1. Introduction
The Sterile Insect Technique (SIT) and biological control programs using hymenopterous parasitoids to control pestiferous fruit flies (Diptera: Tephritidae) worldwide, depend on a continuous supply of millions of high-quality insects that have been reared in cost-effective artificial diets [
1,
2]. These diets constitute the medium in which fruit fly larvae will live, feed and interact with conspecifics before metamorphosing into pupae. The pupal stage is then irradiated to produce sterile adult males that will be released in the field to compete with fertile wild males for opportunities to mate with wild female flies [
1,
3]. Thus, larval diets are critical for the optimal development of larvae and mass production of sexually competitive adults [
1,
3,
4,
5].
The Mexican Fruit Fly or Mexfly,
Anastrepha ludens (Loew), is a major pest of citrus (
Citrus spp.) and mango (
Mangifera indica L.) [
3].
Anastrepha ludens females lay their eggs in the pulp of most hosts (only in the case of
Casimiroa greggii (S. Watson) F. Chiang are eggs inserted in the seeds) and, depending on the temperature, 3–12 days later, larvae hatch and start consuming the pulp [
6]. Larvae go through three stages before abandoning the fruit to pupate in the ground, and adults emerge approximately 12 to 32 days later [
6]. This pest is distributed from southern US throughout Mexico to Central America [
6]. In Mexico, the Moscafrut facility of the National Campaign against Fruit Flies SENASICA-SADER, produces about 200 million
A. ludens sterile flies per week for use in SIT releases [
3]. In addition, millions of
A. ludens larvae are used as hosts of the larval-pupal endoparasitoid
Diachasmimorpha longicaudata (Ashmead) (Hymenoptera: Braconidae) which are released in augmentative biological control programs against
A.
ludens and
A.
obliqua in mango and citrus-producing areas [
3,
7]. Mass production of
A. ludens at Moscafrut is currently based on a diet that includes water, sugar and yeast as nutrients, and corncob powder as a bulking agent [
3,
8]. The major weakness of this formula is that corncob powder is often contaminated with mycotoxins [
9] as most uses of this by-product of maize do not require a high purity substrate. This has become a cause for concern because a contaminated batch of corncob powder can result in a major reduction in the rearing process as yields collapse and the stock population declines, provoking costly time lags during the recovery phase [
4,
9,
10]. In addition, corncob powder promotes high temperatures in the diet with negative effects on larval development [
10]. This highlights the need for corncob substitutes in fruit fly rearing diets as a high priority in diet development [
10].
Gelling agents are another group of ingredients that are used in insect diets to transform high-water content mixtures into solids with a homogeneous distribution of materials [
11]. Gelling agents can also inhibit undesirable reactions among diet ingredients [
11]. Gel diets for artificial rearing of tephritids have been known for many decades [
12]. In the last 13 years, several gel diet formulations have been developed for tephritid flies within the economically important genera
Anastrepha,
Ceratitis,
Rhagoletis and
Bactrocera [
13,
14,
15,
16,
17,
18,
19]. The most common gelling agent in these diets is agar, with the drawback of its high cost [
11,
18]. However, the cost problem can be overcome by lowering the proportion used in the diet [
14]. Gel diets containing agar and a gel diet based on a commercial mixture of pregelatinized starches have shown potential for the mass-rearing of
A. ludens at the Moscafrut facility [
16,
17]. However, the substitution of the standard diet with a gel-based diet has not been implemented given its cost and the impracticalities implicit in the preparation process as agar needs to be heat-activated in boiling water and scaling-up this process requires developing new diet handling technologies [
16,
17]. Despite these drawbacks, the concept of gel-based diets has been integrally adopted for the mass-rearing of
Bactrocera tryoni (Froggatt) as part of a SIT-based program in Australia [
20]. The gel diet developed for this pestiferous fly yields larger numbers of adult flies, generates significantly less diet waste and reduces overall labor costs compared to a traditional diet with a bulking agent [
5,
19,
20].
