Detecting Soil Microarthropods with a Camera-Supported Trap
Abstract
:1. Introduction
2. Materials and Methods
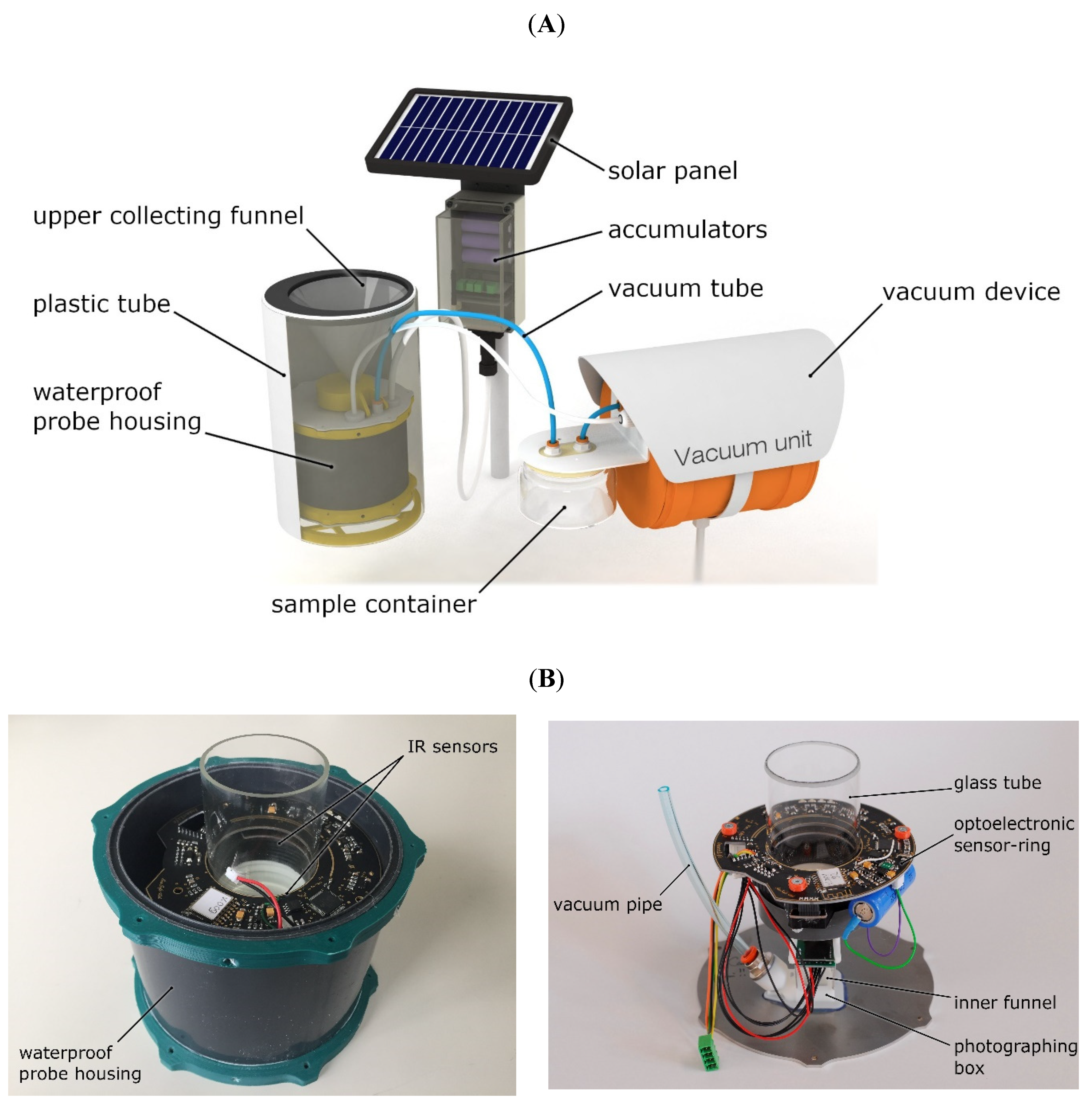
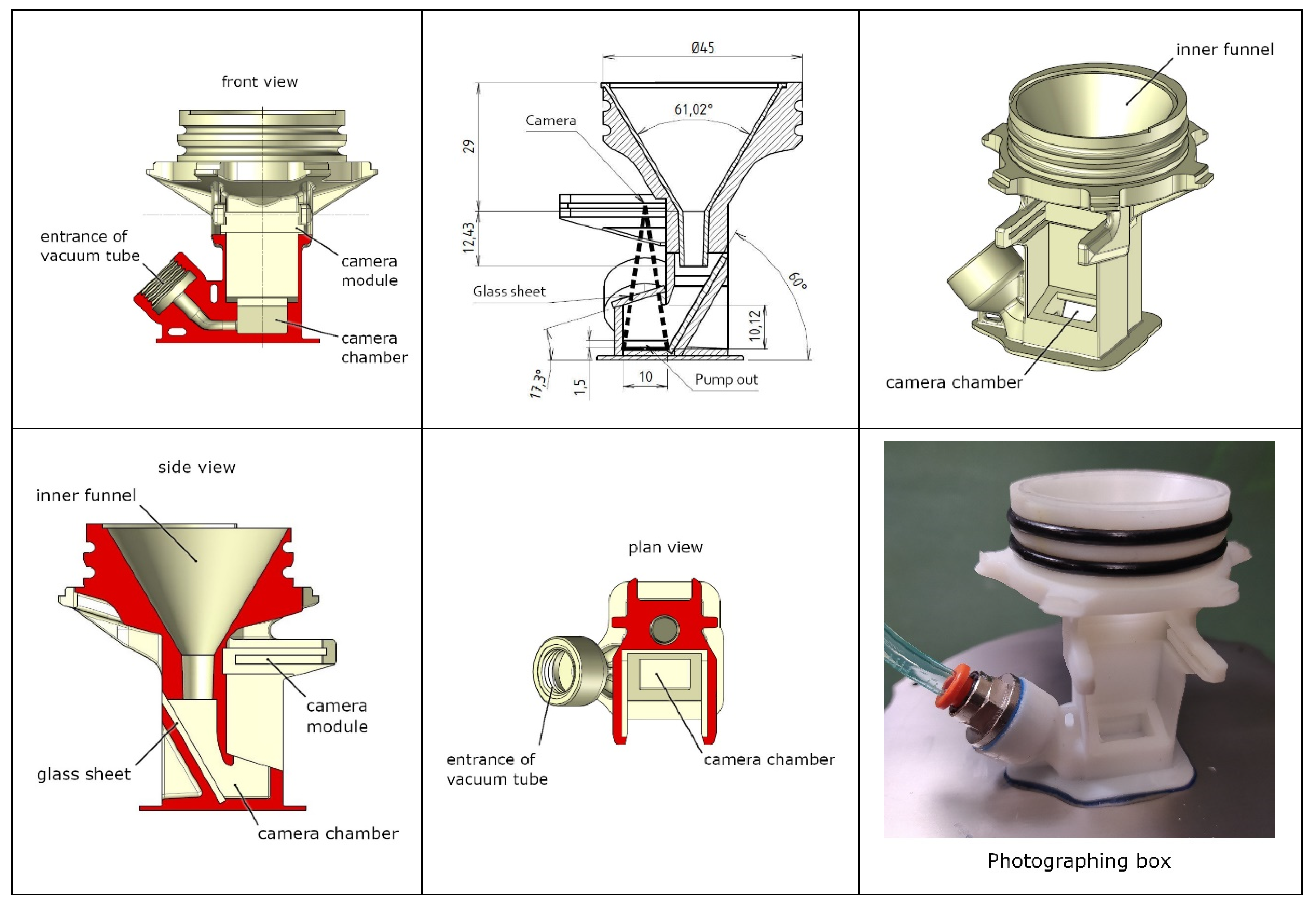
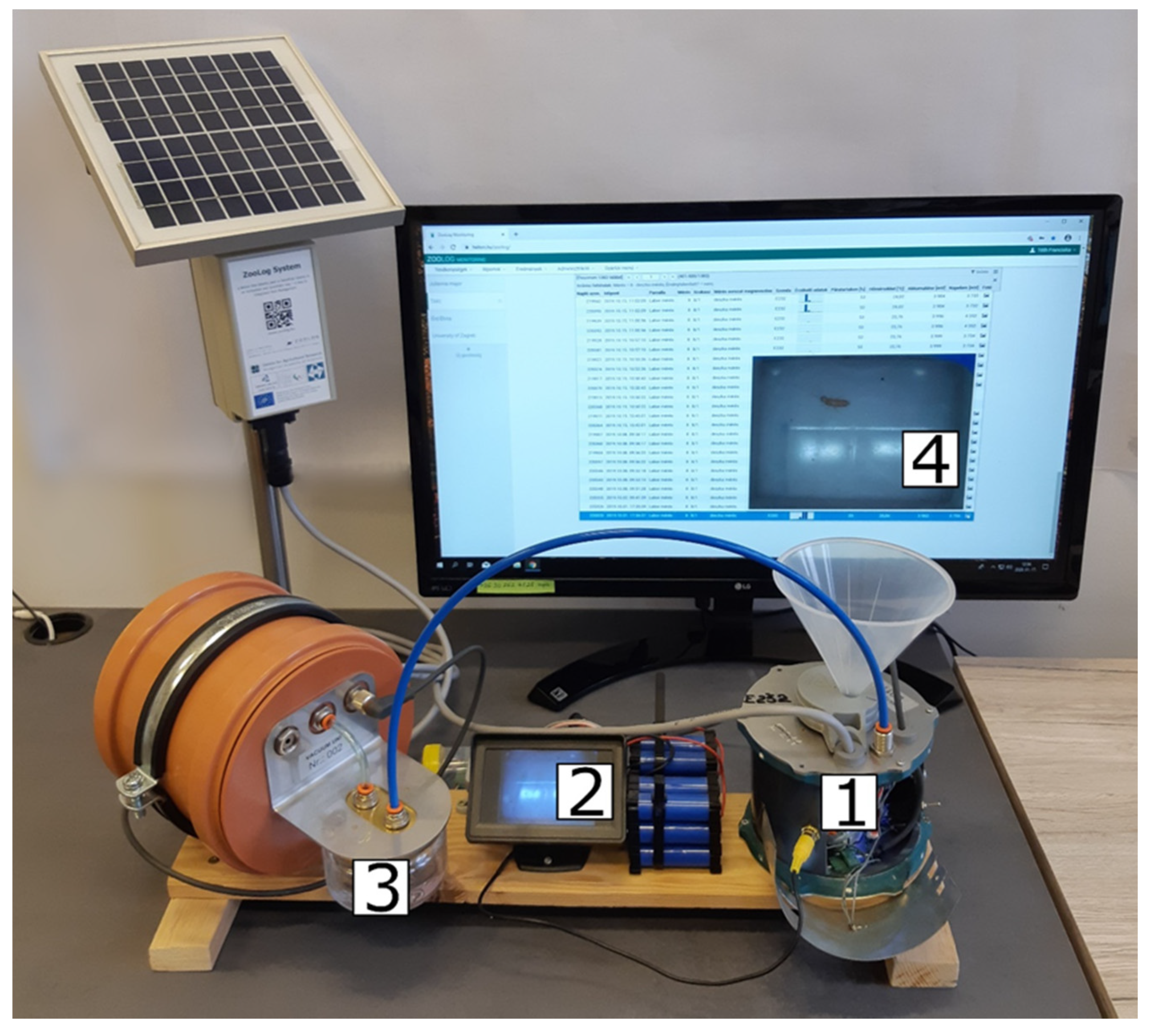
2.1. Description of the Probe
2.2. Tests
2.2.1. Process Test
Detection Accuracy
Microarthropods Getting into the Camera Chamber
Efficiency of Photo Shooting
Efficiency of Vacuum
2.2.2. Testing the Trap Systems under Semi-Natural Conditions
3. Results
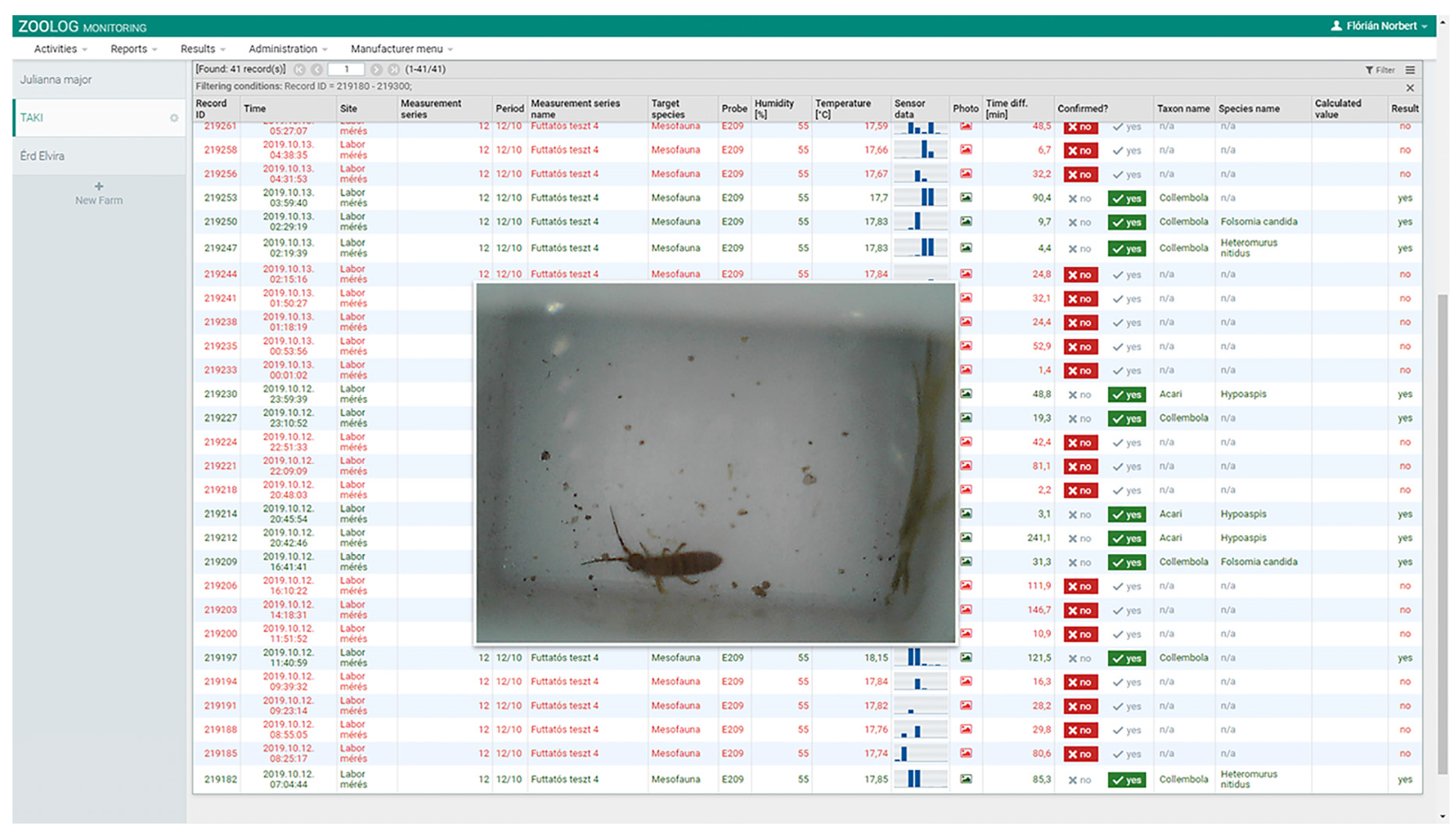
3.1. Process Testing using the Desktop Model
3.2. Semi-Natural Experiment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McCravy, K. A Review of Sampling and Monitoring Methods for Beneficial Arthropods in Agroecosystems. Insects 2018, 9, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.-L.; Li, F.-R.; Liu, L.-L.; Yang, K. Responses of different Collembola and mite taxa to experimental rain pulses in an arid ecosystem. Catena 2017, 155, 53–61. [Google Scholar] [CrossRef]
- Lensing, J.R.; Todd, S.; Wise, D.H. The impact of altered precipitation on spatial stratification and activity-densities of springtails (Collembola) and spiders (Araneae). Ecol. Entomol. 2005, 30, 194–200. [Google Scholar] [CrossRef]
