Spittlebugs of Mediterranean Olive Groves: Host-Plant Exploitation throughout the Year
Abstract
1. Introduction
2. Materials and Methods
2.1. Survey Sites
2.2. Sampling of Nymphal Stages
2.3. Sampling of Adult Stage
2.4. Statistical Analysis
3. Results
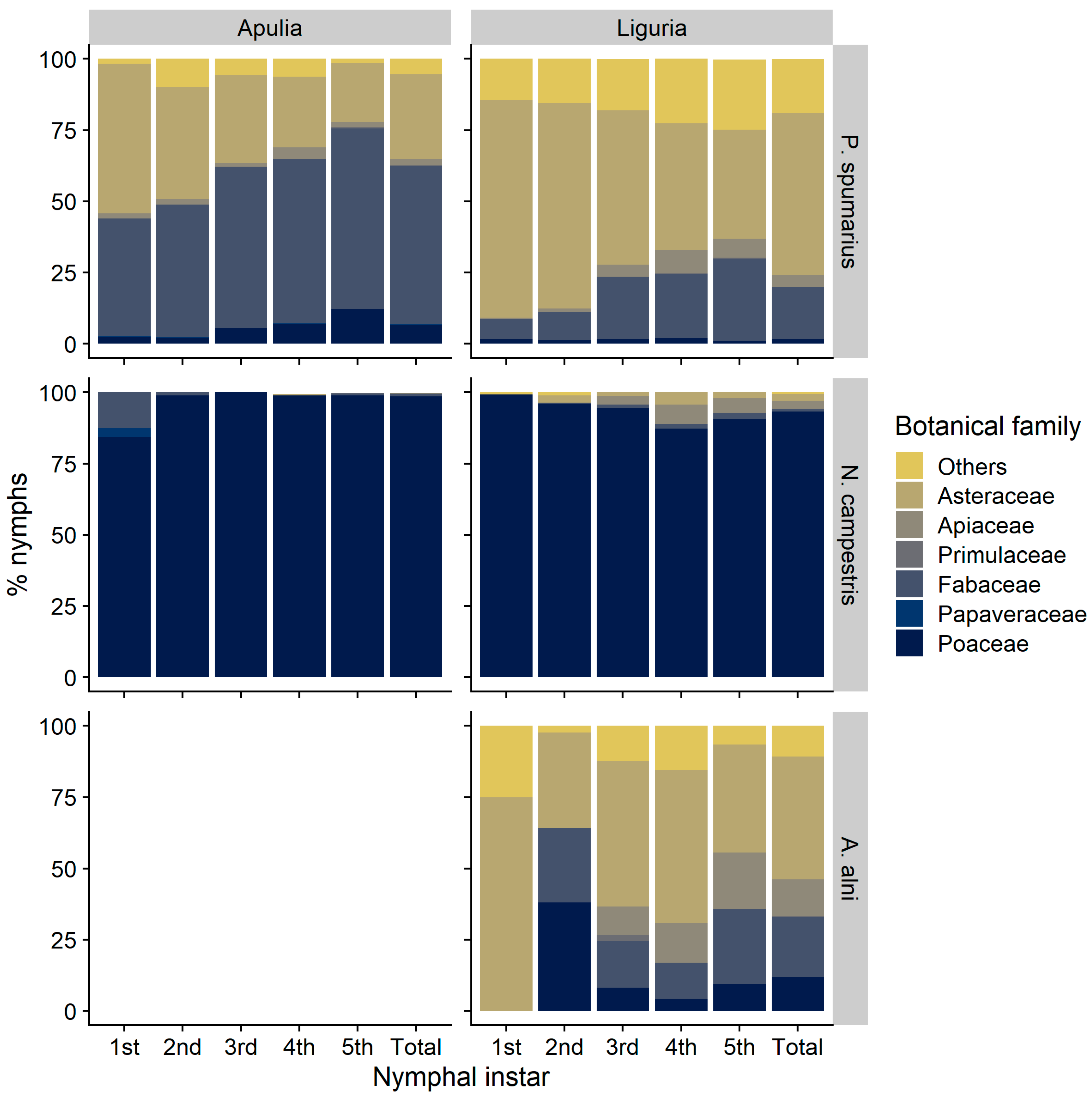
3.1. Nymphal Stages
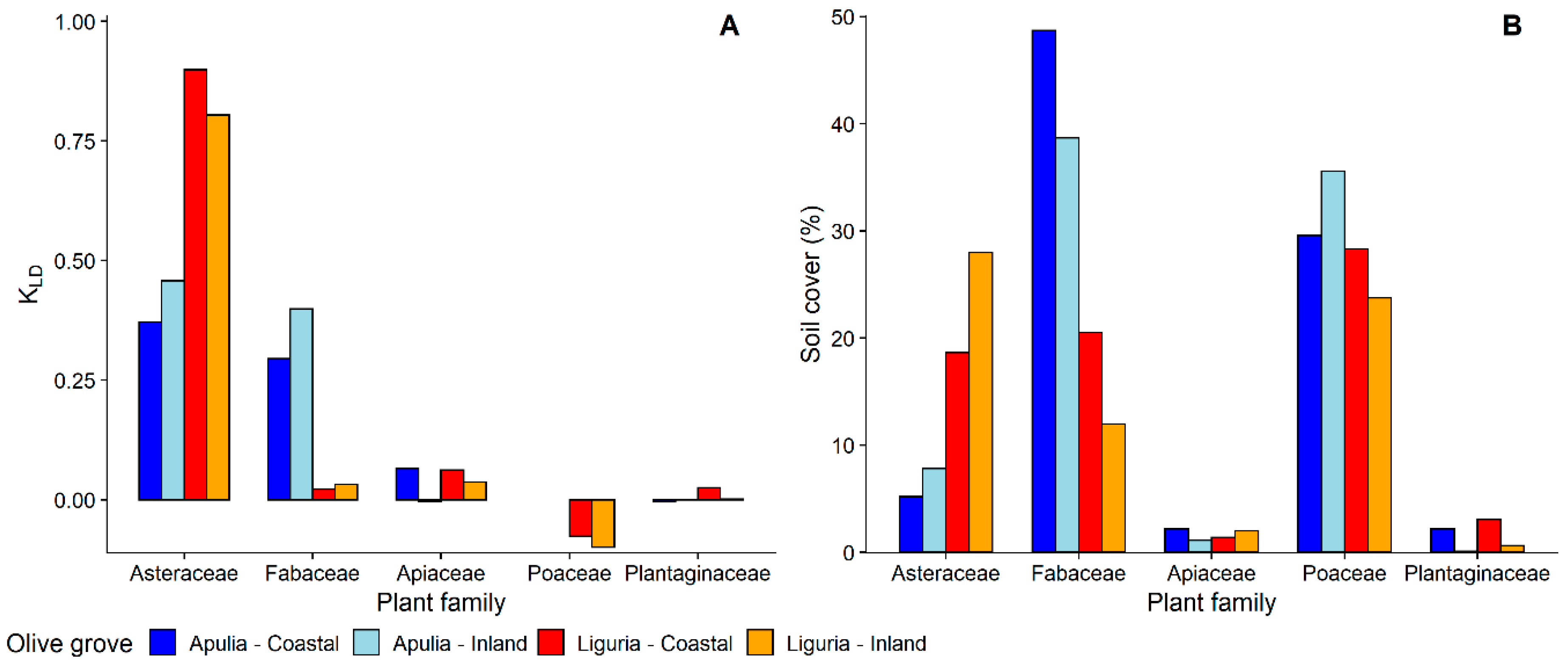
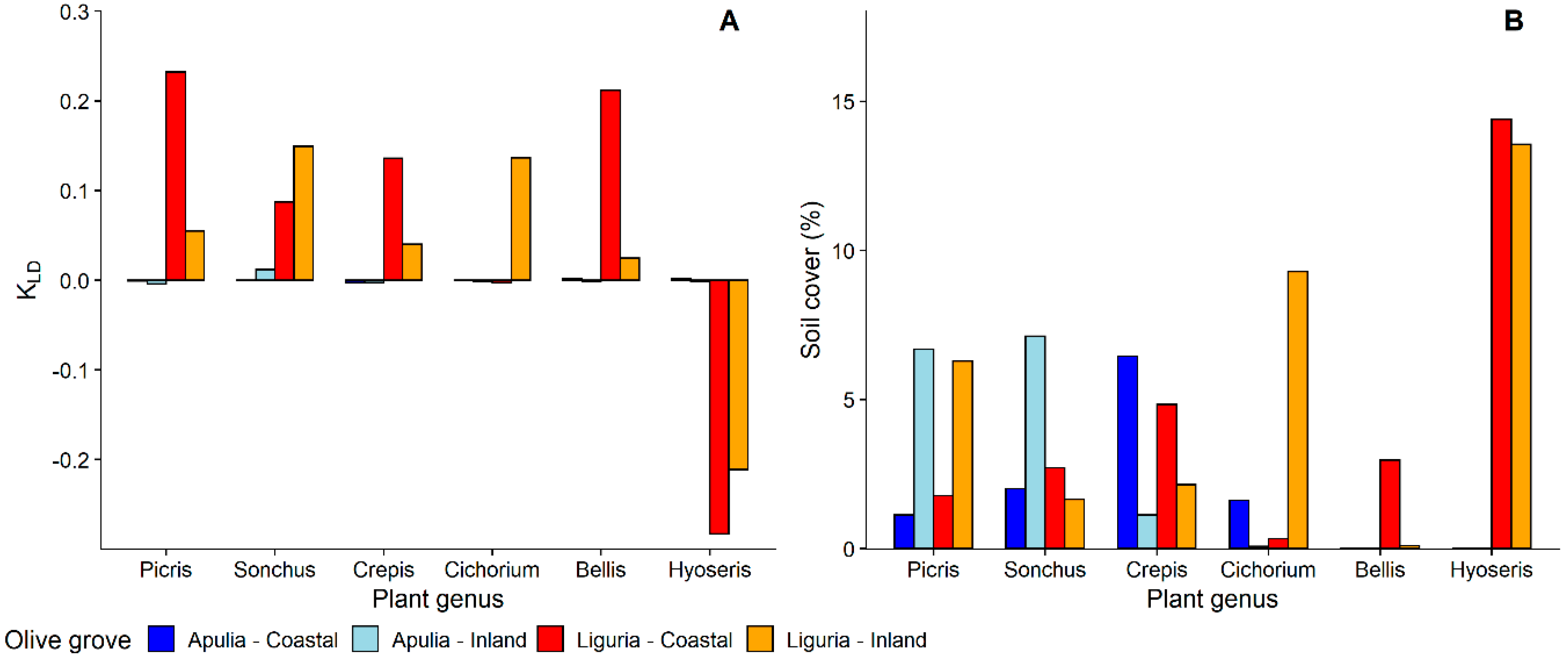
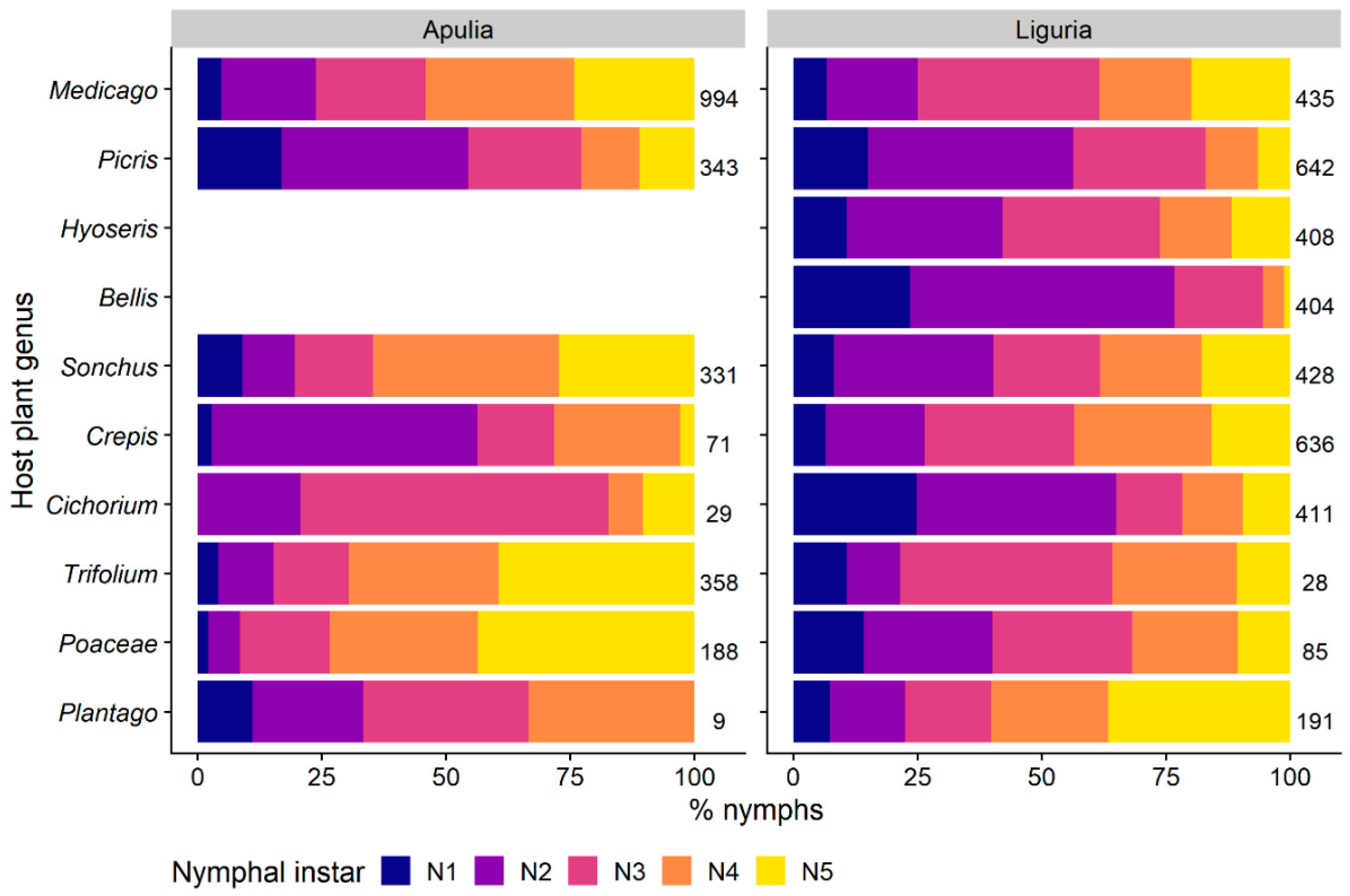
3.1.1. Selection and Exploitation of Host-Plants
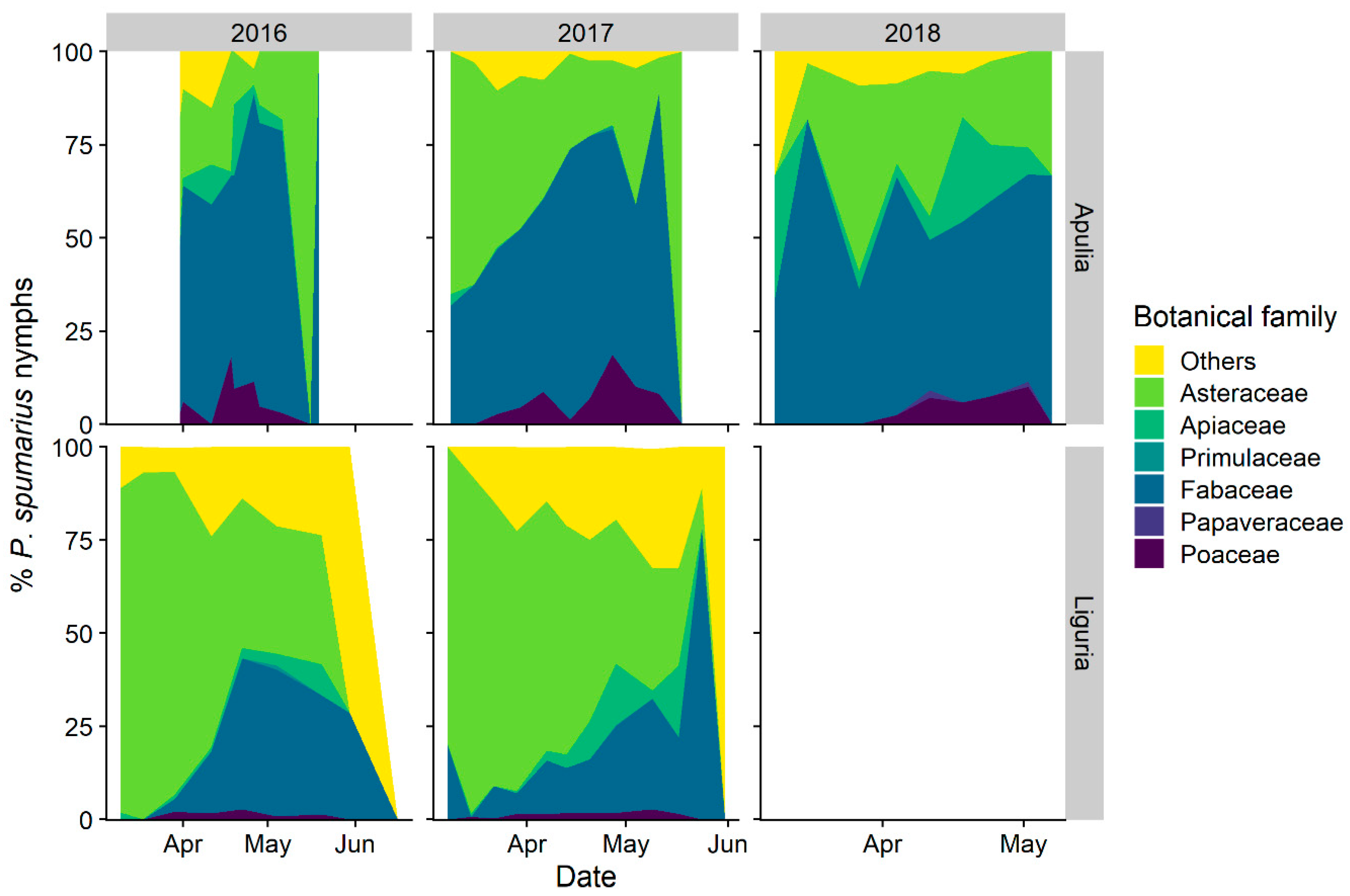
3.1.2. Selection of Host-Plant Taxa during Time
3.1.3. Distribution of Nymphal Instars on Host-Plants
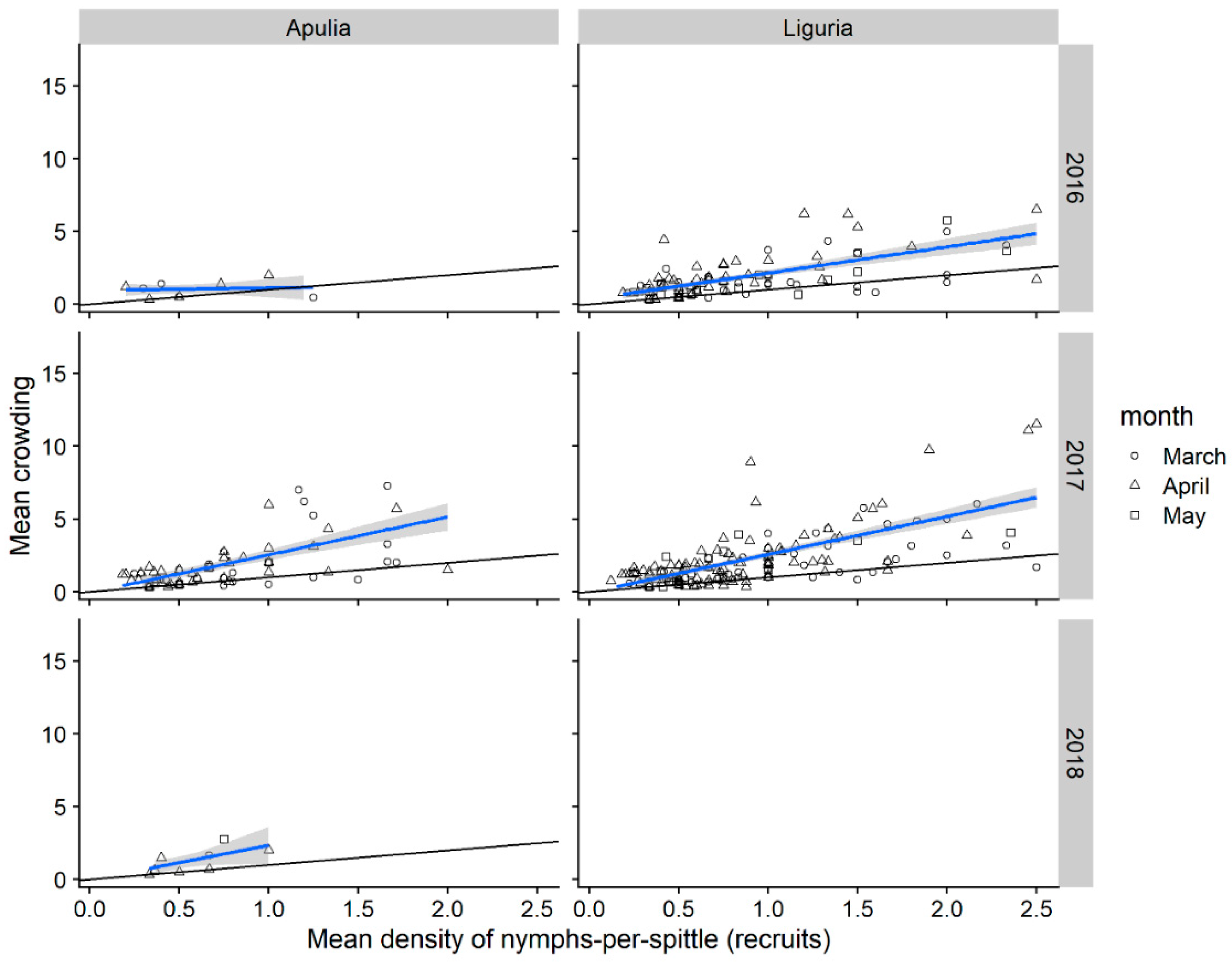
3.1.4. Nymphal Stages Aggregation
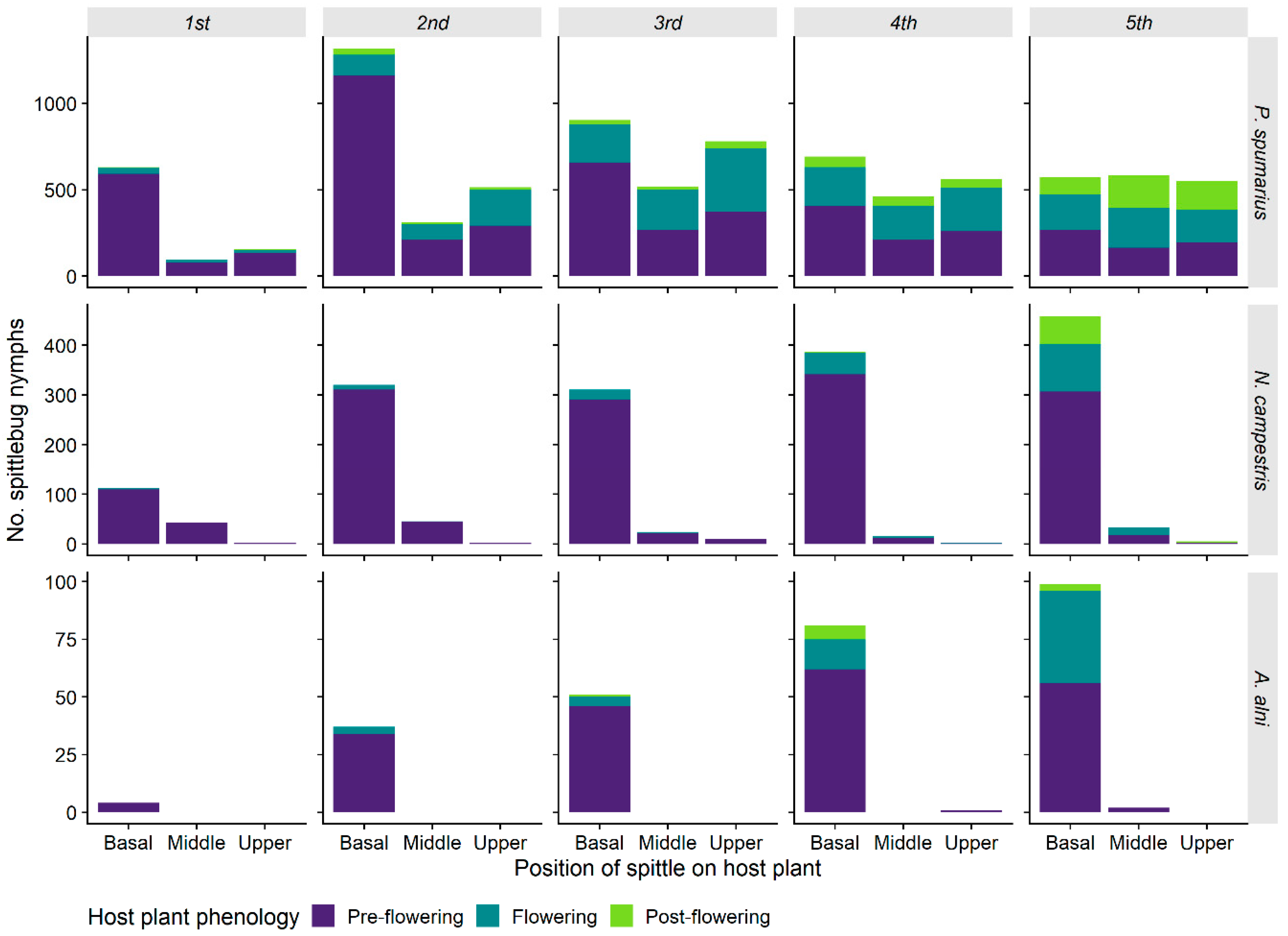
3.1.5. Spittle Position vs. Phenology of the Plant and Preimaginal Instar
3.2. Adults
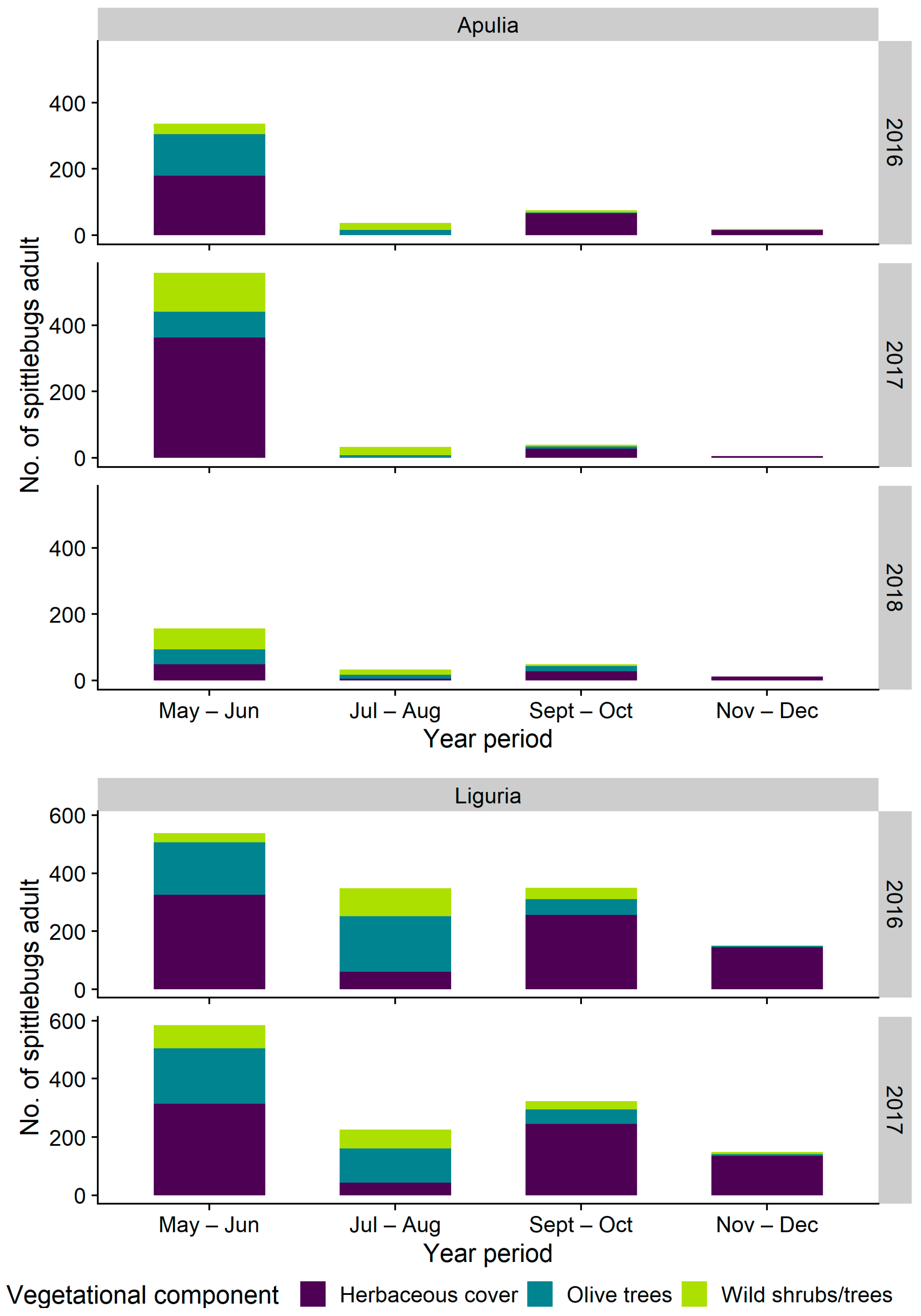
