Interspecific Hybridization and Complete Mitochondrial Genome Analysis of Two Ghost Moth Species
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Morphological and Molecular Characteristics of Thitarodes Insect Populations
2.2. Inbred and Hybrid Thitarodes Populations
2.3. O. sinensis Fungal Isolates
2.4. Larval Infection of Inbred Populations by O. sinensis Isolates
2.5. Analysis of the Mitochondrial Genomes
2.6. Data Analysis
3. Results
3.1. Morphological and Molecular Identification of Two Thitarodes Species
3.2. Development from the Pupae to Next-Generation Pupae in Inbred and Hybrid Populations
3.3. Larval Infection of Inbred Populations by O. sinensis Isolates
3.4. Mitochondrial Genome Analysis
3.5. Phylogenetic Relationships and Taxonomic Relation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paterson, R.R.M. Cordyceps-A traditional Chinese medicine and another fungal therapeutic biofactory? Phytochemisty 2008, 69, 1469–1495. [Google Scholar] [CrossRef] [Green Version]
- Dong, C.H.; Yao, Y.J. On the reliability of fungal materials used in studies on Ophiocordyceps sinensis. J. Ind. Microbiol. Biotechnol. 2011, 38, 1027–1035. [Google Scholar] [CrossRef]
- Shrestha, U.B.; Bawa, K.S. Impact of climate change on potential distribution of Chinese Caterpillar fungus (Ophiocordyceps sinensis) in Nepal Himalaya. PLoS ONE 2014, 9, e106405. [Google Scholar]
- RiChou, H.; Hua, W.; HaiPing, T.; XueHong, Q.; GuiQing, L.; ZhongChen, R.; Li, C. Research on Chinese cordyceps during the past 70 years in China. Chin. J. Appl. Entomol. 2019, 56, 849–883. [Google Scholar]
- Liang, H.H.; Cheng, Z.; Yang, X.L.; Li, S.; Ding, Z.Q.; Zhou, T.S.; Zhang, W.J.; Chen, J.K. Genetic diversity and structure of Cordyceps sinensis populations from extensive geographical regions in China as revealed by inter-simple sequence repeat markers. J. Microbiol. 2008, 5, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Winkler, D. Caterpillar fungus (Ophiocordyceps sinensis) production and sustainability on the Tibetan Plateau and in the Himalayas. Asian Med. 2009, 5, 291–316. [Google Scholar] [CrossRef] [Green Version]
- Holliday, J.; Cleaver, M. Medicinal value of the caterpillar fungi species of the genus Cordyceps (Ascomycetes): A review. Int. J. Med. Mushrooms 2008, 10, 219–234. [Google Scholar] [CrossRef]
- Li, W.J.; Dong, C.H.; Liu, X.Z.; Li, Q.P.; Xia, J.M.; Liang, L. Research advances in artificial cultivation of Chinese cordyceps. Mycosystema 2016, 35, 375–387. [Google Scholar]
- Baral, B. Entomopathogenicity and biological attributes of Himalayan treasured fungus Ophiocordyceps sinensis (Yarsagumba). J. Fungi 2017, 3, 4. [Google Scholar] [CrossRef] [Green Version]
- Pouliota, M.; Pyakurela, D.; Smith-Halla, C. High altitude organic gold: The production network for Ophiocordyceps sinensis from far-western Nepal. J. Ethnopharmacol. 2018, 218, 59–68. [Google Scholar] [CrossRef]
