Mass-Rearing of Drosophila suzukii for Sterile Insect Technique Application: Evaluation of Two Oviposition Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Drosophila suzukii Colony
2.2. Oviposition Systems
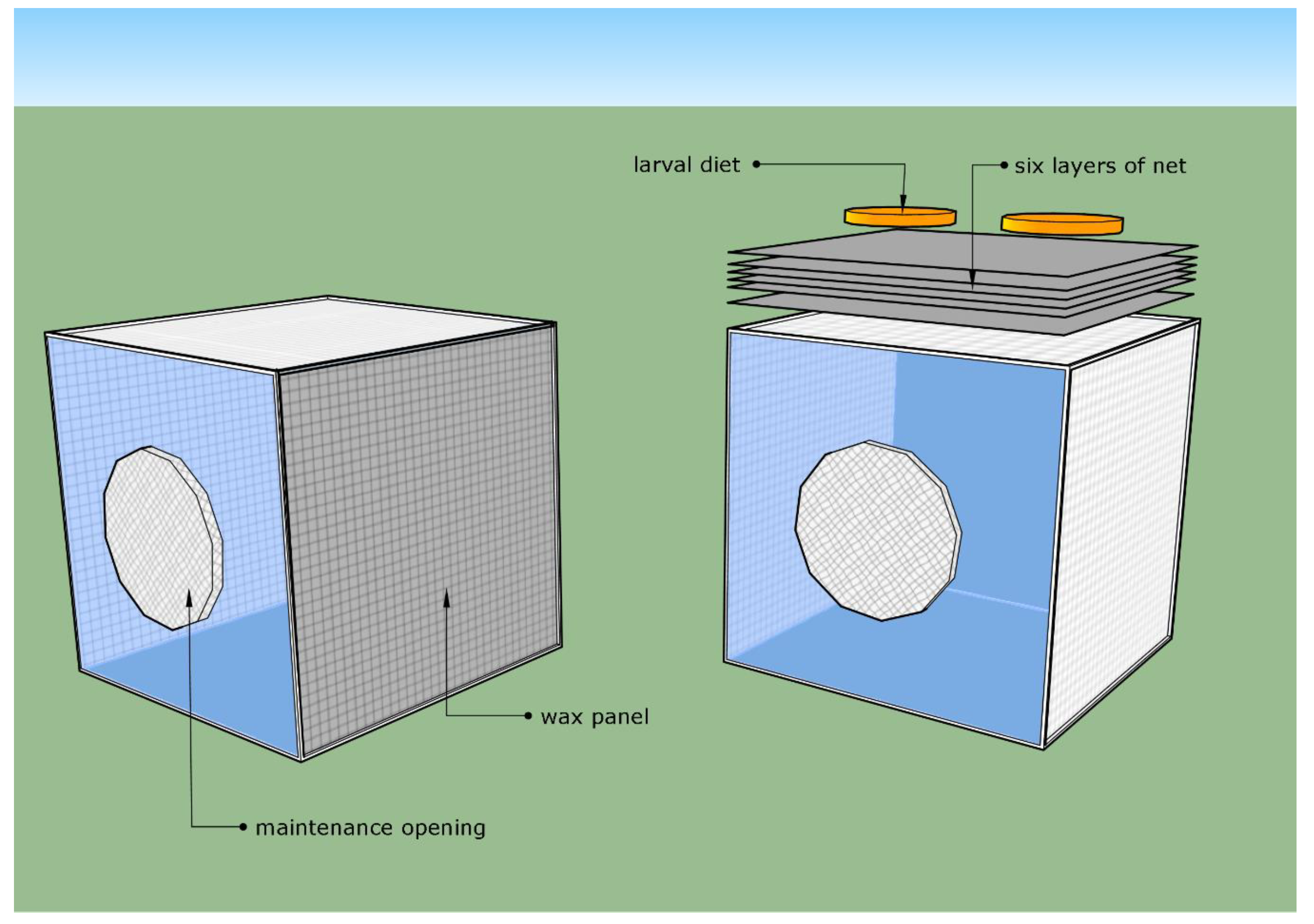
2.2.1. Net System
2.2.2. Wax Panel
2.3. Evaluation of the Artificial Oviposition Systems
2.4. Egg Production and Larvae Rearing
- Egg hatch: closed eggs were counted 48 hours after the collection day;
- Egg to pupa time: 4–5 days after egg collection, Petri dishes were checked for pupae (cryptocephalic pupae) to assess the duration of larval development;
- Pupa recovery: about 11 ± 1 days after the egg-collection day, pupae were removed from the diet, counted, placed on moist paper, and left in sealed boxes without water and food for adult emergence;
- Adult production: once dead, the emerged adults were removed from the boxes and then counted and sexed under stereoscope.
