Life History Evaluation of Ooencyrtus lucidus, a Newly Described Egg Parasitoid of Bagrada hilaris
Abstract
1. Introduction
2. Materials and Methods
2.1. The Host, Bagrada hilaris
2.2. The Parasitoid, Ooencyrtus lucidus
2.3. Experimental Procedure
2.4. Statistical Analyses
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Howard, C.W. The Entomological section: The bagrada bug (Bagrada hilaris). Transvaal Agric. J. 1906, 5, 168–176. [Google Scholar]
- Husain, M.A. Annual report of the entomologist to government, Punjab, Lyallpur, for the year ending 30th June 1924. Rept. Dept. Agric. Punjab 1923–24. 1925, 1, 55–90. [Google Scholar]
- Reed, D.A.; Palumbo, J.C.; Perring, T.M.; May, C. Bagrada hilaris (Hemiptera: Pentatomidae), an invasive stink bug attacking cole crops in the southwestern United States. J. Integr. Pest Manag. 2013, 4, C1–C7. [Google Scholar] [CrossRef]
- Palumbo, J.C.; Perring, T.M.; Millar, J.G.; Reed, D.A. Biology, ecology, and management of an invasive stink bug, Bagrada hilaris, in North America. Annu. Rev. Entomol. 2016, 61, 453–473. [Google Scholar] [CrossRef]
- Palumbo, J.C.; Natwick, E.T. The bagrada bug (Hemiptera: Pentatomidae): A new invasive pest of cole crops in Arizona and California. Plant Health Prog. 2010, 11, 50. [Google Scholar] [CrossRef]
- Huang, T.I.; Reed, D.A.; Perring, T.M.; Palumbo, J.C. Feeding damage by Bagrada hilaris (Hemiptera: Pentatomidae) and impact on growth and chlorophyll content of Brassicaceous plant species. Arthropod-Plant Inte. 2014, 8, 89–100. [Google Scholar] [CrossRef]
- Arakelian, G. Bagrada Bug (Bagrada hilaris); Los Angeles County Agricultural Commissioner/Weights and Measures Department: Arcadia, CA, USA, 2008.
- Bundy, C.S.; Grasswitz, T.R.; Sutherland, C. First report of the invasive stink bug Bagrada hilaris (Burmeister) (Heteroptera: Pentatomidae) from New Mexico, with notes on its biology. Southwest Entomol. 2012, 37, 411–414. [Google Scholar] [CrossRef]
- Vitanza, S. Issues in agriculture. Texas A&M AgriLife Ext. Newsl. 2012, 38, 1–8. [Google Scholar]
- Perring, T.M.; Reed, D.A.; Palumbo, J.C.; Grasswitz, T.; Bundy, C.S.; Jones, W.; Royer, T. National Pest Alert: Bagrada Bug Bagrada hilaris (Burmeister) Family Pentatomidae; USDA-NIFA Regional IPM Centers: East Lansing, MI, USA, 2013.
- Joseph, S.V.; Grettenberger, I.; Godfrey, L. Insecticides applied to soil of transplant plugs for Bagrada hilaris (Burmeister) (Hemiptera: Pentatomidae) management in broccoli. Crop Protect. 2016, 87, 68–77. [Google Scholar] [CrossRef]
- Sánchez-Peña, S.R. First record in Mexico of the invasive stink bug Bagrada hilaris, on cultivated crucifers in Saltillo. Southwest Entomol. 2014, 39, 375–377. [Google Scholar] [CrossRef]
