Compatibility of the Predatory Beetle, Delphastus catalinae, with an Entomopathogenic Fungus, Cordyceps fumosorosea, for Biocontrol of Invasive Pepper Whitefly, Aleurothrixus trachoides, in Florida
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plants
2.2. Insects
2.3. Fungus
2.4. Spray Protocol and Deposition of Blastospores
2.5. Laboratory Protocol for Determining Efficacy of Treatments on the Mortality of Whitefly Populations
2.6. Longevity of Adult Beetles after Exposure to Entomopathogenic Fungus
2.7. Statistical Analysis
3. Results
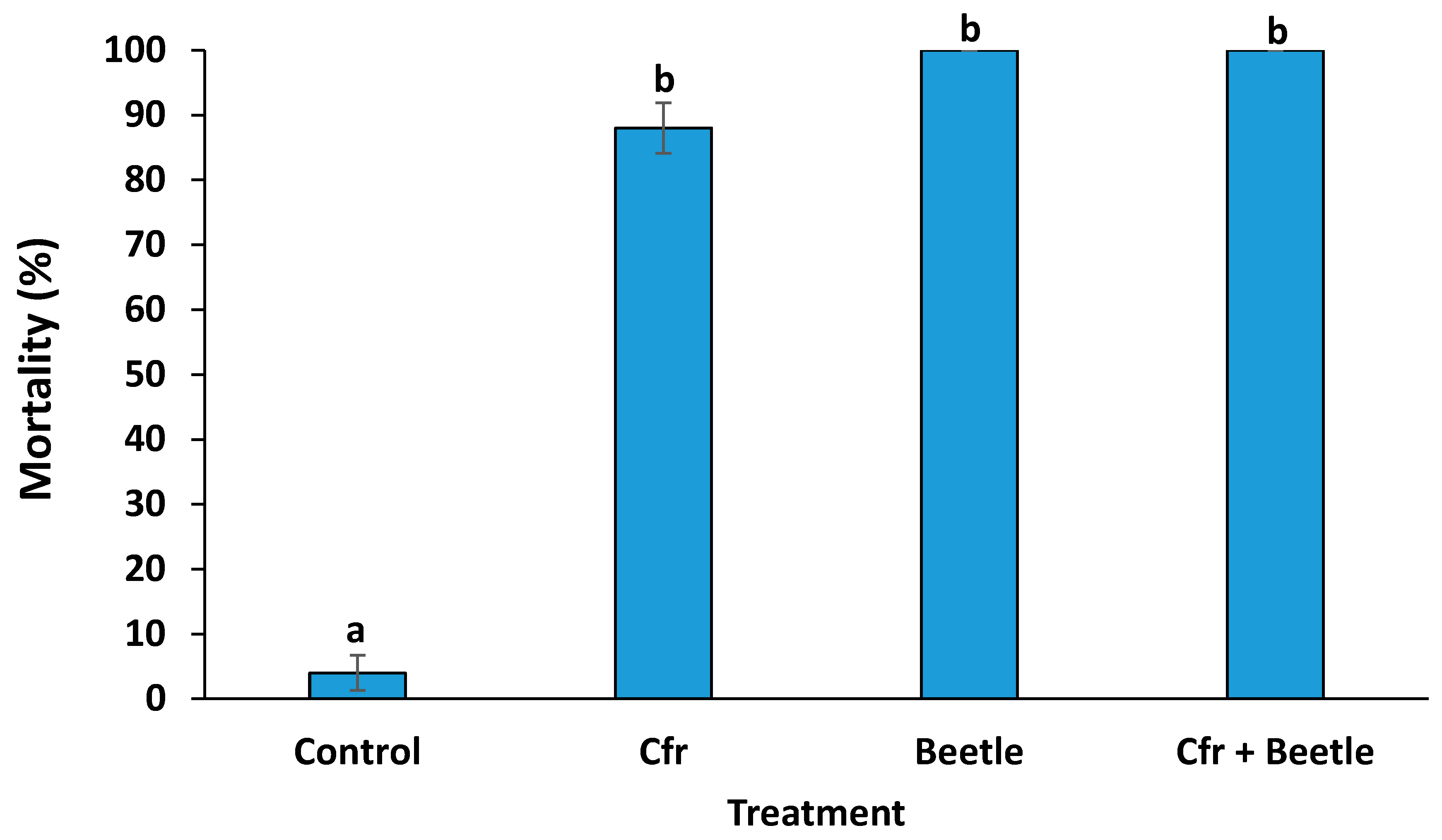
3.1. Efficacy of Treatments on the Mortality of Whitefly Populations
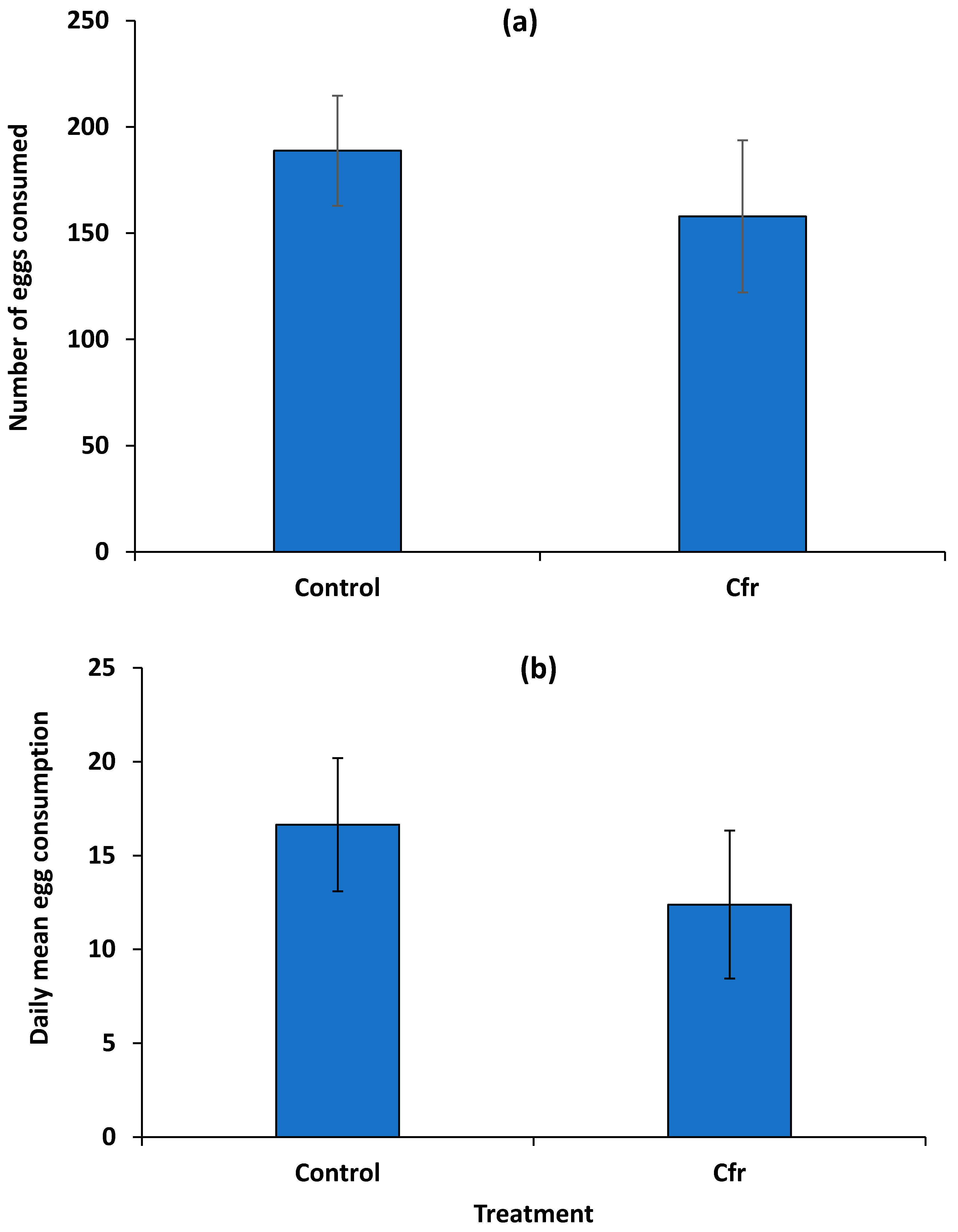
3.2. Consumption of Fungus-Contaminated Whitefly Eggs by Beetles in No-Choice Arenas
3.3. Longevity of Beetles after Exposure to Fungus-Contaminated Whitefly Eggs
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kumar, V.; Francis, A.; Ahmed, M.Z.; Mannion, C.; Stocks, I.; Rohrig, E.; McKenzie, C.L.; Osborne, L.S. Solanum whitefly, pepper whitefly, Aleurotrachelus trachoides Back (Insecta: Hemiptera: Aleyrodidae: Aleyrodinae); IFAS Extension, EENY-662; University of Florida: Gainesville, FL, USA, 2016; Available online: http://entnemdept.ufl.edu/creatures/veg/Aleurotrachelus_trachoides.htm (accessed on 18 November 2019).
- FDACS-DPI Database, Host Records for Pepper Whitefly Aleurotrachelus [Aleurothrixus] trachoides from 1985 to 2015. Florida Department of Agriculture and Consumer Services-Division of Plant Industry, 2019. Available online: https://www.fdacs.gov/Divisions-Offices/Plant-Industry/Plant-Industry-Publications/ (accessed on 21 January 2020).
