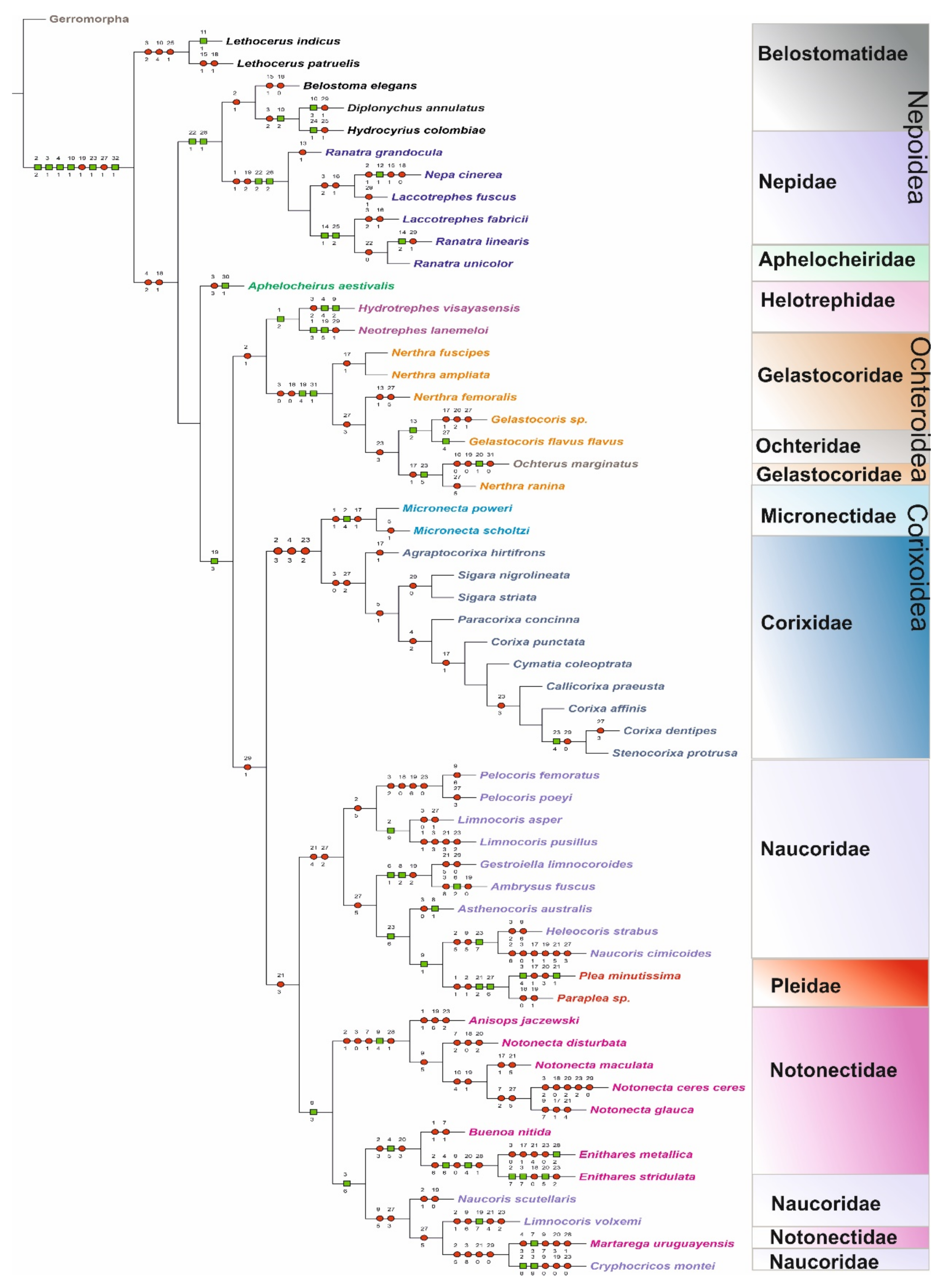
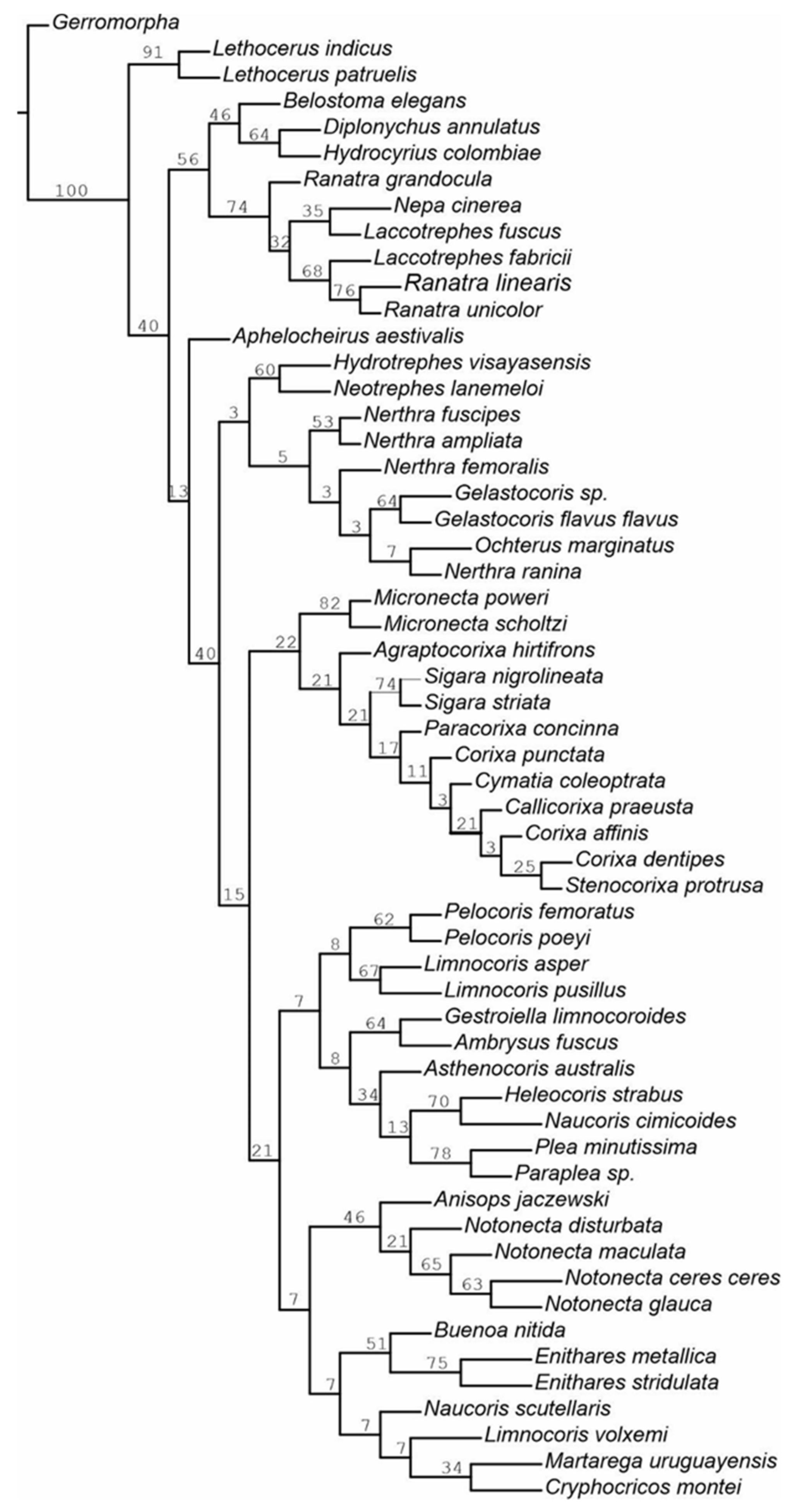
3.2. Cladistic Analysis of the Morphological Characteristics of Antennal Sensilla in Nepomorpha
4. Discussion
4.1. Morphology and Categories of the Antennal Sensilla of Notonectoidea
4.2. Comparison of the Morphological Characters and Distributions of Antennal Sensilla in the Nepomorphan Taxa
4.3. Types of Sensilla in Nepomorphan Families
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rieger, C. Skelett und Muskulatur des Kopfes und Prothorax von Ochterus marginatus Latreille. Zoomorphologie 1976, 83, 109–191. [Google Scholar] [CrossRef]
- Popov, Y.A. Historical development of the hemipterous infraorder Nepomorpha. Tr. Paleontol. Inst. Acad. Sci. Nauk USSR 1971, 129, 1–228. (In Russian) [Google Scholar]
- Mahner, M. Systema cryptoceratum phylogeneticum (Insecta, Heteroptera). Zoologica 1993, 143, 1–302. [Google Scholar]
- Hebsgaard, M.B.; Andersen, N.M.; Damgaard, J. Phylogeny of the true water bugs (Nepomorpha: Hemiptera-Heteroptera) based on 16S and 28S rDNA and morphology. Syst. Entomol. 2004, 29, 488–508. [Google Scholar] [CrossRef]
- Weirauch, C.; Schuh, R.T. Systematics and evolution of Heteroptera: 25 years of progress. Annu. Rev. Entomol. 2011, 56, 487–510. [Google Scholar] [CrossRef] [PubMed]
- Brożek, J. Phylogenetic signals from nepomorpha (Insecta: Hemiptera: Heteroptera) mouthparts: Stylets bundle, sense organs, and labial segments. Sci. World J. 2014, 2014, 1–30. [Google Scholar] [CrossRef] [Green Version]
- Panizzi, A.R.; Grazia, J. (Eds.) Introduction to true bugs (Heteroptera) of the neotropics. In True Bugs (Heteroptera) of the Neotropics; Springer: Dordrecht, The Netherlands, 2015; pp. 3–20. [Google Scholar]
- Ye, Z.; Damgaard, J.; Yang, H.; Hebsgaard, M.B.; Weir, T.; Bu, W. Phylogeny and diversification of the true water bugs (Insecta: Hemiptera: Heteroptera: Nepomorpha). Cladistics 2020, 36, 72–87. [Google Scholar] [CrossRef]
