From a Food Safety Prospective: The Role of Earthworms as Food and Feed in Assuring Food Security and in Valuing Food Waste
Abstract
1. Introduction
2. Materials and Methods
2.1. Pilot Earthworm Rearing, Growth Substrate, and Waste Reduction Efficiency
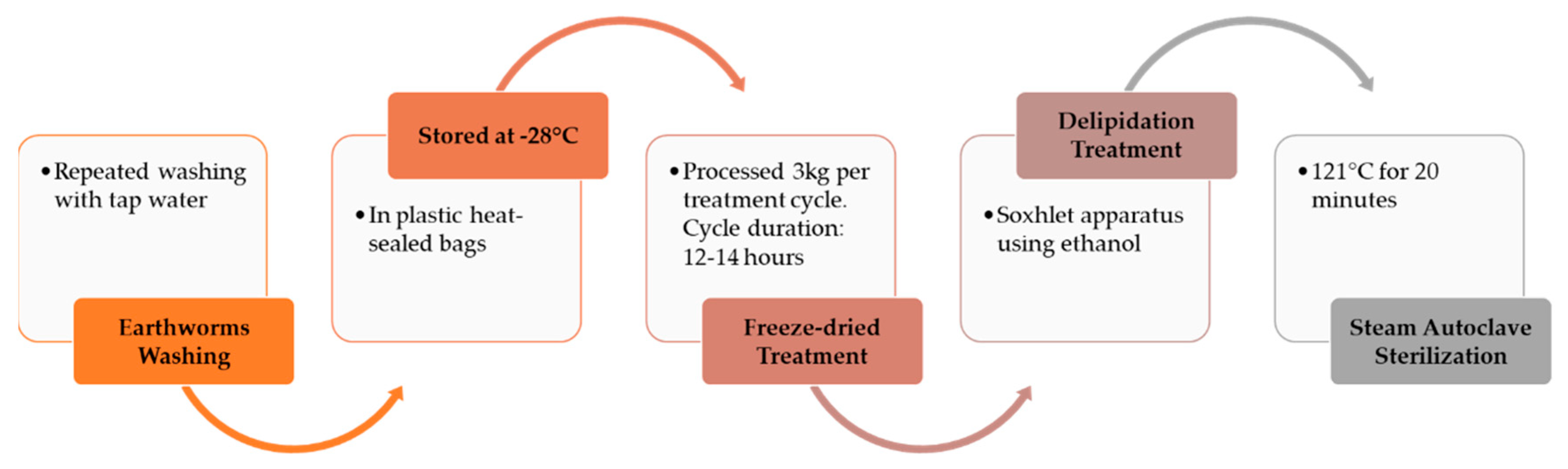
2.2. Processing of Earthworms and Transformation Technology into Meal
2.3. Nutritional, Chemical, and Microbiological Analyses
2.4. Statistical Analysis
3. Results
3.1. Substrate of Growth and Waste Reduction Efficiency Results
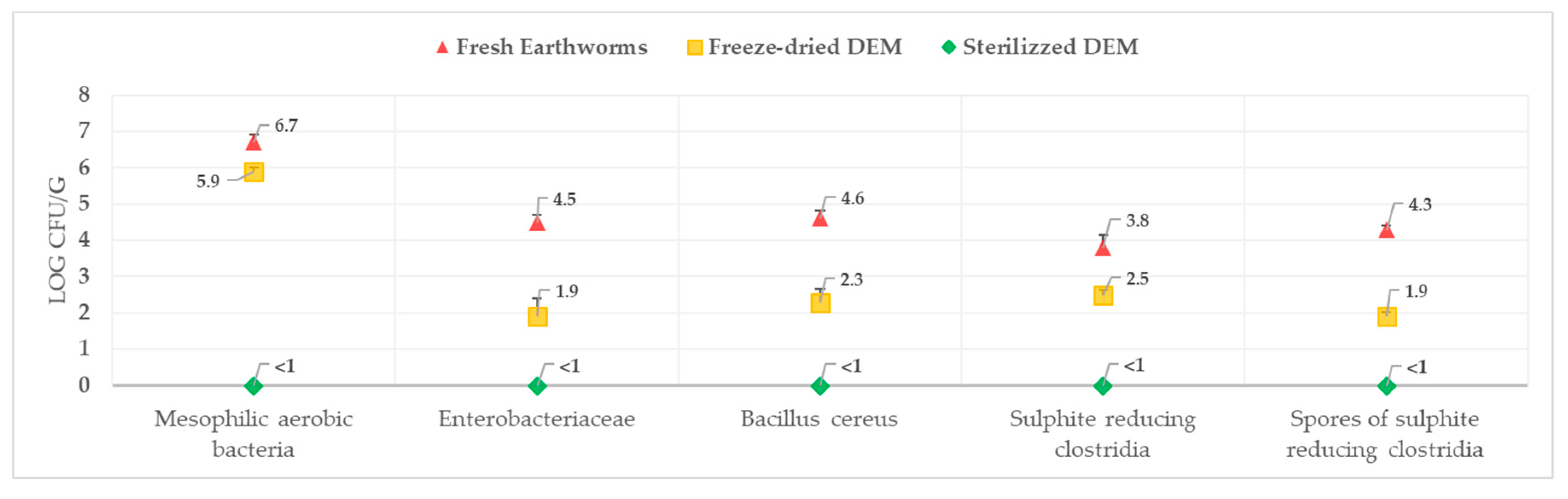
3.2. Nutritional, Chemical, and Microbiological Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Salvia, A.L.; Leal Filho, W.; Brandli, L.L.; Griebeler, J.S. Assessing research trends related to Sustainable Development Goals: Local and global issues. J. Clean. Prod. 2019, 841–849. [Google Scholar] [CrossRef]
- Castrica, M.; Ventura, V.; Panseri, S.; Ferrazzi, G.; Tedesco, D.; Balzaretti, C.M. The Sustainability of Urban Food Systems: The Case of Mozzarella Production in the City of Milan. Sustainability 2020, 12, 682. [Google Scholar] [CrossRef]
- Mourad, M. Recycling, recovering and preventing “food waste”: Competing solutions for food systems sustainability in the United States and France. J. Clean. Prod. 2016, 126, 461–477. [Google Scholar] [CrossRef]
- Castrica, M.; Tedesco, D.E.A.; Panseri, S.; Ferrazzi, G.; Ventura, V.; Frisio, D.G.; Balzaretti, C.M. Pet food as the most concrete strategy for using food waste as feedstuffwithin the European context: A feasibility study. Sustainability 2018, 10, 2035. [Google Scholar] [CrossRef]
- European Commission. COM(2019) 190 Final. On the Implementation of the Circular Economy Action Plan; European Commission: Brussels, Belgium, 2019. [Google Scholar]