2.5. Adult Emergence and Flight Ability
2.6. Data Analysis
3. Results
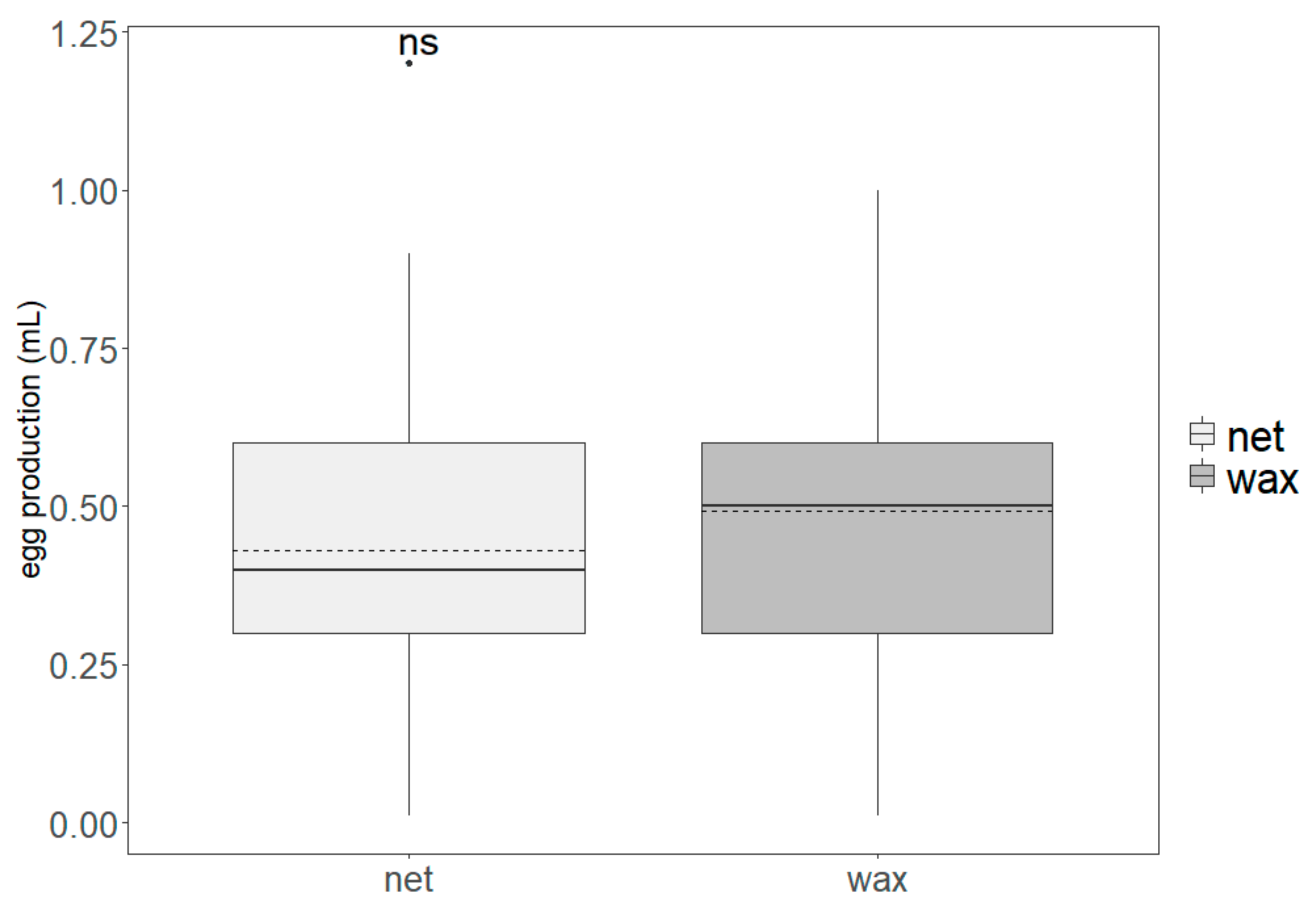
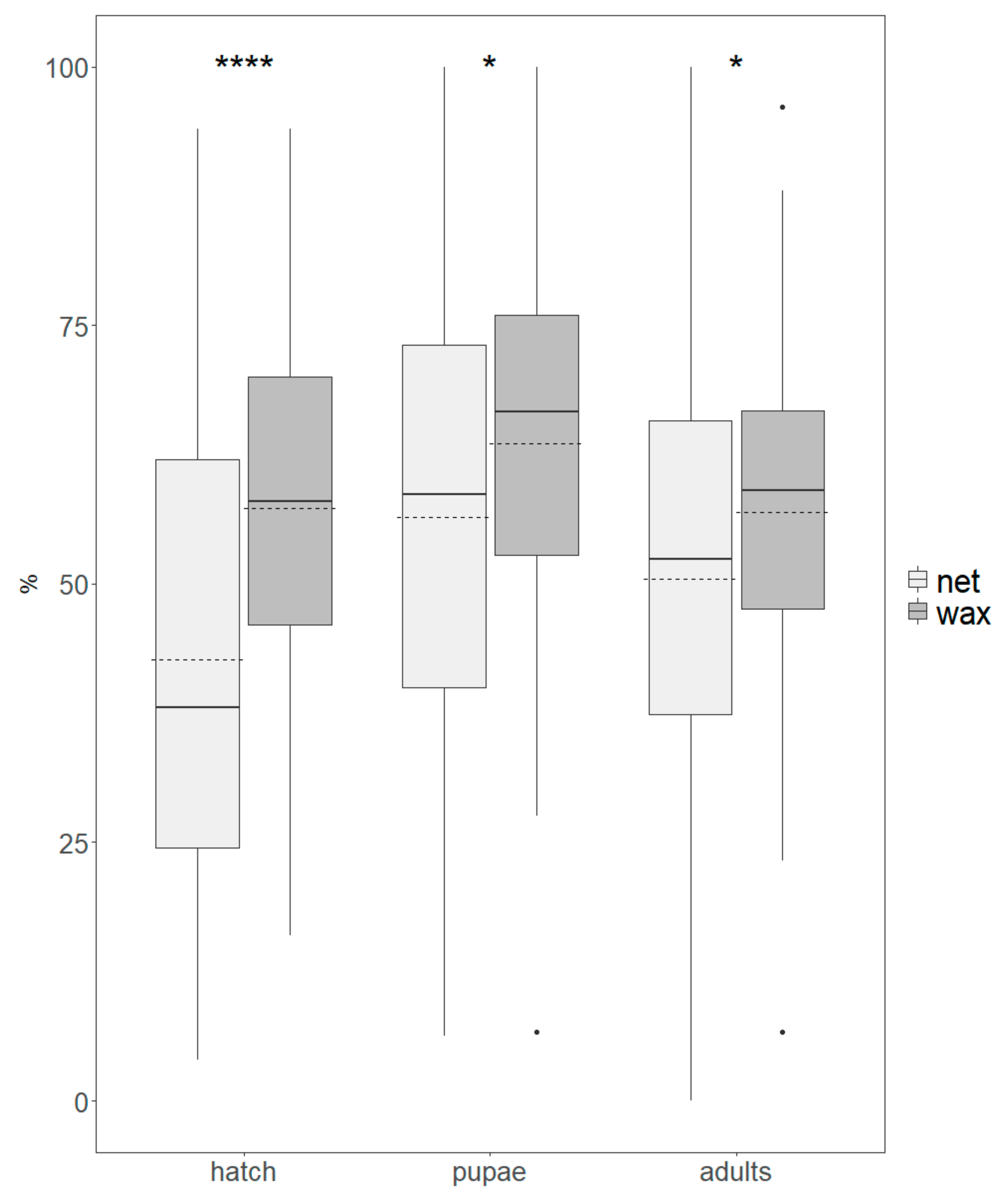