- Hernández-Chávez, L.; Salas-Araiza, M.D.; Martínez-Jaime, O.A.; Flores-Mejía, S. First report of Bagrada hilaris Burmeister, 1835 (Hemiptera: Pentatomidae) in the state of Guanajuato, Mexico. Entomol. News 2018, 128, 72–74. [Google Scholar] [CrossRef]
- Faúndez, E.I.; Lüer, A.; Cuevas, Á.G.; Rider, D.A.; Valdebenito, P. First record of the painted bug Bagrada hilaris (Burmeister, 1835) (Heteroptera: Pentatomidae) in South America. Arquivos Entomol. 2016, 16, 175–179. [Google Scholar]
- Faúndez, E.I.; Lüer, A.; Cuevas, Á.G. The establishment of Bagrada hilaris (Burmeister, 1835) (Heteroptera: Pentatomidae) in Chile, an avoidable situation? Arquivos Entomol. 2017, 17, 239–241. [Google Scholar]
- Carvajal, M.A.; Alaniz, A.J.; Núñez-Hidalgo, I.; González-Césped, C. Spatial global assessment of the pest Bagrada hilaris (Burmeister) (Heteroptera: Pentatomidae): Current and future scenarios. Pest Manag. Sci. 2019, 75, 809–820. [Google Scholar] [CrossRef] [PubMed]
- Bundy, C.S.; Perring, T.M.; Reed, D.A.; Palumbo, J.C.; Grasswitz, T.R.; Jones, W.A. Bagrada hilaris (Burmeister). In Invasive Stink Bugs and Related Species (Pentatomoidea): Biology, Higher Systematics, Semiochemistry, and Management; McPherson, J.E., Ed.; CRC Press: Cleveland, OH, USA, 2018. [Google Scholar]
- Martel, G.; Auge, M.; Talamas, E.; Roche, M.; Smith, L.; Sforza, R.F.H. First laboratory evaluation of Gryon gonikopalense (Hymenoptera: Scelionidae), as potential biological control agent of Bagrada hilaris (Hemiptera: Pentatomidae). Biol. Control 2019, 135, 48–56. [Google Scholar] [CrossRef]
- Power, N.; Ganjisaffar, F.; Perring, T.M. Effect of temperature on the survival and developmental rate of immature Ooencyrtus mirus (Hymenoptera: Encyrtidae). J. Econ. Entomol. 2020, in press. [Google Scholar]
- Ganjisaffar, F.; Talamas, E.J.; Bon, M.C.; Gonzalez, L.; Brown, B.V.; Perring, T.M. Trissolcus hyalinipennis Rajmohana & Narendran (Hymenoptera, Scelionidae), a parasitoid of Bagrada hilaris (Burmeister) (Hemiptera, Pentatomidae), emerges in North America. J. Hymenopt. Res. 2018, 65, 111–130. [Google Scholar]
- Felipe-Victoriano, M.; Talamas, E.; Sánchez-Peña, S. Scelionidae (Hymenoptera) parasitizing eggs of Bagrada hilaris (Hemiptera, Pentatomidae) in Mexico. J. Hymenopt. Res. 2019, 73, 143–152. [Google Scholar] [CrossRef]
- Lomeli-Flores, J.R.; Rodríguez-Rodríguez, S.E.; Rodríguez-Levya, E.; González-Hernández, H.; Gariepy, T.D.; Talamas, E.J. Field studies and molecular forensics identify a new association: Idris elba Talamas, sp. nov. parasitizes the eggs of Bagrada hilaris (Burmeister). J. Hymenopt. Res. 2019, 73, 125–141. [Google Scholar] [CrossRef]