- Tedesco, D.E.A.; Conti, C.; Lovarelli, D.; Biazzi, E.; Bacenetti, J. Bioconversion of fruit and vegetable waste into earthworms as a new protein source: The environmental impact of earthworm meal production. Sci. Total Environ. 2019, 683, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Gómez, M.J.; Nogales, R.; Insam, H.; Romero, E.; Goberna, M. Continuous-feeding vermicomposting as a recycling management method to revalue tomato-fruit wastes from greenhouse crops. Waste Manag. 2010, 30, 2461–2468. [Google Scholar] [CrossRef]
- Lim, S.L.; Lee, L.H.; Wu, T.Y. Sustainability of using composting and vermicomposting technologies for organic solid waste biotransformation: Recent overview, greenhouse gases emissions and economic analysis. J. Clean. Prod. 2016, 111, 262–278. [Google Scholar] [CrossRef]
- Huang, K.; Xia, H.; Li, F.; Wei, Y.; Cui, G.; Fu, X.; Chen, X. Optimal growth condition of earthworms and their vermicompost features during recycling of five different fresh fruit and vegetable wastes. Environ. Sci. Pollut. Res. 2016, 23, 13569–13575. [Google Scholar] [CrossRef]
- Singh, R.P.; Embrandiri, A.; Ibrahim, M.H.; Esa, N. Management of biomass residues generated from palm oil mill: Vermicomposting a sustainable option. Resour. Conserv. Recycl. 2011, 55, 423–434. [Google Scholar] [CrossRef]
- Alexandratos, N.; Bruinsma, J. World Agriculture towards 2030/2050: The 2012 Revision; ESA Working Paper No. 12-03; FAO: Rome, Italy, 2012. [Google Scholar]
- Halloran, A.; Flore, R.; Vantomme, P.; Roos, N. Edible Insects in Sustainable Food Systems; Springer: Cham, Switzerland, 2018; ISBN 978-3-319-74011-9. [Google Scholar]
- Van Huis, A.; Van Itterbeeck, H.; Klunder, E.; Mertens, A.; Halloran, G.; Muir, P.V. Edible Insects—Future Prospects for Food and Feed Security; FAO Forestry Paper 171; FAO: Rome, Italy, 2013. [Google Scholar]