3.1. Production Parameters
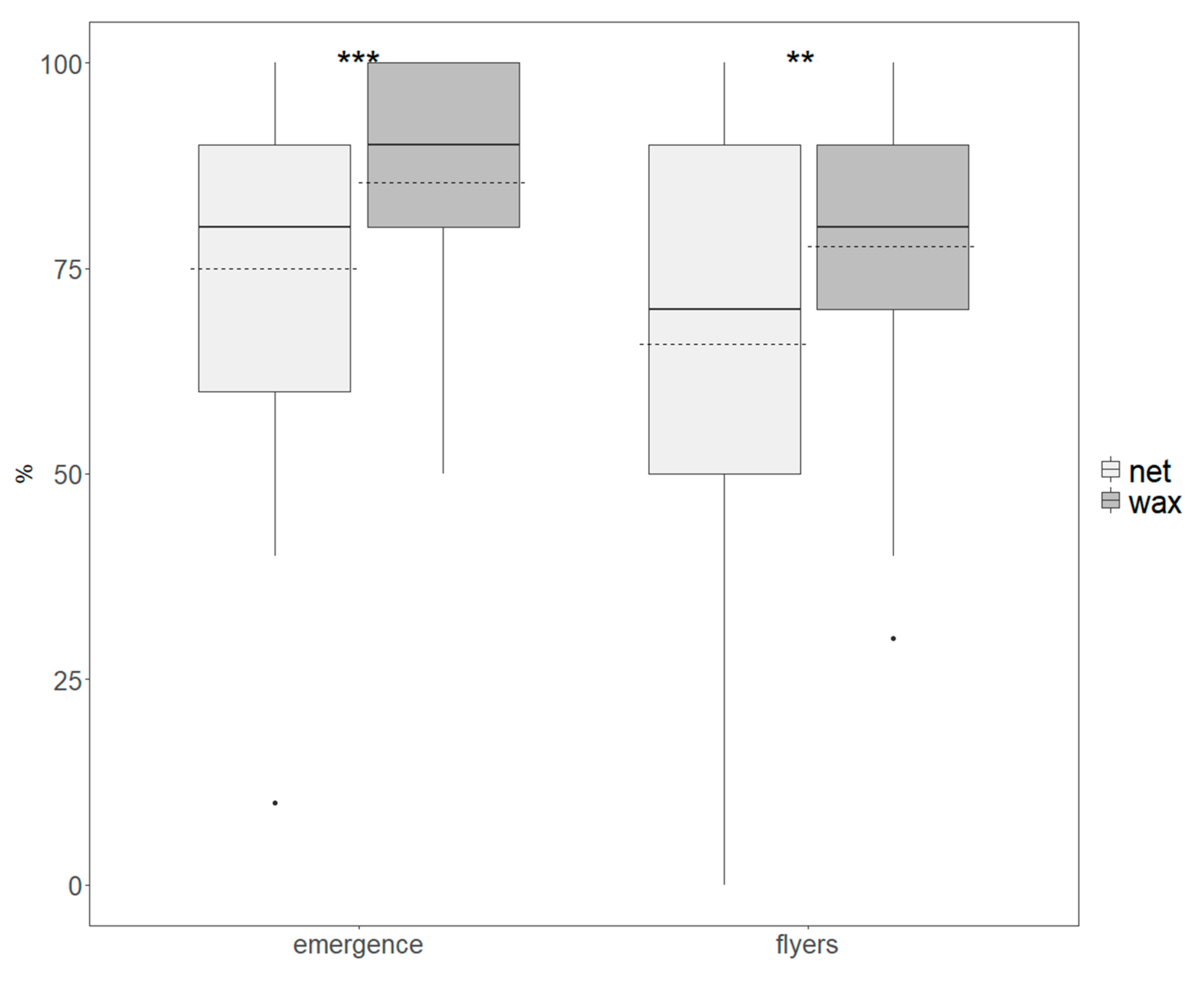
3.2. Quality Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO; IAEA; USDA. Product Quality Control for Sterile Mass-Reared and Released Tephritid Fruit Flies; International Atomic Energy Agency: Vienna, Austria, 2014; p. 164, Version 6.
