Assemblage of the Egg Parasitoids of the Invasive Stink Bug Halyomorpha halys: Insights on Plant Host Associations
Simple Summary
Abstract
1. Introduction
2. Material and methods
2.1. The Search for Naturally Laid Stink Bug Egg Masses
2.2. Rearing of H. halys
2.3. Sentinel Egg Masses Exposure with Cages
2.4. Sentinel Egg Masses Direct Exposure
2.5. Egg Mass Evaluation
2.6. Parasitoid Identification
2.7. mtDNA Haplotype Analysis
2.8. Statistical Analysis
3. Results
3.1. Naturally Laid Egg Masses
3.2. Sentinel Egg Masses
3.3. mtDNA Haplotype Analysis
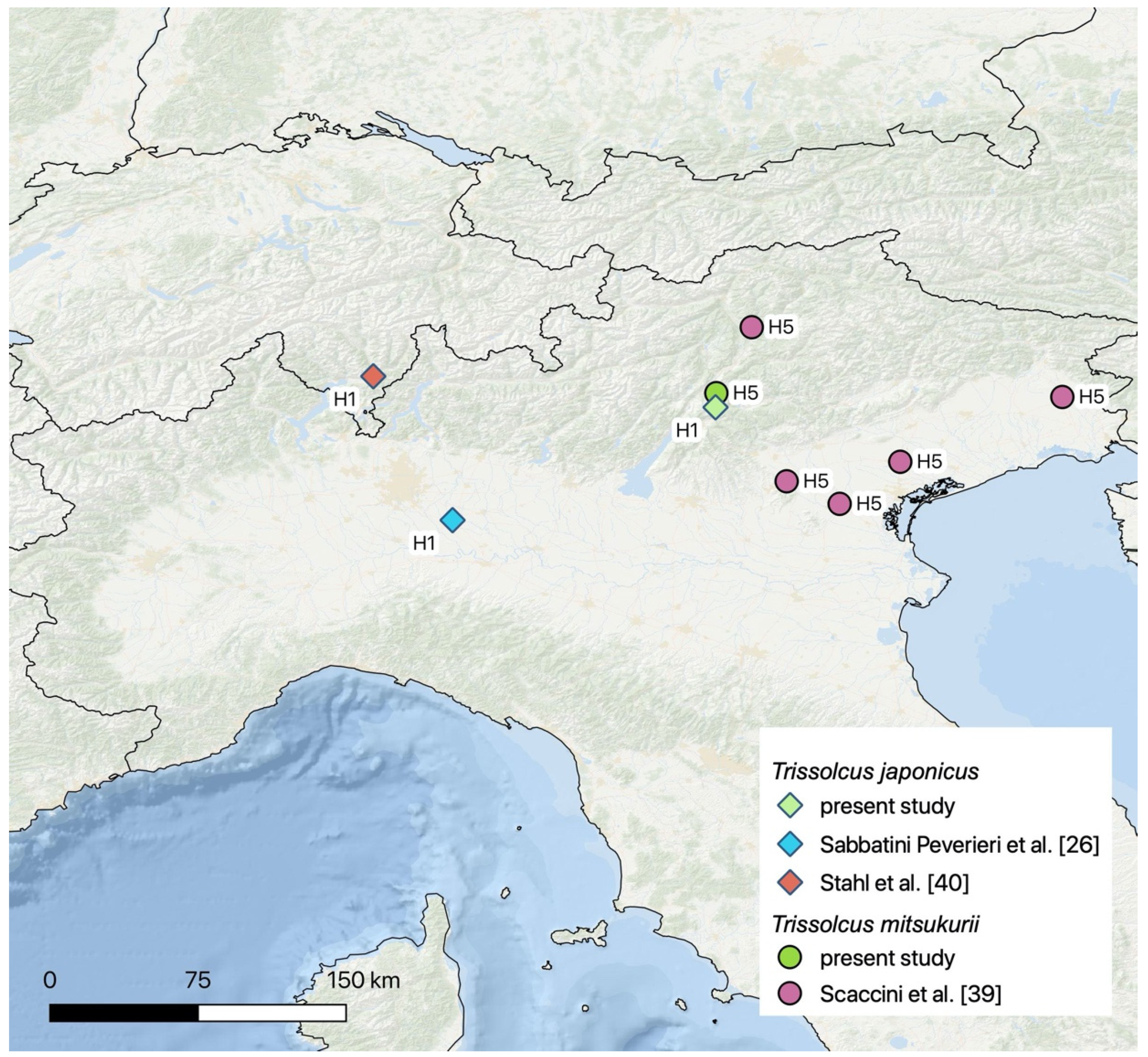
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xu, J.; Fonseca, D.M.; Hamilton, G.C.; Hoelmer, K.A.; Nielsen, A.L. Tracing the origin of US brown marmorated stink bugs, Halyomorpha halys. Biol. Invasions 2014, 16, 153–166. [Google Scholar] [CrossRef]
- Maistrello, L.; Dioli, P.; Dutto, M.; Volani, S.; Pasquali, S.; Gilioli, G. Tracking the spread of sneaking aliens by integrating crowdsourcing and spatial modeling: The Italian invasion of Halyomorpha halys. Bioscience 2018, 68, 979–989. [Google Scholar] [CrossRef]
- Wermelinger, B.; Wyniger, D.; Forster, B. First records of an invasive bug in Europe: Halyomorpha halys Stål (Heteroptera: Pentatomidae), a new pest on woody ornamentals and fruit trees? Mitt. Schweiz. Entomol. Ges. 2008, 81, 1–8. [Google Scholar]
- Gariepy, T.D.; Fraser, H.; Scott-Dupree, C.D. Brown marmorated stink bug (Hemiptera: Pentatomidae) in Canada: Recent establishment, occurrence, and pest status in southern Ontario. Can. Entomol. 2014, 146, 579–582. [Google Scholar] [CrossRef]
- Faúndez, E.I.; Rider, D.A. The brown marmorated stink bug Halyomorpha halys (Stål, 1855)(Heteroptera: Pentatomidae) in Chile. Arq. Entomol. 2017, 17, 305–307. [Google Scholar]
- Lee, D.-H.; Leskey, T.C. Flight behavior of foraging and overwintering brown marmorated stink bug, Halyomorpha halys (Hemiptera: Pentatomidae). Bull. Entomol. Res. 2015, 105, 566–573. [Google Scholar] [CrossRef]
- Haye, T.; Gariepy, T.; Hoelmer, K.; Rossi, J.-P.; Streito, J.-C.; Tassus, X.; Desneux, N. Range expansion of the invasive brown marmorated stinkbug, Halyomorpha halys: An increasing threat to field, fruit and vegetable crops worldwide. J. Pest Sci. 2015, 88, 665–673. [Google Scholar] [CrossRef]
- Hamilton, G.C.; Ahn, J.J.; Bu, W.; Leskey, T.C.; Nielsen, A.L.; Park, Y.L.; Rabitsch, W.; Hoelmer, K.A. Halyomorpha halys (Stål). In Invasive Stink Bugs and Related Species (Pentatomoidea). Biology, Higher Systematics, Semiochemistry and Management; McPherson, J.E., Ed.; CRC Press: Boca Raton, FL, USA, 2018; pp. 243–292. [Google Scholar]
- Lee, D.-H.; Short, B.D.; Joseph, S.V.; Bergh, J.C.; Leskey, T.C. Review of the Biology, Ecology, and Management of Halyomorpha halys (Hemiptera: Pentatomidae) in China, Japan, and the Republic of Korea. Environ. Entomol. 2013, 42, 627–641. [Google Scholar] [CrossRef]
- Leskey, T.C.; Short, B.D.; Butler, B.R.; Wright, S.E. Impact of the invasive brown marmorated stink bug, Halyomorpha halys (Stål), in Mid-Atlantic tree fruit orchards in the United States: Case studies of commercial management. Psyche 2012, 2012, 1–14. [Google Scholar] [CrossRef]