- Qin, Q.L.; Zhou, G.L.; Zhang, H.; Meng, Q.; Zhang, J.H.; Wang, H.T.; Miao, L.; Li, X. Obstacles and approaches in artificial cultivation of Chinese cordyceps. Mycology 2018, 1, 7–9. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.T.; Tang, J.J.; Mao, J.L. A preliminary study on the biology of the “insect herb”, Hepialus armoricanus Oberthür. Acta Entomol. Sin. 1973, 6, 198–202. [Google Scholar]
- Quan, Q.M.; Chen, L.L.; Wang, X.; Li, S.; Yang, X.L.; Zhu, Y.G.; Wang, M.; Cheng, Z. Genetic diversity and distribution patterns of host insects of Caterpillar fungus Ophiocordyceps sinensis in the Qinghai-Tibet plateau. PLoS ONE 2014, 9, e92293. [Google Scholar]
- Zhang, Y.J.; Zhang, S.; Li, Y.L.; Ma, S.L.; Wang, C.S.; Xiang, M.C.; Liu, X.; An, Z.Q.; Xu, J.P.; Liu, X.Z. Phylogeography and evolution of a fungal–insect association on the Tibetan Plateau. Mol. Ecol. 2014, 23, 5337–5355. [Google Scholar] [CrossRef]
- Quan, Q.M.; Wang, Q.X.; Zhou, X.L.; Li, S.; Yang, X.L.; Zhu, Y.G.; Cheng, Z. Comparative phylogenetic relationships and genetic structure of the Caterpillar fungus Ophiocordyceps sinensis and its host insects inferred from multiple gene sequences. J. Microbiol. 2014, 52, 99–105. [Google Scholar] [CrossRef]
- Yang, D.R.; Li, C.D.; Shu, C.; Yang, Y.X. Studies on the Chinese species of the genus Hepialus and their geographical distribution. Acta Entomol. Sin. 1996, 39, 413–422. [Google Scholar]
- Wang, X.L.; Yao, Y.J. Host insect species of Ophiocordyceps sinensis: A review. Zookeys 2011, 127, 43–59. [Google Scholar]
- Li, J.F.; Zhang, G.R. Life table of the experimental population of Thitarodes pui (Lepidoptera, Hepialidae), a host species of Ophiocordyceps sinensis. J. Environ. Entomol. 2012, 34, 386–389. [Google Scholar]
- Li, Q.P.; He, Y.; Liu, J.M.; Xia, J.M.; Li, W.J.; Liu, X.Z. Hybrid breeding of high quality of Hepialus sp., the host of Ophiocordyceps sinensis, and prevention of the host insect reproductive degradation. Mycosystema 2016, 35, 456–466. [Google Scholar]
- Tao, Z.; Cao, L.; Zhang, Y.; Ye, Y.S.; Han, R.C. Laboratory rearing of Thitarodes armoricanus and Thitarodes jianchuanensis (Lepidoptera: Hepialidae), hosts of the Chinese medicinal fungus Ophiocordyceps sinensis (Hypocreales: Ophiocordycipitaceae). J. Econ. Entomol. 2016, 109, 176–181. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, Z.M.; Yin, J.; Zhang, T.M.; Zhang, X.Y.; Yuan, D.W.; Li, T.; Zhong, Y.; Ma, E.B.; Ren, Z.M. Complete mitochondrial genome of two Thitarodes species (Lepidoptera, Hepialidae), the host moths of Ophiocordyceps sinensis and phylogenetic implications. Int. J. Biol. Macromol. 2019, 140, 794–807. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.F.; Fu, S.Q.; Luo, Q.M. Experiments on the feeding habits of Hepialus armoricanus larva in Kangding. Sichuan J. Zool. 1989, 8, 8–10. [Google Scholar]