- EPPO. Pest Risk Analysis for Aleurotrachelus [Aleurothrixus] trachoides (Hemiptera: Aleyrodidae). 2017. Available online: http://www.eppo.int/QUARANTINE/Pest_Risk_Analysis/PRA_intro.htm (accessed on 21 January 2020).
- Chandrashekar, K.; Rao, A.; Gorane, A.; Verma, R.; Tripathi, S. Aleurothrixus trachoides (Back) can transmit begomovirus from Duranta to potato, tomato and bell pepper. J. Biosci. 2020, 45, 36. [Google Scholar] [CrossRef] [PubMed]
- Meyling, N.V.; Eilenberg, J. Ecology of the entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae in temperate agroecosystems: Potential for conservation biological control. Biol. Control 2007, 2, 145–155. [Google Scholar] [CrossRef]
- Vega, F.E.; Goettel, M.S.; Blackwell, H.; Chandler, D.; Jackson, M.A.; Keller, S.; Koike, M.; Maniania, N.K.; Monzón, B.H.; Pell, J.K.; et al. Fungal entomopathogens: New insights on their ecology. Fungal Ecol. 2009, 2, 14–159. [Google Scholar] [CrossRef]
- Jackson, M.A.; Dunlap, C.A.; Jaronski, S.T. Ecological considerations in producing and formulating fungal entomopathogens for use in insect control. BioControl 2010, 55, 129–145. [Google Scholar] [CrossRef]
- Mesquita, A.L.M.; Lacey, L.A.; Leclant, F. Individual and combined effects of the fungus, Paecilomyces fumosoroseus and parasitoid, Aphelinus asychis Walker (Hym., Aphelinidae) on confined populations of Russian wheat aphid, Diuraphis noxia (Mordvilko) (Hom., Aphididae) under field conditions. J. Appl. Entomol. 1997, 121, 155–163. [Google Scholar] [CrossRef]
- Alma, C.R.; Goettel, M.S.; Roitberg, B.D.; Gillespie, D.R. Combined effects of the entomopathogenic fungus, Paecilomyces fumosoroseus Apopka-97, and the generalist predator, Dicyphus hesperus, on whitefly populations. BioControl 2007, 52, 669–681. [Google Scholar] [CrossRef]