- Štys, P.; Jansson, A. Check-list of recent family-group and genus-group names of Nepomorpha (Heteroptera) of the world. Entomol. Fenn. 1988, 50, 1–44. [Google Scholar]
- Hua, J.; Li, M.; Dong, P.; Cui, Y.; Xie, Q.; Bu, W. Phylogenetic analysis of the true water bugs (Insecta: Hemiptera: Heteroptera: Nepomorpha): Evidence from mitochondrial genomes. BMC Evol. Biol. 2009, 9, 134. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.-P.; Nieser, N.; Zettel, H. The Aquatic and Semi-Aquatic Bugs (Heteroptera: Nepomorpha & Gerromorpha) of Malesia; Fauna Malesiana Handbook 5; Brill: Leiden, The Netherlands; Boston, MA, USA, 2005. [Google Scholar]
- Schuh, R.T.; Weirauch, C. (Eds.) Naucoridae in True bugs of the world (Hemiptera: Heteroptera). In Classification and Natural History; Cornell University Press: New York, NY, USA, 2020; pp. 232–236. [Google Scholar]
- Gittelman, S. Locomotion and Predatory Strategy in Backswimmers (Hemiptera: Notonectidae). Am. Midl. Nat. 1974, 92, 496–500. [Google Scholar] [CrossRef]
- Miyamoto, S. Biology of Helotrephes formosanus Esaki et Miyamoto, with descriptions of larval stages. Sieboldia 1952, 1, 1–10. [Google Scholar]
- Papáček, M.K. Problematice Morfologie e Bionomie Vodnich Plastic Nadceledi Pleoidea a Notonectoidea (Heteroptera: Nepomorpha). Ph.D. Thesis, University of South Bohemia, Česke Budejoviče, Czech Republic, 1993. [Google Scholar]
- Papáček, M. Small aquatic and ripicolous bugs (Heteroptera: Nepomorpha) as predators and prey: The question of economic importance. Eur. J. Entomol. 2001, 98, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Todd, J.L.; Baker, T.C. Function of peripheral olfactory organs. In Insect Olfaction; Hansson, B.S., Ed.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 67–96. [Google Scholar]