- Yue, K.; Ye, M.; Lin, X.; Zhou, Z.J. The artificial cultivation of medicinal caterpillar fungus, Ophiocordyceps sinensis (Ascomycetes): A review. Int. J. Med. Mushrooms 2013, 15, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.J.; Xiang, L.; Zhou, Z.J.; Dai, Y.; Han, K.H.; Zhu, T.H. Biological characteristics of four generation artificial swift moth adults. J. Northeast For. Univ. 2014, 42, 112–115. [Google Scholar]
- Cao, L.; Han, R.C. A Method for Artificial Cultivation of Fruiting Bodies of Ophiocordyceps sinensis. Chinese Patent ZL201410289703.0.2014-06-25, 20 May 2015. [Google Scholar]
- Liu, G.Q.; Han, R.C.; Cao, L. Artificial cultivation of the Chinese Cordyceps from injected ghost moth larvae. Environ. Entomol. 2019, 5, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.Q.; Cao, L.; Qiu, X.H.; Han, R.C. Quorum sensing activity and hyphal growth by external stimuli in the entomopathogenic fungus Ophiocordyceps sinensis. Insects 2020, 11, 205. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Herrera, J.; Pérez-Rodríguez, F.; Velez-Haro, J. The signaling mechanisms involved in the dimorphic phenomenon of the Basidiomycota fungus Ustilago maydis. Int. Microbiol. 2020, 23, 123–126. [Google Scholar] [CrossRef]
- Boucias, D.; Liu, S.; Meagher, R.; Baniszewski, J. Fungal dimorphism in the entomopathogenic fungus Metarhizium rileyi: Detection of an in vivo quorum-sensing system. J. Invertebr. Pathol. 2016, 136, 100–108. [Google Scholar] [CrossRef]
- Meng, Q.; Yu, H.Y.; Zhang, H.; Zhu, W.; Wang, M.L.; Zhang, J.H.; Zhou, G.L.; Li, X.; Qin, Q.L.; Hu, S.N.; et al. Transcriptomic insight into the immune defenses in the ghost moth, Hepialus xiaojinensis, during an Ophiocordyceps sinensis fungal infection. Insect Biochem. Mol. Biol. 2015, 64, 1–15. [Google Scholar] [CrossRef]
- Rao, Z.C.; Cao, L.; Qiu, X.H.; Han, R.C. Comparative transcriptome analysis reveals molecular strategies of ghost moth Thitarodes armoricanus in response to hypoxia and anoxia. J. Insect Physiol. 2019, 112, 23–34. [Google Scholar] [CrossRef]
- Krzywinski, J.; Grushko, O.G.; Besansky, N.J. Analysis of the complete mitochondrial DNA from Anopheles funestus: An improved dipteran mitochondrial genome annotation and a temporal dimension of mosquito evolution. Mol. Phylogenet. Evol. 2006, 39, 417–423. [Google Scholar] [CrossRef]
- Wolstenholme, D.R. Genetic novelties in mitochondrial genomes of multicellular animals. Curr. Opin. Genet. Dev. 1992, 2, 918–925. [Google Scholar] [CrossRef]
- Boore, J.L. Animal mitochondrial genomes. Nucleic Acids Res. 1999, 27, 1767–1780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheffield, N.C.; Song, H.; Cameron, S.L.; Whiting, M.F. A Comparative analysis of mitochondrial genomes in Coleoptera (Arthropoda: Insecta) and genome descriptions of six new beetles. Mol. Biol. Evol. 2008, 25, 2499–2509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Y.Q.; Ma, C.; Chen, J.Y.; Yang, D.R. The complete mitochondrial genomes of two ghost moths, Thitarodes renzhiensis and Thitarodes yunnanensis: The ancestral gene arrangement in Lepidoptera. BMC Genom. 2012, 13, 276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, J.Q.; Que, S.Q.; Xin, T.R.; Xia, B.; Zou, Z.W. Complete mitochondrial genome of Thitarodes pui (Lepidoptera: Hepialidae). Mitochondrial DNA 2016, 27, 109–110. [Google Scholar] [CrossRef]