- Rice, K.B.; Bergh, C.J.; Bergmann, E.J.; Biddinger, D.J.; Dieckhoff, C.; Dively, G.; Fraser, H.; Gariepy, T.; Hamilton, G.; Haye, T.; et al. Biology, Ecology, and Management of Brown Marmorated Stink Bug (Hemiptera: Pentatomidae). J. Integr. Pest Manag. 2014, 5, 1–13. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, F.; Gariepy, T.; Mason, P.; Gillespie, D.; Talamas, E.; Haye, T. Seasonal parasitism and host specificity of Trissolcus japonicus in northern China. J. Pest Sci. 2017, 90, 1127–1141. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, R.; Namura, Y. Effects of temperature on development of three Trissolcus spp. (Hymenoptera: Scelionidae), egg parasitoids of the brown marmorated stink bug, Halyomorpha halys (Hemiptera: Pentatomidae). Entomol. Sci. 2002, 5, 215–218. [Google Scholar]
- Arakawa, R.; Miura, M.; Fujita, M. Effects of host species on the body size, fecundity, and longevity of Trissolcus mitsukurii (Hymenoptera: Scelionidae), a solitary egg parasitoid of stink bugs. Appl. Entomol. Zool. 2004, 39, 177–181. [Google Scholar] [CrossRef]
- Talamas, E.J.; Herlihy, M.V.; Dieckhoff, C.; Hoelmer, K.A.; Buffington, M.; Bon, M.-C.; Weber, D.C. Trissolcus japonicus (Ashmead) (Hymenoptera, Scelionidae) emerges in North America. J. Hymenopt. Res. 2015, 43, 119–128. [Google Scholar] [CrossRef]
- Stahl, J.; Tortorici, F.; Pontini, M.; Bon, M.-C.; Hoelmer, K.; Marazzi, C.; Tavella, L.; Haye, T. First discovery of adventive populations of Trissolcus japonicus in Europe. J. Pest Sci. 2019, 92, 371–379. [Google Scholar] [CrossRef]
- Tillman, G.; Toews, M.; Blaauw, B.; Sial, A.; Cottrell, T.; Talamas, E.; Buntin, D.; Joseph, S.; Balusu, R.; Fadamiro, H.; et al. Parasitism and predation of sentinel eggs of the invasive brown marmorated stink bug, Halyomorpha halys (Stål) (Hemiptera: Pentatomidae), in the southeastern US. Biol. Control 2020, 145, 104247. [Google Scholar] [CrossRef]
- Milnes, J.M.; Wiman, N.G.; Talamas, E.J.; Brunner, J.F.; Hoelmer, K.A.; Buffington, M.L.; Beers, E.H. Discovery of an exotic egg parasitoid of the brown marmorated stink bug, Halyomorpha halys (Stål) in the Pacific Northwest. Proc. Entomol. Soc. Washingt. 2016, 118, 466–470. [Google Scholar] [CrossRef]
- Yang, Z.-Q.; Yao, Y.-X.; Qiu, L.-F.; Li, Z.-X. A new species of Trissolcus (Hymenoptera: Scelionidae) parasitizing eggs of Halyomorpha halys (Heteroptera: Pentatomidae) in China with comments on its biology. Ann. Entomol. Soc. Am. 2009, 102, 39–47. [Google Scholar] [CrossRef]
- Quinn, N.F.; Talamas, E.J.; Acebes-Doria, A.L.; Leskey, T.C.; Bergh, J.C. Vertical Sampling in Tree Canopies for Halyomorpha halys (Hemiptera: Pentatomidae) Life Stages and its Egg Parasitoid, Trissolcus japonicus (Hymenoptera: Scelionidae). Environ. Entomol. 2019, 48, 173–180. [Google Scholar] [CrossRef]
- Quinn, N.F.; Talamas, E.J.; Leskey, T.C.; Bergh, J.C. Sampling Methods for Adventive Trissolcus japonicus (Hymenoptera: Scelionidae) in a Wild Tree Host of Halyomorpha halys (Hemiptera: Pentatomidae). J. Econ. Entomol. 2019, 112, 1997–2000. [Google Scholar] [CrossRef]