- Dicke, M.; Grostal, P. Chemical detection of natural enemies by arthropods: An ecological perspective. Annu. Rev. Ecol. Syst. 2001, 32, 1–23. [Google Scholar] [CrossRef]
- Zacharuk, R.Y. Ultrastructure and function of insect chemosensilla. Annu. Rev. Entomol. 1980, 25, 27–47. [Google Scholar] [CrossRef]
- Chapman, R.F. (Ed.) Mechanoreception. Chemoreception. In The Insects: Structure and Function, 4th ed.; Cambridge University Press: New York, NY, USA, 1998; pp. 610–652. [Google Scholar]
- Altner, H.; Prillinger, L. Ultrastructure of Invertebrate Chemo- Thermo- and Hygroreceptors and Its Functional Significance. Int. Rev. Cytol. 1980, 67, 69–139. [Google Scholar]
- Ågren, L. Flagellar sensilla of two species of Andrena (Hymenoptera: Andrena). Int. J. Insect Morphol. Embryol. 1978, 7, 73–79. [Google Scholar] [CrossRef]
- van Baaren, J.; Boivin, G.; Le Lannic, J.; Ne’non, J.P. Comparison of antennal sensilla of Anaphes victus and A. listronoti (Hymenoptera, Mymaridae), egg parasitoids of Curculionidae. Zoomorphology 1999, 119, 1–8. [Google Scholar] [CrossRef]
- Nowińska, A.; Brożek, J. Antennal sensory structures in water bugs of Nepoidea (Insecta: Hemiptera: Nepomorpha), their morphology and function. Zoomorphology 2019, 136, 327–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowińska, A.; Brożek, J. Insect evolution toward aquatic habitats; reassessment of antennal sensilla in the water bug families Ochteridae, Gelastocoridae and Aphelocheiridae (Hemiptera: Heteroptera: Nepomorpha). Contrib. Zool. 2020, 89, 412–433. [Google Scholar] [CrossRef]
- Nowińska, A.; Chen, P.-P.; Brożek, J. Comparative study of antennal sensilla of Corixidae and Micronectidae (Hemiptera: Heteroptera: Nepomorpha: Corixoidea). Insects 2020, 11, 734. [Google Scholar] [CrossRef]
- Nowińska, A.; Brożek, J. The variability of antennal sensilla in Naucoridae (Heteroptera: Nepomorpha). Sci. Rep. 2021, 11, 19651. [Google Scholar] [CrossRef]