- Chen, S.J.; Shi, P.; Zhang, D.L.; Lu, Z.H.; Li, L.Y. Complete mitochondrial genome of Hepialus xiaojinensis (Lepidoptera: Hepialidae). Mitochondrial DNA 2017, 28, 305–306. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Lu, Z.H.; He, Y.C.; Chen, S.J.; Qin, S.R.; Qing, Y.L.; Yan, J. Complete mitochondrial genome of Hepialus gonggaensis (Lepidoptera: Hepialidae), the host insect of Ophiocordyceps sinensis. Mitochondrial DNA 2016, 27, 4205–4206. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.W.; Min, Q.; Cheng, S.Y.; Xin, T.R.; Xia, B. The complete mitochondrial genome of Thitarodes sejilaensis (Lepidoptera: Hepialidae), a host insect of Ophiocordyceps sinensis and its implication in taxonomic revision of Hepialus adopted in China. Gene 2016, 601, 44–55. [Google Scholar] [CrossRef]
- Kang, X.C.; Hu, Y.Q.; Hu, J.; Hu, L.Q.; Wang, F.; Liu, D.B. The mitochondrial genome of the lepidopteran host cadaver (Thitarodes sp.) of Ophiocordyceps sinensis and related phylogenetic analysis. Gene 2017, 598, 32–42. [Google Scholar] [CrossRef]
- Zou, Z.W.; Liu, X.; Zhang, G.R. Revision of taxonomic system of Hepialus (Lepidoptera, Hepialidae) currently adopted in China. J. Hunan Univ. Sci. Technol. 2010, 25, 114–120. [Google Scholar]
- Wang, Z.Y.; Zhuang, H.L.; Wang, M.; Pierce, N.E. Thitarodes shambalaensis sp. nov. (Lepidoptera, Hepialidae): A new host of the caterpillar fungus Ophiocordyceps sinensis supported by genome-wide SNP data. Zookeys 2019, 885, 89–113. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Rao, Z.C.; Cao, L.; De Clercq, P.; Han, R.C. Infection of Ophiocordyceps sinensis fungus causes dramatic changes in the microbiota of its Thitarodes Host. Fornt. Microbiol. 2020, 11, 577268. [Google Scholar] [CrossRef]
- Simon, C.; Frati, F.; Beckenbach, A.; Crespi, B.; Liu, H.; Flook, P. Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann. Entomol. Soc. Am. 1994, 87, 651–701. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar] [PubMed]
- Lunau, S.; Stoessel, S.; Schmidt-Peisker, A.J.; Ehlers, R.-U. Establishment of monoxenic inocula for scaling up in vitro cultures of the entomopathogenic Nematodes Steinernema spp. and Heterorhabditis spp. Nematologica 1993, 39, 385–399. [Google Scholar] [CrossRef] [Green Version]
- Cao, L.; Ye, Y.S.; Han, R.C. Fruiting body production of the medicinal fungus Ophiocordyceps sinensis in artificial medium. Int. J. Med. Mushrooms 2015, 17, 1107–1112. [Google Scholar] [CrossRef]