- Brown, G.R.; Matthews, I.M. A review of extensive variation in the design of pitfall traps and a proposal for a standard pitfall trap design for monitoring ground-active arthropod biodiversity. Ecol. Evol. 2016, 6, 3953–3964. [Google Scholar] [CrossRef] [Green Version]
- Sabu, T.K.; Shiju, R.T. Efficacy of pitfall trapping, Winkler and Berlese extraction methods for measuring ground-dwelling arthropods in moistdeciduous forests in the Western Ghats. J. Insect Sci. 2010, 10. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Lupión, D.; Pascual, J.; Melguizo-Ruiz, N.; Verdeny-Vilalta, O.; Moya-Laraño, J. New Litter Trap Devices Outperform Pitfall Traps for Studying Arthropod Activity. Insects 2019, 10, 147. [Google Scholar] [CrossRef]
- Yi, Z.; Feng, J.; Xue, D.; Sang, W.; Axmacher, J.C. A comparison of terrestrial arthropod sampling methods. J. Resour. Ecol. 2012, 3, 174–183. [Google Scholar] [CrossRef]
- Zaller, J.; Kerschbaumer, G.; Rizzoli, R.; Tiefenbacher, A.; Gruber, E.; Schedl, H. Monitoring arthropods in protected grasslands: Comparing pitfall trapping, quadrat sampling and video monitoring. Web Ecol. 2015, 15, 15–23. [Google Scholar] [CrossRef]
- Yerushalmi, S.; Green, R.M. Evidence for the adaptive significance of circadian rhythms. Ecol. Lett. 2009, 12, 970–981. [Google Scholar] [CrossRef]
- Hopkin, S.P. Biology of the Springtails:(Insecta: Collembola); Oxford University Press: Cary, NC, USA, 1997. [Google Scholar]