- Boyle, S.M.; Weber, D.C.; Hough-Goldstein, J.; Hoelmer, K.A. Host Kairomones Influence Searching Behavior of Trissolcus japonicus (Hymenoptera: Scelionidae), a Parasitoid of Halyomorpha halys (Heteroptera: Pentatomidae). Environ. Entomol. 2020, 49, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Rondoni, G.; Bertoldi, V.; Malek, R.; Foti, M.C.; Peri, E.; Maistrello, L.; Haye, T.; Conti, E. Native egg parasitoids recorded from the invasive Halyomorpha halys successfully exploit volatiles emitted by the plant–herbivore complex. J. Pest Sci. 2017, 90, 1087–1095. [Google Scholar] [CrossRef]
- Morrison, W.R.; Blaauw, B.R.; Nielsen, A.L.; Talamas, E.; Leskey, T.C. Predation and parasitism by native and exotic natural enemies of Halyomorpha halys (Stål) (Hemiptera: Pentatomidae) eggs augmented with semiochemicals and differing host stimuli. Biol. Control 2018, 121, 140–150. [Google Scholar] [CrossRef]
- Jones, A.L.; Jennings, D.E.; Hooks, C.R.R.; Shrewsbury, P.M. Field surveys of egg mortality and indigenous egg parasitoids of the brown marmorated stink bug, Halyomorpha halys, in ornamental nurseries in the mid-Atlantic region of the USA. J. Pest Sci. 2017, 90, 1159–1168. [Google Scholar] [CrossRef] [PubMed]
- Sabbatini Peverieri, G.; Talamas, E.; Bon, M.C.; Marianelli, L.; Bernardinelli, I.; Malossini, G.; Benvenuto, L.; Roversi, P.F.; Hoelmer, K. Two Asian egg parasitoids of Halyomorpha halys (Stål) (Hemiptera, Pentatomidae) emerge in northern Italy: Trissolcus mitsukurii (Ashmead) and Trissolcus japonicus (Ashmead) (Hymenoptera, Scelionidae). J. Hymenopt. Res. 2018, 67, 37–53. [Google Scholar] [CrossRef]
- Bin, F.; Vinson, S.B. Efficacy assesment in egg parasitoids (Hymenoptera): Proposal for a unified terminology. In Proceedings of the 3rd International Symposium on Trichogramma and Other Egg Parasitoids, San Antonio, TX, USA, 23 September 1990; Wajnberg, E., Vinson, S.B., Eds.; Perugia University: Perugia, Italy, 1991. [Google Scholar]
- Moraglio, S.T.; Tortorici, F.; Pansa, M.G.; Castelli, G.; Pontini, M.; Scovero, S.; Visentin, S.; Tavella, L. A 3-year survey on parasitism of Halyomorpha halys by egg parasitoids in northern Italy. J. Pest Sci. 2019, 1–12. [Google Scholar] [CrossRef]
- Talamas, E.J.; Buffington, M.L.; Hoelmer, K. Revision of Palearctic Trissolcus Ashmead (Hymenoptera, Scelionidae). J. Hymenopt. Res. 2017, 56, 79–261. [Google Scholar] [CrossRef]
- Talamas, E.J.; Johnson, N.F.; Buffington, M. Key to Nearctic species of Trissolcus Ashmead (Hymenoptera, Scelionidae), natural enemies of native and invasive stink bugs (Hemiptera, Pentatomidae). J. Hymenopt. Res. 2015, 43, 45–110. [Google Scholar] [CrossRef]
- Tortorici, F.; Talamas, E.J.; Moraglio, S.T.; Pansa, M.G.; Asadi-Farfar, M.; Tavella, L.; Caleca, V. A morphological, biological and molecular approach reveals four cryptic species of Trissolcus Ashmead (Hymenoptera, Scelionidae), egg parasitoids of Pentatomidae (Hemiptera). J. Hymenopt. Res. 2019, 73, 153–200. [Google Scholar] [CrossRef]
- Johnson, N.F. Systematics of Nearctic Telenomus: Classification and revisions of the podisi and phymatae species groups (Hymenoptera: Scelionidae). Bull. Ohio Biol. Surv. New Ser. 1984, 6, x + 113. [Google Scholar]
- Askew, R.R.; Nieves-Aldrey, J.L. Further observations on Eupelminae (Hymenoptera, Chalcidoidea, Eupelmidae) in the Iberian Peninsula and Canary Islands, including descriptions of new species. Graellsia 2004, 60, 27–39. [Google Scholar]