- Labbé, R.M.; Gillespie, D.R.; Cloutier, C.; Brodeur, J. Compatibility of an entomopathogenic fungus with a predator and a parasitoid in the biological control of greenhouse whitefly. Biocontrol Sci. Technol. 2009, 19, 429–446. [Google Scholar] [CrossRef]
- Kumar, V.; Francis, A.; Avery, P.; McKenzie, C.; Osborne, L. Assessing compatibility of Isaria fumosorosea and buprofezin for mitigation of Aleurodicus rugioperculatus (Hemiptera: Aleyrodidae)—An invasive pest in the Florida landscape. J. Econ. Entomol. 2018, 111, 1069–1079. [Google Scholar] [CrossRef]
- Barahona, C.F.S.; Threlkeld, B.S.; Avery, P.B.; Francis, A.W.; Cave, R.D. Compatibility and efficacy of the lady beetle Thalassa montezumae and the entomopathogenic fungus Isaria fumosorosea for biological control of the green croton scale: Laboratory and greenhouse investigations. Arthropod Plant Interact. 2018, 12, 715–723. [Google Scholar] [CrossRef]
- Muma, M.H.; Selhime, A.G.; Denmark, H.A. An annotated list of predators and parasites associated with insects and mites on Florida citrus. Fla. Agric. Exp. Stn. Tech. Bull. 1961, 634, 39. [Google Scholar]
- Meyerdirk, D.E.; Kreasky, J.B.; Hart, G.W. Whiteflies (Aleyrodidae) attacking citrus in southern Texas with notes on natural enemies. Can. Entomol. 1980, 112, 1253–1258. [Google Scholar] [CrossRef]
- Gordon, R.D. South American Coccinellidae (Coleoptera). Part III: Taxonomic revision of the western hemisphere genus Delphastus Casey. Frustula Entomol. 1994, 199417, 1–133. [Google Scholar]
- Hoelmer, K.A.; Pickett, C.H. Geographic origin and taxonomic history of Delphastus spp. (Coleoptera: Coccinellidae) in commercial culture. Biocontrol Sci. Technol. 2003, 13, 529–535. [Google Scholar] [CrossRef]