- Walther, J.R. Antennal patterns of sensilla of the Hymenoptera—A complex character of phylogenetic reconstruction. Verh. Nat. Hambg. 1983, 26, 373–392. [Google Scholar]
- Spaethe, J.; Brockmann, A.; Halbig, C.; Tautz, J. Size determines antennal sensitivity and behavioral threshold to odors in bumblebee workers. Naturwissenschaften 2007, 94, 733–739. [Google Scholar] [CrossRef]
- Chapman, R.F. Chemoreception: The significance of receptor numbers. Adv. Insect Physiol. 1982, 16, 247–356. [Google Scholar]
- Polidori, C.; Nieves-Aldrey, J.L. Diverse Filters to Sense: Great Variability of Antennal Morphology and Sensillar Equipment in Gall-Wasps (Hymenoptera: Cynipidae). PLoS ONE 2014, 9, e101843. [Google Scholar] [CrossRef]
- Chaika, S.Y.; Sinitsina, E.E. Sensory organs of the labium and antennae in water bugs (Heteroptera). Entomol. Obozr. 1999, 78, 40–59. [Google Scholar]
- Garza, C.; Ramos, D.; Cook, J.L. Comparative morphology of antennae in the family Pleidae (Hemiptera: Heteroptera). Zoomorphology 2021, 140, 243–256. [Google Scholar] [CrossRef]
- Shields, V.D.C. High resolution ultrastructural investigation of insect sensory organs using field emission scanning electron microscopy. In Microscopy: Science, Technology, Applications and Education; Mendez, V.A., Diaz, J., Eds.; Formatex: Badajoz, Spain, 2010; pp. 321–328. [Google Scholar]
- Nowińska, A.; Brożek, J. Morphological study of the antennal sensilla in Gerromorpha (Insecta: Hemiptera: Heteroptera). Zoomorphology 2017, 136, 327–347. [Google Scholar] [CrossRef] [Green Version]
- Goloboff, P.; Farris, J.; Nixon, K. TNT, a free program for phylogenetic analysis. Cladistics 2008, 24, 774–786. [Google Scholar] [CrossRef]
- NIXON, K.C. WinClada, ver. 1.00.08; NIXON, K.C.: Ithaca, NY, USA, 2002. Available online: http://www.cladistics.com/aboutWinc.htm(accessed on 4 February 2020).