Here, we incorporated new elements into the development of gel diets for rearing of
A. ludens by testing gelling agents such gelatin and carrageenan that show great promise due to their low cost and that were not considered in previous studies. We also examined the influence of the modified diets on the suitability of
A. ludens larvae for rearing of the parasitoid wasp
D. longicaudata. Given that reducing the cost of the diet is another major challenge in the Moscafrut facility [
3], we followed a diet optimization perspective using a liquid diet formulation without corncob powder and reduced in yeast content to lower diet cost [
8,
21]. Our main goal was to determine the feasibility of developing a rearing system for
A. ludens involving gel diets, to support the decision-making process related to the mass-rearing of this fly pest at the Moscafrut facility.
4. Discussion
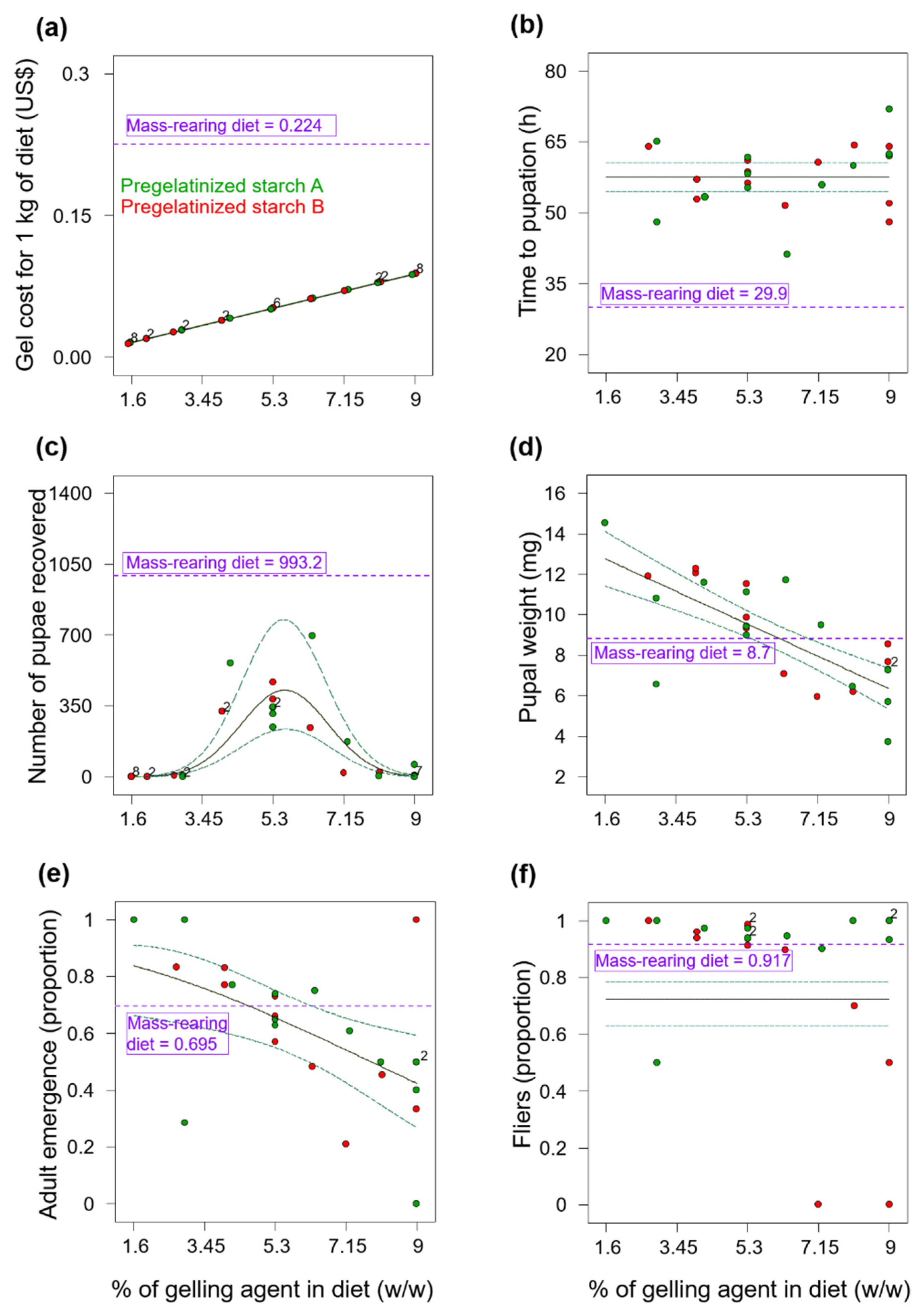
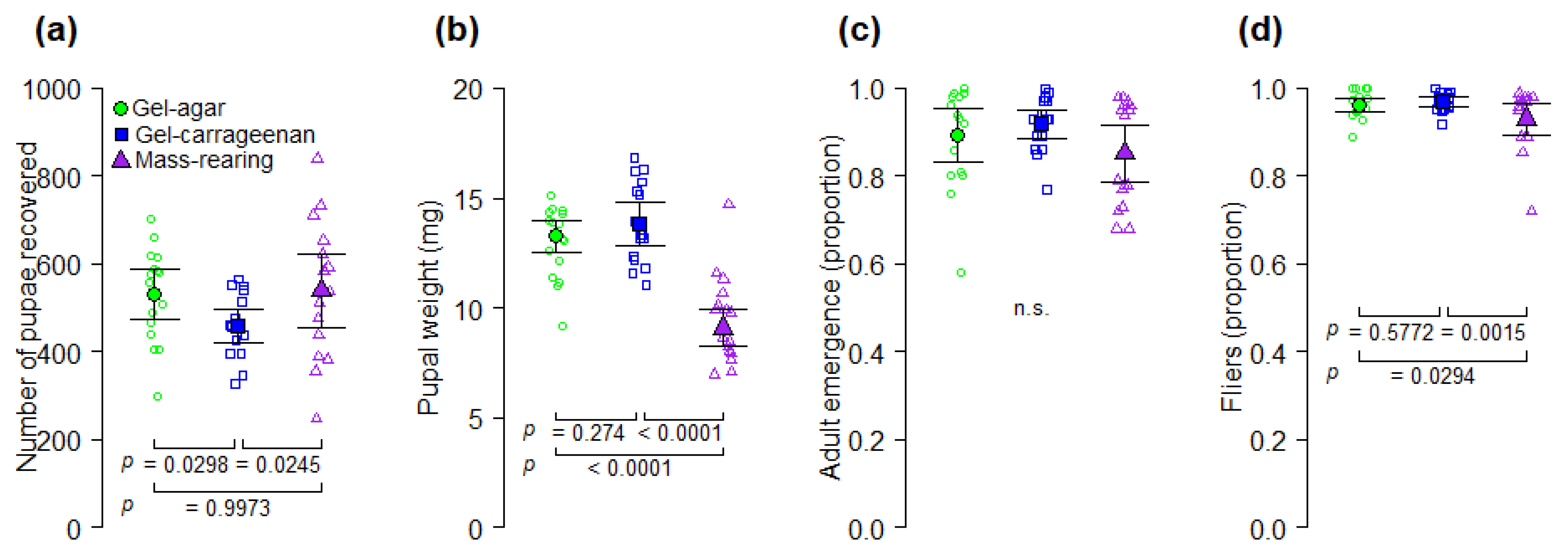
Although the use of gel diets for rearing the fruit fly pest
A. ludens had been previously reported [
16,
17], this is the first study in which several gelling agents (carrageenan, agar, gelatin and pregelatinized corn starch) have been tested from a multivariate optimization perspective, considering production and quality parameters of both flies and parasitoids, as well as diet costs. In addition, this is the first report on the use of carrageenan and gelatin as gelling agents in an
A. ludens larval gel diet. Our results indicate that yeast-reduced gel diet formulations based on carrageenan and agar as gelling agents can be used for more cost-effective rearing of
A. ludens (
Figure 2 and
Figure 3). Even though agar and carrageenan are relatively expensive [
11], the low percentages of these ingredients and the reduction in the yeast content in the diets we tested in Experiment 3 (
Appendix A,
Table A3), made our gel diets 24% less expensive than the mass-rearing diet. More importantly, our analyses indicate that the production of one million flying insects in the gel-agar and gel-carrageenan diets could be 27% and 17%, respectively, less expensive than with the mass-rearing formulation (
Table 4). By reducing the cost of the diet, we addressed one of the most pressing priorities in mass-rearing facilities of fruit flies worldwide such as the Moscafrut facility [
1,
3,
37]. On the other hand, although gelatin and pregelatinized corn starches were quite inexpensive, gel diets based on these ingredients did not produce sufficient numbers of flies (