- Razze, J.; Liburd, O. Delphastus catalinae (Horn) (Insecta: Coleoptera: Coccinellidae). EENY-677, IFAS Extension; University of Florida: Gainesville, FL, USA, 2017. [Google Scholar]
- Ahmed, M.Z.; Hernandez, Y.V.; Kumar, V.; Francis, A.; Skelley, P.; Rohrig, E.; McKenzie, C.; Osborne, L.S.; Mannion, C. Pallidus beetle, Delphastus Pallidus LeConte (Insecta: Coleoptera: Coccinellidae), a Native Predatory Beetle of Whitefly Species in Florida; Florida Dept. of Agric. & Consumer Services: Gainesville, FL, USA, 2017.
- Hoelmer, K.A.; Osborne, L.S.; Yokomi, R.K. Biological control of sweet potato whitefly in Florida. In Pest Management in the Subtropics: Biological Control-Florida Perspective; Intercept: Andover, UK, 1994; pp. 101–113. [Google Scholar]
- Legaspi, J.C.; Simmons, A.M.; Legaspi, B.C., Jr. Prey preference by Delphastus catalinae (Coleoptera: Coccinellidae) on Bemisia argentifolii (Homoptera: Aleyrodidae): Effects of plant species and prey stages. Fla. Entomol. 2006, 33, 218–222. [Google Scholar] [CrossRef]
- Razze, J.M.; Liburd, O.E.; McSorley, R. Preference of the silverleaf whitefly, Bemisia tabaci B biotype, on zucchini squash and buckwheat and the effect of Delphastus catalinae on whitefly populations. Pest Manag. Sci. 2016, 72, 1335–1339. [Google Scholar] [CrossRef] [PubMed]
- Avery, P.B.; Kumar, V.; Xiao, Y.; Powell, C.A.; McKenzie, C.L.; Osborne, L.S. Selecting an ornamental pepper banker plant for Amblyseius swirskii in floriculture crops. Arthropod Plant Interact. 2014, 8, 49–56. [Google Scholar] [CrossRef]
- Delane, B. (Certis USA, Columbia, MD, USA). Personal communication, 2020.