- Triapitsyn, S.; Andreason, S.; Power, N.; Ganjisaffar, F.; Fusu, L.; Dominguez, C.; Perring, T.M. Two new species of Ooencyrtus (Hymenoptera, Encyrtidae), egg parasitoids of the bagrada bug Bagrada hilaris (Hemiptera, Pentatomidae), with taxonomic notes on Ooencyrtus telenomicida. J. Hymenopt. Res. 2020, 76, 57–98. [Google Scholar] [CrossRef]
- Jones, T.S.; Bilton, A.R.; Mak, L.; Sait, S.M. Host switching in a generalist parasitoid: Contrasting transient and transgenerational costs associated with novel and original host species. Ecol. Evol. 2015, 5, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Matošević, D.; Melika, G. Recruitment of native parasitoids to a new invasive host: First results of Dryocosmus kuriphilus parasitoid assemblage in Croatia. Bull. Insectol. 2013, 66, 231–238. [Google Scholar]
- Maple, J.D. The Eggs and First Instar Larvae of Encyrtidae and Their Morphological Adaptations for Respiration; University of California Press: Berkeley, CA, USA, 1947. [Google Scholar]
- Reed, D.A.; Ganjisaffar, F.; Palumbo, J.C.; Perring, T.M. Effects of temperatures on immature development and survival of the invasive stink bug Bagrada hilaris (Hemiptera: Pentatomidae). J. Econ. Entomol. 2017, 110, 2497–2503. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Carey, J.R. Applied Demography for Biologists: With Special Emphasis on Insects; Oxford University Press: Oxford, UK, 1993. [Google Scholar]
- Meyer, J.S.; Ingersoll, C.G.; McDonald, L.L.; Boyce, M.S. Estimating uncertainty in population growth rates: Jackknife vs. bootstrap techniques. Ecology 1986, 67, 1156–1166. [Google Scholar] [CrossRef]
- SAS Institute. SAS 9.4 SQL Procedure User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2016. [Google Scholar]
- Bin, F. Biological Control with Egg Parasitoids other than Trichogramma. In Biological Control with Egg Parasitoids; Wajnberg, E., Hassan, S.A., Eds.; CAB International: Wallingford, UK, 1994. [Google Scholar]
- Conti, E.; Colazza, S. Chemical ecology of egg parasitoids associated with true bugs. Psyche 2012, 2012, 11. [Google Scholar] [CrossRef]
- Noyes, J.S. Universal Chalcidoidea database world wide web electronic publication. 2012. Acesso em 2015, 1. Available online: https://www.nhm.ac.uk/our-science/data/chalcidoids/ (accessed on 2 May 2020).
- Huang, D.W.; Noyes, J.S. A revision of the Indo-Pacific species of Ooencyrtus (Hymenoptera: Encyrtidae), parasitoids of the immature stages of economically important insect species (mainly Hemiptera and Lepidoptera). Bull. Br Mus (Nat Hist) Entomol. Ser. 1994, 63, 1–136. [Google Scholar]
- Cornelius, M.L.; Vinyard, B.T.; Gates, M.W. Use of flowering plants to enhance parasitism and predation rates on two squash bug species Anasa tristis and Anasa armigera (Hemiptera: Coreidae). Insects 2019, 10, 318. [Google Scholar] [CrossRef] [PubMed]