- McIver, S.B. Structure of Cuticular Mechanoreceptors of Arthropods. Annu. Rev. Entomol. 1975, 20, 381–397. [Google Scholar] [CrossRef] [PubMed]
- Keil, T.A.; Steinbrecht, R.A. Mechanosensitive and olfactory sensilla of insects. In Insect Ultrastructure; King, R.C., Akai, H., Eds.; Plenum: New York, NY, USA; London, UK, 1984; Volume 2, pp. 477–516. [Google Scholar]
- Slifer, E.H.; Sekhon, S.S. Circumfila and other sense organs on the antenna of the sorghum midge (Diptera, Cecidomyiidae). J. Morphol. 1971, 133, 281–301. [Google Scholar] [CrossRef] [PubMed]
- Steinbrecht, R.A.; Müller, B. Fine structure of the antennal receptors of the bed bug, Cimex lectularius L. Tissue Cell 1976, 8, 615–636. [Google Scholar] [CrossRef] [PubMed]
- Tichy, H.; Kallina, W. The Evaporative Function of Cockroach Hygroreceptors. PLoS ONE 2013, 8, e53998. [Google Scholar] [CrossRef]
- Altner, H.; Schaller-Selzer, L.; Setter, H.; Wohlrab, I. Poreless sensilla with inflexible sockets. A comparative study of a fundamental type of insect sensilla probably comprising thermo- and hygroreceptors. Cell Tissue Res. 1983, 234, 279–307. [Google Scholar] [CrossRef]
- Wheeler, W.C.; Schuh, R.T.; Bang, R. Cladistic relation-ships among higher groups of Heteroptera: Congruence between morphological and molecular data sets. Entomol. Scand. 1993, 24, 121–137. [Google Scholar] [CrossRef]
- Scherbakov, D.E.; Popov, Y.A. Superorder Cimicidea Laicharting, 1781. Order Hemiptera Linne, 1758. The bugs, cicadas, plantlice, scale insects, etc. In History of Insects; Rasnitsyn, A.P., Quicke, D.L.J., Eds.; Kluwer Academic: Dordrecht, The Netherlands, 2002; pp. 143–157. [Google Scholar]
- Xie, Q.; Tian, Y.; Zheng, L.; Bu, W. 18S rRNA hyper-elongation and the phylogeny of Euhemiptera (Insecta: Hemiptera). Mol. Phylogenet. Evol. 2008, 47, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Nieser, N. Guide to aquatic Heteroptera of Singapore and Peninsular Malaysia. IV. Corixoidea. Raes. Bull. Zool. 2002, 50, 263–274. [Google Scholar]
- Brożek, J. Comparative analysis and systematic mapping of the labial sensilla in the Nepomorpha (Heteroptera: Insecta). Sci. World J. 2013, 2013, 518034. [Google Scholar] [CrossRef]
- Schneider, D. Insect antennae. Annu. Rev. Entomol. 1964, 9, 103–122. [Google Scholar] [CrossRef]
- Isidoro, N.; Bin, F.; Colazza, S.; Vinson, S.B. Morphology of antennal gustatory sensilla and glands in some parasitoid Hymenoptera with hypothesis on their role in sex and host recognition. J. Hymen. Res. 1996, 5, 206–239. [Google Scholar]
- Rebora, M.; Salerno, G.; Piersanti, S. Aquatic Insect Sensilla: Morphology and Function. In Aquatic Insects; Del-Claro, K., Guillermo, R., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Steinbrecht, R.A. Pore structures in insect olfactory sensilla: A review of data and concepts. Int. J. Insect Morphol. Embryol. 1997, 26, 229–245. [Google Scholar] [CrossRef]
- Chinta, S.; Dickens, J.C.; Baker, G.T. Morphology and distribution of antennal sensilla of the tarnished plant bug, Lygus lineolaris (Palisot de Beauvois) (Hemiptera: Miridae). Int. J. Insect Morphol. Embryol. 1997, 26, 21–26. [Google Scholar] [CrossRef]
- Slu, C. Ultrastructure of the antennal sensillae of the bug, Rhodnius prolixus (Hemiptera, Reduviidae). Parazitologiia 1980, 14, 92–486. [Google Scholar]
- Akent’eva, N.A. The formation of the antenna sensory apparatus in some bug (Heteroptera) species in the course of their postembryonic development. Entomol. Rev. 2008, 88, 381–390. [Google Scholar] [CrossRef]
- Ahmad, A.; Parveena, S.; Brożek, J.; Dey, D. Antennal sensilla of phytophagous and predatory pentatomids (Hemiptera: Pentatomidae): A comparative study of four genera. Zool. Anz. 2016, 261, 48–55. [Google Scholar] [CrossRef]
