Comparative Ecology of Hyalomma lusitanicum and Hyalomma marginatum Koch, 1844 (Acarina: Ixodidae)
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Host Affinity
3.2. Life Cycle
3.3. Sex Ratio
3.4. Questing/Host Seeking Strategies
3.5. Relationships to Climatic Parameters
3.6. Habitats and Geographical Distribution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guglielmone, A.A.; Robbins, R.G.; Apanaskevich, D.A.; Petney, T.N.; Estrada-Peña, A.; Horak, I.G.; Shao, R.; Barker, S.C. The Argasidae, Ixodidae and Nuttalliellidae (Acari: Ixodida) of the world: A list of valid species names. Zootaxa 2010, 2528, 1. [Google Scholar] [CrossRef]
- ABC. Las «garrapatas gigantes» se expanden por el norte de Europa. Available online: https://www.abc.es/sociedad/abci-garrapatas-gigantes-expanden-norte-europa-201907301343_noticia.html (accessed on 6 August 2019).
- Dutch News. Available online: https://www.dutchnews.nl/news/2019/07/giant-ticks-which-hunt-their-prey-confirmed-in-the-netherlands/ (accessed on 6 August 2019).
- I am Expat. Giant tropical ticks overwinter in Germany for the first time. Available online: https://www.iamexpat.de/expat-info/german-expat-news/giant-tropical-ticks-overwinter-germany-first-time (accessed on 6 August 2019).
- The Local. Giant “Hunter” Tick Found in Sweden for First Time. 2019. Available online: https://www.thelocal.se/20180825/giant-hunter-tic-found-for-first-time-in-sweden (accessed on 6 August 2019).
- Hornok, S.; Horváth, G. First report of adult Hyalomma marginatum rufipes (vector of Crimean-Congo haemorrhagic fever virus) on cattle under a continental climate in Hungary. Parasites & vectors 2012, 5, 170. [Google Scholar]
- Kampen, H.; Poltz, W.; Hartelt, K.; Wolfel, R.; Faude, M. Detection of a questing Hyalomma marginatum marginatum adult female (Acari, Ixodidae) in southern Germany. Exp. Appl. Acarol. 2007, 43, 227–231. [Google Scholar] [CrossRef]
- Vial, L.; Stachurski, F.; Leblond, A.; Huber, K.; Vourc’h, G.; René-Martellet, M.; Desjardins, I.; Balança, G.; Grosbois, V.; Pradier, S.; et al. Strong evidence for the presence of the tick Hyalomma marginatum Koch, 1844 in southern continental France. Ticks and Tick-borne Diseases 2016, 7, 1162–1167. [Google Scholar] [CrossRef] [PubMed]
- Chitimia-Dobler, L.; DE Araujo, B.C.; Ruthensteiner, B.; Pfeffer, T.; Dunlop, J.A. Amblyomma birmitum a new species of hard tick in Burmese amber. Parasitology 2017, 144, 1441–1448. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cull, B.; Pietzsch, M.E.; Hansford, K.M.; Gillingham, E.L.; Medlock, J.M. Surveillance of British ticks: An overview of species records, host associations, and new records of Ixodes ricinus distribution. Ticks and Tick-Borne Dis. 2018, 9, 605–614. [Google Scholar] [CrossRef] [PubMed]
- ECDC. Hyalomma marginatum—Factsheet for experts. Available online: https://ecdc.europa.eu/en/disease-vectors/facts/tick-factsheets/Hyalomma-marginatum (accessed on 12 September 2019).
- ECDC. Hyalomma marginatum—current known distribution: January 2019. Available online: https://ecdc.europa.eu/en/publications-data/<italic>Hyalomma</italic>-<italic>marginatum</italic>-current-known-distribution-january-2019 (accessed on 16 September 2019).
- ECDC. Hyalomma marginatum—current known distribution in Europe, April 2017. Available online: https://ecdc.europa.eu/en/publications-data/Hyalomma-marginatum-current-known-distribution-europeapril-2017 (accessed on 16 September 2019).