- Cruaud, A.; Jabbour-Zahab, R.; Genson, G.; Cruaud, C.; Couloux, A.; Kjellberg, F.; van Noort, S.; Rasplus, J.-Y. Laying the foundations for a new classification of Agaonidae (Hymenoptera: Chalcidoidea), a multilocus phylogenetic approach. Cladistics 2009, 26, 359–387. [Google Scholar] [CrossRef]
- Simon, C.; Buckley, T.R.; Frati, F.; Stewart, J.B.; Beckenbach, A.T. Incorporating molecular evolution into phylogenetic analysis, and a new compilation of conserved polymerase chain reaction primers for animal mitochondrial DNA. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 545–579. [Google Scholar] [CrossRef]
- Ganjisaffar, F.; Talamas, E.J.; Bon, M.-C.; Brown, B.V.; Gonzalez, L.; Perring, T.M. Trissolcus hyalinipennis Rajmohana & Narendran (Hymenoptera, Scelionidae), a parasitoid of Bagrada hilaris (Burmeister) (Hemiptera, Pentatomidae), emerges in North America. J. Hymenopt. Res. 2018, 65, 111–130. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Scaccini, D.; Falagiarda, M.; Tortorici, F.; Martinez-Sañudo, I.; Tirello, P.; Reyes-Domínguez, Y.; Gallmetzer, A.; Tavella, L.; Zandigiacomo, P.; Duso, C.; et al. An insight into the role of Trissolcus mitsukurii as biological control agent of Halyomorpha halys in Northeastern Italy. Insects 2020, 11, 306. [Google Scholar] [CrossRef] [PubMed]
- Abram, P.K.; Brodeur, J.; Burte, V.; Boivin, G. Parasitoid-induced host egg abortion: An underappreciated component of biological control services provided by egg parasitoids. Biol. Control 2016, 98, 52–60. [Google Scholar] [CrossRef]
- Konopka, J.K.; Gariepy, T.D.; Haye, T.; Zhang, J.; Rubin, B.D.; McNeil, J.N. Exploitation of pentatomids by native egg parasitoids in the native and introduced ranges of Halyomorpha halys: A molecular approach using sentinel egg masses. J. Pest Sci. 2019, 92, 609–619. [Google Scholar] [CrossRef]
- Streito, J.-C.; Clouet, C.; Hamdi, F.; Gauthier, N. Population genetic structure of the biological control agent Macrolophus pygmaeus in Mediterranean agroecosystems. Insect Sci. 2017, 24, 859–876. [Google Scholar] [CrossRef]
- Hebert, P.D.N.; Cywinska, A.; Ball, S.L.; deWaard, J.R. Biological identifications through DNA barcodes. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef]
- Park, D.-S.; Suh, S.-J.; Oh, H.-W.; Hebert, P.D. Recovery of the mitochondrial COI barcode region in diverse Hexapoda through tRNA-based primers. BMC Genom. 2010, 11, 423. [Google Scholar] [CrossRef] [PubMed]
- Vinson, S.B. The general host selection behavior of parasitoid Hymenoptera and a comparison of initial strategies utilized by larvaphagous and oophagous species. Biol. Control 1998, 11, 79–96. [Google Scholar] [CrossRef]
- Bertoldi, V.; Rondoni, G.; Brodeur, J.; Conti, E. An egg parasitoid efficiently exploits cues from a coevolved host but not those from a novel host. Front. Physiol. 2019, 10, 746. [Google Scholar] [CrossRef] [PubMed]
- Leskey, T.; Hamilton, G.; Biddinger, D.; Buffington, M.; Dieckhoff, C.; Fraser, H.; Gariepy, T.; Herbert, D.; Hoelmer, K.; Hooks, C.; et al. Datasheet Halyomorpha halys, CABI Invasive Species Compendium, 2014. Available online: http://www.cabi.org/isc/datasheet/27377 (accessed on 20 April 2020).