- Zhenjun, S.; Xianchun, L.; Lihui, S.; Chunyang, S. Earthworm as a potential protein resource. Ecol. Food Nutr. 1997, 36, 221–236. [Google Scholar] [CrossRef]
- Vielma, R.A.; Carrero, P.; Rondon, C.; Medina, A.L. Comparison of the content of minerals and trace-elements in flour of earthworm (Eisenia foetida) using two drying procedures. Saber Rev. Multidiscip. Cons. Investig. Univ. Oriente 2007, 19, 83–89. [Google Scholar]
- Medina, A.L.; Cova, J.A.; Vielma, R.A.; Pujic, P.; Carlos, M.P.; Torres, J.V. Immunological and chemical analysis of proteins from Eisenia foetida earthworm. Food Agric. Immunol. 2003, 15, 255–263. [Google Scholar] [CrossRef]
- Sun, Z.; Jiang, H. Nutritive Evaluation of Earthworms as Human Food. Future Food 2017, 37. [Google Scholar] [CrossRef]
- Kennedy, J.F.; Jin, M. International Food Information Service, Dictionary of Food Science and Technology; Blackwell Publishing: Oxford, UK, 2009. [Google Scholar]
- Cayot, N.; Cayot, P.; Bou-Maroun, E.; Laboure, H.; Abad-Romero, B.; Pernin, K.; Seller-Alvarez, N.; Hernández, A.V.; Marquez, E.; Medina, A.L. Physico-chemical characterisation of a non-conventional food protein source from earthworms and sensory impact in arepas. Int. J. Food Sci. Technol. 2009, 44, 2303–2313. [Google Scholar] [CrossRef]
- Sabine, J.R. Earthworms as a source of food and drugs. Earthworm Ecol. 1983, 285–296. [Google Scholar]
- Bou-Maroun, E.; Cayot, N. Odour-active compounds of an Eisenia foetida protein powder. Identification and effect of delipidation on the odour profile. Food Chem. 2011, 124, 889–894. [Google Scholar] [CrossRef]
- Commission Directive 2010/59/EU of 26 August 2010 amending Directive 2009/32/EC of the European Parliament and of the Council on the approximation of the laws of the Member States on extraction solvents used in the production of foodstuffs and food ingredients. Off. J. EU 2010, 225, 10–12.