- Oehme, R.; Bestehorn, M.; Wölfel, S.; Chitimia-Dobler, L. Hyalomma marginatum in Tübingen, Germany. Syst. Appl. Acarol. 2017, 22, 1. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Venzal, J.M. A GIS framework for the assessment of tick impact on human health in a changing climate. Geospat. Health 2007, 1, 157–168. [Google Scholar] [CrossRef]
- EFSA Panel on Animal and Welfare (AHAW) Scientific Opinion on the Role of Tick Vectors in the Epidemiology of Crimean-Congo Hemorrhagic Fever and African Swine Fever in Eurasia. EFSA J. 2010, 8, 156. [CrossRef]
- Viseras, J.; Hueli, L.E.; Adroher, F.J.; García-Fernández, P. Studies on the transmission of Theileria annulata to cattle by the tick Hyalomma lusitanicum. Zoonoses and Public Health 1999, 46, 505–509. [Google Scholar] [CrossRef]
- Bolaños-Rivero, M.; Carranza-Rodríguez, C.; Rodríguez, N.F.; Gutiérrez, C.; Pérez-Arellano, J.-L. Detection of Coxiella burnetii DNA in Peridomestic and Wild Animals and Ticks in an Endemic Region (Canary Islands, Spain). Vector Borne Zoonotic Dis. 2017, 17, 630–634. [Google Scholar] [CrossRef] [PubMed]
- González, J.; González, M.G.; Valcárcel, F.; Sánchez, M.; Martín-Hernández, R.; Tercero, J.M.; Olmeda, A.S. Prevalence of Coxiella burnetii (Legionellales: Coxiellaceae) Infection Among Wildlife Species and the Tick Hyalomma lusitanicum (Acari: Ixodidae) in a Meso-Mediterranean Ecosystem. J. Med. Entomol. 2019, tjz169. [Google Scholar] [CrossRef] [PubMed]
- Apanaskevich, D.A.; Horak, I.A. The genus Hyalomma. VI. Systematics of H. (EuHyalomma) truncatum and the closely related species, H. (E.) albiparmatum and H. (E.) nitidum (Acari: Ixodidae). Exp. Appl. Acarol. 2008, 44, 115–136. [Google Scholar] [CrossRef] [PubMed]
- Habela, M.; Peña, J.; Corchero, E.; Sevilla, R. Manual práctico para la identificación de garrapatas y hemoparásitos por ellas transmitidos de interés veterinario en España; Facultad Veterinaria de Cáceres, Parasitología y Enfermedades Parasitarias: Cáceres, España, 2000. [Google Scholar]
- Chisu, V.; Foxi, C.; Masala, G. First molecular detection of the human pathogen Rickettsia raoultii and other spotted fever group rickettsiae in Ixodid ticks from wild and domestic mammals. Parasitol. Res. 2018, 117, 3421–3429. [Google Scholar] [CrossRef]
- Chisu, V.; Foxi, C.; Mannu, R.; Satta, G.; Masala, G. A five-year survey of tick species and identification of tick-borne bacteria in Sardinia, Italy. Ticks Tick-borne Dis. 2018, 9, 678–681. [Google Scholar] [CrossRef]
- Chisu, V.; Zobba, R.; Lecis, R.; Sotgiu, F.; Masala, G.; Foxi, C.; Pisu, D.; Alberti, A. GroEL typing and phylogeny of Anaplasma species in ticks from domestic and wild vertebrates. Ticks Tick-Borne Dis. 2018, 9, 31–36. [Google Scholar] [CrossRef]
- Toledo, Á.; Olmeda, A.S.; Escudero, R.; Jado, I.; Valcárcel, F.; Casado-Nistal, M.A.; Rodríguez-Vargas, M.; Gil, H.; Anda, P. Tick-borne zoonotic bacteria in ticks collected from central Spain. Am. J. Trop. Med. Hyg. 2009, 81, 67–74. [Google Scholar] [CrossRef]
- Kasi, K.K.; von Arnim, F.; Schulz, A.; Rehman, A.; Chudhary, A.; Oneeb, M.; Sas, M.A.; Jamil, T.; Maksimov, P.; Sauter-Louis, C.; et al. Crimean-Congo haemorrhagic fever virus in ticks collected from livestock in Balochistan, Pakistan. Transboundary Emerg. Dis. 2020, 1–10. [Google Scholar] [CrossRef]
- Scoles, G.A.; Ueti, M.W. Vector Ecology of Equine Piroplasmosis. Annu. Rev. Entomol. 2015, 60, 561–580. [Google Scholar] [CrossRef]
- Fernández, G.; González, C.; Alarcón, V. Detection of Theileria annulata in the parasitic ixodids of the Spanish fightingbull by the nested-PCR technique. Rev. Ibérica Parasitol. 2006, 66, 13–16. [Google Scholar]
- Valcarcel, F.; González, J.; González, M.; Sánchez, M.; Elhachimi, L.; Carbonell, J.D.; Tercero, J.; Olmeda, A.S. Comparative Study of Morphological Characteristics of Hyalomma lusitanicum and Hyalomma marginatum Adult Ticks, Koch 1844 (Acarina: Ixodidae). Acarologia. 2020. submitted. [Google Scholar]
- Walker, A.; Bouattour, A.; Camicas, J.-L.; Estrada-Pena, A.; Horak, I.; Latif, A.; Pegram, R.G.; Preston, P.M. Ticks of Domestic Animals in Africa: A Guide to Identification of Species; Bioscience Reports: Edinburgh, UK, 2003; ISBN 0-9545173-0-X. [Google Scholar]
- Barandika, J.F.; Olmeda, S.A.; Casado-Nistal, M.A.; Hurtado, A.; Juste, R.A.; Valcárcel, F.; Anda, P.; García-Pérez, A.L. Differences in Questing Tick Species Distribution Between Atlantic and Continental Climate Regions in Spain. J. Med. Entomol. 2011, 48, 13–19. [Google Scholar] [CrossRef] [PubMed]
- González, J.; Valcárcel, F.; Pérez-Sánchez, J.L.; Tercero-Jaime, J.M.; Olmeda, A.S. Seasonal dynamics of ixodid ticks on wild rabbits Oryctolagus cuniculus (Leporidae) from Central Spain. Exp. Appl Acarol 2016, 70, 369–380. [Google Scholar] [CrossRef] [PubMed]