Figure 1c and
Figure 2c) to be considered as candidate diets for large scale production of
A. ludens.
Our results contrast with those of Rivera et al. [
17], who found that a pregelatinized starch diet based on a commercial product (Nutrifly
®) increased yields of
A. ludens compared to a standard mass-rearing diet with corncob fractions. The exact content of yeast and the composition of pregelatinized starches in this pregelatinized starch diet were not stated [
17], but it is possible that it contained starches from various sources [
38]. Mixing corn starch with other types of starches or gelling agents in the diet might provide positive results for the rearing of
A. ludens but increasing the number of ingredients would rise the cost of the diet and add unwanted complexity to the diet preparation process [
10].
Overall, larvae from the gel diets in Experiments 1 and 2, took much longer to pupate than larvae from the mass-rearing diets (
Figure 1b and
Figure 2b). This delayed larval development time could be associated with the reduction of yeast (i.e., the source of protein) content in gel diets compared to the yeast content in the mass-rearing diet (
Appendix A,
Table A1 and
Table A2). In fact, the duration of tephritid larval development is highly dependent on the quantity and quality of protein in the larval diet [
39]. However, if the reduction of yeast content in the gel diets would have caused a nutritional deficiency in the larvae, then individuals from the mass-rearing diet with a higher level of yeast would have had higher pupal weights than individuals reared on the gel diets, but that was not what we observed. Pupal weight of flies reared on gel diets was significantly higher than that of flies reared on the mass-rearing diet (
Figure 3b). Alternatively, larvae from the mass-rearing diet may have pupated earlier due to an increase in the diet temperature promoted by corncob powder as the larvae grew [
10]. In any case, we note that longer larval development times could delay production times at the mass-rearing facility level, but this could be overcome by increasing the rearing temperature [
8].
Despite the delay in the time to pupation of flies reared on the gelatin diets in Experiment 2 (
Figure 2b) and the low numbers of pupae recovered from these diets (
Figure 2c), we note that gelatin diets produced the heaviest
A. ludens pupae and that the pupal weight increased as the percentage of gelatin in the diet increased (
Figure 2d). Thus, although gelatin apparently has no essential amino acids for insects [
11], it is a protein with positive effects on pupal weight of
A. ludens. Overall, pupal weights were atypically low in our study, likely because the volume of eggs we inoculated into the experimental diets was rather high. Indeed, it was previously reported that pupal weight of
A. ludens was reduced when flies were reared at a density of 15 larvae per g of diet compared to flies reared at a density of 10 larvae per g of diet [
17], and the volume of eggs we inoculated to all the experimental diets was equivalent to approximately 14 larvae per g of diet. This high larval density inoculated to the diets and the overall positive effects of agar and carrageenan gel diets on
A. ludens production and quality compared to the mass rearing diet (
Figure 2 and
Figure 3), suggest that agar and carrageenan increase the production capacity of
A. ludens larval diet. Further studies are warranted to examine the effects of larval density in gel-agar and gel-carrageenan diets on survival and quality of
A. ludens and to determine the optimal larval density at which the production and quality (e.g., pupal weight) of flies is maximized.
Thanks to our modeling procedure, the percentage of agar (0.234%) and carrageenan (0.434%) used in Experiment 3 was lower than the percentages of agar used in many other tephritid larval gel diets, greatly reducing the cost of this expensive ingredient. For example, gel diets for
B. tryoni [
5],
A. fraterculus [
18] and previous gel diets for
A. ludens [
16], contained 0.77%, 0.17% and 0.77–2.27% more agar, respectively, than the agar content of 0.234% we used in Experiment 3. This highlights the benefit of using RSM [
28,
29] to design diet experiments and, in general, to optimize insect diet formulations. These methods allow for the design of experiments involving many variables, optimizing the critical ones simultaneously [
28]. Here for example, we were able to test 21 different gel diets in a single experiment (Experiment 2,
Figure 2,
Appendix A,
Table A2) and identified optimal formulations reaching multiple optimization criteria simultaneously.