- Lobry, J.R. Asymmetric substitution patterns in the two DNA strands of bacteria. Mol. Biol. Evol. 1996, 13, 660–665. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.F.; Su, T.J.; Luo, A.R.; Zhu, C.D.; Wu, C.S. Characterization of the complete mitochondrion genome of diurnal moth Amata emma (Butler) (Lepidoptera: Erebidae) and its phylogenetic implications. PLoS ONE 2013, 8, e72410. [Google Scholar] [CrossRef]
- Dai, L.S.; Qian, C.; Zhang, C.F.; Wang, L.; Wei, G.Q.; Li, J.; Zhu, B.J.; Liu, C.L. Characterization of the complete mitochondrial genome of Cerura menciana and comparison with other lepidopteran insects. PLoS ONE 2015, 10, e0132951. [Google Scholar]
- Cong, Q.; Grishin, N.V. The complete mitochondrial genome of Lerema accius and its phylogenetic implications. PeerJ 2016, 4, e1546. [Google Scholar] [CrossRef] [Green Version]
- Cameron, S.L.; Whiting, M.F. The complete mitochondrial genome of the tobacco hornworm, Manduca sexta, (Insecta: Lepidoptera: Sphingidae), and an examination of mitochondrial gene variability within butterflies and moths. Gene 2008, 408, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Liao, F.; Wang, L.; Wu, S.; Li, Y.P.; Zhao, L.; Huang, G.M.; Niu, C.J.; Liu, Y.Q.; Li, M.G. The complete mitochondrial genome of the fall webworm, Hyphantria cunea (Lepidoptera: Arctiidae). Int. J. Biol. Sci. 2010, 6, 172–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salvato, P.; Simonato, M.; Battisti, A.; Negrisolo, E. The complete mitochondrial genome of the bag-shelter moth Ochrogaster lunifer (Lepidoptera, Notodontidae). BMC Genom. 2008, 9, 331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotzé, A.; Grobler, J.P.; Dalton, D.L.; Labuschagne, C. The complete sequence of the mitochondrial genome of the African Penguin (Spheniscus demersus). Gene 2014, 534, 113–118. [Google Scholar]
- Zhao, X.C.; Dong, J.F.; Tang, Q.B.; Yan, Y.H.; Gelibic, I.; Van Loon, J.J.A.; Wang, C.Z. Hybridization between Helicoverpa armigera and Helicoverpa assulta (Lepidoptera: Noctuidae): Development and morphological characterization of F1 hybrids. Bull. Entomol. Res. 2005, 95, 409–416. [Google Scholar] [CrossRef] [Green Version]
- Hartke, T.R.; Rosengaus, R.B. Heterospecific pairing and hybridization between Nasutitermes corniger and N. ephratae. Naturwissenschaften 2011, 98, 745–753. [Google Scholar] [CrossRef]
- Chouvence, T.; Helmick, E.E.; Su, N.Y. Hybridization of two major termite invaders as a consequence of human activity. PLoS ONE 2015, 10, e0120745. [Google Scholar]
- Pandey, M.; Addesso, K.M.; Archer, R.S.; Valles, S.M.; Baysal-Gurel, F.; Ganter, P.F.; Youssef, N.N.; Oliver, J.B. Worker size, geographical distribution, and introgressive hybridization of invasive Solenopsis invicta and Solenopsis richteri (Hymenoptera: Formicidae) in Tennessee. Environ. Entomol. 2019, 48, 727–732. [Google Scholar] [CrossRef]
- Ernst, M. The biological meaning of species. Biol. J. Linn. Soc. 1969, 1, 311–320. [Google Scholar]