- González, J.; Valcárcel, F.; Pérez-Sánchez, J.L.; Tercero-Jaime, J.M.; Cutuli, M.T.; Olmeda, A.S. Control of Hyalomma lusitanicum (Acari: Ixodidade) Ticks Infesting Oryctolagus cuniculus (Lagomorpha: Leporidae) Using the Entomopathogenic Fungus Beauveria bassiana (Hyocreales: Clavicipitaceae) in Field Conditions. J. Med. Entomol. 2016, 53, 1396–1402. [Google Scholar] [CrossRef]
- Requena-García, F.; Cabrero-Sañudo, F.; Olmeda-García, S.; González, J.; Valcárcel, F. Influence of environmental temperature and humidity on questing ticks in central Spain. Exp. Appl. Acarol. 2017, 71, 277–290. [Google Scholar] [CrossRef]
- Valcárcel, F.; González, J.; Tercero, J.M.; Olmeda, A.S. Long term study of ixodid ticks feeding on red deer (Cervus elaphus) in a meso-Mediterranean climate. Exp. Appl. Acarol. 2016, 69, 61–72. [Google Scholar] [CrossRef]
- Valcárcel, F.; González, J.; Pérez Sánchez, J.L.; Tercero, J.M.; Olmeda, A.S. Long-Term Ecological Study of Host-Seeking Adults of Hyalomma lusitanicum (Acari: Ixodidae) in a Meso-Mediterranean Climate. J. Med. Entomol. 2016, 53, 221–224. [Google Scholar] [CrossRef]
- Cota, S. Control biológico e integrado de la garrapata Hyalomma lusitanicum en explotaciones silvo-agro-cinegéticas de ecosistema mesomediterráneo. UCM: Madrid, Spain, 2015. [Google Scholar]
- González, J.; Valcárcel, F.; Aguilar, A.; Olmeda, A.S. In vitro feeding of Hyalomma lusitanicum ticks on artificial membranes. Exp Appl Acarol 2017, 72, 449–459. [Google Scholar] [CrossRef]
- Julio, L.F.; Díaz, C.E.; Aissani, N.; Valcarcel, F.; Burillo, J.; Olmeda, S.; González-Coloma, A. Ixodicidal compounds from pre-domesticated Lavandula luisieri. Ind. Crops Prod 2017, 110, 83–87. [Google Scholar] [CrossRef]
- Valcárcel, F.; González, J.; Tercero-Jaime, J.M.; Olmeda, A.S. The effect of excluding ungulates on the abundance of ixodid ticks on wild rabbit (Oryctolagus cuniculus). Exp. Appl. Acarol. 2017, 72, 439–447. [Google Scholar] [CrossRef]
- Valcárcel, F.; Sánchez, J.; Jaime, J.; Basco-Basco, P.; Guajardo, S.; Cutuli, M.-T.; Martín-Hernández, R.; Olmeda, A.-S. Control of Host-seeking Adults of Hyalomma lusitanicum with Oxalic Acid under Field Conditions. Int. J. Vet. Med. Res. Rep. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Valcárcel, F.; Sánchez, J.L.P.; Jaime, J.M.T.; Basco-Basco, P.I.; Guajardo, S.C.C.; Cutuli, M.T.; GonzAlez, J.; Olmeda, A.S. Control of Tick Infestations in Oryctolagus cuniculus (Lagomorpha: Leporidae) With Spinosad Under Laboratory and Field Conditions. J. Med. Entomol. 2015, 52, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Tendeiro, J. Revisiao sistemática ds ixodídeos portugueses. Bol. Pec. 1962, 2, 1–131. [Google Scholar]
- Diehl, P.A.; Aeschlimann, A.; Obenchain, F.D. CHAPTER 9—Tick Reproduction: Oogenesis and Oviposition. In Physiology of Ticks; Obenchain, F.D., Galun, R., Eds.; Current Themes in Tropical Science; Pergamon Press: Pergamon, Turkey, 1982; Volume 1, pp. 277–350. ISBN 978-0-08-024937-7. [Google Scholar]
- Dias, J.A. Travassos Santos (Jaime Augusto Travassos Santos) & Instituto de Investigação Científica Tropical (Portugal). As carraças (Acarina-Ixodoidea) da Península Ibérica: Algumas considerações sobre a sua biogeografia e relacionamento com a ixodofauna afropaleárctica e afrotropical; Estudos, ensaios e documentos, 0870¨C001X; 158; Ministério do Planeamento e da Administração do Território, Secretaria de Estado da Ciência e Tecnologia, Instituto de Investigação Científica Tropical: Lisboa, Portugal, 1994. [Google Scholar]
- García Fernández, P.; Hueli, L.E. Garrapatas (Acarina, Ixodidae) parasitas del ganado bovino en el sur de España. Identificación, distribución geográfica y estacional. Rev. Ibér. Parasitol. 1984, 129–138. [Google Scholar]
- Perez-Eid, C.; Cabrita, J. La larve et la nymphe de Hyalomma (Hyalomma) lusitanicum Koch, 1844 (Acari: Ixodida): Description morphologique, habitats, hotes. Acarologia 2003, 4, 327–335. [Google Scholar]
- Vatansever, Z.; DVM, R.U.; Estrada-Pena, A.; Ergonul, O. Crimean-Congo Hemorrhagic Fever in Turkey. In Crimean-Congo Hemorrhagic Fever: A Global Perspective; Ergonul, O., Whitehouse, C.A., Eds.; Springer Netherlands: Dordrecht, 2007; pp. 59–74. ISBN 978-1-4020-6106-6. [Google Scholar]
- Choubdar, N.; Oshaghi, M.A.; Rafinejad, J.; Pourmand, M.R.; Maleki-Ravasan, N.; Salehi-Vaziri, M.; Telmadarraiy, Z.; Karimian, F.; Koosha, M.; Rahimi-Foroushani, A.; et al. Effect of Meteorological Factors on Hyalomma Species Composition and Their Host Preference, Seasonal Prevalence and Infection Status to Crimean-Congo Haemorrhagic Fever in Iran. J. Arthropod Borne Dis. 2019, 13, 268–283. [Google Scholar] [CrossRef]
- Monsonis, G.S. Parasitofauna del zorro rojo (Vulpes vulpes) en la Comunidad Valenciana. Available online: http://purl.org/dc/dcmitype/Text (accessed on 2 February 2020).