We believe that gel diets better simulate the natural conditions experienced by fruit fly larvae, as pulp is a moist medium with a smooth consistency that allows larvae to move with relative ease and ingest food. In fact, we noticed that larvae reared on the gel-agar and gel-carrageenan diets we tested, leave very little or no residues as they eat almost all the food provided (
Appendix A,
Figure A1), as was previously reported for
B. tryoni reared on a gel diet with agar [
5]. This contrasts with the large amounts of residues observed in diets prepared with corncob powder as a bulking agent which can comprise some 32% (
w/
w) of the starting amount of diet (C. Pascacio-Villafán, personal observation). This represents not only a waste of food resources and money, but also a source of pollution as the trays with diet waste are washed with water that flows into water treatment facilities or sometimes directly into the sewage system. Thus, the gel diets reported here could contribute to reduce pollution in mass-rearing facilities.
Perhaps the reason why
A. ludens larvae eat almost all the food in the gel diets and waste so much in the mass-rearing diet (
Appendix A,
Figure A1) is because the mass-rearing diet using corncob powder as a bulking agent make swallowing more difficult for larvae, thus reducing the feeding rate. As a result, larvae reach the third instar having ingested less food, a fact that does not necessarily cause a drop in weight due to the high protein and carbohydrate content of artificial diets. Another possibility is that gel diets facilitate larval movement and reduce metabolic heat allowing larvae to group and via a social facilitation phenomenon increase per capita ingestion which could explain why almost no residues are left. These ideas merit further investigation.
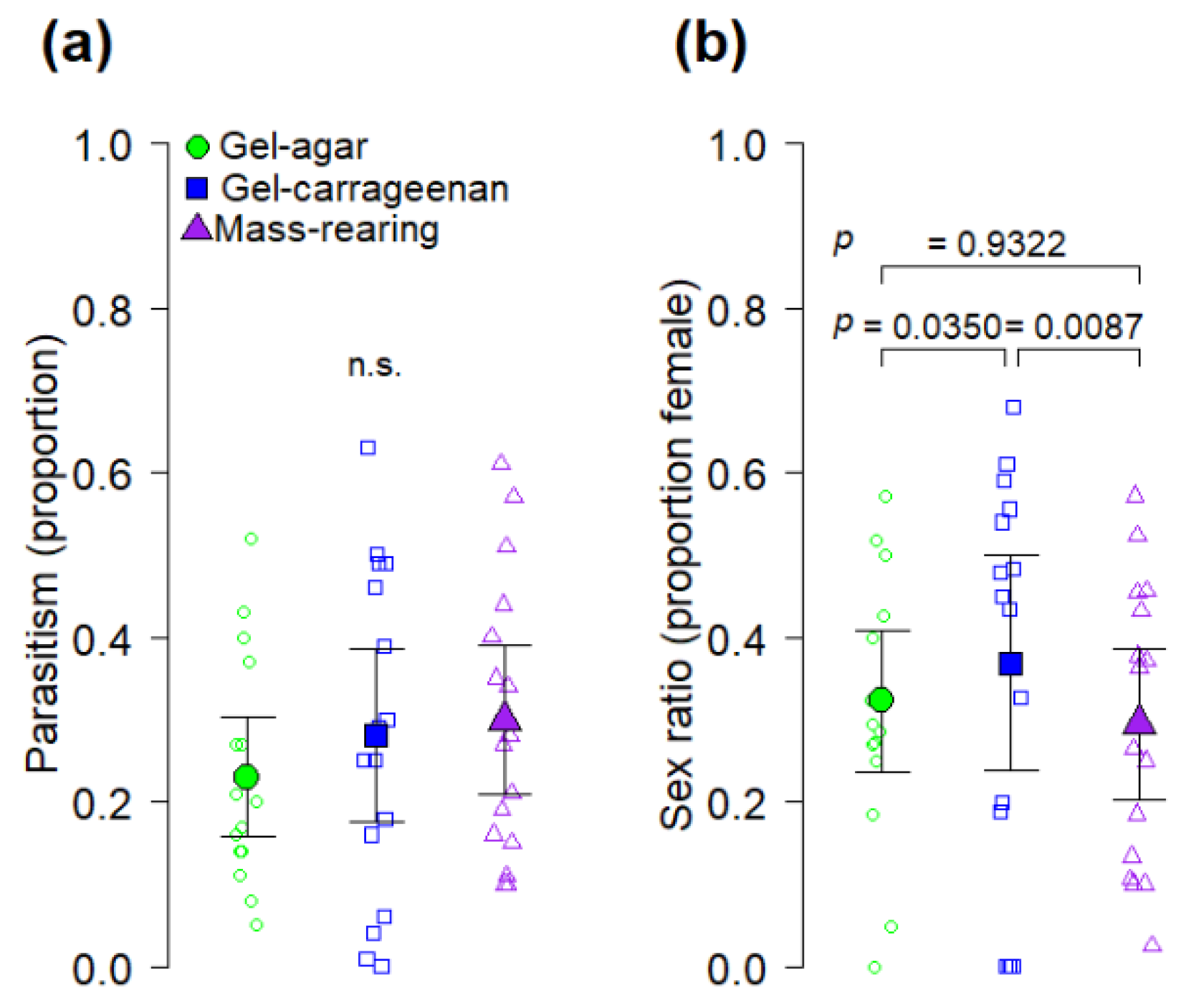
Another novel aspect of our study is represented by the fact that, in addition of having addressed the fly perspective, we also examined the quality of
A. ludens larvae reared on gel diets as hosts of the parasitoid wasp
D. longicaudata. This parasitoid species is the most widely used biocontrol agent against tephritid pests worldwide [
40,
41]. We found that the gel-carrageenan diet produced
A. ludens hosts from which a significantly higher proportion of
D. longicaudata females emerged compared to the proportion of females emerging from hosts reared on the gel-agar and the mass-rearing diets (
Figure 4b). The reason why