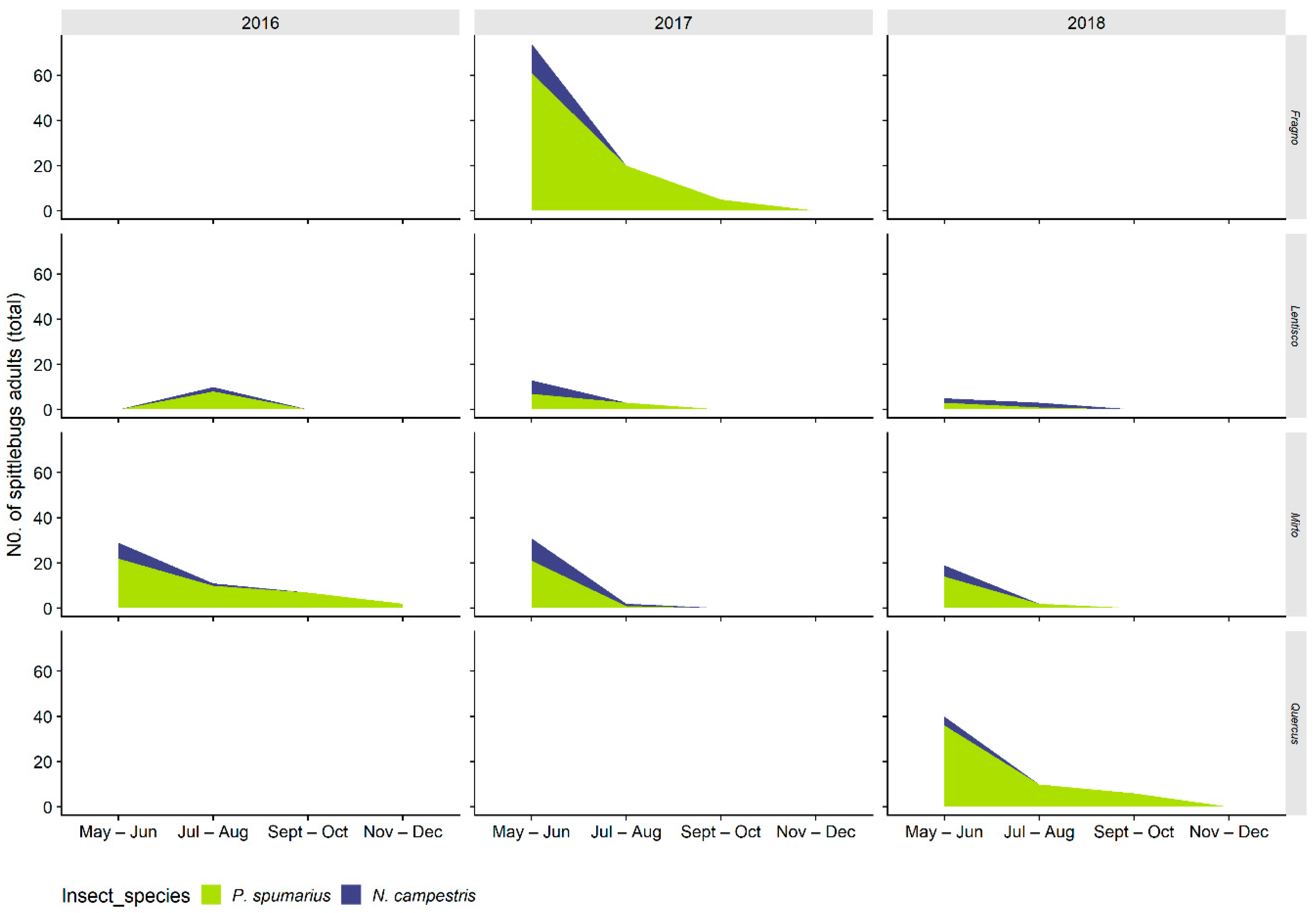
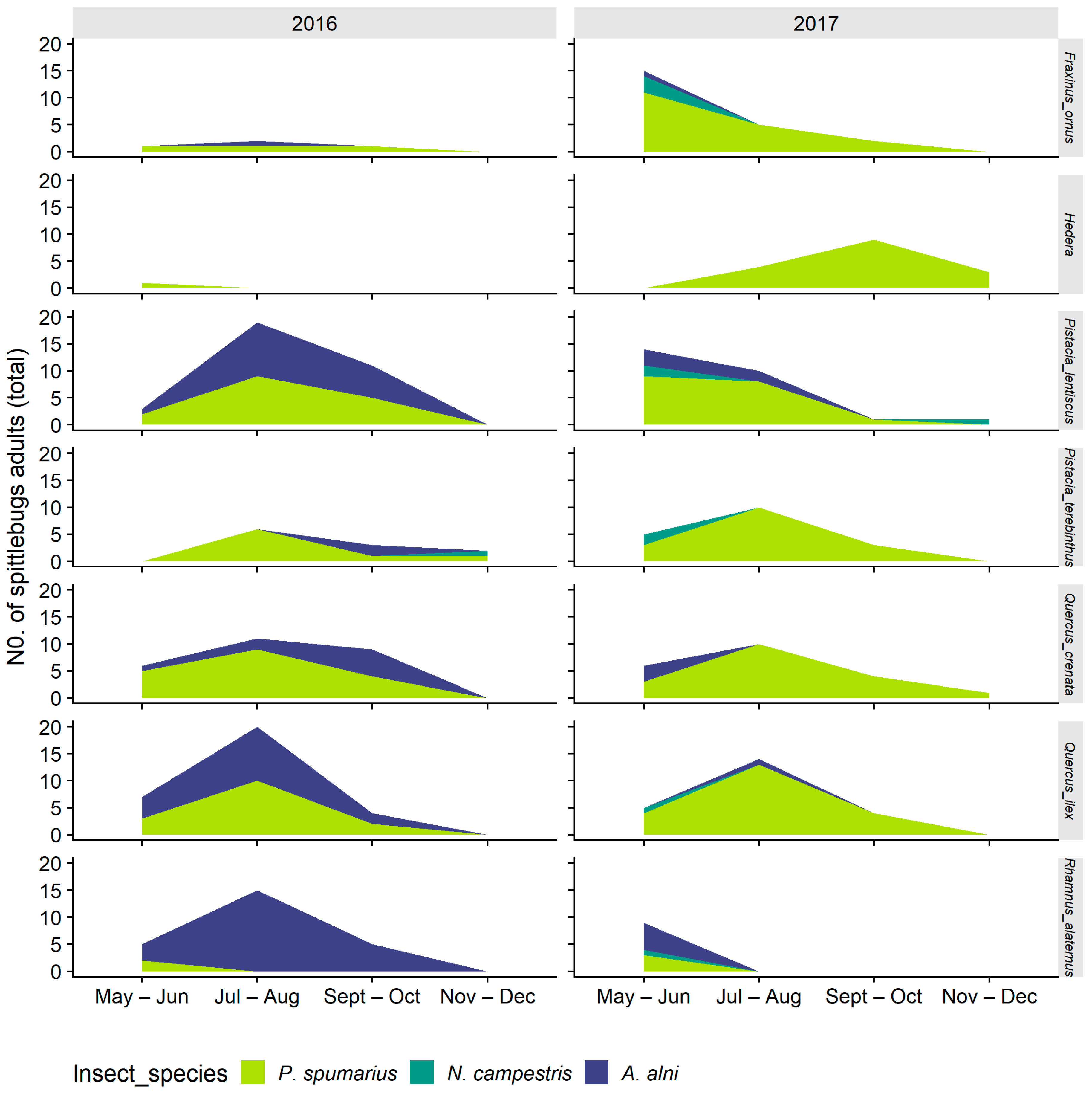
3.2.1. Distribution on Woody Plants—Olive vs. Wild Shrubs/Trees
3.2.2. Abundance of Spittlebugs on Olive According to Crop Phenology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cornara, D.; Saponari, M.; Zeilinger, A.R.; de Stradis, A.; Boscia, D.; Loconsole, G.; Bosco, D.; Martelli, G.P.; Almeida, R.P.P.; Porcelli, F. Spittlebugs as vectors of Xylella fastidiosa in olive orchards in Italy. J. Pest Sci. 2017, 90, 521–530. [Google Scholar] [CrossRef]
- Cornara, D.; Morente, M.; Markheiser, A.; Bodino, N.; Tsai, C.-W.; Fereres, A.; Redak, R.A.; Perring, T.M.; Lopes, J.R.S. An overview on the worldwide vectors of Xylella fastidiosa. Entomol. Gen. 2019, 39, 157–181. [Google Scholar] [CrossRef]