- Rol Díaz, J. Nuevas aportaciones al conocimiento epidemiológico de la theileriosis (Theileria annulata) en Extremadura; Universidad de Extremadura: Cáceres, Spain, 1996. [Google Scholar]
- Adeli-Sardou, M.; Azizi, K.; Soltani, A.; Moemenbellah-Fard, M.D. First record of hard tick species, Hyalomma marginatum marginatum and H. marginatum rufipes (Acari: Ixodidae), as probable vectors of Crimean-Congo hemorrhagic fever virus, from the spur-thighed tortoise, Testudo graeca (Reptilia: Testudinidae), SE Iran. Persian J. Acarol. 2019, 8, 281–286. [Google Scholar] [CrossRef]
- MSSSI Informe de situación y evaluación del riesgo de transmisión del virus de Fiebre Hemorrágica de Crimea-Congo (FHCC) en España; 2019.
- Torina, A.; Blanda, V.; Blanda, M.; Auteri, M.; La Russa, F.; Scimeca, S.; D’Agostino, R.; Disclafani, R.; Villari, S.; Currò, V.; et al. A Geographical Information System Based Approach for Integrated Strategies of Tick Surveillance and Control in the Peri-Urban Natural Reserve of Monte Pellegrino (Palermo, Southern Italy). Int J. Environ. Res. Public Health 2018, 15. [Google Scholar] [CrossRef]
- Apanaskevich, D.A. Host-parasite relationships of the genus Hyalomma Koch, 1844 (Acari, Ixodidae) and their connection with microevolutionary process. Parazitologia 2004, 38(6), 515–523. [Google Scholar]
- García Romero, C.; Valcárcel, F.; Corchero, J.; Olmeda, A.S.; Pérez Jimenez, J. Contribución al estudio de las parasitosis del ciervo (Cervus elaphus) en las provincias de Toledo y Ciudad Real (Castilla-La Mancha, España). Ecología 2000, 14, 235–249. [Google Scholar]
- Aktas, M.; Dumanli, N.; Angin, M. Cattle infestation by Hyalomma ticks and prevalence of Theileria in Hyalomma species in the east of Turkey. Vet. Parasitol. 2004, 119, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kotti, B.K.; Shaposhnikova, L.I.; Evchenko, I.M.; Levchenko, B.I.; Surkhaev, D.B.; Korzhov, P.N.; Tokhov, I.M. Hyalomma marginatum Koch in Stavropol’ region. Zh. Mikrobiol. Epidemiol. Immunobiol. 2001, 105–108. [Google Scholar]
- Estrada-Peña, A.; Bouattour, A.; Camicas, J.L.; Walker, A.R. Ticks of Domestic Animals in the Mediterranean Region.: A Guide to Identification of Species.; Universidad de Zaragoza: Zaragoza, Spain, 2004. [Google Scholar]
- Ruiz-Fons, F.; Fernández-de-Mera, I.G.; Acevedo, P.; Höfle, U.; Vicente, J.; de la Fuente, J.; Gortazár, C. Ixodid ticks parasitizing Iberian red deer (Cervus elaphus hispanicus) and European wild boar (Sus scrofa) from Spain: Geographical and temporal distribution. Vet. Parasitol. 2006, 140, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Calvete, C.; Estrada, R.; Lucientes, J.; Estrada, A. Ectoparasite ticks and chewing lice of red-legged partridge, Alectoris rufa, in Spain. Med. Vet. Entomol. 2003, 17, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Norte, A.C.; de Carvalho, I.L.; Ramos, J.A.; Gonçalves, M.; Gern, L.; Núncio, M.S. Diversity and seasonal patterns of ticks parasitizing wild birds in western Portugal. Exp. Appl. Acarol. 2012, 58, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Caeiro, V.; Simoes, A. Ixodoidea da fauna silvestre de Portugal continental. Interesse do seu conhecimento. Rev. Port. Cienc. Vet. 1991, 497, 20–30. [Google Scholar]
- Apanaskevich, D.A.; Santos-Silva, M.M.; Horak, I.G. The genus Hyalomma Koch, 1844. IV. Redescription of all parasitic stages of H.(EuHyalomma) lusitanicum Koch, 1844 and the adults of H.(E.) franchinii Tonelli Rondelli, 1932 (Acari: Ixodidae) with a first description of its immature stages. Folia parasitologica 2008, 55, 61–74. [Google Scholar] [CrossRef]
- Papadopoulos, B.; Morel, P.C.; Aeschlimann, A. Ticks of domestic animals in the Macedonia region of Greece. Vet. Parasitol. 1996, 63, 25–40. [Google Scholar] [CrossRef]
- Tokhov, I.M.; Sysoliatina, G.V.; Chumakova, I.V.; Popova, E.V. Specific features of the parasitic system of Crimean haemorrhagic fever in Stavropol’ region during epidemic season of 2000. Zh. Mikrobiol. Epidemiol. Immunobiol. 2001, 98–99. [Google Scholar]
- Belan, I.; Bull, C.M. Host-seeking behaviour by Australian ticks (Acari: Ixodidae) with differing host specificities. Exp. Appl. Acarol. 1995, 19, 221–232. [Google Scholar] [CrossRef]
- Uspensky, I. Low air humidity increases aggressiveness of ixodid ticks (Acari_ Ixodidae) under high ambient temperatures (a preliminary hypothesis). Ticks and Tick-borne Diseases 2019, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Hagan, R.W.; Didion, E.M.; Rosselot, A.E.; Holmes, C.J.; Siler, S.C.; Rosendale, A.J.; Hendershot, J.M.; Elliot, K.S.B.; Jennings, E.C.; Nine, G.A.; et al. Dehydration prompts increased activity and blood feeding by mosquitoes. Sci. Rep. 2018, 8, 6804. [Google Scholar] [CrossRef] [PubMed]
- Ouhelli, H.; Pandey, V.S. Development of Hyalomma lusitanicum under laboratory conditions. Vet. Parasitol. 1984, 15, 57–66. [Google Scholar] [CrossRef]
- Ouhelli, H. Theilériose bovine à Theileria annulata (Dschunkowsky & Luhs, 1904). Recherche sur la biologie des vecteurs (Hyalomma spp.) et sur les intéractions hôte-parasite; Institut Polytechnique de Toulouse: Toulouse, France, 1985. [Google Scholar]