- Deriaz, R.E. Routine analysis of carbohydrates and lignin in herbage. J. Sci. Food Agric. 1961, 12, 152–160. [Google Scholar] [CrossRef]
- Confalonieri, M.; Carelli, M.; Tava, A.; Borrelli, L. Overexpression of MtTdp2α (tyrosyl-DNA phosphodiesterase 2) gene confers salt tolerance in transgenic Medicago truncatula. Plant Cell Tissue Organ Cult. 2019, 137, 157–172. [Google Scholar] [CrossRef]
- Arioli, F.; Ceriani, F.; Nobile, M.; Vigano’, R.; Besozzi, M.; Panseri, S.; Chiesa, L.M. Presence of organic halogenated compounds, organophosphorus insecticides and polycyclic aromatic hydrocarbons in meat of different game animal species from an Italian subalpine area. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2019, 36, 1244–1252. [Google Scholar] [CrossRef]
- Chiesa, L.M.; Nobile, M.; Ceriani, F.; Malandra, R.; Arioli, F.; Panseri, S. Risk characterisation from the presence of environmental contaminants and antibiotic residues in wild and farmed salmon from different FAO zones. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2019, 36, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Man, Y.; Liang, G.; Li, A.; Pan, L. Recent advances in mycotoxin determination for food monitoring via microchip. Toxins 2017, 9, 324. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, L.; Panseri, S.; Pasquale, E.; Malandra, R.; Pavlovic, R.; Arioli, F. Validated multiclass targeted determination of antibiotics in fish with high performance liquid chromatography–benchtop quadrupole orbitrap hybrid mass spectrometry. Food Chem. 2018, 258, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Faustini, M.; Pastorino, G.Q.; Colombani, C.; Chiesa, L.M.; Panseri, S.; Vigo, D.; Curone, G. Volatilome in Milk for Grana Padano and Parmigiano Reggiano Cheeses: A first survey. Vet. Sci. 2019, 6, 41. [Google Scholar] [CrossRef]
- Salomone, R.; Saija, G.; Mondello, G.; Giannetto, A.; Fasulo, S.; Savastano, D. Environmental impact of food waste bioconversion by insects: Application of Life Cycle Assessment to process using Hermetia illucens. J. Clean. Prod. 2017, 140, 890–905. [Google Scholar] [CrossRef]
- Mondello, G.; Salomone, R.; Ioppolo, G.; Saija, G.; Sparacia, S.; Lucchetti, M.C. Comparative LCA of alternative scenarios for waste treatment: The case of food waste production by the mass-retail sector. Sustainbility 2017, 9, 827. [Google Scholar] [CrossRef]
- Conti, C.; Costa, A.; Balzaretti, C.M.; Russo, V.; Tedesco, D.E.A. Survey on food preferences of university students: From tradition to new food customs? Agriculture 2018, 8, 155. [Google Scholar] [CrossRef]
- Verneau, F.; La Barbera, F.; Kolle, S.; Amato, M.; Del Giudice, T.; Grunert, K. The effect of communication and implicit associations on consuming insects: An experiment in Denmark and Italy. Appetite 2016, 106, 30–36. [Google Scholar] [CrossRef]
- World Health Organization. Protein and Amino Acid Requirements in Human Nutrition: Report of a Joint WHO/FAO/UNU Expert Consultation; WHO Technical Report Series; WHO: Geneva, Switzerland, 2007; pp. 1–265. [Google Scholar]