- EFSA Panel on Plant Health (EFSA PLH Panel); Jeger, M.; Caffier, D.; Candresse, T.; Chatzivassiliou, E.; Dehnen-Schmutz, K.; Gilioli, G.; Grégoire, J.; Jaques, M.J.A.; MacLeod, A.; et al. Updated pest categorisation of Xylella fastidiosa. EFSA J. 2018, 16, 5357. [Google Scholar] [CrossRef]
- Cornara, D.; Bosco, D.; Fereres, A. Philaenus spumarius: When an old acquaintance becomes a new threat to European agriculture. J. Pest Sci. 2018, 91, 957–972. [Google Scholar] [CrossRef]
- Ben Moussa, I.E.; Mazzoni, V.; Valentini, F.; Yaseen, T.; Lorusso, D.; Speranza, S.; Digiaro, M.; Varvaro, L.; Krugner, R.; D’Onghia, A.M. Seasonal fluctuations of sap-feeding insect species infected by Xylella fastidiosa in apulian olive groves of southern Italy. J. Econ. Entomol. 2016, 109, 1512–1518. [Google Scholar] [CrossRef] [PubMed]
- Morente, M.; Cornara, D.; Plaza, M.; Durán, J.M.; Capiscol, C.; Trillo, R.; Ruiz, M.; Ruz, C.; Sanjuan, S.; Pereira, J.A.; et al. Distribution and