- Wang, M.; Zhang, W.; Xia, H.; Huang, J.; Wu, Z.; Xu, G. Effect of Collembola on mineralization of litter and soil organic matter. Biol. Fertil. Soils 2017, 53, 563–571. [Google Scholar] [CrossRef]
- Reddy, G.; Shrestha, G.; Miller, D.; Oehlschlager, A. Pheromone-Trap Monitoring System for Pea Leaf Weevil, Sitona lineatus: Effects of Trap Type, Lure Type and Trap Placement within Fields. Insects 2018, 9, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Why, A.; Batista, G.; Mafra-Neto, A.; Keogh, E. Flying insect detection and classification with inexpensive sensors. JoVE (J. Vis. Exp.) 2014, e52111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eliopoulos, P.; Tatlas, N.-A.; Rigakis, I.; Potamitis, I. A “Smart” Trap Device for Detection of Crawling Insects and Other Arthropods in Urban Environments. Electronics 2018, 7, 161. [Google Scholar] [CrossRef] [Green Version]
- Guarnieri, A.; Maini, S.; Molari, G.; Rondelli, V. Automatic trap for moth detection in integrated pest management. Bull. Insectology 2011, 64, 247–251. [Google Scholar]
- McMunn, M.S. A time-sorting pitfall trap and temperature datalogger for the sampling of surface-active arthropods. HardwareX 2017, 1, 38–45. [Google Scholar] [CrossRef]