- FAO; IAEA; USDA. Manual for Product Quality Control and Shipping Procedures for Sterile Mass-Reared Tephritid Fruit Flies; International Atomic Energy Agency: Vienna, Austria, 2003; p. 85, Version 5.
- Cáceres, C.; Mohammad Islam, S.; Ahmad, S.; Ramírez, E.; Wornoayporn, V. A protocol for storage and long-distance shipment of Mediterranean fruit fly (Diptera: Tephitidae) eggs. I. Effect of temperature, embryo age, and storage time on survival and quality. Fla. Entomol. 2007, 90, 103–109. [Google Scholar] [CrossRef]
- Thistlewood, H.M.A.; Judd, G.J.R. Twenty-five years of research experience with the sterile insect technique and area-wide management of codling moth, Cydia pomonella (L.), in Canada. Insects 2019, 10, 292. [Google Scholar] [CrossRef] [PubMed]
- Gast, R.T. Mass rearing of insects: Its concept, methods, and problems. In Radiation, Radioisotopes, and Rearing Methods in the Control of Insect Pests; International Atomic Energy Agency: Vienna, Austria, 1968; pp. 59–67. [Google Scholar]
- Shelly, T.E.; Whittier, T.S.; Kaneshiro, K.Y. Sterile Insect Release and the Natural Mating System of the Mediterranean Fruit Fly, Ceratitis capitata (Diptera: Tephritidae). Ann. Entomological Soc. Am. 1994, 87, 470–481. [Google Scholar]
- Boller, E. Behavioral aspects of mass-rearing of insects. Entomophaga 1972, 17, 9–25. [Google Scholar] [CrossRef]
- Calkins, C.O.; Parker, A.G. Sterile insect quality. In Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 269–296. [Google Scholar] [CrossRef]
- Economopoulos, A.P.; Judt, S. Effect of oviposition net hole size and treatment of net with sugar solution or lubricant-release agent on medfly egg production and collection. In Fruit Flies: Proceedings of the second International Symposium, 16–21 September 1986, Colymbari, Crete, Greece/edited by AP Economopoulos; Symposium Organizing Committee: Athens, Greece, Elsevier Science Publ. Co. distributor for USA and Canada; 1987. [Google Scholar]
- Economopoulos, A.P.; Judt, S. Artificial rearing of the Mediterranean fruit fly (Diptera: Tephritidae): Size of oviposition holes. J. Econ. Entomol. 1989, 82, 668–674. [Google Scholar] [CrossRef]
- Boller, E.F. An artificial oviposition device for the european cherry fruit fly, Rhagoletis cerasi. J. Econ. Entomol. 1968, 61, 850–852. [Google Scholar] [CrossRef]
- Roff, D.A.; Mousseau, T.A. Quantitative genetics and fitness: Lessons from Drosophila. Heredity 1987, 58, 103–118. [Google Scholar] [CrossRef]
- Hoffmann, A.A.; Ross, P.A.; Rašić, G. Wolbachia strains for disease control: Ecological and evolutionary considerations. Evol. Appl. 2015, 8, 751–768. [Google Scholar] [CrossRef]
- Bartlett, A.C. Genetic changes during insect domestication. In Advances and Challanges in Insect Rearing; King, E.G., Leppla, N.C., Eds.; Agricultural Research Service: Washington, DC, USA, 1984; pp. 2–8. [Google Scholar]
- Franz, G. Genetic sexing strains in mediterranean fruit fly, an example for other species amenable to large-scale rearing for the sterile insect technique. In Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management; Springer: Dordrecht, The Netherlands, 2005. [Google Scholar] [CrossRef]
- Dyck, V.A.; Hendrichs, J.; Robinson, A.S. Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management; Springer: Dordrecht, The Netherlands, 2005. [Google Scholar] [CrossRef]