- Rani, P.U.; Madhavendra, S.S. Morphology and distribution of antennal sense organs and diversity of mouthpart structures in Odontopus nigricornis (Stall) and Nezara viridula L. (Hemiptera). Int. J. Insect Morphol. Embryol. 1995, 24, 119–132. [Google Scholar] [CrossRef]
- Rani, P.U.; Madhavendra, S.S. External morphology of antennal and rostral sensillae in four hemipteran insects and their possible role in host plant selection. Int. J. Trop. Insect Sci. 2005, 25, 198–207. [Google Scholar] [CrossRef]
- Brézot, P.; Tauban, D.; Renou, M. Sense organs on the antennal flagellum of the green stink bug, Nezara viridula (L.) (Heteroptera: Pentatomidae): Sensillum types and numerical growth during the post-embryonic development. Int. J. Insect Morphol. Embryol. 1997, 25, 427–441. [Google Scholar] [CrossRef]
| Gerromorpha | 00000000000000000000000000000000 |
| Belostoma_elegans | 01120000010000100010011001100001 |
| Diplonychus_annulatus | 01220000030000000110011001101001 |
| Hydrocyrius_colombiae | 01220000020000000110011111100001 |
| Lethocerus_indicus | 02210000041000000010001010100001 |
| Lethocerus_patruelis | 02210000040000100110001010100001 |
| Nepa_cinerea | 11220000010100110020021002100001 |
| Laccotrephes_fuscus | 12220000010000010120021002101001 |
| Laccotrephes_fabricii | 12220000010001010120021022100001 |
| Ranatra_grandocula | 12120000010010000120021002100001 |
| Ranatra_linearis | 12120000010002000120001022101001 |
| Ranatra_unicolor | 12120000010001000120001022100001 |
| Agraptocorixa_hirtifrons | 03030000010000001130002000201001 |
| Callicorixa_praeusta | 03021000010000001130003000201001 |
| Corixa_affinis | 03021000010000001130003000201001 |
| Corixa_dentipes | 03021000010000001130004000300001 |
| Corixa_punctata | 03021000010000001130002000201001 |
| Paracorixa_concinna | 03021000010000000130002000201001 |
| Sigara_nigrolineata | 03031000010000000130002000200001 |
| Sigara_striata | 03031000010000000130002000200001 |
| Cymatia_coleoptrata | 03021000010000001130002000201001 |
| Stenocorixa_protrusa | 03021000010000001130004000200001 |
| Micronecta_poweri | 14130000010000001130002000101001 |
| Micronecta_scholtzi | 14131000010000001130002000101001 |
| Aphelocheirus_aestivalis_ | 02320000010000000110001000100101 |
| Ochterus_marginatus | 01020000000000001001005000300001 |
| Gelastocoris_sp. | 01020000010020001042003000100011 |
| Gelastocoris_flavus_flavus | 01020000010020000040003000400011 |
| Nerthra_femoralis | 01020000010010000040001000500011 |
| Nerthra_fuscipes | 01020000010000001040001000100011 |
| Nerthra_ampliata | 01020000010000001040001000100011 |
| Nerthra_ranina | 01020000010000001040005000500011 |
| Plea_minutissima | 11420000110000001133106000601001 |
| Paraplea_sp. | 11120000110000000010206000601001 |
| Hydrotrephes_visayasensis | 21240000210000000130001000100001 |
| Neotrephes_lanemeloi | 31120000010000000150001000101001 |
| Anisops_jaczewski | 11020010410000000160302000111001 |
| Buenoa_nitida | 13650010310000000133301000101001 |
| Enithares_metallica | 06060000010000001134400000121001 |
| Enithares_stridulata | 07760000010000000035302000111001 |
| Notonecta_ceres_ceres | 01220020540000000012302000510001 |
| Notonecta_disturbata | 01020020510000000032301000111001 |
| Notonecta_glauca | 01020020740000001110401000511001 |
| Notonecta_maculata | 01020010540000001110501000111001 |
| Martarega_uruguayensis | 05830030710000000133001000510001 |
| Asthenocoris_australis | 02020001010000000130406000501001 |
| Gestroiella_limnocoroides | 02120102010000000120501000500001 |
| Ambrysus_fuscus | 02820202010000000100401000501001 |
| Cryphocricos_montei | 08920000010000000100000000500001 |
| Heleocoris_strabus | 05220000610000000130407000501001 |
| Limnocoris_asper | 09020000010000000130401000101001 |
| Limnocoris_pusillus | 19320000010000000130302000201001 |