- Zhong, Y.; Gao, J.; Lei, Q.; Zhou, Y. A vision-based counting and recognition system for flying insects in intelligent agriculture. Sensors 2018, 18, 1489. [Google Scholar] [CrossRef] [Green Version]
- Buchholz, S. Design of a time-sorting pitfall trap for surface-active arthropods. Entomol. Exp. Appl. 2009, 133, 100–103. [Google Scholar] [CrossRef]
- Collett, R.A.; Fisher, D.O. Time-lapse camera trapping as an alternative to pitfall trapping for estimating activity of leaf litter arthropods. Ecol. Evol. 2017, 7, 7527–7533. [Google Scholar] [CrossRef]
- Dombos, M.; Kosztolányi, A.; Szlávecz, K.; Gedeon, C.; Flórián, N.; Groó, Z.; Dudás, P.; Bánszegi, O. EDAPHOLOG monitoring system: Automatic, real-time detection of soil microarthropods. Methods Ecol. Evol. 2017, 8, 313–321. [Google Scholar] [CrossRef]
- Gedeon, C.; Flórián, N.; Liszli, P.; Hambek-Oláh, B.; Bánszegi, O.; Schellenberger, J.; Dombos, M. An Opto-electronic sensor for detecting soil microarthropods and estimating their size in field conditions. Sensors 2017, 17, 1757. [Google Scholar] [CrossRef] [Green Version]
- Balla, E.; Flórián, N.; Gergócs, V.; Gránicz, L.; Tóth, F.; Németh, T.; Dombos, M. An Opto-electronic Sensor-ring to Detect Arthropods of Significantly Different Body Sizes. Sensors 2020, 20, 982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bánszegi, O.; Kosztolányi, A.; Bakonyi, G.; Szabó, B.; Dombos, M. New method for automatic body length measurement of the collembolan, Folsomia candida Willem 1902 (insecta: Collembola). PLoS ONE 2014, 9, e98230. [Google Scholar] [CrossRef] [PubMed]