- Hedstrom, C.; Lowenstein, D.; Andrews, H.; Bai, B.; Wiman, N. Pentatomid host suitability and the discovery of introduced populations of Trissolcus japonicus in Oregon. J. Pest Sci. 2017, 90, 1169–1179. [Google Scholar] [CrossRef]
- Meiners, T.; Peri, E. Chemical ecology of insect parasitoids: Essential elements for developing effective biological control programmes. In Chemical Ecology of Insect Parasitoids; John Wiley & Sons, Ltd.: Chichester, UK, 2013; pp. 191–224. [Google Scholar]
- Wang, Z.; Liu, Y.; Shi, M.; Huang, J.; Chen, X. Parasitoid wasps as effective biological control agents. J. Integr. Agric. 2019, 18, 705–715. [Google Scholar] [CrossRef]
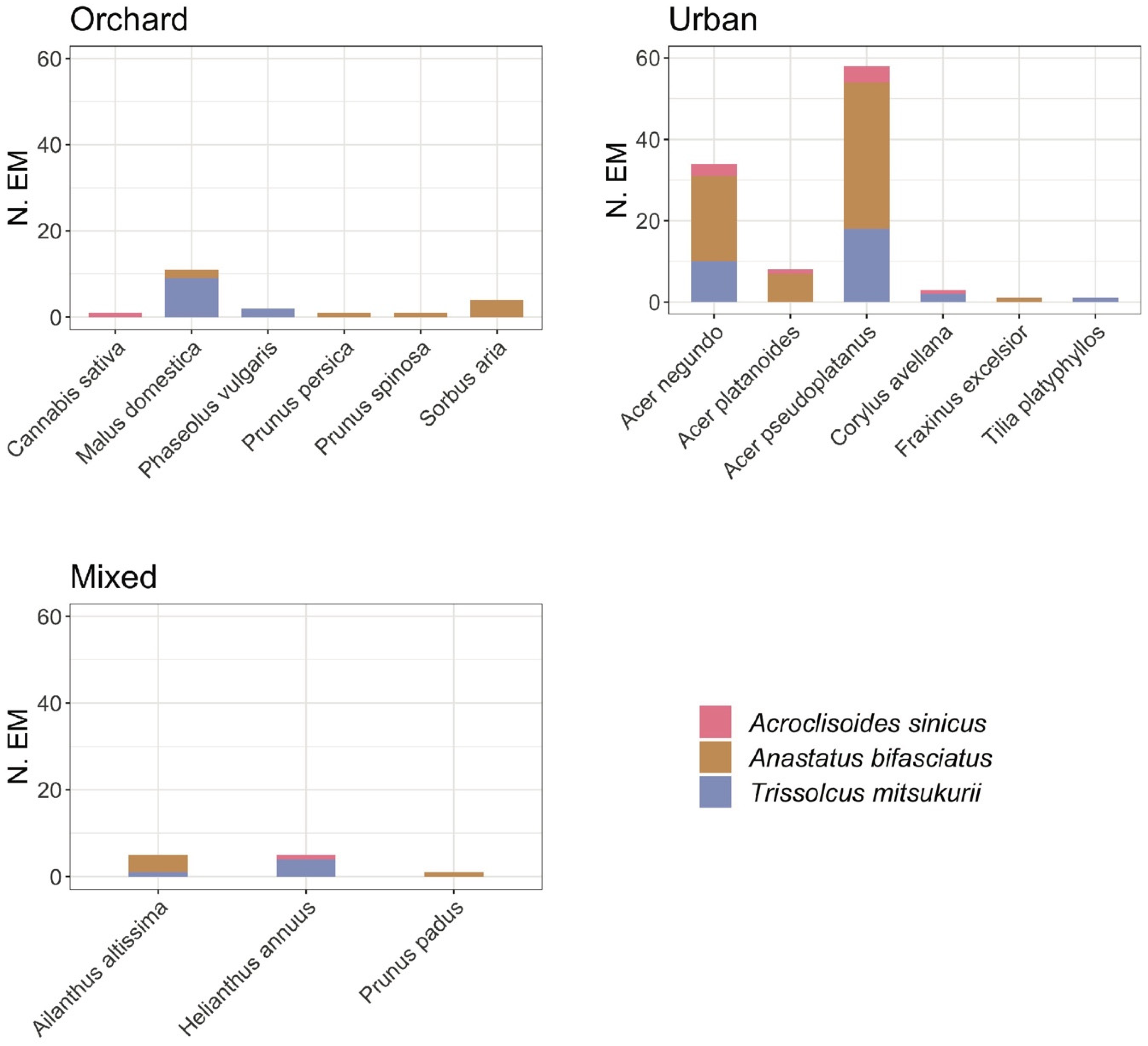
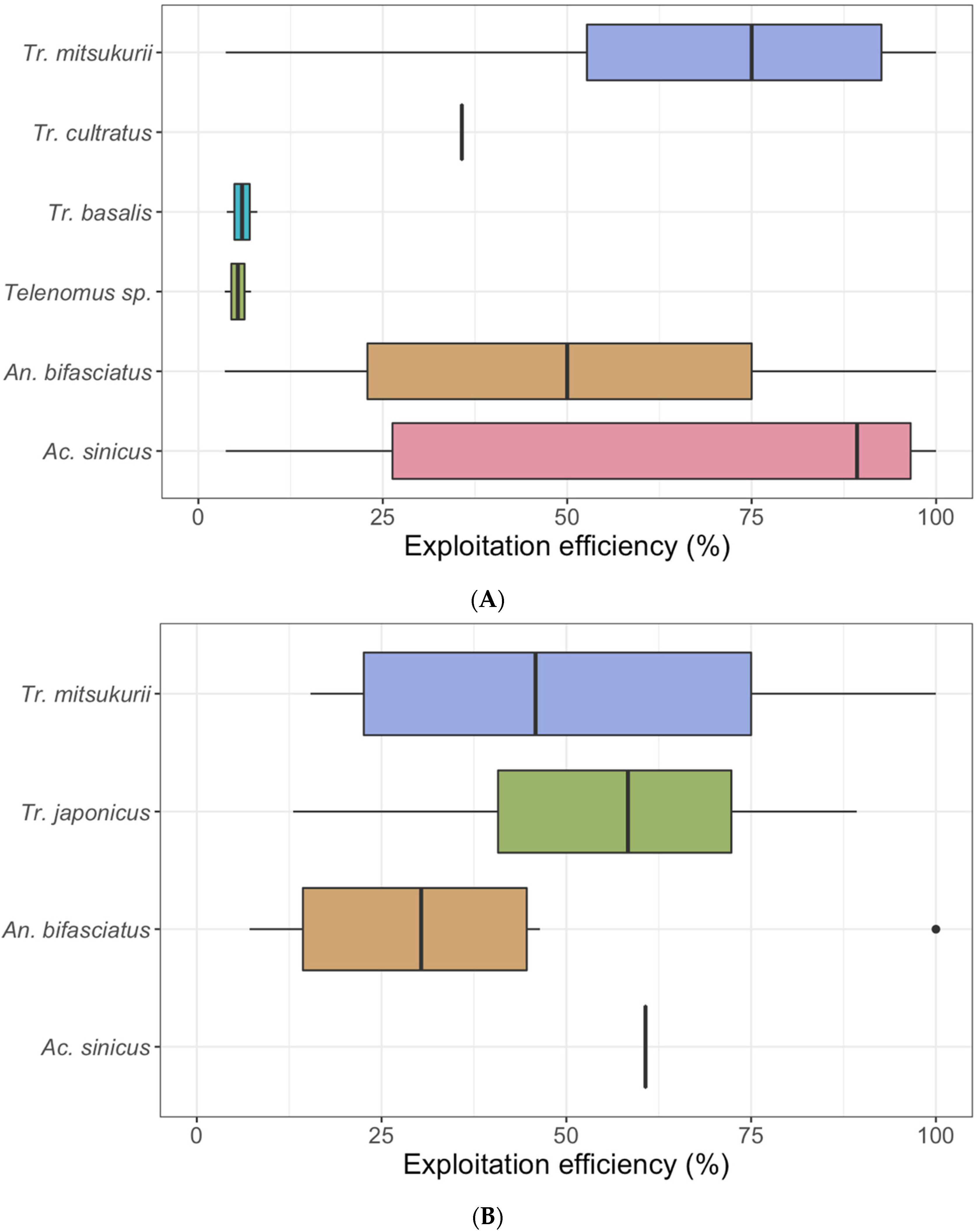
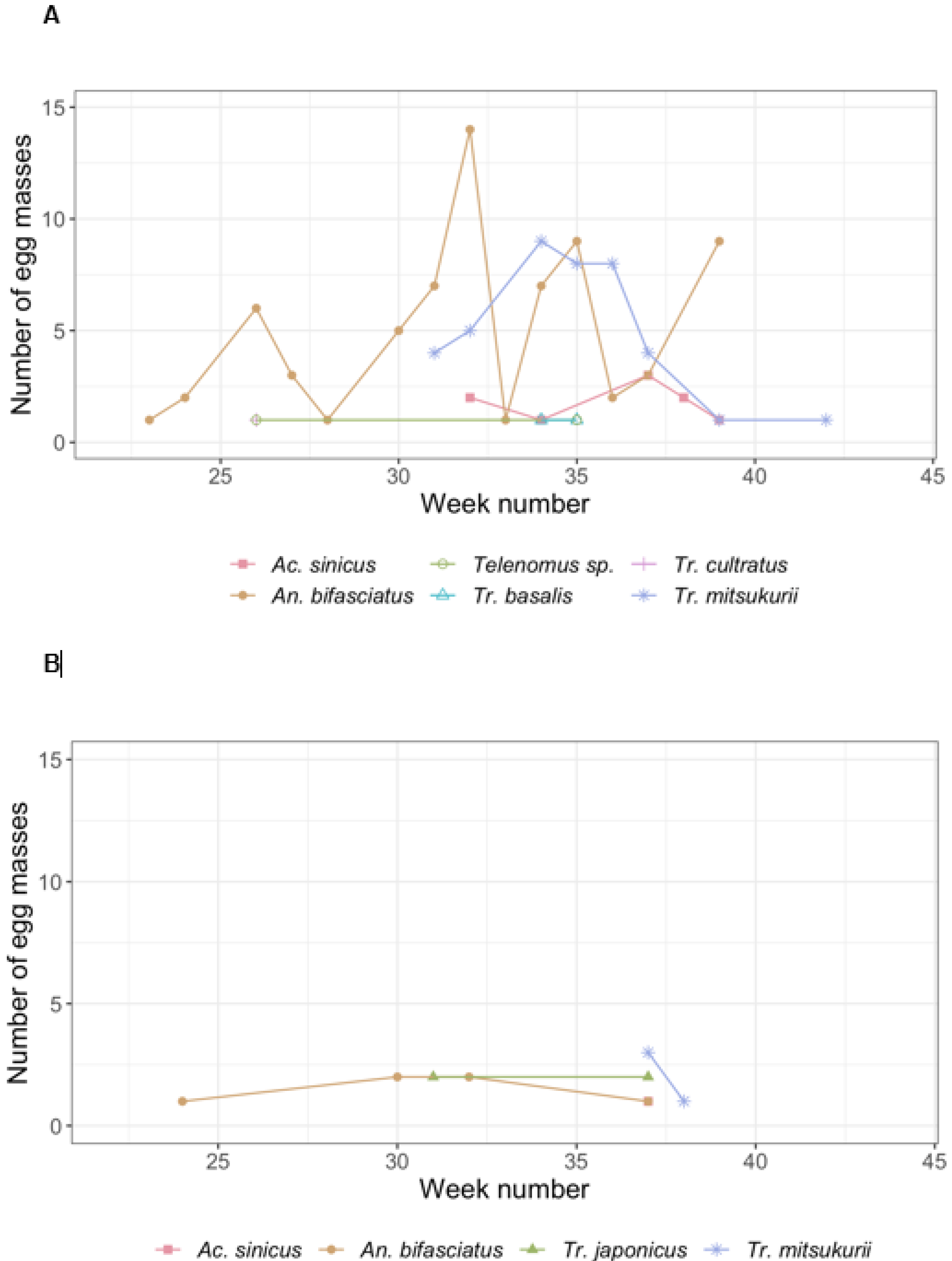
| Stink Bug Species | EM | E | Par. EM | Par. E * (%) | Un. E (%) | Number of Emerged Individuals for Each Species | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acroclisoides sinicus | Anastatus bifasciatus | Trissolcus basalis | Trissolcus belenus | Trissolcus cultratus | Trissolcus mitsukurii | Telenomus sp. | ||||||
| Pentatomidae | ||||||||||||
| Halyomorpha halys | 581 | 15,244 | 158 | 2541 (16.7) | 2889 (18.9) | 201 | 957 | 4 | 15 | 14 | 871 | 3 |