- Hueli, L.E. Biological cycle of Hyalomma marginatum marginatum Koch, 1844 (Acarina, Ixodidae) under standard laboratory conditions. Rev. Iber. Parasitol. 1979, 39, 143–152. [Google Scholar]
- Hueli, L.E.; Guevara Benitez, D.C.; García Fernández, P. Estudio de la incubacion y eclosion de los huevos de Hyalomma (Hyalomma) lusitanicum Koch, 1844 (Acarina Ixodidae) en condiciones de laboratorio. Rev. Iber. Parasitol. 1984, 309–314. [Google Scholar]
- Yukarı, B.A.; Nalbantoğlu, S.; Karaer, Z.; Inci, A.; Eren, H.; Sayın, F. Some biological features of Hyalomma marginatum in the laboratory. Turkiye Parazitol Derg 2011, 35, 40–42. [Google Scholar] [CrossRef]
- Gargili, A.; Thangamani, S.; Bente, D. Influence of laboratory animal hosts on the life cycle of Hyalomma marginatum and implications for an in vivo transmission model for Crimean-Congo hemorrhagic fever virus. Front. Cell. Infect. Microbiol. 2013, 3. [Google Scholar] [CrossRef]
- Abdigoudarzi, M. Tick rearing studies of Hyalomma anatolicum anatolicum (Acari: Ixodidac) and preparing of its life cycle data at laboratory conditions. Vet. J. 2011, 90, 1–12. [Google Scholar]
- Chen, Z.; Li, Y.; Liu, Z.; Yang, J.; Yin, H. The life cycle of Hyalomma rufipes (Acari: Ixodidae) under laboratory conditions. Exp. Appl. Acarol. 2012, 56, 85–92. [Google Scholar] [CrossRef]
- Al-Asgah, N.A. Biology of Hyalomma schulzei (Acari: Ixodidae) on Rabbits Under Laboratory Conditions. J. Med. Entomol. 1992, 29, 19–24. [Google Scholar] [CrossRef]
- Encinas, A.; Oleaga, A.; Pérez Sánchez, R. Garrapatas duras. In Parasitología Veterinaria; McGraw Hill Interamericana: Madrid, Spain, 1999; pp. 420–429. ISBN 84-486-0236-6. [Google Scholar]
- Herrmann, C.; Gern, L. Do the level of energy reserves, hydration status and Borrelia infection influence walking by Ixodes ricinus (Acari: Ixodidae) ticks? Parasitology 2012, 139, 330–337. [Google Scholar] [CrossRef] [PubMed]
- McClure, M.; Diuk-Wasser, M.A. Climate impacts on blacklegged tick host-seeking behavior. Int. J. Parasitol. 2019, 49, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Gale, P.; Estrada-Peña, A.; Martinez, M.; Ulrich, R.G.; Wilson, A.; Capelli, G.; Phipps, P.; de la Torre, A.; Muñoz, M.J.; Dottori, M.; et al. The feasibility of developing a risk assessment for the impact of climate change on the emergence of Crimean-Congo haemorrhagic fever in livestock in Europe: A review. J. Appl. Microbiol. 2010, 108, 1859–1870. [Google Scholar] [CrossRef] [PubMed]
- Maltezou, H.C.; Andonova, L.; Andraghetti, R.; Bouloy, M.; Ergonul, O.; Jongejan, F.; Kalvatchev, N.; Nichol, S.; Niedrig, M.; Platonov, A.; et al. Crimean-Congo hemorrhagic fever in Europe: Current situation calls for preparedness. Euro Surveill. 2010, 15, 19504. [Google Scholar] [PubMed]
- López-Vélez, R.; Molina Moreno, R. Cambio climático en España y riesgo de enfermedades infecciosas y parasitarias transmitidas por artrópodos y roedores. Rev. Española Salud Pública 2005, 79, 177–190. [Google Scholar] [CrossRef]
- ECDC. Consultation on Crimean-Congo haemorragic fever prevention and control. Available online: https://www.ecdc.europa.eu/en/publications-data/consultation-crimean-congo-haemorragic-fever-prevention-and-control (accessed on 29 November 2019).
- Hoogstraal, H. The epidemiology of tick-borne Crimean-Congo hemorrhagic fever in Asia, Europe, and Africa. J. Med. Entomol. 1979, 15(4), 307–417. [Google Scholar] [CrossRef]
- Hoogstraal, H. African Ixodoidea. I. Ticks of the Sudan (with special reference to Equatoria Province and with preliminary reviews of the genera Boophilus, Margaropus, and Hyalomma); Department of the Navy: Washington, DC, USA, 1956. [Google Scholar]
- Carroll, J.F. How specific are host-produced kairomones to host-seeking ixodid ticks? In Ticks and Tick-Borne Pathogens: Proceedings of the 4th International Conference on Ticks and Tick-Borne Pathogens The Banff Centre Banff, Alberta, Canada 21–26 July 2002; Jongejan, F., Kaufman, W.R., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2003; pp. 155–161. ISBN 978-94-017-3526-1. [Google Scholar]
- Latif, A.A.; Walker, A.R. An introduction to the biology and control of ticks in Africa. ICTTD-2 Proj. 2004, 1–29. [Google Scholar]
- Strong, A. Tick Host-Seeking Behavior. Insect Behav. 2005, 1, 1–15. [Google Scholar]
- Schulze, T.L.; Jordan, R.A. Influence of meso-and microscale habitat structure on focal distribution of sympatric Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae). J. Med. Entomol. 2005, 42, 285–294. [Google Scholar] [CrossRef]
- Romanenko, V.N. Visual potentialities of the tick Hyalomma asiaticum asiaticum (Ixodidae). Parazitologiia 2005, 39, 186–190. [Google Scholar]
- Akimov, I.; Nebogatkin, I. Distribution of the Ixodid Tick Hyalomma marginatum (Ixodoidea, Ixodidae) in Ukraine. Vestn. Zool. 2011, 45, e-25–e-28. [Google Scholar] [CrossRef]
- EFSA Field sampling methods for mosquitoes, sandflies, biting midges and ticks: VectorNet project 2014-2018; EFSA: Parma, Italy, 2018.