relative abundance of insect vectors of Xylella fastidiosa in olive groves of the iberian Peninsula. Insects 2018, 9, 175. [Google Scholar] [CrossRef] [PubMed]
- Tsagkarakis, A.E.; Afentoulis, D.G.; Matared, M.; Thanou, Z.N.; Stamatakou, G.D.; Kalaitzaki, A.P.; Tzobanoglou, D.K.; Goumas, D.; Trantas, E.; Zarboutis, I.; et al. Identification and seasonal abundance of Auchenorrhyncha with a focus on potential insect vectors of Xylella fastidiosa in olive orchards in three regions of Greece. J. Econ. Entomol. 2018, 111, 2536–2545. [Google Scholar] [CrossRef] [PubMed]
- Dongiovanni, C.; Cavalieri, V.; Bodino, N.; Tauro, D.; Di Carolo, M.; Fumarola, G.; Altamura, G.; Lasorella, C.; Bosco, D. Plant selection and population trend of spittlebug immatures (Hemiptera: Aphrophoridae) in olive groves of the Apulia region of Italy. J. Econ. Entomol. 2019, 112, 67–74. [Google Scholar] [CrossRef]
- Redak, R.A.; Purcell, A.H.; Lopes, J.R.S.; Blua, M.J.; Mizell, R.F., III; Andersen, P.C. The biology of xylem fluid-feeding insect vectors of Xylella fastidiosa and their relation to disease epidemiology. Annu. Rev. Entomol. 2004, 49, 243–270. [Google Scholar] [CrossRef]
- Esteves, M.B.; Kleina, H.T.; Sales, T.d.M.; Oliveira, T.P.; de Lara, I.A.R.; Almeida, R.; Coletta-Filho, H.; Lopes, J.S. Transmission efficiency of Xylella fastidiosa subsp. pauca sequence types by sharpshooter vectors after in vitro acquisition. Phytopathology 2018, 109, 286–293. [Google Scholar] [CrossRef]
- Sicard, A.; Zeilinger, A.R.; Vanhove, M.; Schartel, T.E.; Beal, D.J.; Daugherty, M.P.; Almeida, R.P.P. Xylella fastidiosa: Insights into an Emerging Plant Pathogen. Annu. Rev. Phytopathol. 2018, 56, 181–202. [Google Scholar] [CrossRef] [PubMed]
- Halkka, O.; Raatikainen, M.; Vasarainen, A.; Heinonen, L. Ecology and ecological genetics of Philaenus spumarius (L.) (Homoptera). Ann. Zool. Fenn. 1967, 4, 1–18. [Google Scholar]
- Nickel, H.; Remane, R. Check list of the planthoppers and leafhoppers of Germany, with notes on food plants, diet width, life cycles, geographic range and conservation status (Hemiptera, Fulgoromorpha and Cicadomorpha). Beitr. Zur. Zikadenkunde 2002, 5, 27–64. [Google Scholar]
- Eyre, M.D. Habitat diversity in the conservation of the grassland Auchenorrhyncha (Homoptera: Cercopidae, Cicadellidae, Cixidae, Delphacidae) of Northern Britain. J. Insect Conserv. 2005, 9, 309–317. [Google Scholar] [CrossRef]
- Capinera, J.L.; Capinera, J.L. Encyclopedia of entomology. Volume 4, S-Z. Available online: http://www.cabdirect.org/abstracts/20083298254.html?resultNumber=4&q=capinera+yr%3A2008 (accessed on 24 February 2012).