- FAO; World Health Organization. Vitamin and Mineral Requirements in Human Nutrition, 2nd ed.; World Health Organization: Geneva, Switzerland, 1998; pp. 1–20. [Google Scholar]
- Suzuki, K.T.; Yamamura, M.; Mori, T. Cadmium-binding proteins induced in the earthworm. Arch. Environ. Contam. Toxicol. 1980, 9, 415–424. [Google Scholar] [CrossRef]
- Yang, G.; Li, J.; Wang, Y.; Chen, C.; Zhao, H.; Shao, K. Quantitative ecotoxicity analysis for pesticide mixtures using benchmark dose methodology. Ecotoxicol. Environ. Saf. 2018, 159, 94–101. [Google Scholar] [CrossRef]
- Conti, C.; Castrica, M.; Balzaretti, C.M.; Tedesco, D.E.A. Edible earthworms in a food safety perspective: Preliminary data. Ital. J. Food Saf. 2019, 8, 7695. [Google Scholar] [CrossRef] [PubMed]
- Cappellozza, S.; Leonardi, M.G.; Savoldelli, S.; Carminati, D.; Rizzolo, A.; Cortellino, G.; Terova, G.; Moretto, E.; Badaile, A.; Concheri, G.; et al. A first attempt to produce proteins from insects by means of a circular economy. Animals 2019, 9, 278. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Pegg, R.B. Hexanal as an Indicator of the Flavor Deterioration of Meat and Meat Products. In Lipids in Food Flavors; Ho, C.T., Hartman, T.G., Eds.; ACS Symposium Series 558; American Chemical Society: Washington, DC, USA, 1994; pp. 256–279. [Google Scholar]
- Ullrich, F.; Grosch, W. Identification of the most intense volatile flavour compounds formed during autoxidation of linoleic acid. Z. Lebensm. Unters. Forsch. 1987, 184, 277–282. [Google Scholar] [CrossRef]
- Romero, B.A.; Bou-Maroun, E.; Reparet, J.M.; Blanquet, J.; Cayot, N. Impact of lipid extraction on the dearomatisation of an Eisenia foetida protein powder. Food Chem. 2010, 119, 459–466. [Google Scholar] [CrossRef]
- Montel, M.C.; Masson, F.; Talon, R. Bacterial role in flavour development. Meat Sci. 1998, 49, S111–S123. [Google Scholar] [CrossRef]
- Caparros Megido, R.C.; Desmedt, S.; Blecker, C.; Béra, F.; Haubruge, É.; Alabi, T.; Francis, F. Microbiological load of edible insects found in Belgium. Insects 2017, 8, 12. [Google Scholar] [CrossRef]
- Byambas, P.; Hornick, J.L.; Marlier, D.; Francis, F. Vermiculture in animal farming: A review on the biological and nonbiological risks related to earthworms in animal feed. Cogent Environ. Sci. 2019, 5, 1591328. [Google Scholar] [CrossRef]
- Gall, J.E.; Boyd, R.S.; Rajakaruna, N. Transfer of heavy metals through terrestrial food webs: A review. Environ. Monit. Assess. 2015, 187, 201. [Google Scholar] [CrossRef]
- Yeung, C.Y.; Lee, H.C.; Lin, S.P.; Yang, Y.C.; Huang, F.Y.; Chuang, C.K. Negative effect of heat sterilization on the free amino acid concentrations in infant formula. Eur. J. Clin. Nutr. 2006, 60, 136–141. [Google Scholar] [CrossRef]
- Yu, T.Y.; Morton, J.D.; Clerens, S.; Dyer, J.M. Cooking-Induced Protein Modifications in Meat. Compr. Rev. Food Sci. Food Saf. 2017, 16, 141–159. [Google Scholar] [CrossRef]