- Estrada-Peña, A.; Ayllón, N.; de la Fuente, J. Impact of Climate Trends on Tick-Borne Pathogen Transmission. Front. Physiol 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Peña, A.; Martínez Avilés, M.; Muñoz Reoyo, M.J. A population model to describe the distribution and seasonal dynamics of the tick Hyalomma marginatum in the Mediterranean Basin. Transbound. Emerg. Dis. 2011, 58, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Peña, A.; Pfäffle, M.; Baneth, G.; Kleinerman, G.; Petney, T.N. Ixodoidea of the Western Palaearctic: A review of available literature for identification of species. Ticks Tick-Borne Dis. 2017, 8, 512–525. [Google Scholar] [CrossRef]
- Santos-Silva, M.M.; Vatansever, Z. Hyalomma marginatum Koch, 1844 (Figs. 139–141). In Ticks of Europe and North Africa; Estrada-Peña, A., Mihalca, A.D., Petney, T.N., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 349–354. ISBN 978-3-319-63759-4. [Google Scholar]
- Ginsberg, H.S. Tick Control: Trapping, Biocontrol, Host Management and Other Alternative Strategies. In Biology of Ticks, 2nd ed.; Sonenshine, D.E., Roe, R.M., Eds.; Oxford University Press: New York, NY, USA, 2014; Volume 2. [Google Scholar]
- Estrada-Peña, A.; Jameson, L.; Medlock, J.; Vatansever, Z.; Tishkova, F. Unraveling the ecological complexities of tick-associated Crimean-Congo hemorrhagic fever virus transmission: A gap analysis for the western Palearctic. Vector Borne Zoonotic Dis. 2012, 12, 743–752. [Google Scholar] [CrossRef]
- Jameson, L.J.; Morgan, P.J.; Medlock, J.M.; Watola, G.; Vaux, A.G.C. Importation of Hyalomma marginatum, vector of Crimean-Congo haemorrhagic fever virus, into the United Kingdom by migratory birds. Ticks Tick-Borne Dis. 2012, 3, 95–99. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; de la Fuente, J. The ecology of ticks and epidemiology of tick-borne viral diseases. Antivir. Res. 2014, 108, 104–128. [Google Scholar] [CrossRef]
- Olmeda, A.S.; González, J.; Toledo, A.; González, M.; Sánchez-Sánchez, M.; Valcárcel, F. Expansión en la distribución de las garrapatas del género Hyalomma (Koch 1844) en la Comunidad de Madrid.; XVIII Congreso Ibérico de Entomología.; Facultad de CC. Biológicas: Madrid, Spain, 2019. [Google Scholar]
- EFSA Scientific Opinion on Geographic Distribution of Tick-borne Infections and their Vectors in Europe and the other Regions of the Mediterranean Basin: Geographic distribution of ticks and tick-borne diseases. EFSA Journal 2010, 8, 1723. [CrossRef]
- Capek, M.; Literak, I.; Kocianova, E.; Sychra, O.; Najer, T.; Trnka, A.; Kverek, P. Ticks of the Hyalomma marginatum complex transported by migratory birds into Central Europe. Ticks Tick-Borne Dis. 2014, 5, 489–493. [Google Scholar] [CrossRef]
- Lindeborg, M.; Barboutis, C.; Ehrenborg, C.; Fransson, T.; Jaenson, T.G.T.; Lindgren, P.-E.; Lundkvist, Å.; Nyström, F.; Salaneck, E.; Waldenström, J.; et al. Migratory Birds, Ticks, and Crimean-Congo Hemorrhagic Fever Virus. Emerg Infect. Dis 2012, 18, 2095–2097. [Google Scholar] [CrossRef]
- Cetin, H.; Cilek, J.E.; Oz, E.; Aydin, L.; Deveci, O.; Yanikoglu, A. Acaricidal activity of Satureja thymbra L. essential oil and its major components, carvacrol and γ-terpinene against adult Hyalomma marginatum (Acari: Ixodidae). Vet. Parasitol. 2010, 170, 287–290. [Google Scholar] [CrossRef] [PubMed]



| Hyalomma lusitanicum | Hyalomma marginatum | |
|---|---|---|
| Vector demonstrated of | Coxiella burnetii (Q fever) Theileria equi Theileria annulata (Mediterranean theileriosis) | Babesia caballi (babesiosis) Coxiella burnetii Crimean-Congo Haemorrhagic Fever virus Rickettsia conorii (botonouse fever) Theileria annulata |