| Limnocoris_volxemi | 01620000610000000170402000501001 |
| Naucoris_cimicoides | 06020000510000001110507000301001 |
| Naucoris_scutellaris_ | 01620000510000000100301000301001 |
| Pelocoris_femoratus | 05220000610000000060400000201001 |
| Pelocoris_poeyi | 05220000010000000060400000301001 |
| Sensilla Types | Present Study Plea minutissima | Study of Garza et al. (2021) Plea minutissima |
|---|---|---|
| Mechanoreceptive sensilla | ST1—hairlike sensillum with a smooth surface and a flexible socket ST2—hairlike sensillum with a ribbed surface and a flexible socket ST5—leaflike sensillum with a flexible surface | ST1—long (40–70 µm) hairlike structures with an inflexible socket ST3—sensilla with the length of 40–55 µm, curled towards the apex with an inflexible socket SL2—leaflike sensilla, wide and long with a flexible socket SL3—the widest leaflike sensilla with a ribbed surface and a flexible socket SCh—short, thick and hairlike sensilla with a flexible socket |
| Chemoreceptive sensilla | SB3—long porous structures with an inflexible socket SB4—short cones with a smooth surface on the base and the rest of the sensillum ribbed SB5—short flattened cones with an inflexible surface | SUT—hairlike sensilla with a single pore on the surface with an inflexible socket SB—cones with an apical pore, smooth at the base with channels extending to the apical pore |
| Thermo-hygroreceptive sensilla | SCo1—pegs arising from the cuticle, sometimes on a protuberance of the cuticle with an inflexible socket | SCo1—pegs arising from a pit in the center of a cone with a movable socket SCo3—pegs arising from a simple pit |
| Family/Species | Mechanoreception | Chemoreception | Thermo-Hygroreception | Unknown Function | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ST1 | ST2 | ST3 | ST4a | ST4b | ST4c | ST5a | ST5b | SCh | SCoL | SBL | SClL | SPL1 | SPL2 | SSq | SCa | SB1 | SB2 | SB3a | SB3b | SB3c | SB4 | SCo1a | SCo2a | SCo3 | SCo1b | SCo2b | SA | SPl | SPM | ||
| Belostomatidae | Belostoma elegans | + | + | + | + | + | + | + | + | ||||||||||||||||||||||
| Diplonychus annulatus | + | + | + | + | + | + | + | ||||||||||||||||||||||||
| Hydrocyrius colombiae | + | + | + | + | + | + | + | + | + | + | |||||||||||||||||||||
| Lethocerus indicus | + | + | + | + | + | + | + | + | + | ||||||||||||||||||||||
| Lethocerus patruelis | + | + | + | + | + | + | + | + | |||||||||||||||||||||||
| Nepidae | Nepa cinerea | + | + | + | + | + | + | + | + | + | + | + | |||||||||||||||||||
| Laccotrephes fuscus | + | + | + | + | + | + | |||||||||||||||||||||||||
| Laccotrephes fabricii | + | + | + | + | + | + | + | + | + | ||||||||||||||||||||||
| Ranatra grandocula | + | + | + | + | + | + | |||||||||||||||||||||||||
| Ranatra linearis | + | + | + | + | + | ||||||||||||||||||||||||||
| Ranatra unicolor | + | + | + | + | + | + | |||||||||||||||||||||||||
| Aphelo cheiridae | Aphelocheirus aestivalis | + | + | + | + | + | + | ||||||||||||||||||||||||
| Ochteridae | Ochterus marginatus | + | + | + | + | + | + | + | + | + | |||||||||||||||||||||
| Gelastocoridae | Gelastocoris sp. | + | + | + | + | + | + | + | + | + | |||||||||||||||||||||
| Gelastocoris flavus flavus | + | + | + | + | + | + | + | + | + | + | |||||||||||||||||||||
| Nerthra femoralis | + | + | + | + | + | + | + | + | + | ||||||||||||||||||||||
| Nerthra fuscipes | + | + | + | + | + | + | |||||||||||||||||||||||||
| Nerthra ampliata | + | + | + | + | + | + | + | ||||||||||||||||||||||||
| Nerthra ranina | + | + | + | + | + | + | + | + | |||||||||||||||||||||||
| Corixidae | Agraptocorixa hirtifrons | + | + | + | + | + | |||||||||||||||||||||||||
| Callicorixa praeusta | + | + | + | + | + | ||||||||||||||||||||||||||
| Corixa affinis | + | + | + | + | + | ||||||||||||||||||||||||||
| Corixa dentipes | + | + | + | + | + | + | |||||||||||||||||||||||||