- Avery, P.B.; Kumar, V.; Skvarch, E.A.; Mannion, C.M.; Powell, C.A.; McKenzie, C.L.; Osborne, L.S. An ecological assessment of Isaria fumosorosea applications compared to a neonicotinoid treatment for regulating invasive ficus whitefly. J. Fungi 2019, 5, 36. [Google Scholar] [CrossRef]
- Hoelmer, K.A.; Osborne, L.S.; Yokomi, R.K. Interactions of the whitefly predator Delphastus pusillus (Coleoptera: Coccinellidae) with parasitized sweetpotato whitefly (Homoptera: Aleyrodidae). Environ. Entomol. 1994, 23, 136–139. [Google Scholar] [CrossRef]
- Kumar, V.; Mehra, L.; McKenzie, C.; Osborne, L. Functional response and prey stage preference of Delphastus catalinae and D. pallidus (Coleoptera: Coccinellidae) on Bemisia tabaci (Hemiptera: Aleyrodidae). Biocontrol Sci. Technol. 2020, 30, 581–591. [Google Scholar] [CrossRef]
- Osborne, L.; Landa, Z. Biological control of whiteflies with entomopathogenic fungi. Fla. Entomol. 1992, 75, 456–471. [Google Scholar] [CrossRef]
- Vidal, C.; Fargues, J.; Lacey, L. Intraspecific variability of Paecilomyces fumosoroseus: Effect of the temperature on the vegetative growth. J. Invertebr. Pathol. 1997, 70, 18–26. [Google Scholar] [CrossRef]
- Lacey, L.A.; Frutos, R.; Kaya, H.K.; Vail, P. Insect pathogens as biological control agents: Do they have a future? Biol. Control 2001, 21, 230–248. [Google Scholar] [CrossRef]
- Feng, M.G.; Chen, B.; Ying, S.H. Trials of Beauveria bassiana, Paecilomyces fumosoroseus and imidacloprid for management of Trialeurodes vaporariorum (Homoptera: Aleyrodidae) on greenhouse grown lettuce. Biocontrol Sci. Technol. 2004, 14, 531–544. [Google Scholar] [CrossRef]
- Avery, P.B.; Faull, J.; Simmonds, M.S.J. Effect of different photoperiods on the growth, infectivity and colonization of Trinidadian strains of Paecilomyces fumosoroseus on the greenhouse whitefly, Trialeurodes, using a glass slide bioassay. J. Insect Sci. 2004, 4, 38. [Google Scholar] [CrossRef] [PubMed]
- Avery, P.B.; Faull, J.; Simmonds, M.S.J. Effects of Paecilomyces fumosoroseus and Encarsia formosa on the control of the greenhouse whitefly: Preliminary assessment of a compatibility study. BioControl 2008, 53, 303–316. [Google Scholar] [CrossRef]
- Zimmermann, G. The entomopathogenic fungi Isaria farinosa (formerly Paecilomyces farinosus) and the Isaria fumosorosea species complex (formerly Paecilomyces fumosoroseus): Biology, ecology and use in biological control. Biocontrol Sci. Technol. 2008, 18, 865–901. [Google Scholar] [CrossRef]
- Kim, J.S.; Yeon, H.J.; Skinner, M.; Parker, B.L. An oil-based formulation of Isaria fumosorosea blastospores for management of greenhouse whitefly Trialeurodes vaporariorum (Homoptera: Aleyrodidae). Pest Manag. Sci. 2013, 69, 576–581. [Google Scholar] [CrossRef]
- Lacey, L.A.; Grzywacz, D.; Shapiro-Ilan, D.I.; Frutos, R.; Brownbridge, M.; Goettel, M.S. Insect pathogens as biological control agents: Back to the future. J. Invertebr. Pathol. 2015, 132, 1–41. [Google Scholar] [CrossRef]