- Dobzhansky Theodosius, D. Genetic Organization. BioScience 1970, 20, 777. [Google Scholar]
- Adams, S.A.; Tsutsui, N.D. The evolution of species recognition labels in insects. Philos. Trans. R. Soc. B 2020, 375, 20190476. [Google Scholar] [CrossRef] [PubMed]
- Kartavtsev, Y.P. Sequence divergence at mitochondrial genes in animals: Applicability of DNA data in genetics of speciation and molecular phylogenetics. Mar. Genom. 2011, 4, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.Z.; Xiong, X.M.; Zhang, X.J.; Wan, S.M.; Guan, N.N.; Nie, C.H.; Zhao, B.W.; Hsiao, C.D.; Wang, W.M.; Gao, Z.X. Mitochondrial genome variation after hybridization and differences in the first and second generation hybrids of bream fishes. PLoS ONE 2016, 11, e0158915. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Guo, W.T.; Liu, X.Q.; Qu, H.T.; Guan, M.; Su, W. Complete mitochondrial genome of the hybrid of Acipenser schrenckii (♀) × Huso dauricus (♂). Mitochondrial DNA 2016, 27, 2887–2888. [Google Scholar] [CrossRef] [PubMed]
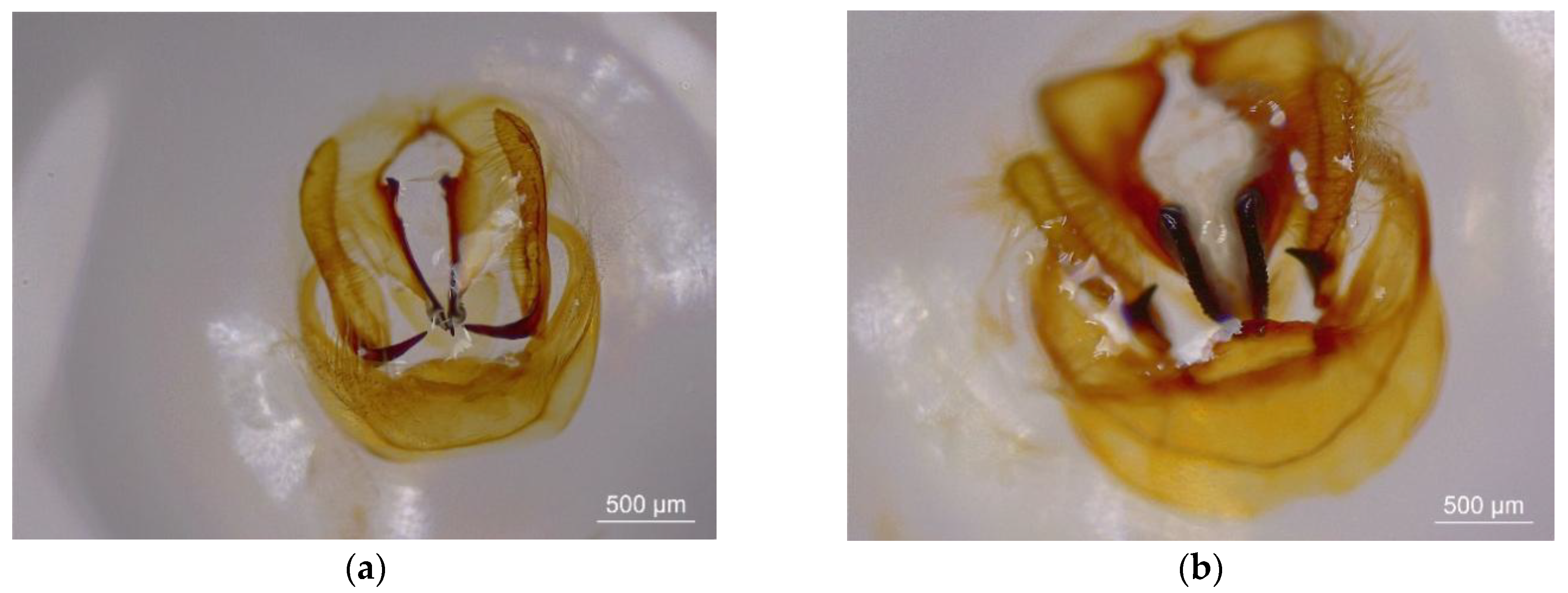


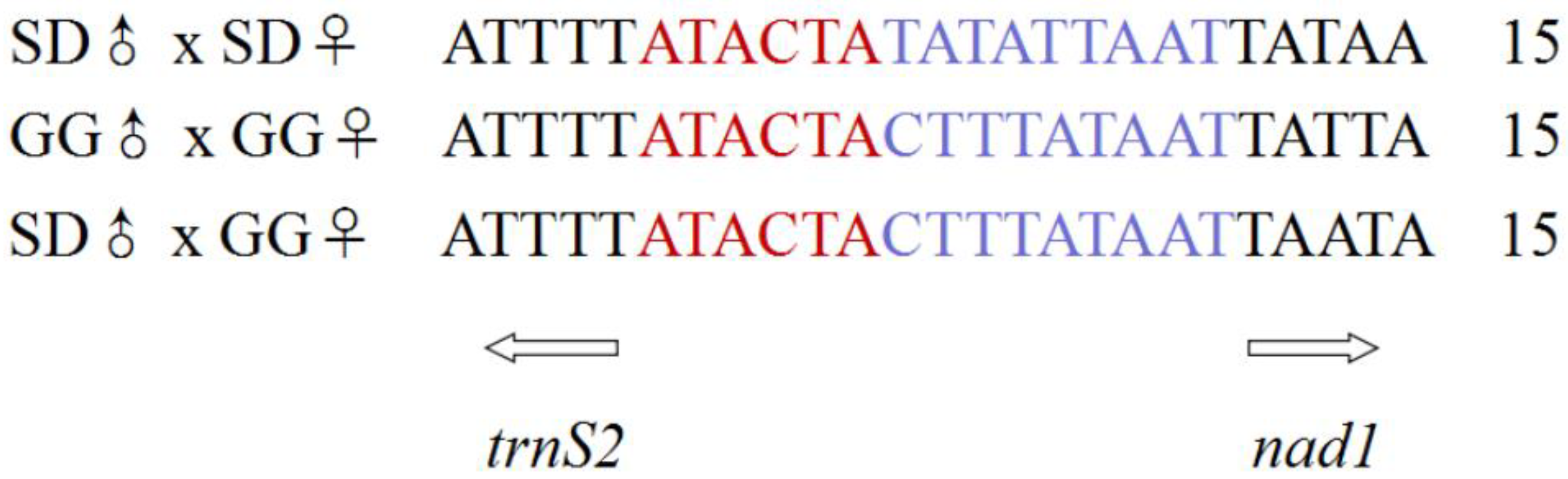

| Strains | Thitarodes sp. | T.shambalaensis | p-Values | |
|---|---|---|---|---|
| Pupae | Female fresh weight (g) | 0.88 ± 0.04 a | 0.67 ± 0.03 b | p = 0.014 |
| Male fresh weight (g) | 0.59 ± 0.05 a | 0.45 ± 0.03 a | p = 0.271 | |
| Female body length (cm) | 2.73 ± 0.21 a | 2.50 ± 0.05 a | p = 0.335 | |
| Male body length (cm) | 2.46 ± 0.08 a | 2.25 ± 0.04 a | p = 0.404 | |
| Ratio of females and males | 1.27 ± 0.16 a | 1.04 ± 0.06 a | p = 0.254 | |
| Female emergence rate (%) | 38.38 ± 5.44 a | 48.96 ± 2.75 a | p = 0.231 | |
| Male emergence rate (%) | 43.57 ± 6.79 a | 57.58 ± 2.19 a | p = 0.188 | |
| Color | Light to black yellow | Light to dark yellow | ||
| Adults | Female fresh weight (g) | 0.48 ± 0.02 a | 0.42 ± 0.04 a | p = 0.223 |
| Male fresh weight (g) | 0.20 ± 0.01 b | 0.22 ± 0.01 a | p = 0.013 | |
| Female body length (cm) | 2.77 ± 0.03 a | 2.77 ± 0.07 a | p = 1.000 | |
| Male body length (cm) | 2.13 ± 0.03 b | 2.43 ± 0.03 a | p = 0.003 | |
| Ratio of females and males | 0.89 ± 0.06 a | 0.87 ± 0.03 a | p = 0.800 | |
| Female longevity (day) | 6.0 ± 0.6 a | 5.3 ± 0.3 a | p = 0.374 | |
| Male longevity (day) | 6.0 ± 0.6 a | 5.3 ± 0.3 a | p = 0.374 | |
| Oviposition period (day) | 5.3 ± 1.2 a | 4.3 ± 0.3 a | p = 0.621 | |
| Mating time | Evening and night | All day and night | ||
| Females |  |  | ||
| Males |  |  | ||
| Mitochondrial Genome | SD♂ | GG♂ | SD♂ × GG♀ | |||||||||||||
| Overall length (bp) | 15,389 | 15,612 | 15,496 | |||||||||||||
| A% | 41.51 | 41.26 | 41.22 | |||||||||||||
| T% | 40.84 | 39.66 | 39.64 | |||||||||||||
| C% | 10.43 | 11.75 | 11.78 | |||||||||||||
| G% | 7.22 | 7.33 | 7.36 | |||||||||||||
| A + T% | 82.35 | 80.92 | 80.87 | |||||||||||||
| AT skew = (A − T)/(A + T) | 0.008 | 0.020 | 0.020 | |||||||||||||
| GC skew = (G − C)/(G + C) | −0.182 | −0.232 | −0.231 | |||||||||||||