- Montoya, P.; Cancino, J.; Pérez-Lachaud, G.; Liedo, P. Host size, superparasitism and sex ratio in mass-reared Diachasmimorpha longicaudata, a fruit fly parasitoid. BioControl 2011, 56, 11–17. [Google Scholar] [CrossRef]
- Wang, Z.Y.; He, K.L.; Zhang, F.; Lu, X.; Babendreier, D. Mass rearing and release of Trichogramma for Biological control of insect pests of corn in China. Biol. Control 2014, 68, 136–144. [Google Scholar] [CrossRef]
- Balestrino, F.; Puggioli, A.; Carrieri, M.; Bouyer, J.; Bellini, R. Quality control methods for Aedes albopictus sterile male production. PLoS Negl. Trop. Dis. 2017, 11, e0005881. [Google Scholar] [CrossRef]
- Greene, G.L.; Leppla, N.C.; Dickerson, W.A. Velvetbean caterpillar: A rearing procedure and artificial medium. J. Econ. Entomol. 2015, 69, 487–488. [Google Scholar] [CrossRef]
- Dyck, V.A. Rearing codling moth for SIT. In FAO Plant Production and Protection Paper; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2010; p. 50. [Google Scholar]
- Giroud, L.; Thomas, M.; Tomberlin, J.K.K.; Ruiz, A.T.T.; Yi, L.; Morales-Ramos, J.A.; Jullien, R.L.; Han, R.; Cortes Ortiz, J.A.; Rojas, M.G.G.; et al. Insect mass production technologies. In Insects as Sustainable Food Ingredients; Academic Press: Cambridge, MA, USA, 2016; pp. 153–201. [Google Scholar] [CrossRef]
- Cáceres, C.; Hendrichs, J.; Vreysen, M.J.B. Development and improvement of rearing techniques for fruit flies (Diptera: Tephritidae) of economic importance. Int. J. Trop. Insect Sci. 2014, 34, S1–S12. [Google Scholar] [CrossRef]
- Prokopy, R.J.; Boller, E.F. Artificial egging system for the European cherry fruit fly. J. Econ. Entomol. 1970, 63, 1413–1417. [Google Scholar] [CrossRef]
- Ahmad, S.; ul Haq, I.; Rempoulakis, P.; Orozco, D.; Jessup, A.; Cáceres, C.; Paulus, H.; Vreysen, M.J.B. Artificial rearing of the olive fruit fly Bactrocera oleae (Rossi) (Diptera: Tephritidae) for use in the sterile insect technique: Improvements of the egg collection system. Int. J. Ind. Entomol. 2016, 33, 15–23. [Google Scholar] [CrossRef]
- Orozco-Dávila, D.; Quintero, L.; Hernández, E.; Solís, E.; Artiaga, T.; Hernández, R.; Ortega, C.; Montoya, P. Mass rearing and sterile insect releases for the control of Anastrepha spp. pests in Mexico—A review. Entomol. Exp. Appl. 2017, 164, 176–187. [Google Scholar] [CrossRef]
- Vargas, R.I. Alternative egg collection system for mass production of mediterranean fruit fly (Diptera: Tephritidae). J. Econ. Entomol. 1984, 77, 1064–1069. [Google Scholar] [CrossRef]
- Fisher, K.; Cáceres, C. A filter rearing system for mass reared genetic sexing strains of mediterranean fruit fly (Diptera: Tephritidae). In Area-Wide Control of Fruit Flies and Other Insect Pests. Joint Proceedings of the International Conference on Area-Wide Control of Insect Pests; Tan, K.-H., Ed.; Penebrit Universiti Sains: Pulau Pinang, Malaysia, 2000; pp. 543–550. [Google Scholar]
- Bolda, M.P.; Goodhue, R.E.; Zalom, F.G. Spotted wing drosophila: Potential economic impact of a newly established pest. Agric. Resour. Econ. Update 2010, 13, 5–8. [Google Scholar] [CrossRef]
- Lee, J.C.; Bruck, D.J.; Dreves, A.J.; Ioriatti, C.; Vogt, H.; Baufeld, P. In focus: Spotted wing Drosophila, Drosophila suzukii, across perspectives. Pest Manag. Sci. 2011, 67, 1349–1351. [Google Scholar] [CrossRef]