- Sandhu, S.S.; Sharma, A.K.; Beniwal, V.; Goel, G.; Batra, P.; Kumar, A.; Jaglan, S.; Sharma, A.; Malhotra, S. Myco-biocontrol of insect pests: Factors involved, mechanism, and regulation. J. Pathog. 2012, 126819, 10. [Google Scholar] [CrossRef]
- Kumar, V.; Avery, P.B.; Ahmed, J.; Cave, R.D.; McKenzie, C.L.; Osborne, L.S. Compatibility and efficacy of Isaria fumosorosea with horticultural oils for mitigation of the Asian citrus psyllid, Diaphorina citri (Hemiptera: Liviidae). Insects 2017, 8, 119. [Google Scholar] [CrossRef]
- Meyer, J.M.; Hoy, M.A.; Boucias, D.G. Isolation and characterization of an Isaria fumosorosea isolate infecting the Asian citrus psyllid in Florida. J. Invertebr. Pathol. 2008, 99, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Avery, P.B. (University of Florida, IFAS, Indian River Research and Education Center, Ft. Pierce, FL, USA). Personal communication, 2019.
- Poprawski, T.J.; Legaspi, J.C.; Parker, P.E. Influence of entomopathogenic fungi on Serangium parcesetosum (Coleoptera: Coccinellidae), an important predator of whiteflies (Homoptera: Aleyrodidae). Biol. Control 1998, 27, 785–795. [Google Scholar] [CrossRef]
- Zhou, F.; Ali, S.; Huang, Z. Influence of the entomopathogenic fungus Isaria fumosorosea on Axinoscymnus cardilobus (Coleoptera: Coccinellidae) under laboratory conditions. Biocontrol Sci. Technol. 2010, 20, 709–722. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avery, P.B.; Kumar, V.; Francis, A.; McKenzie, C.L.; Osborne, L.S. Compatibility of the Predatory Beetle, Delphastus catalinae, with an Entomopathogenic Fungus, Cordyceps fumosorosea, for Biocontrol of Invasive Pepper Whitefly, Aleurothrixus trachoides, in Florida. Insects 2020, 11, 590. https://doi.org/10.3390/insects11090590
Avery PB, Kumar V, Francis A, McKenzie CL, Osborne LS. Compatibility of the Predatory Beetle, Delphastus catalinae, with an Entomopathogenic Fungus, Cordyceps fumosorosea, for Biocontrol of Invasive Pepper Whitefly, Aleurothrixus trachoides, in Florida. Insects. 2020; 11(9):590. https://doi.org/10.3390/insects11090590
Chicago/Turabian StyleAvery, Pasco B., Vivek Kumar, Antonio Francis, Cindy L. McKenzie, and Lance S. Osborne. 2020. "Compatibility of the Predatory Beetle, Delphastus catalinae, with an Entomopathogenic Fungus, Cordyceps fumosorosea, for Biocontrol of Invasive Pepper Whitefly, Aleurothrixus trachoides, in Florida" Insects 11, no. 9: 590. https://doi.org/10.3390/insects11090590
APA StyleAvery, P. B., Kumar, V., Francis, A., McKenzie, C. L., & Osborne, L. S. (2020). Compatibility of the Predatory Beetle, Delphastus catalinae, with an Entomopathogenic Fungus, Cordyceps fumosorosea, for Biocontrol of Invasive Pepper Whitefly, Aleurothrixus trachoides, in Florida. Insects, 11(9), 590. https://doi.org/10.3390/insects11090590