- Prodhan, U.K.; Pundir, S.; Chiang, V.S.C.; Milan, A.M.; Barnett, M.P.G.; Smith, G.C.; Markworth, J.F.; Knowles, S.O.; Cameron-smith, D. Comparable postprandial amino acid and gastrointestinal hormone responses to beef steak cooked using different methods: A randomised crossover trial. Nutrients 2020, 12, 380. [Google Scholar] [CrossRef] [PubMed]
- Villate, D. Maladies des Volailles, 2nd ed.; Agricole: Paris, France, 2001. [Google Scholar]

| Investigated Parameters | Reference Methods | |
|---|---|---|
| Nutrients | Dry matter (DM), ash, crude proteins, ether extract | AOAC, 2012. Official Methods of Analysis, 18th ed. Association of Official Analytical Chemists International, Washington, DC, USA. |
| Total carbohydrates Water-soluble carbohydrates | Anthrone colorimetric method using UV–Vis spectrophotometer as reported by Deriaz [23]. | |
| Minerals | Potassium, sodium, calcium, phosphorus, magnesium, iron, aluminium, silicate, chloride, zinc, bromide, manganese, copper, iodine, nickel, barium, chromium, cadmium and lead | Inductive coupled-plasma mass spectrometry (ICP-MS) analyses as reported by Confalonieri et al. [24]. |
| Pesticides | Atrazin, azinphos-ethyl, azinphos-methyl, azoxystrobin, benalaxyl, bitertanol, bupirimate, buprofezin, cadusafos, chlorfenvinphos, cyproconazol, cyprodinil, diazinon, ethoprophos, ethoxyquin, fenamiphos, fenarimol, fludioxonil, flusilazole, furalaxyl, kresoxim-methyl, malathion, metalaxyl, methidathion, oxadixyl, paraoxon-methyl, phosalone, piperonyl butoxide, pirimicarb, pirimiphos-ethyl, pirimiphos-methyl, profenophos, propachlor, propargite, pyrazophos, quinalphos, simazine, tetrachlorvinphos, tetraconazole, and triazophos. | Method of analysis as reported by Arioli et al. [25] and Chiesa et al. [26]. |
| Mycotoxins | Aflatoxin B1 and B2 and ochratoxin A | Method of analysis as reported by Man et al. [27] |
| Antibiotic residues | Amoxicillin, ampicillin, cloxacillin, dicloxacillin, benzylpenicillin, oxolinic acid, nalidixic acid, cefalexin, cefquinome, ciprofloxacin, enrofloxacin, lomefloxacin, marbofloxacin, florfenicol, florfenicol-amine, chloramphenicol, flumequine, chlortetracycline, doxycycline, oxytetracycline, tetracycline, lincomycin, sulfathiazole, sulfadimidine, sulfadiazine, sulphadimethoxin, trimethoprim, erythromycin, tylosin, thiamphenicol | Method of analysis as reported by Chiesa et al. [28]. |
| Volatile Organic Compounds (VOCs) | Alcohols, Aldehydes, Ketones, Sulfur compounds, Free Fatty Acids, Esters, Nitrogen compounds, Hydrocarbons | Method of analysis as reported by Faustini et al. [29]. |
| Microbiological analyses | Research of Salmonella spp. | UNI EN ISO 6579-1:2017 |
| Research of Listeria monocytogenes | AFNOR BRD 07/04- 09/98 | |
| Enumeration of mesophilic aerobic bacteria | AFNOR 3M 01/1-09/89 | |
| Enumeration of Enterobacteriaceae | AFNOR 3M 01/06-09/97 | |
| Enumeration of E. coli | AFNOR 3M 01/08-06/01 | |
| Enumeration of total coliforms bacteria | AFNOR 3M 01/2-09/89 A | |
| Enumeration of coag. + Staphylococci | AFNOR 3M 01/09-04/03 A | |
| Enumeration of presumed Bacillus cereus | UNI EN ISO 7932:2005 | |
| Enumeration of sulphite reducing clostridia | ISO 15213:2003 | |