- Azfar, S.; Nadeem, A.; Alkhodre, A.; Ahsan, K.; Mehmood, N.; Alghmdi, T.; Alsaawy, Y. Monitoring, Detection and Control Techniques of Agriculture Pests and Diseases using Wireless Sensor Network: A Review. Int. J. Adv. Comput. Sci. Appl. 2018, 9, 424–433. [Google Scholar] [CrossRef] [Green Version]
- Gibb, R.; Browning, E.; Glover-Kapfer, P.; Jones, K.E. Emerging opportunities and challenges for passive acoustics in ecological assessment and monitoring. Methods Ecol. Evol. 2019, 10, 169–185. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Chen, P.; Wang, B.; Xie, C. Automatic localization and count of agricultural crop pests based on an improved deep learning pipeline. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Flórián, N.; Ladányi, M.; Ittzés, A.; Kröel-Dulay, G.; Ónodi, G.; Mucsi, M.; Szili-Kovács, T.; Gergócs, V.; Dányi, L.; Dombos, M. Effects of single and repeated drought on soil microarthropods in a semi-arid ecosystem depend more on timing and duration than drought severity. PLoS ONE 2019, 14, e0219975. [Google Scholar] [CrossRef] [Green Version]
- Schirmel, J.; Lenze, S.; Katzmann, D.; Buchholz, S. Capture efficiency of pitfall traps is highly affected by sampling interval. Entomol. Exp. Appl. 2010, 136, 206–210. [Google Scholar] [CrossRef]
| Microarthropod Species | 100% = Total Specimens | 100% = Detected Specimens | The Whole Process Is Perfect | ||
|---|---|---|---|---|---|
| Detection Accuracy | Appears in the Camera Chamber | Vacuum Function | Seen in the Sent Picture | ||
| F. candida (50) | 88% | 78% | 75% | 61% | 42% |
| <1 mm (22) | 73% | 82% | 100% | 63% | 45% |
| >1 mm (28) | 100% | 75% | 61% | 61% | 39% |
| H. nitidus (50) | 94% | 74% | 74% | 66% | 46% |
| <1 mm (17) | 82% | 71% | 71% | 57% | 35% |
| >1 mm (33) | 100% | 76% | 76% | 70% | 52% |
| H. aculeifer (50) <1 mm | 100% | 92% | 46% | 88% | 44% |
| Oribatida (50) <1 mm | 100% | 78% | 50% | 78% | 48% |
| Species/Taxonomic Groups | N. of Extraction | Sum of Specimens in Sample Container | Sum of Specimens in Pictures | % of Specimens in Photos (Mean ± SD) |
|---|---|---|---|---|
| Collembola | 13 | 269 | 166 | 56.5 ± 28.9 |
| Heteromurus nitidus | 13 | 113 | 68 | 60.6 ± 21.5 |
| Folsomia candida | 13 | 95 | 60 | 66.2 ± 20.7 |
| Entomobrya | 8 | 13 | 11 | 75.0 ± 46.3 |
| Orchesella | 8 | 10 | 7 | 58.3 ± 49.6 |
| Tomocerus | 2 | 6 | 5 | 83.3 ± 23.6 |
| Lepidocyrtus | 4 | 6 | 5 | 68.8 ± 47.3 |
| Symphypleona | 2 | 2 | 0 | 0.0 ± 0.0 |
| Proisotoma | 3 | 24 | 10 | 39.6 ± 22.0 |