- Weaver, C.; King, D. Meadow spittlebug Philaenus leucophthalmus (L.). Ohio Agric. Exp. Stn. Res. Bull. 1954, 741, 1–99. [Google Scholar]
- Whittaker, J.B. Cercopid spittle as a microhabitat. Oikos 1970, 21, 59. [Google Scholar] [CrossRef]
- Chen, X.; Meyer-Rochow, V.B.; Fereres, A.; Morente, M.; Liang, A.-P. The role of biofoam in shielding spittlebug nymphs (Insecta, Hemiptera, Cercopidae) against bright light. Ecol. Entomol. 2018, 43, 273–281. [Google Scholar] [CrossRef]
- Burrows, M. Jumping performance of froghopper insects. J. Exp. Biol. 2006, 209, 4607–4621. [Google Scholar] [CrossRef]
- Horsfield, D. Evidence for xylem feeding by Philaenus spumarius (L.) (Homoptera: Cercopidae). Entomol. Exp. Appl. 1978, 24, 95–99. [Google Scholar] [CrossRef]
- Ponder, K.L.; Watson, R.J.; Malone, M.; Pritchard, J. Mineral content of excreta from the spittlebug Philaenus spumarius closely matches that of xylem sap. New Phytol. 2002, 153, 237–242. [Google Scholar] [CrossRef]
- Saponari, M.; Loconsole, G.; Cornara, D.; Yokomi, R.K.; Stradis, A.D.; Boscia, D.; Bosco, D.; Martelli, G.P.; Krugner, R.; Porcelli, F. Infectivity and transmission of Xylella fastidiosa by Philaenus spumarius (Hemiptera: Aphrophoridae) in Apulia, Italy. J. Econ. Entomol. 2014, 107, 1316–1319. [Google Scholar] [CrossRef]
- Cornara, D.; Cavalieri, V.; Dongiovanni, C.; Altamura, G.; Palmisano, F.; Bosco, D.; Porcelli, F.; Almeida, R.P.P.; Saponari, M. Transmission of Xylella fastidiosa by naturally infected Philaenus spumarius (Hemiptera, Aphrophoridae) to different host plants. J. Appl. Entomol. 2017, 141, 80–87. [Google Scholar] [CrossRef]
- Drosopoulos, S.; Maryańska-Nadachowska, A.; Kuznetsova, V.G. The Mediterranean: Area of origin of polymorphism and speciation in the spittlebug Philaenus (Hemiptera, Aphrophoridae). Zoosystematics Evol. 2010, 86, 125–128. [Google Scholar] [CrossRef]
- Bodino, N.; Cavalieri, V.; Dongiovanni, C.; Plazio, E.; Saladini, M.A.; Volani, S.; Simonetto, A.; Fumarola, G.; Carolo, M.D.; Porcelli, F.; et al. Phenology, seasonal abundance and stage-structure of spittlebug (Hemiptera: Aphrophoridae) populations in olive groves in Italy. Sci. Rep. 2019, 9, 17725. [Google Scholar] [CrossRef] [PubMed]
- EFSA (European Food Safety Authority); Vos, S.; Camilleri, M.; Diakaki, M.; Lázaro, E.; Parnell, S.; Schenk, M.; Schrader, G.; Vicent, A. Pest survey card on Xylella fastidiosa. EFSA Supporting Publ. 2019, 16, 1667E. [Google Scholar]
- Halkka, O.; Raatikainen, M.; Halkka, L.; Raatikainen, T. Coexistence of four species of spittle-producing Homoptera. Ann. Zool. Fenn. 1977, 16, 228–231. [Google Scholar]
- Thompson, V. Spittlebug indicators of nitrogen-fixing plants. Ecol. Entomol. 1994, 19, 391–398. [Google Scholar] [CrossRef]
- Raven, J.A. Phytophages of xylem and phloem: A comparison of animal and plant sap-feeders. In Advances in Ecological Research; Elsevier: Amsterdam, The Netherlands, 1983; Volume 13, pp. 135–234. [Google Scholar]
- Owen, D.F. native and alien plants in the diet of Philaenus spumarius (L.) (homoptera: Cercopidae). Entomol. Gaz. 1998, 39, 2. [Google Scholar]
- Ossiannilsson, F. The Auchenorrhyncha (Homoptera) of Fennoscandia and Denmark, 2: The families Cicadidae, Cercopidae, Membracidae, and Cicadellidae (excl. Deltocephalinae) [Sweden, Norway, Finland]. Fauna Entomol. Scand. Den. V 72 1981, 7, 223–593. [Google Scholar]
- Wiegert, R.G. The ingestion of xylem sap by meadow spittlebugs, Philaenus spumarius (L.). Am. Midl. Nat. 1964, 71, 422. [Google Scholar] [CrossRef]
- Latini, A.; Foxi, C.; Borfecchia, F.; Lentini, A.; De Cecco, L.; Iantosca, D.; Serafini, M.; Laneri, U.; Citterio, M.; Campiotti, A.; et al. Tacking the vector of Xylella fastidiosa: Geo-statistical analysis of long-term field observations on host plants influencing the distribution of Phylaenus spumarius nymphs. Environ. Sci. Pollut. Res. 2019, 26, 6503–6516. [Google Scholar] [CrossRef] [PubMed]
- Maryańska-Nadachowska, A.; Drosopoulos, S.; Lachowska, D.; Kajtoch, Ł.; Kuznetsova, V.G. Molecular phylogeny of the Mediterranean species of Philaenus (Hemiptera: Auchenorrhyncha: Aphrophoridae) using mitochondrial and nuclear DNA sequences. Syst. Entomol. 2010, 35, 318–328. [Google Scholar] [CrossRef]
- Cavalieri, V.; Altamura, G.; Fumarola, G.; di Carolo, M.; Saponari, M.; Cornara, D.; Bosco, D.; Dongiovanni, C. Transmission of Xylella fastidiosa subspecies pauca sequence type 53 by different insect species. Insects 2019, 10, 324. [Google Scholar] [CrossRef] [PubMed]
- Panzavolta, T.; Bracalini, M.; Croci, F.; Ghelardini, L.; Luti, S.; Campigli, S.; Goti, E.; Marchi, R.; Tiberi, R.; Marchi, G. Philaenus italosignus a potential vector of Xylella fastidiosa: Occurrence of the spittlebug on olive trees in Tuscany (Italy). Bull. Insectology 2019, 72, 317–320. [Google Scholar]
- Lopes, J.R.S.; Landa, B.B.; Fereres, A. A survey of potential insect vectors of the plant pathogenic bacterium Xylella fastidiosa in three regions of Spain. Span. J. Agric. Res. 2014, 12, 795–800. [Google Scholar] [CrossRef]
- Mazzoni, V. Contribution to the knowledge of the Auchenorrhyncha (Hemiptera Fulgoromorpha and Cicadomorpha) of Tuscany (Italy). Redia 2005, 88, 85–102. [Google Scholar]
- Whittaker, J.B. Density regulation in a population of Philaenus spumarius (L.) (Homoptera: Cercopidae). J. Anim. Ecol. 1973, 42, 163–172. [Google Scholar] [CrossRef]
- Biedermann, R. Aggregation and survival of Neophilaenus albipennis (Hemiptera: Cercopidae) spittlebug nymphs. Eur. J. Entomol. 2003, 100, 493–500. [Google Scholar] [CrossRef]
- Vilbaste, J. Preliminary key for the identification of the nymphs of North European Homoptera Cicadinea. II. Cicadelloidea. Ann. Zool. Fenn. 1982, 19, 1–20. [Google Scholar]
- Pignatti, S. Flora d’Italia; 3 vols; Bologna Edagricole: Bologna, Italy, 1982. [Google Scholar]
- Bodino, N.; Plazio, E.; Picciau, L.; Cavalieri, V.; Dongiovanni, C.; Di Carolo, M.; Tauro, D.; Volani, S.; Salerno, M.; Russo, V.; et al. Phenology population dynamics and host plants of Philaenus spumarius in Italian olive groves. In Proceedings of the European Conference on Xylella Fastidiosa: Finding Answers To a Global Problem, Palma de Mallorca, Spain, 13–15 November 2017; p. 63. [Google Scholar]
- Sanz-Cortés, F.; Martinez-Calvo, J.; Badenes, M.L.; Bleiholder, H.; Hack, H.; Llacer, G.; Meier, U. Phenological growth stages of olive trees (Olea europaea). Ann. Appl. Biol. 2002, 140, 151–157. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Kullback, S.; Leibler, R.A. On information and sufficiency. Ann. Math. Stat. 1951, 22, 79–86. [Google Scholar] [CrossRef]
- Canty, A. Ripley Brian Boot: Bootstrap R (S-Plus) Functions, R Package Version 1.3-22; Available online: https://cran.r-project.org/web/packages/boot/citation.html (accessed on 1 January 2020).