| Enumeration of spores of sulphite reducing clostridia |
| Parameter | Substrate of Growth |
|---|---|
| Moisture (%) | 84–88 |
| Temperature (°C) | 20–25 |
| pH | 6.07–8.02 |
| C 1 (%DM) | 29.5–44 |
| N 2 (%DM)) | 1.3–1.6 |
| C/N | 22.7–27.5 |
| Parameter | Sterilized Defatted Earthworm Meal |
|---|---|
| Ash (%DM) | 4.1 ± 0.1 |
| Crude proteins (%DM) | 73.2 ± 0.6 |
| Ether extract (%DM) | ≤0.1 |
| Total carbohydrates (%DM) | 19.5 ± 0.4 |
| Water soluble carbohydrates (% DM) | 6.6 ± 0.3 |
| Minerals (mg kg−1) | |
| Potassium | 4723 ± 46 |
| Sodium | 2202 ± 23 |
| Calcium | 4014 ± 130 |
| Phosphorus | 3082 ± 163 |
| Magnesium | 1106 ± 18 |
| Iron | 330 ± 22 |
| Aluminium | 71 ± 7 |
| Silicate | 339 ± 8 |
| Chloride | 184 ± 8 |
| Zinc | 141 ± 13 |
| Bromide | 8 ± 1 |
| Manganese | 37 ± 1 |
| Copper | 12 ± 1 |
| Iodine | 9 ± 1 |
| Nickel | 2 ± 0 |
| Barium | ND 1 |
| Chromium | 4 ± 0 |
| Cadmium | ND 1 |
| Lead | ND 1 |
| Rt. (min) | Volatile Compounds | ng/g |
|---|---|---|
| Hydrocarbons | ||
| 1.32 | Hexane | 9.37 |
| 3.84 | 2.2-dimethyl decane | 241.60 |
| 8.84 | Undecane | 184.47 |
| 17.95 | Tetradecane | 92.07 |
| Tot | 527.52 | |
| Alcohols | ||
| 3.47 | Ethanol | 9183.74 |
| Tot | 9183.74 | |
| Free fatty acids | ||
| 21.63 | Acetic | 85.39 |
| 23.69 | Propionic | 17.31 |
| 29.84 | Pentanoic | 4.52 |
| Tot | 107.22 | |
| Aldehydes | ||
| 1.46 | Acetaldehyde | 40.96 |
| 2.82 | 2-methyl butanal | 270.24 |
| 7.66 | Hexanal | 35.41 |
| 23.2 | Benzaldehyde | 188.27 |
| Tot | 534.88 | |
| Ketones | ||
| 2.63 | 2-butanone | 63.06 |
| Tot | 63.06 | |
| Esters | ||
| 2.01 | Acetic acid ethyl ester | 11.56 |
| 2.53 | Acetic acid methyl ester | 36.21 |
| 3.7 | Propanoic acid ethyl ester | 33.39 |
| 25.14 | Dodecanoic acid methyl ester | 17.15 |
| Tot | 98.31 | |
| Sulfur compounds | ||
| 7.35 | Dimethyl sulfide | 411.27 |
| Tot | 411.27 | |
| Nitrogen compounds | ||
| 28.52 | Formamide | 2.24 |
| Tot | 2.24 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tedesco, D.E.A.; Castrica, M.; Tava, A.; Panseri, S.; Balzaretti, C.M. From a Food Safety Prospective: The Role of Earthworms as Food and Feed in Assuring Food Security and in Valuing Food Waste. Insects 2020, 11, 293. https://doi.org/10.3390/insects11050293
Tedesco DEA, Castrica M, Tava A, Panseri S, Balzaretti CM. From a Food Safety Prospective: The Role of Earthworms as Food and Feed in Assuring Food Security and in Valuing Food Waste. Insects. 2020; 11(5):293. https://doi.org/10.3390/insects11050293
Chicago/Turabian StyleTedesco, Doriana Eurosia Angela, Marta Castrica, Aldo Tava, Sara Panseri, and Claudia Maria Balzaretti. 2020. "From a Food Safety Prospective: The Role of Earthworms as Food and Feed in Assuring Food Security and in Valuing Food Waste" Insects 11, no. 5: 293. https://doi.org/10.3390/insects11050293
APA StyleTedesco, D. E. A., Castrica, M., Tava, A., Panseri, S., & Balzaretti, C. M. (2020). From a Food Safety Prospective: The Role of Earthworms as Food and Feed in Assuring Food Security and in Valuing Food Waste. Insects, 11(5), 293. https://doi.org/10.3390/insects11050293