A. ludens from the gel-carrageenan diet produced higher proportions of female wasps than the other diets is not clear in our study, but results suggest that larvae reared on this diet were of higher quality as hosts for
D. longicaudata. Perhaps the gel-carrageenan diet improved nutrient assimilation by
A. ludens larvae making them more nutritionally adequate for the development of female parasitoids. In fact, an in vitro study showed that some types of carrageenan modulate the digestion of macronutrients such as lipids [
42]. If true for
A. ludens, future studies should find that
A. ludens larvae fed on the gel-carrageenan diet have a higher nutrient content than larvae from the gel-agar or mass-rearing diets. Another relevant point is that the heaviest flies in our study were obtained from the gel-carrageenan diet from which the highest proportion of female parasitoids were also obtained (
Figure 3b and
Figure 4b) However, we note that sex ratio of artificially reared
D. longicaudata is apparently not affected by host size [
43,
44], but larger
A. ludens hosts are known to produce female-biased sex ratios under superparasitism conditions [
44]. Several studies have addressed the role of host quality (i.e., fruit fly larvae) on the fitness and performance of adult parasitoids, and how in turn the rearing media used to produce the fly larvae determines to a large extend their quality [
45,
46,
47,
48]. Future studies on the effects of the artificial larval diet of tephritid flies on hymenopteran parasitoids used in integrated pest management programs, should consider the examination of other quality parameters of parasitoids such as fecundity and longevity in the absence of food [
43].
Our research provides novel gel diet formulations devoid of the troublesome corncob powder bulking agent and reduced in yeast content that could contribute to improving the cost-effectiveness of the SIT and biological control programs against
A. ludens in Mexico. Gel diets developed in this study could also be used as cost-effective generic diets for rearing other pestiferous
Anastrepha spp. [
10,
49]. Having cost-effective diets for mass-rearing fruit flies becomes particularly relevant under current climate change conditions that is expanding the distribution and host range of pestiferous flies [
6,
50], as this may require increasing the mass production of artificially-reared sterile flies and parasitoids to protect fruit growing areas that were not previously attacked.