| PCGs: length (bp) | 11,073 | 11,067 | 11,067 | |||||||||||||
| PCGs: A + T% | 80.78 | 79.00 | 78.97 | |||||||||||||
| tRNA: length (bp) | 1474 | 1478 | 1477 | |||||||||||||
| tRNA: A + T% | 84.46 | 83.42 | 83.41 | |||||||||||||
| rRNA: length (bp) | 2132 | 2114 | 2114 | |||||||||||||
| rRNA: A + T% | 85.60 | 85.10 | 85.10 | |||||||||||||
| A + T-rich length (bp) | 554 | 787 | 673 | |||||||||||||
| A + T-rich A + T% | 91.70 | 89.83 | 90.64 | |||||||||||||
| A + T-rich base constitution | No repeat sequence. | Two repeat base sequences. A = 113 bp, B = 114 bp, C = 114 bp  | Base sequences. A = 113 bp, B = 114 bp 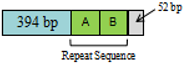 | |||||||||||||
| Composition | 13PCGs +22tRNAs + 2rRNAs + non-coding region | |||||||||||||||
| intergenic arrangement | 9 PCGs and 14 tRNAs were in the F chain, and 4 PCGs, 8 tRNAs and 2rRNAs were in the R chain. The arrangement of each gene in the three self-measured mitochondrial genomes was consistent. | |||||||||||||||
| Gene | Chain | Location | Spacer | Length | Initiation code | Termination code | Location | Spacer | Length | Initiation code | Termination code | Location | Spacer | Length | Initiation code | Termination code |
| trnI | F | 1–67 | - | 67 | 1–65 | - | 65 | 1–64 | - | 64 | ||||||
| trnQ | R | 78–146 | 10 | 69 | 63–131 | −3 | 69 | 62–130 | −3 | 69 | ||||||
| trnM | F | 158–227 | 11 | 70 | 151–220 | 19 | 70 | 148–217 | 17 | 70 | ||||||
| nad2 | F | 264–1247 | 36 | 984 | ATA | TAA | 257–1240 | 36 | 984 | ATA | TAA | 254–1237 | 36 | 984 | ATA | TAA |
| trnW | F | 1249–1314 | 1 | 66 | 1239–1304 | −2 | 66 | 1236–1301 | −2 | 66 | ||||||
| trnC | R | 1307–1373 | −8 | 67 | 1297–1367 | −8 | 71 | 1294–1364 | −8 | 71 | ||||||
| trnY | R | 1379–1444 | 5 | 66 | 1374–1440 | 6 | 67 | 1371–1437 | 6 | 67 | ||||||
| cox1 | F | 1447–2977 | 2 | 1531 | CGA | T | 1443–2973 | 2 | 1531 | CGA | T | 1440–2970 | 2 | 1531 | CGA | T |
| trnL2 | F | 2978–3046 | 0 | 69 | 2974–3042 | 0 | 69 | 2971–3039 | 0 | 69 | ||||||
| cox2 | F | 3049–3730 | 2 | 682 | ATG | T | 3045–3726 | 2 | 682 | ATG | T | 3042–3723 | 2 | 682 | ATG | T |
| trnK | F | 3731–3801 | 0 | 71 | 3727–3797 | 0 | 71 | 3724–3794 | 0 | 71 | ||||||
| trnD | F | 3801–3866 | −1 | 66 | 3797–3861 | −1 | 65 | 3794–3858 | −1 | 65 | ||||||
| atp8 | F | 3867–4031 | 0 | 165 | ATA | TAA | 3862–4023 | 0 | 162 | ATA | TAA | 3859–4020 | 0 | 162 | ATA | TAA |
| atp6 | F | 4028–4702 | −4 | 675 | ATA | TAA | 4020–4694 | −4 | 675 | ATA | TAA | 4017–4691 | −4 | 675 | ATA | TAA |
| cox3 | F | 4705–5490 | 2 | 786 | ATA | TAA | 4697–5482 | 2 | 786 | ATA | TAA | 4694–5479 | 2 | 786 | ATA | TAA |
| trnG | F | 5493–5558 | 2 | 66 | 5485–5550 | 2 | 66 | 5482–5547 | 2 | 66 | ||||||
| nad3 | F | 5559–5912 | 0 | 354 | ATT | TAG | 5551–5904 | 0 | 354 | ATT | TAG | 5548–5901 | 0 | 354 | ATT | TAG |
| trnA | F | 5911–5978 | −2 | 68 | 5903–5971 | −2 | 69 | 5900–5968 | −2 | 69 | ||||||