| Palomena prasina | 42 | 971 | 28 | 508 (52.3) | 265 (27.3) | 82 | 200 | 3 | 120 | |||
| Nezara viridula | 23 | 2023 | 7 | 234 (11.6) | 502 (24.8) | 20 | 55 | 95 | 6 | |||
| Dolycoris baccarum | 8 | 206 | 1 | 7 (3.4) | 21 (10.2) | 7 | ||||||
| Pentatoma rufipes | 4 | 54 | 2 | 27 (50) | 26 (48.1) | 25 | ||||||
| Carpocoris sp. | 4 | 54 | 4 | 48 (88.9) | 6 (11.1) | 48 | ||||||
| Rhaphigaster nebulosa | 3 | 41 | 1 | 14 (34.1) | 2 (4.9) | 14 | ||||||
| Piezodorus lituratus | 1 | 12 | 1 | 2 (16.7) | 9 (75) | 2 | ||||||
| Eurydema sp. | 2 | 24 | 0 | 0 (0) | 4 (16.7) | |||||||
| Coreidae | ||||||||||||
| Gonocerus acuteangulatus | 3 | 15 | 1 | 3 (20) | 3 (20) | 3 | ||||||
| No | Model Formulation | AIC | BIC | logLik | Dev. | df |
|---|---|---|---|---|---|---|
| (1) | parasitized ~ plant + week n ° + altitude + longitude + context + (1 | district) | 523.7 | 630.7 | −236.9 | 473.7 | 508 |
| (2) | parasitized ~ plant + week n ° + plant * week n ° + altitude + longitude + context + (1 | district) | 518.4 | 698.1 | −217.2 | 434.4 | 491 |
| (3) | parasitized ~ plant * week n ° + (1 | district) | 517.2 | 679.8 | −220.6 | 441.2 | 495 |
| Fixed effects: | Est. | Std. Error | z value | Pr (>|z|) | ||
| (1) | (Intercept) | −6.67 | 1.56 | −4.28 | <0.001 | *** |
| week n ° | 0.15 | 0.03 | 4.71 | <0.001 | *** | |