- Sousa, K.K.A.; Silva, N.N.P.; Querino, R.B.; Silva, P.H.S.; Grazia, J. Diversity, seasonality, and egg parasitism of Hemipteran (Coreidae and Pentatomidae) from a cowpea crop in northeastern Brazil. Fla Entomol. 2019, 102, 29–35. [Google Scholar]
- Parker, B.L.; Amir-Maafi, M.; Skinner, M.; Kim, J.S.; El Bouhssini, M. Distribution of sunn pest, Eurygaster integriceps Puton (Hemiptera: Scutelleridae), in overwintering sites. J. Asia-Pac. Entomol. 2011, 14, 83–88. [Google Scholar] [CrossRef]
- Ahmadpour, S.; Iranipour, S.; Asgari, S. Effects of superparasitism on reproductive fitness of Ooencyrtus fecundus Ferriere & Foegele (Hym. Encyrtidae), egg parasitoid of sunn pest, Eurygaster integriceps Puton (Hem. Scutelleridae). Biol. Control Pests Plant Dis. 2014, 2, 97–105. [Google Scholar]
- Brown, M.W. Literature review of Ooencyrtus kuvanae [Hym.: Encyrtidae], an egg parasite of Lymantria dispar [Lep: Lymantriidae]. Entomophaga 1984, 29, 249–265. [Google Scholar] [CrossRef]
- Liu, H.; Mottern, J. An old remedy for a new problem? identification of Ooencyrtus kuvanae (Hymenoptera: Encyrtidae), an egg parasitoid of Lycorma delicatula (Hemiptera: Fulgoridae) in North America. J. Insect Sci. 2017, 17, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Takasu, K.; Hirose, Y. Host acceptance behavior by the host-feeding egg parasitoid, Ooencyrtus nezarae (Hymenoptera: Encyrtidae): Host age effects. Ann. Entomol. Soc. Am. 1993, 86, 117–121. [Google Scholar] [CrossRef]
- Ademokoya, B.; Balusu, R.; Ray, C.; Mottern, J.; Fadamiro, H. The first record of Ooencyrtus nezarae (Hymenoptera: Encyrtidae) on kudzu bug (Hemiptera: Plataspidae) in North America. J. Insect Sci. 2018, 18, 1–7. [Google Scholar] [CrossRef]
- Hayat, M.; Mehrnejad, M. Ooencyrtus (Hymenoptera: Encyrtidae), egg parasitoids of the pistachio green stink bugs (Hemiptera: Pentatomidae) in Iran. Zootaxa 2016, 4117, 198–210. [Google Scholar] [CrossRef]
- Samra, S.; Cascone, P.; Noyes, J.; Ghanim, M.; Protasov, A.; Guerrieri, E.; Mendel, Z. Diversity of Ooencyrtus spp. (Hymenoptera: Encyrtidae) parasitizing the eggs of Stenozygum coloratum (Klug) (Hemiptera: Pentatomidae) with description of two new species. PLoS ONE 2018, 13, e0205245. [Google Scholar] [CrossRef]
- Mohammadpour, M.; Jalali, M.A.; Ziaaddini, M.; Hashemirad, H. Some biological characteristics of Ooencyrtus pityocampae Mercet parasitoid of Brachynema signatum Jakovlev under laboratory conditions. J. Pistachio Health 2018, 1, 20–25. [Google Scholar]
- Tabone, E.; Tunca, H.; Colombel, E.A.; Venard, M.; Defferier, T.; Martin, J.C. Performance of egg parasitoid Ooencyrtus pityocampae (Mercet) (Hymenoptera: Encyrtidae) on three substitute hosts in laboratory. In Proceedings of the II International Symposium on Innovative Plant Protection in Horticulture, Istanbul, Turkey, 12–16 August 2018; pp. 135–140. [Google Scholar]