| trnR | F | 5982–6047 | 3 | 66 | 5975–6040 | 3 | 66 | 5972–6037 | 3 | 66 | ||||||
| trnN | F | 6055–6120 | 7 | 66 | 6045–6110 | 4 | 66 | 6042–6107 | 4 | 66 | ||||||
| trnS1 | F | 6121–6181 | 0 | 61 | 6111–6170 | 0 | 60 | 6108–6167 | 0 | 60 | ||||||
| trnE | F | 6182–6247 | 0 | 66 | 6171–6235 | 0 | 65 | 6168–6232 | 0 | 65 | ||||||
| trnF | R | 6258–6324 | 10 | 67 | 6238–6304 | 2 | 67 | 6235–6301 | 2 | 67 | ||||||
| nad5 | R | 6325–8020 | 0 | 1696 | ATA | T | 6305–7997 | 0 | 1693 | ATA | T | 6302–7994 | 0 | 1693 | ATA | T |
| trnH | R | 8063–8129 | 42 | 67 | 8043–8109 | 45 | 67 | 8040–8106 | 45 | 67 | ||||||
| nad4 | R | 8131–9471 | 1 | 1341 | ATG | TAA | 8111–9451 | 1 | 1341 | ATG | TAA | 8108–9448 | 1 | 1341 | ATG | TAA |
| nad4L | R | 9471–9746 | −1 | 276 | ATA | TAA | 9451–9726 | −1 | 276 | ATA | TAA | 9448–9723 | −1 | 276 | ATA | TAA |
| trnT | F | 9767–9832 | 20 | 66 | 9747–9812 | 20 | 66 | 9744–9809 | 20 | 66 | ||||||
| trnP | R | 9833–9897 | 0 | 65 | 9813–9876 | 0 | 64 | 9810–9873 | 0 | 64 | ||||||
| nad6 | F | 9900–10,424 | 2 | 525 | ATA | TAA | 9879–10,403 | 2 | 525 | ATA | TAA | 9876–10,400 | 2 | 525 | ATA | TAA |
| cytb | F | 10,424–11,569 | −1 | 1146 | ATG | TAA | 10,403–11,548 | −1 | 1146 | ATG | TAA | 10,400–11,545 | −1 | 1146 | ATG | TAA |
| trnS2 | F | 11,575–11,645 | 5 | 71 | 11,557–11,629 | 8 | 73 | 11,554–11,626 | 8 | 73 | ||||||
| nad1 | R | 11,661–12,572 | 15 | 912 | ATA | TAA | 11,645–12,556 | 15 | 912 | ATA | TAA | 11,642–12,553 | 15 | 912 | ATA | TAA |
| trnL1 | R | 12,594–12,662 | 21 | 69 | 12,578–12,648 | 21 | 71 | 12,575–12,645 | 21 | 71 | ||||||
| rrnL | R | 12,638–13,992 | −25 | 1355 | 12,649–13,984 | 0 | 1336 | 12,646–13,981 | 0 | 1336 | ||||||
| trnV | R | 13,993–14,057 | 0 | 65 | 13,984–14,048 | −1 | 65 | 13,982–14,046 | −1 | 65 | ||||||
| rrnS | R | 14,059–14,835 | 1 | 777 | 14,048–14,825 | −1 | 778 | 14,046–14,823 | −1 | 778 | ||||||
| A + T-rich | 14,836–15,389 | 554 | 14,826–15,612 | 787 | 14,824–15,496 | 673 | ||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Cao, L.; He, M.; Han, R.; De Clercq, P. Interspecific Hybridization and Complete Mitochondrial Genome Analysis of Two Ghost Moth Species. Insects 2021, 12, 1046. https://doi.org/10.3390/insects12111046
Wu H, Cao L, He M, Han R, De Clercq P. Interspecific Hybridization and Complete Mitochondrial Genome Analysis of Two Ghost Moth Species. Insects. 2021; 12(11):1046. https://doi.org/10.3390/insects12111046
Chicago/Turabian StyleWu, Hua, Li Cao, Meiyu He, Richou Han, and Patrick De Clercq. 2021. "Interspecific Hybridization and Complete Mitochondrial Genome Analysis of Two Ghost Moth Species" Insects 12, no. 11: 1046. https://doi.org/10.3390/insects12111046
APA StyleWu, H., Cao, L., He, M., Han, R., & De Clercq, P. (2021). Interspecific Hybridization and Complete Mitochondrial Genome Analysis of Two Ghost Moth Species. Insects, 12(11), 1046. https://doi.org/10.3390/insects12111046