| (2) | (Intercept) | −15.32 | 2.96 | −5.18 | <0.001 | *** |
| Acer pseudoplatanus | 11.12 | 2.93 | 3.80 | <0.001 | *** | |
| Ailanthus altissima | 10.68 | 4.82 | 2.22 | 0.03 | * | |
| Sorbus aria | 24.86 | 12.66 | 1.96 | 0.05 | * | |
| week n ° | 0.37 | 0.08 | 4.91 | <0.001 | *** | |
| Acer pseudoplatanus: week n ° | −0.34 | 0.09 | −3.90 | <0.001 | *** | |
| Ailanthus altissima: week n ° | −0.31 | 0.16 | −2.01 | 0.04 | * | |
| (3) | (Intercept) | −12.90 | 2.55 | −5.05 | <0.001 | *** |
| Acer pseudoplatanus | 11.02 | 2.92 | 3.77 | <0.001 | *** | |
| Ailanthus altissima | 10.42 | 4.46 | 2.34 | 0.02 | * | |
| week n ° | 0.37 | 0.08 | 4.91 | <0.001 | *** | |
| Acer pseudoplatanus: week n ° | −0.34 | 0.09 | −3.85 | <0.001 | *** | |
| Ailanthus altissima: week n ° | −0.31 | 0.15 | −2.14 | 0.03 | * |
| Survey Method | Number of Emerged Individuals for Each Species | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Egg Mass | Number of Egg Masses | Number of Eggs Per Egg Mass | Discovery Efficiency | Exploitation Efficiency | Acroclisoides sinicus | Anastatus bifasciatus | Trissolcus japonicus | Trissolcus mitsukurii | |
| With cages | Fresh | 192 | 26.4 ± 0.15 | 0% | 0.00 ± 0.00 | 0 | 0 | 0 | 0 |
| Frozen | 192 | 27.2 ± 0.13 | 1.60% | 0.21 ± 0.13 | 0 | 8 | 3 | 0 | |