- Tunca, H.; Venard, M.; Colombel, E.A.; Capelli, M.; Tabone, E. Life history traits of Ooencyrtus pityocampae (Hymenoptera: Encyrtidae) reared on Halyomorpha halys eggs (Hemiptera: Pentatomidae). Entomol. Gen. 2019, 39, 93–101. [Google Scholar] [CrossRef]
- Foti, M.C.; Peri, E.; Wajnberg, E.; Colazza, S.; Rostás, M. Contrasting olfactory responses of two egg parasitoids to buckwheat floral scent are reflected in field parasitism rates. J. Pest Sci. 2019, 92, 747–756. [Google Scholar] [CrossRef]
- Rondoni, G.; Bertoldi, V.; Malek, R.; Foti, M.C.; Peri, E.; Maistrello, L.; Haye, T.; Conti, E. Native egg parasitoids recorded from the invasive Halyomorpha halys successfully exploit volatiles emitted by the plant—Herbivore complex. J. Pest Sci. 2017, 90, 1087–1095. [Google Scholar] [CrossRef]
- Roversi, P.F.; Maltese, M.; Simoni, S.; Cascone, P.; Binazzi, F.; Strangi, A.; Sabbatini Peverieri, G.; Guerrieri, E. Graphosoma lineatum (Hemiptera: Pentatomidae): A suitable host for mass rearing Ooencyrtus telenomicida (Hymenoptera: Encyrtidae). Int. J. Pest Manag. 2018, 64, 294–302. [Google Scholar] [CrossRef]
- Doyon, J.; Boivin, G. The effect of development time on the fitness of female Trichogramma evanescens. J. Insect Sci. 2005, 5, 4. [Google Scholar] [CrossRef]
- Pompanon, F.; Fouillet, P.; Bouletréau, M. Emergence rhythms and protandry in relation to daily patterns of locomotor activity in Trichogramma species. Evol. Ecol. 1995, 9, 467–477. [Google Scholar] [CrossRef]
- Morbey, Y.E.; Ydenberg, R.C. Protandrous arrival timing to breeding areas: A review. Ecol. Lett. 2001, 4, 663–673. [Google Scholar] [CrossRef]
- Guerrieri, E.; Gitau, C.W.; Fletcher, M.J.; Noyes, J.S.; Dewhurst, C.F.; Gurr, G.M. Description and biological parameters of Ooencyrtus isabellae Guerrieri and Noyes sp. nov. (Hymenoptera: Chalcidoidea: Encyrtidae), a potential biocontrol agent of Zophiuma butawengi (Heller) (Hemiptera: Fulgoromorpha: Lophopidae) in Papua New Guinea. J. Nat. Hist. 2011, 45, 2747–2755. [Google Scholar] [CrossRef]
- Heimpel, G.E.; Jervis, M.A. Does floral nectar improve biological control by parasitoids? In Plant-Provided Food for Carnivorous Insects: A Protective Mutualism and Its Applications; Cambridge University Press: Cambridge, UK, 2005; pp. 267–304. [Google Scholar]
- Aung, K.S.D.; Takasu, K.; Ueno, T.; Takagi, M. Effect of temperature on egg maturation and longevity of the egg parasitoids Ooencyrtus nezarae (Ishii) (Hymenoptera: Encyrtidae). J. Fac. Agric. Kyushu U. 2010, 55, 87–89. [Google Scholar]