- Lloyd, M. Mean crowding. J. Anim. Ecol. 1967, 36, 1–30. [Google Scholar] [CrossRef]
- Southwood, R.; Henderson, P. Ecological Methods; Blackwell Science: Oxford, UK, 2000. [Google Scholar]
- Iwao, S. A new regression method for analyzing the aggregation pattern of animal populations. Res. Popul. Ecol. 1968, 10, 1–20. [Google Scholar] [CrossRef]
- Mangan, R.L.; Wutz, A. Aggregation patterns of meadow spittlebugs, Philaenus spumarius L. (Homoptera: Cercopidae), on old-field alfalfa plants. Environ. Entomol. 1983, 12, 151–157. [Google Scholar] [CrossRef]
- Raatikainen, M.; Vasarainen, A. Froghoppers (Hom., Cercopidae) in strawberry plantations. Ann. Agric. Fenn. 1970, 9, 290–292. [Google Scholar]
- Yurtsever, S. On the polymorphic meadow spittlebug, Philaenus spumarius (L.) (Homoptera: Cercopidae). Turk. J. Zool. 2000, 24, 447–459. [Google Scholar]
- EFSA Panel on Plant Health (PLH) Scientific Opinion on the risks to plant health posed by Xylella fastidiosa in the EU territory, with the identification and evaluation of risk reduction options. EFSA J. 2015, 13, 3989. [CrossRef]
- Saponari, M.; Giampetruzzi, A.; Loconsole, G.; Boscia, D.; Saldarelli, P. Xylella fastidiosa in Olive in Apulia: Where We Stand. Phytopathology 2018, 109, 175–186. [Google Scholar] [CrossRef]
- White, S.M.; Bullock, J.M.; Hooftman, D.A.P.; Chapman, D.S. Modelling the spread and control of Xylella fastidiosa in the early stages of invasion in Apulia, Italy. Biol. Invasions 2017, 19, 1825–1837. [Google Scholar] [CrossRef]
- Cruaud, A.; Gonzalez, A.-A.; Godefroid, M.; Nidelet, S.; Streito, J.-C.; Thuillier, J.-M.; Rossi, J.-P.; Santoni, S.; Rasplus, J.-Y. Using insects to detect, monitor and predict the distribution of Xylella fastidiosa: A case study in Corsica. Sci. Rep. 2018, 8, 15628. [Google Scholar] [CrossRef]
- Soubeyrand, S.; de Jerphanion, P.; Martin, O.; Saussac, M.; Manceau, C.; Hendrikx, P.; Lannou, C. Inferring pathogen dynamics from temporal count data: The emergence of Xylella fastidiosa in France is probably not recent. New Phytol. 2018, 219, 824–836. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, P.B. Niche partitioning in spittlebugs (Homoptera: Cercopidae) sharing shelters on host plants. Ecology 1986, 67, 465–478. [Google Scholar] [CrossRef]
- Hoffman, G.; McEvoy, P.B. Mechanical limitations on feeding by meadow spittlebugs Philaenus spumarius (Homoptera: Cercopidae) on wild and cultivated host plants. Ecol. Entomol. 1985, 10, 415–426. [Google Scholar] [CrossRef]
- Wise, M.J.; Kieffer, D.L.; Abrahamson, W.G. Costs and benefits of gregarious feeding in the meadow spittlebug, Philaenus spumarius. Ecol. Entomol. 2006, 31, 548–555. [Google Scholar] [CrossRef]
- Pires, C.S.S.; Price, P.W.; Fontes, E.G. Preference–performance linkage in the neotropical spittlebug Deois flavopicta, and its relation to the Phylogenetic Constraints Hypothesis. Ecol. Entomol. 2000, 25, 71–80. [Google Scholar] [CrossRef]
- Craig, T.P.; Ohgushi, T. Preference and performance are correlated in the spittlebug Aphrophora pectoralis on four species of willow. Ecol. Entomol. 2002, 27, 529–540. [Google Scholar] [CrossRef]
- Drossopoulos, J.B.; Niavis, C.A. Seasonal changes of the metabolites in the leaves, bark and xylem tissues of olive tree (Olea europaea L.) I. Nitrogenous compounds. Ann. Bot. 1988, 62, 313–320. [Google Scholar] [CrossRef]
- Santoiemma, G.; Tamburini, G.; Sanna, F.; Mori, N.; Marini, L. Landscape composition predicts the distribution of Philaenus spumarius, vector of Xylella fastidiosa, in olive groves. J. Pest Sci. 2019, 92, 1101–1109. [Google Scholar] [CrossRef]
- Kairis, O.; Karavitis, C.; Kounalaki, A.; Salvati, L.; Kosmas, C. The effect of land management practices on soil erosion and land desertification in an olive grove. Soil Use Manag. 2013, 29, 597–606. [Google Scholar] [CrossRef]


| Apulia | Liguria | ||||
|---|---|---|---|---|---|
| 2016 | 2017 | 2018 | 2016 | 2017 | |
| Nymps-per-spittle | |||||
| Mean crowdedness (x) | 0.107 | 1.18 | 0.229 | 0.812 | 0.758 |
| Patchiness (P) | 0.1 | 0.849 | 0.204 | 0.545 | 0.522 |
| intercept | 0.315 ± 0.475 | −0.388 ± 0.175 | −0.360 ± 0.424 | −0.208 ± 0.119 | −0.170 ± 0.116 |
| slope | 0.733 ± 0.259 | 2.526 ± 0.143 | 2.488 ± 0.190 | 2.244 ± 0.119 | 2.373 ± 0.118 |
| R2 Adjusted | 0.085 | 0.579 | 0.593 | 0.671 | 0.634 |
| p value | 0.144 | >0.001 | 0.003 | >0.001 | >0.001 |
| Nymps-per-quadrat | |||||
| Mean crowdedness (x) | 1.93 | 5.02 | 3.67 | 8.08 | 10.1 |
| Patchiness (P) | 0.84 | 1.2 | 1.11 | 1.17 | 1.43 |
| intercept | −3 ± 0.468 | −1.41 ± 0.081 | 0.517 ± 0.331 | 0.064 ± 0.125 | 0.811 ± 0.103 |
| slope | 2.596 ± 1.304 | 1.722 ± 0.424 | 1.309 ± 1.289 | 1.541 ± 0.983 | 1.485 ± 0.951 |
| R2 Adjusted | 0.768 | 0.964 | 0.53 | 0.914 | 0.916 |
| p value | 0.001 | >0.001 | 0.002 | >0.001 | >0.001 |
| Fixed Effects | χ2 | df | p-Value |
|---|---|---|---|
| Spittle position | 707.05 | 2 | <0.001 |
| Phenology host-plant | 1403.3 | 2 | <0.001 |
| Instar | 365.17 | 4 | <0.001 |
| Spittle position × henology host-plant | 342.62 | 4 | <0.001 |
| Spittle position × instar | 316.88 | 8 | <0.001 |
| Phenology host-plant × instar | 971.1 | 8 | <0.001 |
| Spittle position × phenology host-plant × instar | 121.5 | 16 | <0.001 |
| N | 225 | ||
| AIC | 4406.85 | ||
| R2 (fixed) | 0.730 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bodino, N.; Cavalieri, V.; Dongiovanni, C.; Saladini, M.A.; Simonetto, A.; Volani, S.; Plazio, E.; Altamura, G.; Tauro, D.; Gilioli, G.; et al. Spittlebugs of Mediterranean Olive Groves: Host-Plant Exploitation throughout the Year. Insects 2020, 11, 130. https://doi.org/10.3390/insects11020130
Bodino N, Cavalieri V, Dongiovanni C, Saladini MA, Simonetto A, Volani S, Plazio E, Altamura G, Tauro D, Gilioli G, et al. Spittlebugs of Mediterranean Olive Groves: Host-Plant Exploitation throughout the Year. Insects. 2020; 11(2):130. https://doi.org/10.3390/insects11020130
Chicago/Turabian StyleBodino, Nicola, Vincenzo Cavalieri, Crescenza Dongiovanni, Matteo Alessandro Saladini, Anna Simonetto, Stefania Volani, Elisa Plazio, Giuseppe Altamura, Daniele Tauro, Gianni Gilioli, and et al. 2020. "Spittlebugs of Mediterranean Olive Groves: Host-Plant Exploitation throughout the Year" Insects 11, no. 2: 130. https://doi.org/10.3390/insects11020130
APA StyleBodino, N., Cavalieri, V., Dongiovanni, C., Saladini, M. A., Simonetto, A., Volani, S., Plazio, E., Altamura, G., Tauro, D., Gilioli, G., & Bosco, D. (2020). Spittlebugs of Mediterranean Olive Groves: Host-Plant Exploitation throughout the Year. Insects, 11(2), 130. https://doi.org/10.3390/insects11020130