- Zalom, F.G.; Goodhue, R.E.; Lee, J.; Bruck, D.J.; Dreves, A.J.; Walsh, D.B.; O’Neal, S.D.; Walton, V.M.; Bolda, M.P. Drosophila suzukii (Diptera: Drosophilidae): Invasive pest of ripening soft fruit expanding its geographic range and damage potential. J. Integr. Pest Manag. 2011, 2, G1–G7. [Google Scholar] [CrossRef]
- Briem, F.; Dominic, A.R.; Golla, B.; Hoffmann, C.; Englert, C.; Herz, A.; Vogt, H. Explorative data analysis of Drosophila suzukii trap catches from a seven-year monitoring program in southwest Germany. Insects 2018, 9, 125. [Google Scholar] [CrossRef]
- Kanzawa, T. Studies on Drosophila suzukii mats. J. Plant Prot. 1939, 23, 66–70. [Google Scholar]
- De Ros, G.; Anfora, G.; Grassi, A.; Ioriatti, C. The potential economic impact of Drosophila suzukii on small fruits production in Trentino (Italy). IOBC-WPRS Bull 2013, 91, 317–322. [Google Scholar] [CrossRef]
- Mazzi, D.; Bravin, E.; Meraner, M.; Finger, R.; Kuske, S. Economic impact of the introduction and establishment of Drosophila suzukii on sweet cherry production in Switzerland. In Insects; Multidisciplinary Digital Publishing Institute: Basel, Switzerland, 2017; p. 18. [Google Scholar] [CrossRef]
- Nikolouli, K.; Colinet, H.; Renault, D.; Enriquez, T.; Mouton, L.; Gibert, P.; Sassu, F.; Cáceres, C.; Stauffer, C.; Pereira, R.; et al. Sterile insect technique and Wolbachia symbiosis as potential tools for the control of the invasive species Drosophila suzukii. J. Pest Sci. 2018, 91, 489–503. [Google Scholar] [CrossRef] [PubMed]
- Farnsworth, D.; Hamby, K.A.; Bolda, M.; Goodhue, R.E.; Williams, J.C.; Zalom, F.G. Economic analysis of revenue losses and control costs associated with the Spotted Wing Drosophila, Drosophila suzukii (Matsumura), in the California raspberry industry. Pest Manag. Sci. 2017, 73, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Haye, T.; Girod, P.; Cuthbertson, A.G.S.; Wang, X.G.; Daane, K.M.; Hoelmer, K.A.; Baroffio, C.; Zhang, J.P.; Desneux, N. Current SWD IPM tactics and their practical implementation in fruit crops across different regions around the world. J. Pest Sci. 2016, 89, 643–651. [Google Scholar] [CrossRef]
- Bruck, D.J.; Stanley, C.A.; Walsh, D.B.; Isaacs, R.; Gut, L.J.; Barrantes, L.D.; Dreves, A.J.; Beers, E.H.; Walton, V.M.; Rodriguez-Saona, C.R.; et al. Trap designs for monitoring Drosophila suzukii (Diptera: Drosophilidae). Environ. Entomol. 2013, 42, 1348–1355. [Google Scholar] [CrossRef]
- Harmon, D.S.; Haseeb, M.; Kanga, L.H.B.; Liburd, O.E. Evaluation of monitoring traps and lures for Drosophila suzukii (Diptera: Drosophilidae) in berry plantings in Florida. Insects 2019, 10, 313. [Google Scholar] [CrossRef]
- Rogers, M.A.; Burkness, E.C.; Hutchison, W.D. Evaluation of high tunnels for management of Drosophila suzukii in fall-bearing red raspberries: Potential for reducing insecticide use. J. Pest Sci. 2016, 89, 815–821. [Google Scholar] [CrossRef]
- Cormier, D.; Veilleux, J.; Firlej, A. Exclusion net to control Spotted Wing Drosophila in blueberry fields. IOBC-WPRS Bull 2015, 109, 181–184. [Google Scholar]
- Follett, P.A.; Swedman, A.; Price, D.K. Postharvest irradiation treatment for quarantine control of Drosophila suzukii (Diptera: Drosophilidae) in fresh commodities. J. Econ. Entomol. 2014, 107, 964–969. [Google Scholar] [CrossRef]