- Baggen, L.R.; Gurr, G.M.; Meats, A. Flowers in tri-trophic systems: Mechanisms allowing selective exploitation by insect natural enemies for conservation biological control. In Proceedings of the 10th International Symposium on Insect-Plant Relationships; Springer: Berlin, Germany, 1999; pp. 155–161. [Google Scholar]
- Takasu, K.; Hirose, Y. Host searching behavior in the parasitoid Ooencyrtus nezarae Ishii (Hymenoptera: Encyrtidae) as influenced by non-host food deprivation. Appl. Entomol. Zool. 1991, 26, 415–417. [Google Scholar] [CrossRef][Green Version]
- Teraoka, T.; Numata, H. Effects of feeding on reproduction and overwintering in female adults of Ooencyrtus nezarae Ishii (Hymenoptera: Encyrtidae). Appl. Entomol. Zool. 2000, 35, 361–367. [Google Scholar] [CrossRef][Green Version]
- Tunca, H.; Colombel, E.A.; Ben Soussan, T.; Buradino, M.; Galio, F.; Tabone, E. Optimal biological parameters for rearing Ooencyrtus pityocampae on the new laboratory host Philosamia ricini. J. Appl. Entomol. 2016, 140, 527–535. [Google Scholar] [CrossRef]
- Ball, G. The cost of reproduction. Oxf. Surv. Evolut. Biol. 1986, 3, 83–131. [Google Scholar]
- Weseloh, R.M. Host and microhabitat preferences of forest parasitic Hymenoptera: Inferences from captures on colored sticky panels. Environ. Entomol. 1986, 15, 64–70. [Google Scholar] [CrossRef]
- Maple, J.D. The biology of Ooencyrtus johnsoni (Howard), and the role of the egg shell in the respiration of certain encyrtid larvae (Hymenoptera). Ann. Entomol. Soc. Am. 1937, 30, 123–154. [Google Scholar] [CrossRef]
- Crossman, S.S. Two imported egg parasites of the gipsy moth, Anastatus bifasciatus Fonsc and Schedius kuvanae Howard. J. Agric. Res. 1925, 30, 643–675. [Google Scholar]
- Aung, K.S.D.; Takagi, M.; Myint, Y.Y.; Yun, K.M.; Ueno, T. Effect of host density on the progeny production of the egg parasitoids Ooencyrtus nezarae (Ishii) (Hymenoptera: Encyrtidae). J. Fac. Agric. Kyushu U 2011, 56, 71–74. [Google Scholar]
- Uçkan, F.; Gülel, A. Age-related fecundity and sex ratio variation in Apanteles galleriae (Hym., Braconidae) and host effect on fecundity and sex ratio of its hyperparasitoid Dibrachys boarmiae (Hym., Pteromalidae). J. Appl. Entomol. 2002, 126, 534–537. [Google Scholar] [CrossRef]
- Gündüz, E.A.; Gülel, A. Investigation of fecundity and sex ratio in the parasitoid Bracon hebetor Say (Hymenoptera: Braconidae) in relation to parasitoid age. Turkish J. Zool. 2005, 29, 291–294. [Google Scholar]
- Liu, P.C.; Wei, H.X.; Cao, D.D.; Wei, J.R. Relationships amongst sex ratio of progeny in Anastatus disparis (Hymenoptera: Eupelmidae), sperm depletion and decreased fecundity. Appl. Entomol. Zool. 2020, 55, 25–30. [Google Scholar] [CrossRef]
- King, B.H. Offspring sex ratios in parasitoid wasps. Q. Rev. Biol. 1987, 62, 367–396. [Google Scholar] [CrossRef]