| Acari | 13 | 215 | 153 | 66.4 ± 27.1 |
| Oribatid mites | 13 | 101 | 73 | 77.6 ± 21.6 |
| Hypoaspis aculeifer | 13 | 100 | 70 | 71.4 ± 16.5 |
| Other Acari | 4 | 5 | 2 | 25.0 ± 50.0 |
| Trombidiidae | 6 | 9 | 8 | 91.7 ± 20.4 |
| Isopoda | 10 | 12 | 7 | 43.3 ± 47.3 |
| Diplopoda | 4 | 3 | 1 | 12.5 ± 25.0 |
| Diptera larvae | 4 | 1 | 0 | 0.0 ± 0.0 |
| Coleoptera | 6 | 5 | 3 | 50.0 ± 57.7 |
| other Coleoptera | 3 | 2 | 1 | 33.3 ± 57.7 |
| Curcolionidae | 3 | 3 | 2 | 66.7 ± 57.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flórián, N.; Gránicz, L.; Gergócs, V.; Tóth, F.; Dombos, M. Detecting Soil Microarthropods with a Camera-Supported Trap. Insects 2020, 11, 244. https://doi.org/10.3390/insects11040244
Flórián N, Gránicz L, Gergócs V, Tóth F, Dombos M. Detecting Soil Microarthropods with a Camera-Supported Trap. Insects. 2020; 11(4):244. https://doi.org/10.3390/insects11040244
Chicago/Turabian StyleFlórián, Norbert, Laura Gránicz, Veronika Gergócs, Franciska Tóth, and Miklós Dombos. 2020. "Detecting Soil Microarthropods with a Camera-Supported Trap" Insects 11, no. 4: 244. https://doi.org/10.3390/insects11040244
APA StyleFlórián, N., Gránicz, L., Gergócs, V., Tóth, F., & Dombos, M. (2020). Detecting Soil Microarthropods with a Camera-Supported Trap. Insects, 11(4), 244. https://doi.org/10.3390/insects11040244