| Presence reported but transmission should be determined: | Anaplasma phagocytophilum Anaplasma platys Borrelia burgdorferi (Lyme disease) Crimean-Congo Haemorrhagic Fever virus Francisella tularensis (Tularemia) Rickettsia aeschlimannii Babesia caballi | Anaplasma phagocytophilum Anaplasma platys Babesia caballi Bahig virus Dhori virus Matruh virus Rickettsia aeschlimannii Theileria equi |
| Transmission ways | Transovarial Transstadial | Co-feeding Intrastadial Transovarial Transstadial Venereal |
| Characteristics | Hyalomma lusitanicum | Hyalomma marginatum |
|---|---|---|
| Behavior | Three-host tick Ditropic/telotropic * Immature ticks are endophilic/exophilic * Adults are exophilic One generation per year | Two-host tick Ditropic/monotropic Immature ticks are endophilic/exophilic Adults are endophilic/exophilic One generation per year |
| Host for immature stages | ||
| Main hosts | Wild rabbits, hares | Wild rabbits, hares, passerine birds (blackbird, thrush bird, great tit, common accentor, red tailed bird) |
| Secondary hosts | ||
| Larvae | Cattle, red deer, domestic dog, red fox, European polecat, rodents, garden dormouse, house rat, least weasel, red partridge and passerine birds | Rodents, hedgehog, passerine (larks and corvids) and galliform birds. |
| Nymph | Red deer, domestic dog, red fox, hares, house rat, least weasel, hedgehogs, bustard, red partridge, passerine bird | |
| Host for adults | ||
| Main hosts | Red deer, cattle, camel, cattle, fallow deer, roe deer, sheep, wild and domestic goat, horse, domestic pig, wild boar | Camels, cattle, deer, goats, horses, sheep, wild boar |
| Secondary hosts | Leporids, wild rabbit, eagle owl, fox, hedgehog, mongoose, dog, human, bustard, ostrich, nocturnal birds of prey, gallifom and passerine birds | Donkey, Spur-thighed tortoise, weasel, fox, birds, human |
| Feeding sites | Immature specimens and adults on whole body More evident in areas with little hair: In small mammals on ears, belly or around eyes. In ungulates on hind quarters, inner side of the thigh, anus, udders * Congregations are very rare* | Immature specimens on birds and small-medium-sized mammals: head (mainly in and around the ears), face, neck and around the eyes Adults on ungulates only on hind quarters (mainly udders, scrotum, inguinal area and perineum). Typically appears in congregations |
| Life Cycle Phases | H. Lusitanicum | H. marginatum |
|---|---|---|
| Pre-oviposition | 8–47 | 3–28 |
| Oviposition | 15–26 | 8–24 |
| Hatching | 22–40 | 20–40 |
| Oviposition + hatching | 34 | |
| Larvae cuticle hardening ** | 8 * | 5 |
| Larvae feeding | 4–8 | 26 |
| Molt to nymph | 12–16 | 15–30 |
| Nymph feeding | 5–13 | |
| Larvae feeding+ molt to nymph + nymph feeding | 12–26 | |
| Nymph cuticle hardening ** | 8 | |
| Molt to adult | 11–22 | 15–30 |
| Resting period of immatures ** | 21 * | 21 |
| Adult cuticle hardening ** | 30 | 8 |
| Adult feeding | 5–30 | 7–30 |
| Whole cycle | 101–196 | 90–167 |
| Eggs laid per engorged female | 629–14,519 | 2662–15,500 |
| Hyalomma lusitanicum | Hyalomma marginatum |
|---|---|
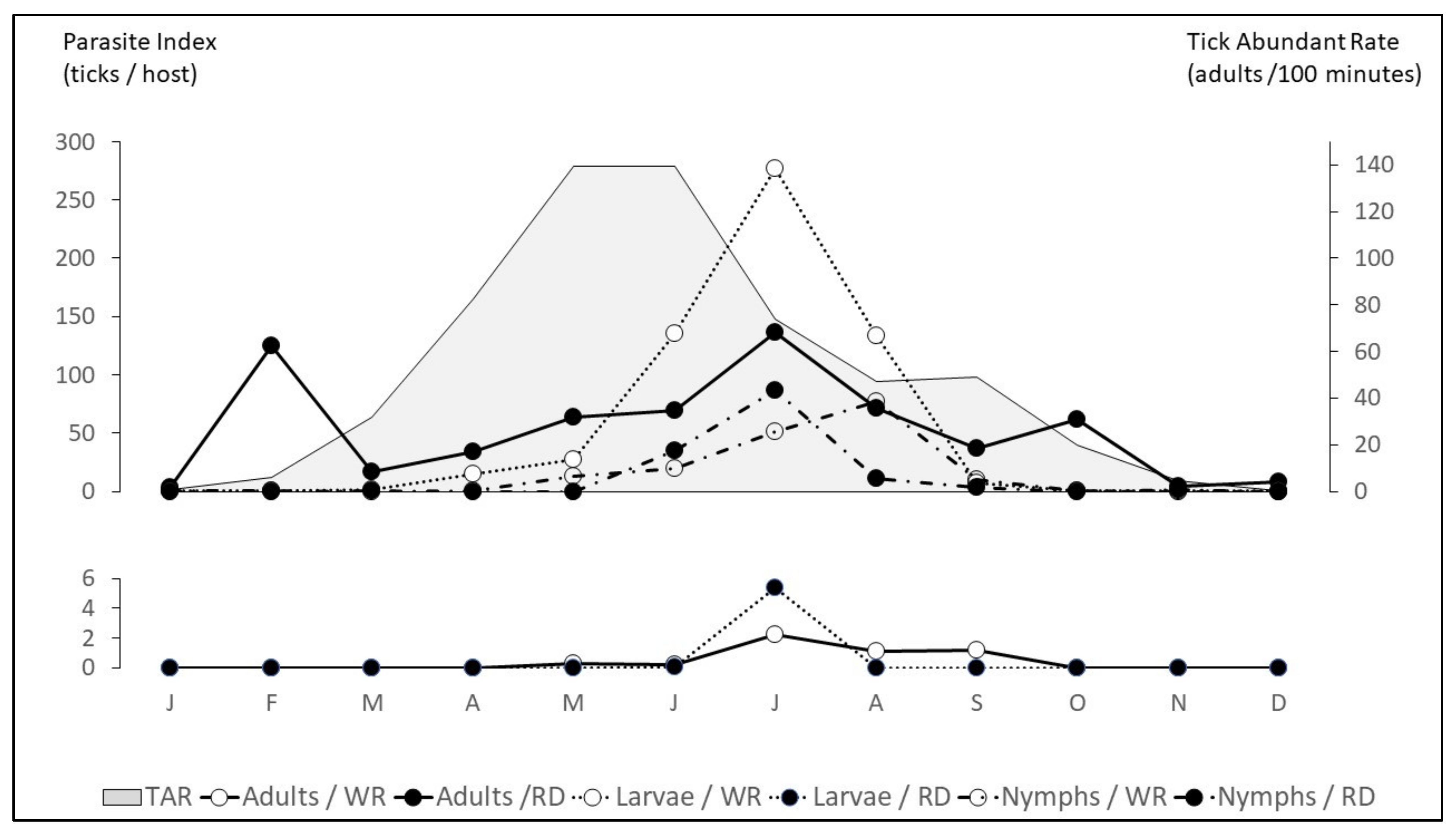
| Adult Host Seeking * | Complete annual pattern is unknown |
| Start to quest in March Main peak in May–June Later decline progressively Small increase in September–October | In Africa, peak of questing adults in April–May |
| Host infestation: | |
| Larvae: April-September (peak in May–June) Nymphs: May-November (peak in June–July) Adults: April to October (peaks in May–June and September–October) | Larvae-nymphs: May-June to September–October Adults: March to November (peak from April to June) |