- Ueno, T. Age-dependent constraints of sex allocation in a parasitoid wasp. Psyche 2014, 2014, 363174. [Google Scholar] [CrossRef]
- Pandey, A.K.; Tripathi, S.; Tripathi, C. Effects of parental age at mating on the fecundity and progeny sex ratio of Campoletis chlorideae, an endolarval parasitoid of the pod borer, Helicoverpa armigera. BioControl 2009, 54, 47–53. [Google Scholar] [CrossRef]
- Santolamazza-Carbone, S.; Nieto, M.P.; Rivera, A.C. Maternal size and age affect offspring sex ratio in the solitary egg parasitoid Anaphes nitens. Entomol. Exp. Appl. 2007, 125, 23–32. [Google Scholar] [CrossRef]
- Hardy, I.C.; Dijkstra, L.J.; Gillis, J.E.; Luft, P.A. Patterns of sex ratio, virginity and developmental mortality in gregarious parasitoids. Biol. J. Linn. Soc. 1998, 64, 239–270. [Google Scholar] [CrossRef]
- Kapranas, A.; Wajnberg, E.; Luck, R.F. Sequences of sex allocation and mortality in clutches of Metaphycus parasitoids of soft scale insects and the prevalence of all-female broods. Ecol. Entomol. 2009, 34, 652–662. [Google Scholar] [CrossRef]
- Santolamazza-Carbone, S.; Rivera, A.C. Superparasitism and sex ratio adjustment in a wasp parasitoid: Results at variance with local mate competition? Oecologia 2003, 136, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Van Alphen, J.J.; Visser, M.E. Superparasitism as an adaptive strategy for insect parasitoids. Annu. Rev. Entomol. 1990, 35, 59–79. [Google Scholar] [CrossRef]
- Stouthamer, R.; Hurst, G.; Breeuwer, J. Sex Ratios: Concepts and Research Methods; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Lewis, S.M. Multiple mating and repeated copulations: Effects on male reproductive success in red flour beetles. Anim. Behav. 2004, 67, 799–804. [Google Scholar] [CrossRef]
- Takasu, K.; Hirose, Y. Host discrimination in the parasitoid Ooencyrtus nezarae: The role of the egg stalk as an external marker. Entomol. Exp. Appl. 1988, 47, 45–48. [Google Scholar] [CrossRef]
- Mohammadpour, M.; Jalali, M.A.; Michaud, J.P.; Ziaaddini, M.; Hashemirad, H. Multiparasitism of stink bug eggs: Competitive interactions between Ooencyrtus pityocampae and Trissolcus agriope. BioControl 2014, 59, 279–286. [Google Scholar] [CrossRef]
- Mackauer, M. Host discrimination and larval competition in solitary endoparasitoids. In Critical Issues in Biological Control; Mackauer, M., Ehler, L., Roland, J., Eds.; Intercept Limited: Andover, UK, 1990; pp. 41–62. [Google Scholar]
- Boivin, G. Phenotypic plasticity and fitness in egg parasitoids. Neotrop. Entomol. 2010, 39, 457–463. [Google Scholar] [CrossRef]
- Price, P.W. Reproductive strategies of parasitoids. In Evolutionary Strategies of Parasitic Insects and Mites; Springer: Berlin, Germany, 1975; pp. 87–111. [Google Scholar]