- Deutscher, A.T.; Reynolds, O.L.; Chapman, T.A. Yeast: An overlooked component of Bactrocera tryoni (Diptera: Tephritidae) larval gut microbiota. J. Econ. Entomol. 2017, 110, 298–300. [Google Scholar] [CrossRef] [PubMed]
- R Core Team; R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Bates, D.; Maechler, M.; Bolker, B.M.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Vargas, R.I. Mass production of tephritid fruit flies. In Fruit Flies their Biology, Natural Enemies and Control; Elsevier: Oxford, UK, 1989; Volume 3, pp. 141–150. [Google Scholar]
- Maïga, H.; Mamai, W.; Bimbilé Somda, N.S.; Konczal, A.; Wallner, T.; Herranz, G.S.; Herrero, R.A.; Yamada, H.; Bouyer, J. Reducing the cost and assessing the performance of a novel adult mass-rearing cage for the dengue, chikungunya, yellow fever and zika vector, Aedes aegypti (L.). PLoS Negl. Trop. Dis. 2019, 13, e0007775. [Google Scholar] [CrossRef] [PubMed]
- Balestrino, F.; Puggioli, A.; Bellini, R.; Petric, D.; Gilles, J.R.L. Mass production cage for Aedes albopictus (Diptera: Culicidae). J. Med. Entomol. 2014, 51, 155–163. [Google Scholar] [CrossRef]
- Schwarz, A.J.; Zambada, A.; Orozco, D.H.S.; Zavala, J.L. Mass production of the mediterranean fruit fly at Metapa, Mexico. Fla. Entomol. 1985, 68, 467–477. [Google Scholar] [CrossRef]
- Calkins, C.O.; Ashley, T.R. The impact of poor quality of mass-reared mediterranean fruit flies on the sterile insect technique used for eradication. J. Appl. Entomol. 1989, 108, 401–408. [Google Scholar] [CrossRef]
- Gallardo-Ortiz, U.; Pérez-Staples, D.; Liedo, P.; Toledo, J. Sexual competitiveness of fertile and sterile, wild and mass-reared males of Anastrepha obliqua. Entomol. Exp. Appl. 2018, 166, 771–777. [Google Scholar] [CrossRef]
- Yadav, P.; Borges, R.M. Why resource history matters: Age and oviposition history affect oviposition behaviour in exploiters of a mutualism. Ecol. Entomol. 2018, 43, 473–482. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sassù, F.; Nikolouli, K.; Caravantes, S.; Taret, G.; Pereira, R.; Vreysen, M.J.B.; Stauffer, C.; Cáceres, C. Mass-Rearing of Drosophila suzukii for Sterile Insect Technique Application: Evaluation of Two Oviposition Systems. Insects 2019, 10, 448. https://doi.org/10.3390/insects10120448
Sassù F, Nikolouli K, Caravantes S, Taret G, Pereira R, Vreysen MJB, Stauffer C, Cáceres C. Mass-Rearing of Drosophila suzukii for Sterile Insect Technique Application: Evaluation of Two Oviposition Systems. Insects. 2019; 10(12):448. https://doi.org/10.3390/insects10120448
Chicago/Turabian StyleSassù, Fabiana, Katerina Nikolouli, Silvana Caravantes, Gustavo Taret, Rui Pereira, Marc J. B. Vreysen, Christian Stauffer, and Carlos Cáceres. 2019. "Mass-Rearing of Drosophila suzukii for Sterile Insect Technique Application: Evaluation of Two Oviposition Systems" Insects 10, no. 12: 448. https://doi.org/10.3390/insects10120448
APA StyleSassù, F., Nikolouli, K., Caravantes, S., Taret, G., Pereira, R., Vreysen, M. J. B., Stauffer, C., & Cáceres, C. (2019). Mass-Rearing of Drosophila suzukii for Sterile Insect Technique Application: Evaluation of Two Oviposition Systems. Insects, 10(12), 448. https://doi.org/10.3390/insects10120448