| Parameter | Hyalomma lusitanicum | Hyalomma marginatum |
|---|---|---|
| Overwintering | unfed adults hidden in the field males on host engorged females hidden in the field | unfed adults hidden in the field engorged nymphs (high mortality) |
| Climate | Mediterranean steppe meso-Mediterranean | Mediterranean climate of North Africa and southern Europe Steppe, savannah and scrubland hill and valley biotypes |
| Preferences | partly sunny days dry soil no wind days tall grass or shrubbery | populations are regulated by rainfall and evapo-transpiration in summer (in Mediterranean basin) no strong wind |
| Best range of temperature | larvae/nymphs unknown adults: 20–35 °C | populations are regulated by the minimum temperatures in late autumn (in Eastern Europe and the Caucasus) larvae 14‒16 °C engorged nymphs tolerate 7–42 °C adults 22‒27 °C |
| Best range of Relative Humidity | adults 10–60% | engorged nymphs tolerate 0–100% adults 75‒100% although they may survive in low to moderate levels of humidity and long dry season during the summer months (but no survival in desert conditions) |
| Factor | Hyalomma lusitanicum | Hyalomma marginatum |
|---|---|---|
| Habitat | Mediterranean forests Woodlands Scrublands | Arid open habitats, marsh, steppe, savannah and scrubland hill and valley biotypes They are absent from contemporary and former European deciduous and mixed forest biotypes |
| Geographical distribution | Europe (France, Italy, Portugal and Spain, including Canary Islands) North Africa (Algeria and Morocco) Occasionally in the United Kingdom | North Africa and Asia (Algeria, Armenia, Azerbaijan, Egypt, Ethiopia, Georgia, Iran, Iraq, Israel, Morocco, Sudan, Syria, Tunisia and Turkey) Europe (Albania, Bosnia and Herzegovina, Bulgaria, Croatia, Cyprus, France, Greece, Italy, Kosovo, the former Yugoslav Republic of Macedonia, Moldova, Montenegro, Portugal, Romania, Russia, Serbia, Spain and Ukraine) Occasionally in Germany, Hungary, Finland, Netherlands and the United Kingdom |
| Risk factors of spreading | Human and animal movement Reforestation of livestock farms | Human and animal movement Migratory birds (short distances) Degradation of agricultural lands especially after cattle pasture |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valcárcel, F.; González, J.; González, M.G.; Sánchez, M.; Tercero, J.M.; Elhachimi, L.; Carbonell, J.D.; Olmeda, A.S. Comparative Ecology of Hyalomma lusitanicum and Hyalomma marginatum Koch, 1844 (Acarina: Ixodidae). Insects 2020, 11, 303. https://doi.org/10.3390/insects11050303
Valcárcel F, González J, González MG, Sánchez M, Tercero JM, Elhachimi L, Carbonell JD, Olmeda AS. Comparative Ecology of Hyalomma lusitanicum and Hyalomma marginatum Koch, 1844 (Acarina: Ixodidae). Insects. 2020; 11(5):303. https://doi.org/10.3390/insects11050303
Chicago/Turabian StyleValcárcel, Félix, Julia González, Marta G. González, María Sánchez, José María Tercero, Latifa Elhachimi, Juan D. Carbonell, and A. Sonia Olmeda. 2020. "Comparative Ecology of Hyalomma lusitanicum and Hyalomma marginatum Koch, 1844 (Acarina: Ixodidae)" Insects 11, no. 5: 303. https://doi.org/10.3390/insects11050303
APA StyleValcárcel, F., González, J., González, M. G., Sánchez, M., Tercero, J. M., Elhachimi, L., Carbonell, J. D., & Olmeda, A. S. (2020). Comparative Ecology of Hyalomma lusitanicum and Hyalomma marginatum Koch, 1844 (Acarina: Ixodidae). Insects, 11(5), 303. https://doi.org/10.3390/insects11050303