- Taylor, M.E.; Bundy, C.S.; McPherson, J.E. Unusual ovipositional behavior of the stink bug Bagrada hilaris (Hemiptera: Heteroptera: Pentatomidae). Ann. Entomol. Soc. Am. 2014, 107, 872–877. [Google Scholar] [CrossRef]
- Jervis, M.A.; Ellers, J.; Harvey, J.A. Resource acquisition, allocation, and utilization in parasitoid reproductive strategies. Annu. Rev. Entomol. 2008, 53, 361–385. [Google Scholar] [CrossRef] [PubMed]
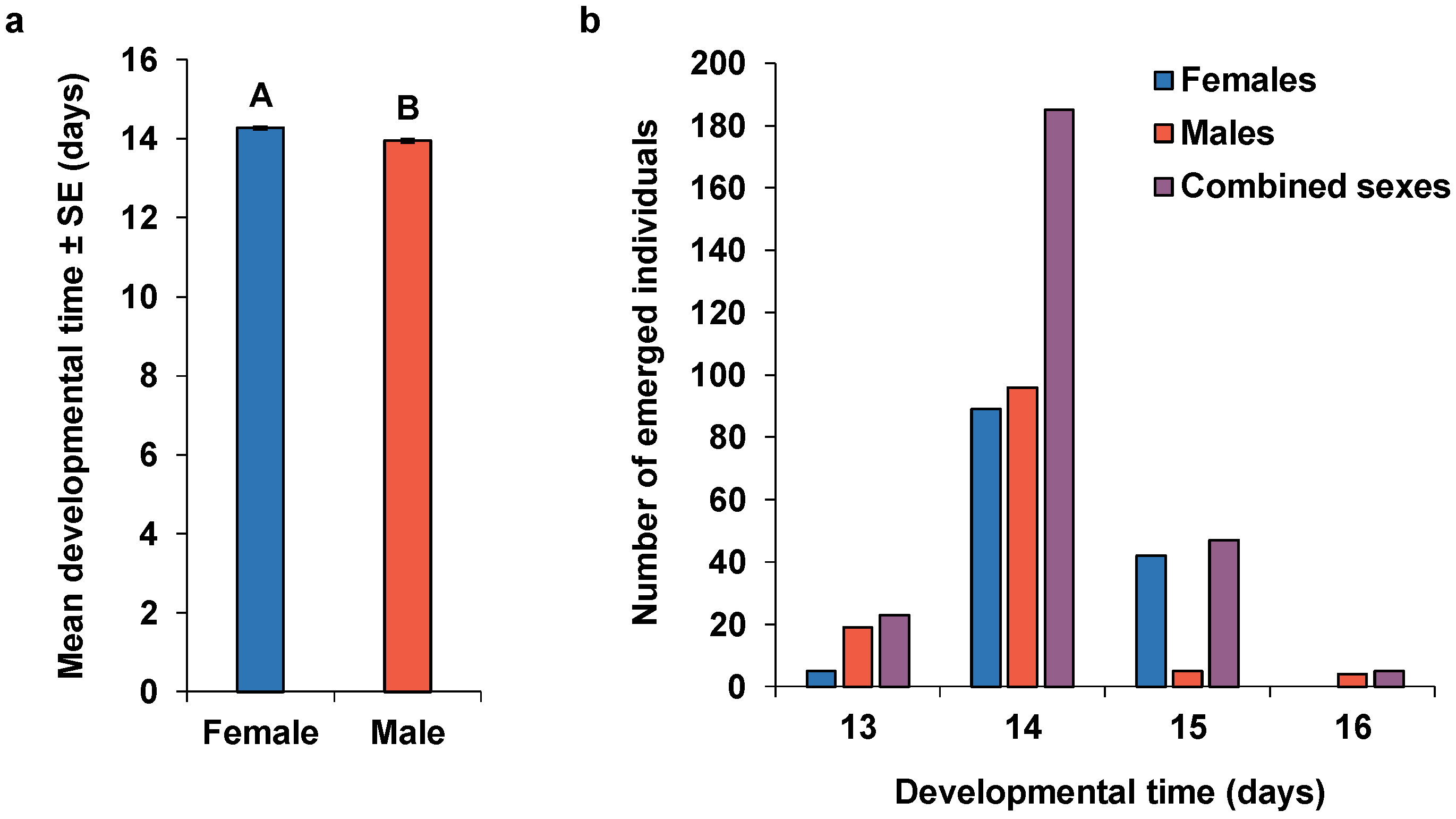
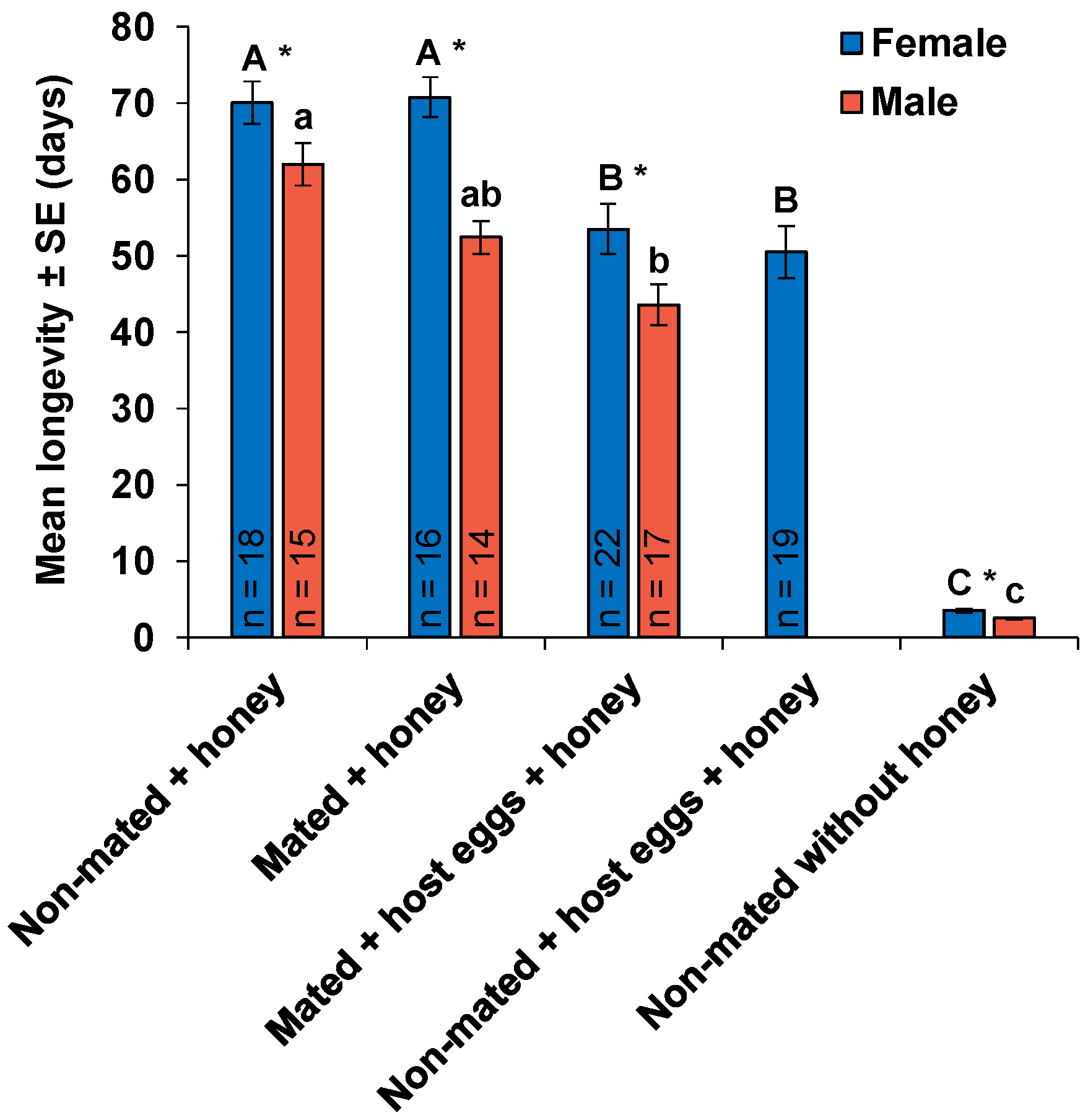
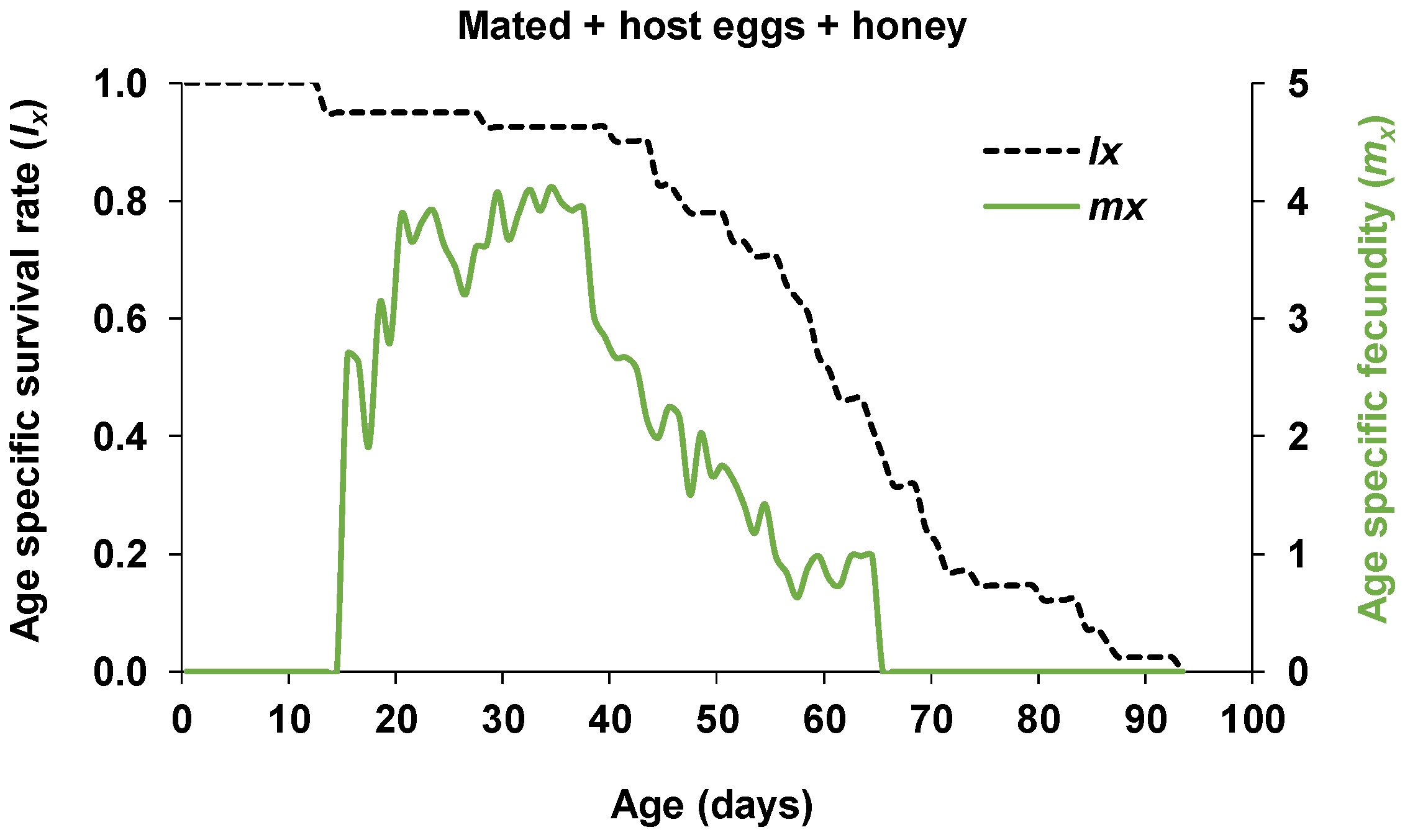
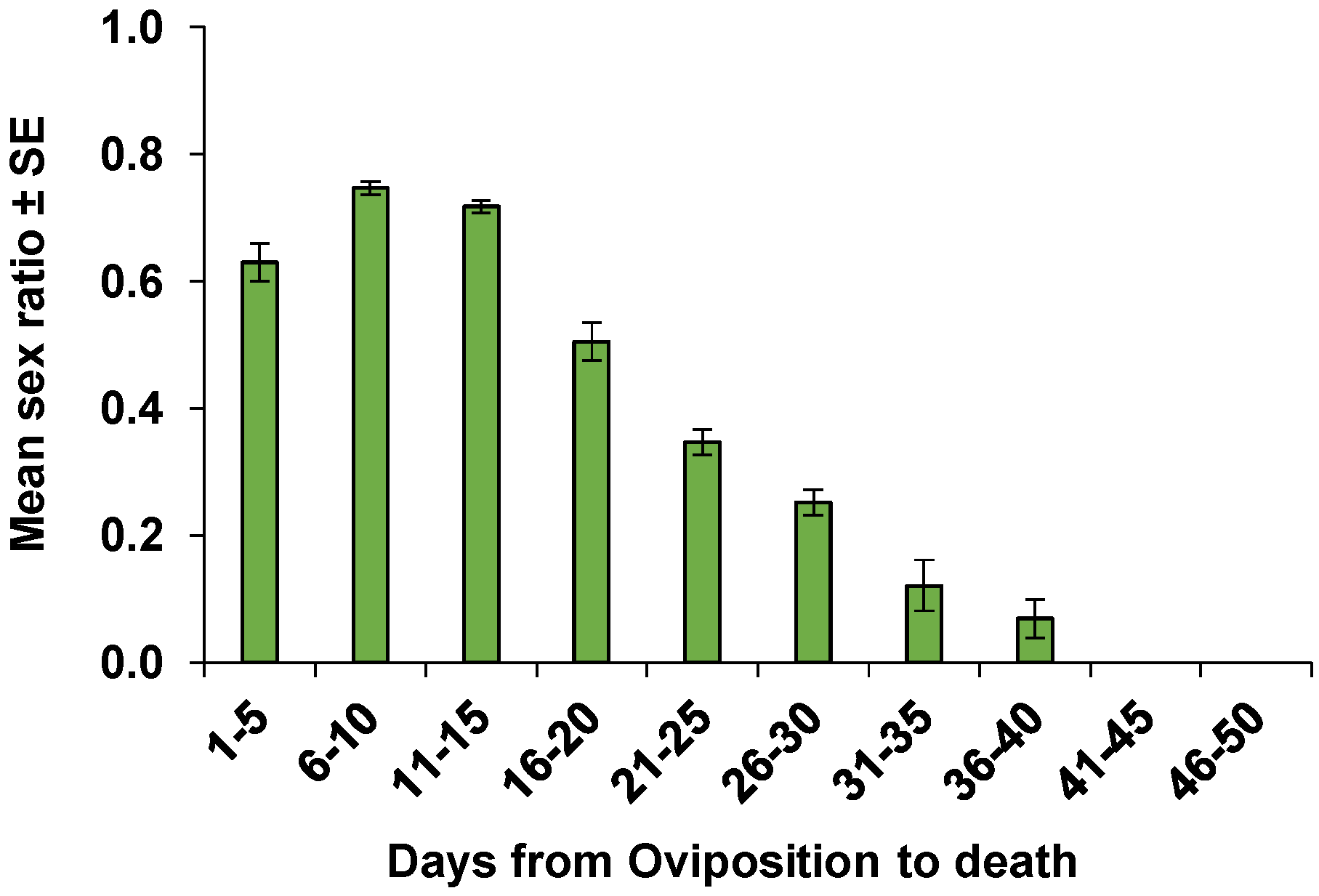
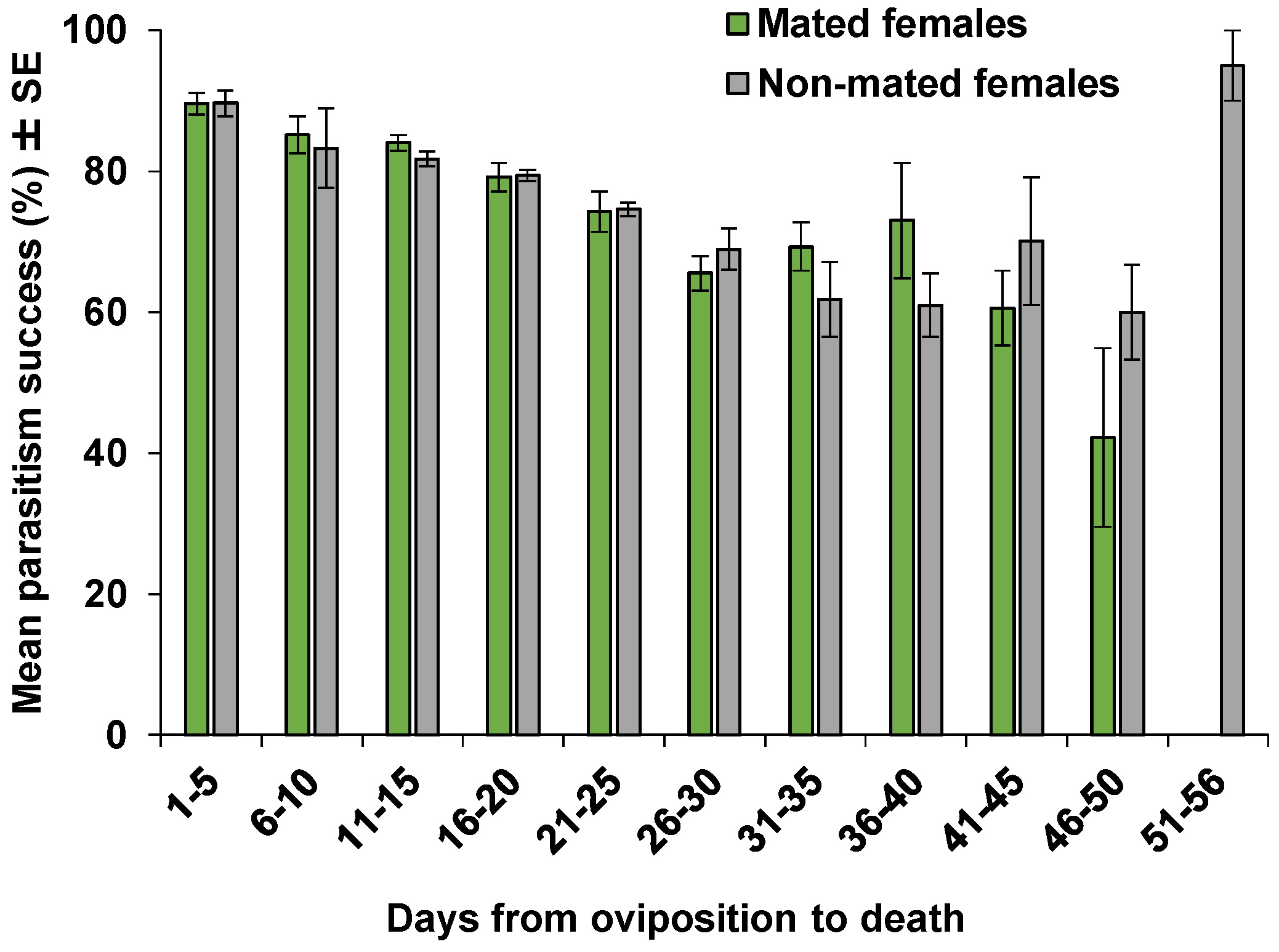
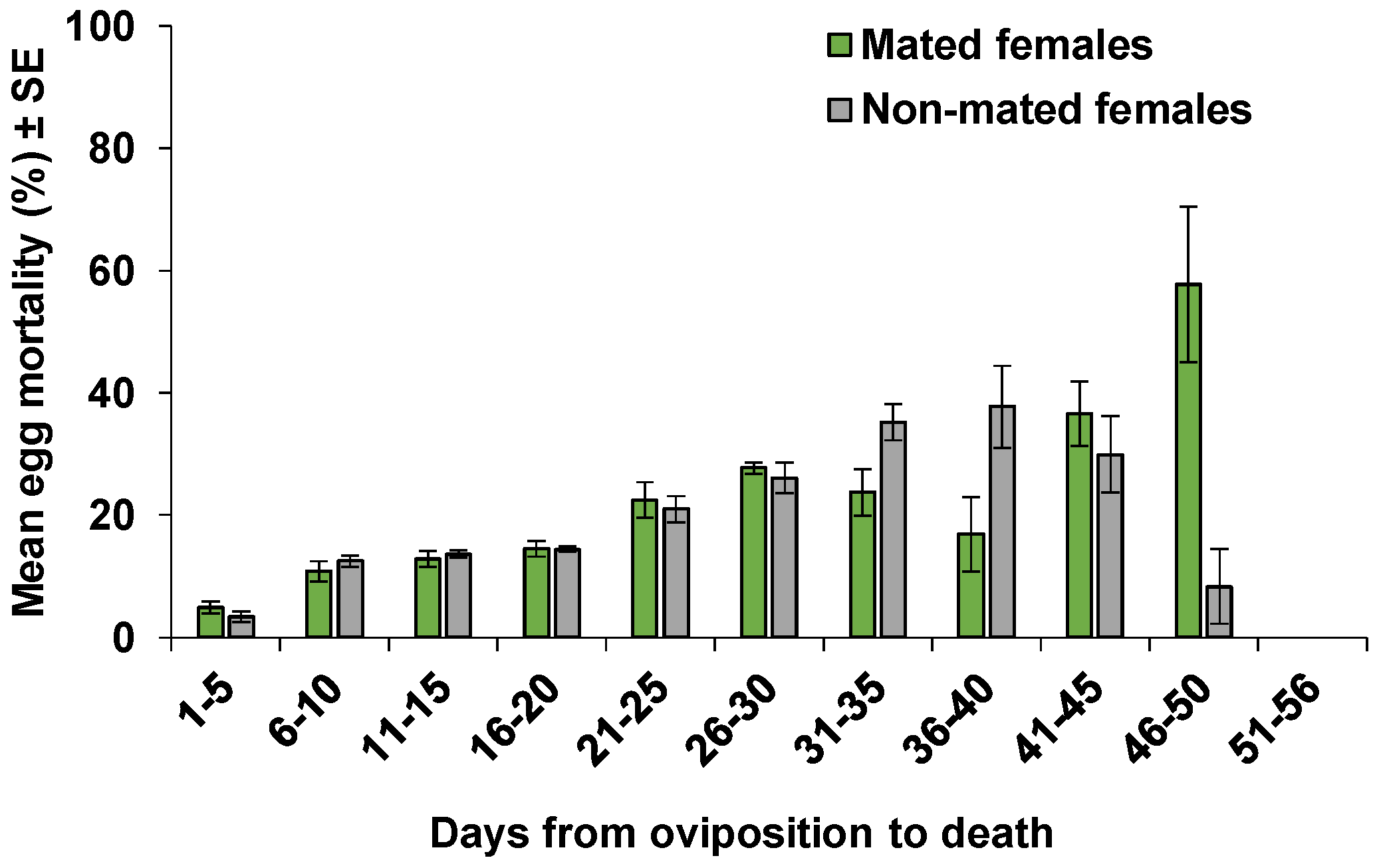
| Parameters | Mated Females | Non-Mated Females | p |
|---|---|---|---|
| Pre-oviposition period (days) | 0.0066 | ||
| Oviposition period (days) | 0.7819 * | ||
| Post-oviposition period (days) | 0.5037 | ||
| Total fecundity (eggs/female) | 0.7807 | ||
| Fecundity rate (eggs/female/day) | 0.8614 * | ||
| Parasitism success rate (progeny/eggs laid by a female/day) | 0.7120 | ||
| Sex ratio ([females/progeny]/female/day) | 0.00 (all male) |
| Parameters | Equations | Mated Females |
|---|---|---|
| Net reproductive rate () | ||
| Intrinsic rate of increase () | ||
| Finite rate of increase () | ||
| Mean generation time (T) | ||
| Doubling time (DT) |
| Life Stages | Mated Females | Non-Mated Females | X | p | ||||
|---|---|---|---|---|---|---|---|---|
| Number of Dead | Number of Dead at each Stage | Mortality (%) | Number of Dead | Number of Dead at each Stage | Mortality (%) | |||
| Eggs | 911 | 716 | 78.6 | 869 | 679 | 78.1 | 0.06 | 0.7980 |
| Larvae | 20 | 2.2 | 38 | 4.4 | 6.79 | 0.0091 * | ||
| Pupae | 50 | 5.5 | 53 | 6.1 | 0.29 | 0.5882 | ||
| Adults | 125 | 13.7 | 99 | 11.4 | 2.14 | 0.1436 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganjisaffar, F.; Perring, T.M. Life History Evaluation of Ooencyrtus lucidus, a Newly Described Egg Parasitoid of Bagrada hilaris. Insects 2020, 11, 292. https://doi.org/10.3390/insects11050292
Ganjisaffar F, Perring TM. Life History Evaluation of Ooencyrtus lucidus, a Newly Described Egg Parasitoid of Bagrada hilaris. Insects. 2020; 11(5):292. https://doi.org/10.3390/insects11050292
Chicago/Turabian StyleGanjisaffar, Fatemeh, and Thomas M. Perring. 2020. "Life History Evaluation of Ooencyrtus lucidus, a Newly Described Egg Parasitoid of Bagrada hilaris" Insects 11, no. 5: 292. https://doi.org/10.3390/insects11050292
APA StyleGanjisaffar, F., & Perring, T. M. (2020). Life History Evaluation of Ooencyrtus lucidus, a Newly Described Egg Parasitoid of Bagrada hilaris. Insects, 11(5), 292. https://doi.org/10.3390/insects11050292