| Direct exposure | Fresh | 57 | 25.5 ± 0.37 | 10.40% | 5.68 ± 2.44 | 0 | 24 | 43 | 10 |
| Frozen | 64 | 26.7 ± 0.23 | 9.40% | 5.52 ± 2.53 | 17 | 21 | 14 | 35 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zapponi, L.; Bon, M.C.; Fouani, J.M.; Anfora, G.; Schmidt, S.; Falagiarda, M. Assemblage of the Egg Parasitoids of the Invasive Stink Bug Halyomorpha halys: Insights on Plant Host Associations. Insects 2020, 11, 588. https://doi.org/10.3390/insects11090588
Zapponi L, Bon MC, Fouani JM, Anfora G, Schmidt S, Falagiarda M. Assemblage of the Egg Parasitoids of the Invasive Stink Bug Halyomorpha halys: Insights on Plant Host Associations. Insects. 2020; 11(9):588. https://doi.org/10.3390/insects11090588
Chicago/Turabian StyleZapponi, Livia, Marie Claude Bon, Jalal Melhem Fouani, Gianfranco Anfora, Silvia Schmidt, and Martina Falagiarda. 2020. "Assemblage of the Egg Parasitoids of the Invasive Stink Bug Halyomorpha halys: Insights on Plant Host Associations" Insects 11, no. 9: 588. https://doi.org/10.3390/insects11090588
APA StyleZapponi, L., Bon, M. C., Fouani, J. M., Anfora, G., Schmidt, S., & Falagiarda, M. (2020). Assemblage of the Egg Parasitoids of the Invasive Stink Bug Halyomorpha halys: Insights on Plant Host Associations. Insects, 11(9), 588. https://doi.org/10.3390/insects11090588