| Corixa punctata | + | + | + | + | + | ||||||||||||||||||||||||||
| Paracorixa concinna | + | + | + | + | + | + | |||||||||||||||||||||||||
| Sigara nigrolineata | + | + | + | + | + | + | + | + | |||||||||||||||||||||||
| Sigara striata | + | + | + | + | + | + | + | + | |||||||||||||||||||||||
| Cymatia coleoptrata | + | + | + | + | + | ||||||||||||||||||||||||||
| Stenocorixa protrusa | + | + | + | + | + | + | |||||||||||||||||||||||||
| Micronecidae | Micronecta poweri | + | + | + | |||||||||||||||||||||||||||
| Micronecta scholtzi | + | + | + | + | |||||||||||||||||||||||||||
| Naucoridae | Asthenocoris australis | + | + | + | + | + | + | ||||||||||||||||||||||||
| Gestroiella limnocoroides | + | + | + | + | + | + | + | ||||||||||||||||||||||||
| Ambrysus fuscus | + | + | + | + | + | + | + | ||||||||||||||||||||||||
| Cataractocoris marginiventris | + | + | + | + | + | + | + | ||||||||||||||||||||||||
| Heleocoris strabus | + | + | + | + | + | + | + | ||||||||||||||||||||||||
| Limnocoris asper | + | + | + | + | |||||||||||||||||||||||||||
| Limnocoris pusillus | + | + | + | + | + | + | |||||||||||||||||||||||||
| Limnocoris volxemi | + | + | + | + | + | + | + | + | |||||||||||||||||||||||
| Naucoris cimicoides | + | + | + | + | + | + | + | + | |||||||||||||||||||||||
| Naucoris scutellaris | + | + | + | + | + | + | + | ||||||||||||||||||||||||
| Pelocoris femoratus | + | + | + | + | + | + | + | + | + | ||||||||||||||||||||||
| Pelocoris poeyi | + | + | + | + | + | + | + | + | + | ||||||||||||||||||||||
| Notonectidae | Anisops jaczewski | + | + | + | + | + | + | + | + | + | |||||||||||||||||||||
| Buenoa nitida | + | + | + | + | + | + | + | + | |||||||||||||||||||||||
| Enithares metallica | + | + | + | + | + | + | + | ||||||||||||||||||||||||
| Enithares stridulata | + | + | + | + | + | + | + | + | + | + | |||||||||||||||||||||
| Notonecta ceres ceres | + | + | + | + | + | + | + | + | + | + | + | + | + | + | |||||||||||||||||
| Notonecta disturbata | + | + | + | + | + | + | + | + | + | ||||||||||||||||||||||
| Notonecta glauca | + | + | + | + | + | + | + | + | + | ||||||||||||||||||||||
| Notonecta maculata | + | + | + | + | + | + | + | + | |||||||||||||||||||||||
| Martarega uruguayensis | + | + | + | + | + | + | + | + | + | + | |||||||||||||||||||||
| Pleidae | Plea minutissima | + | + | + | + | + | + | + | |||||||||||||||||||||||
| Paraplea sp. | + | + | + | + | + | + | + | + | |||||||||||||||||||||||
| Helotrephidae | Hydrotrephes visayasensis | + | + | + | + | + | + | ||||||||||||||||||||||||
| Neotrephes lanemeloi | + | + | + | ||||||||||||||||||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowińska, A.; Brożek, J. Morphology of the Antennal Sensilla of Notonectoidea and Comparison of Evolutionary Changes in Sensilla Types and Distribution in Infraorder Nepomorpha (Insecta: Heteroptera). Insects 2021, 12, 1121. https://doi.org/10.3390/insects12121121
Nowińska A, Brożek J. Morphology of the Antennal Sensilla of Notonectoidea and Comparison of Evolutionary Changes in Sensilla Types and Distribution in Infraorder Nepomorpha (Insecta: Heteroptera). Insects. 2021; 12(12):1121. https://doi.org/10.3390/insects12121121
Chicago/Turabian StyleNowińska, Agnieszka, and Jolanta Brożek. 2021. "Morphology of the Antennal Sensilla of Notonectoidea and Comparison of Evolutionary Changes in Sensilla Types and Distribution in Infraorder Nepomorpha (Insecta: Heteroptera)" Insects 12, no. 12: 1121. https://doi.org/10.3390/insects12121121
APA StyleNowińska, A., & Brożek, J. (2021). Morphology of the Antennal Sensilla of Notonectoidea and Comparison of Evolutionary Changes in Sensilla Types and Distribution in Infraorder Nepomorpha (Insecta: Heteroptera). Insects, 12(12), 1121. https://doi.org/10.3390/insects12121121






