Resolving the Taxonomic Status of Potential Biocontrol Agents Belonging to the Neglected Genus Lipolexis Förster (Hymenoptera, Braconidae, Aphidiinae) with Descriptions of Six New Species †
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Molecular Analysis
2.3. Phylogenetic Analysis
3. Results
3.1. Molecular Analysis
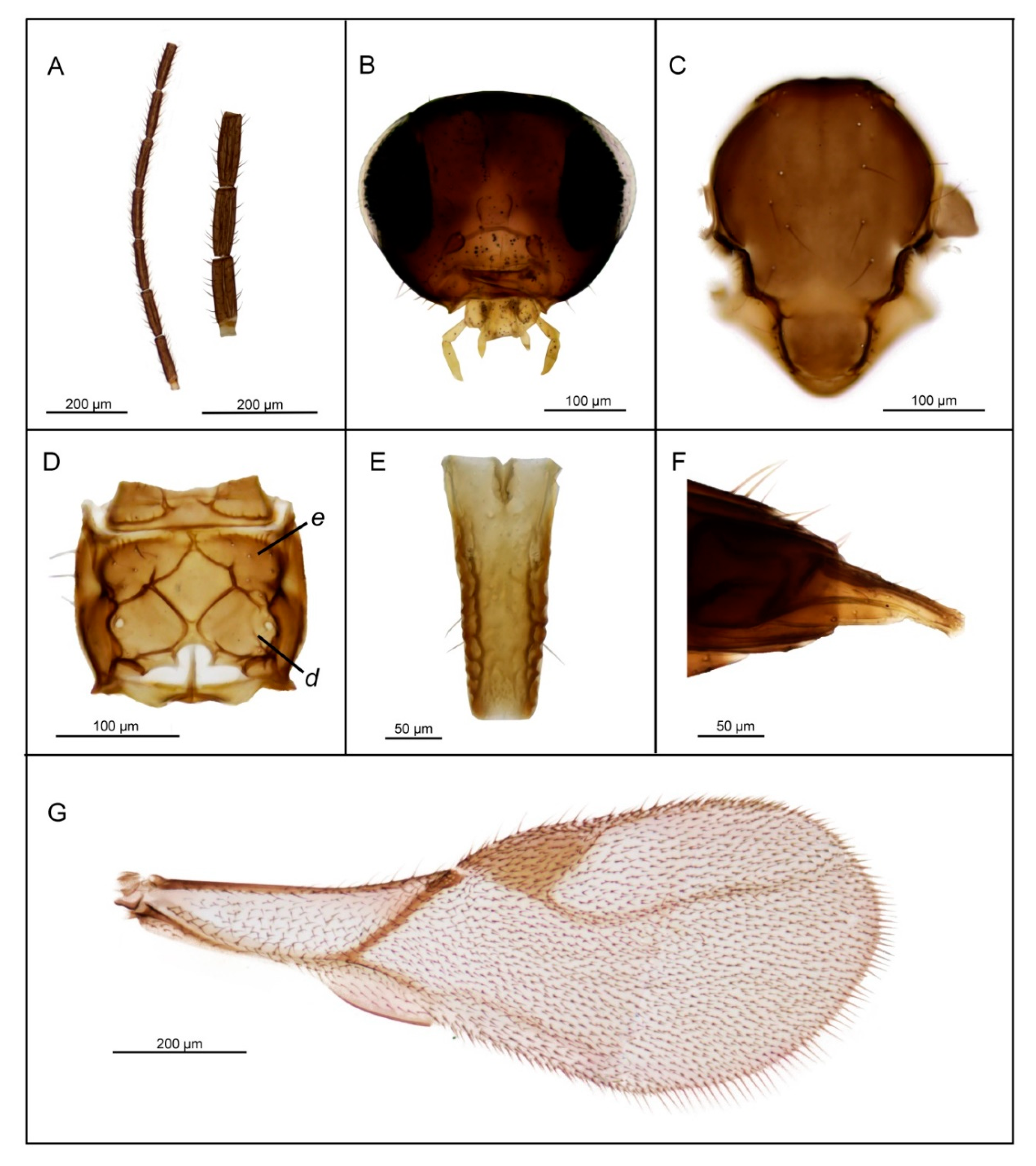
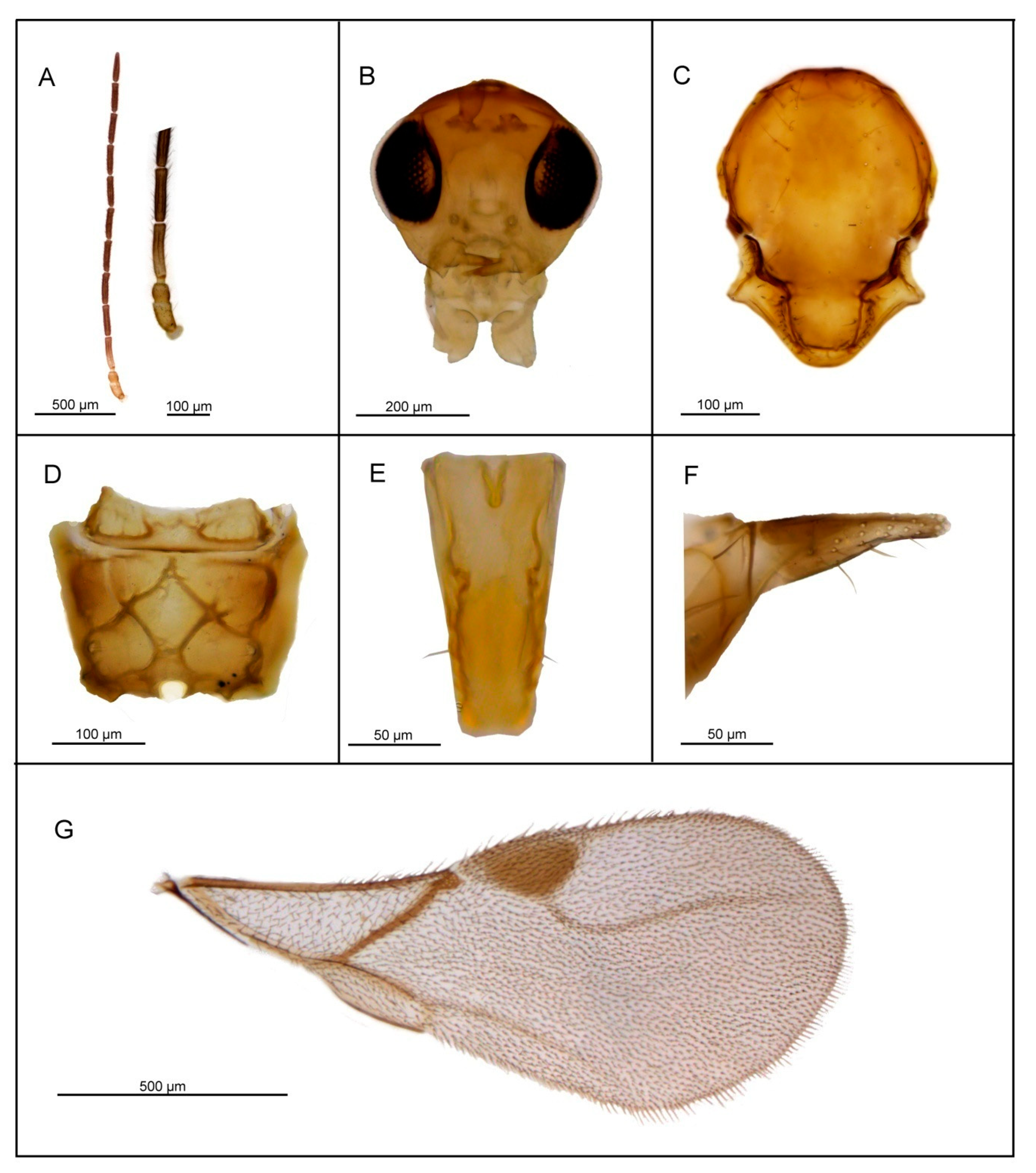
3.2. Description of Six New Species
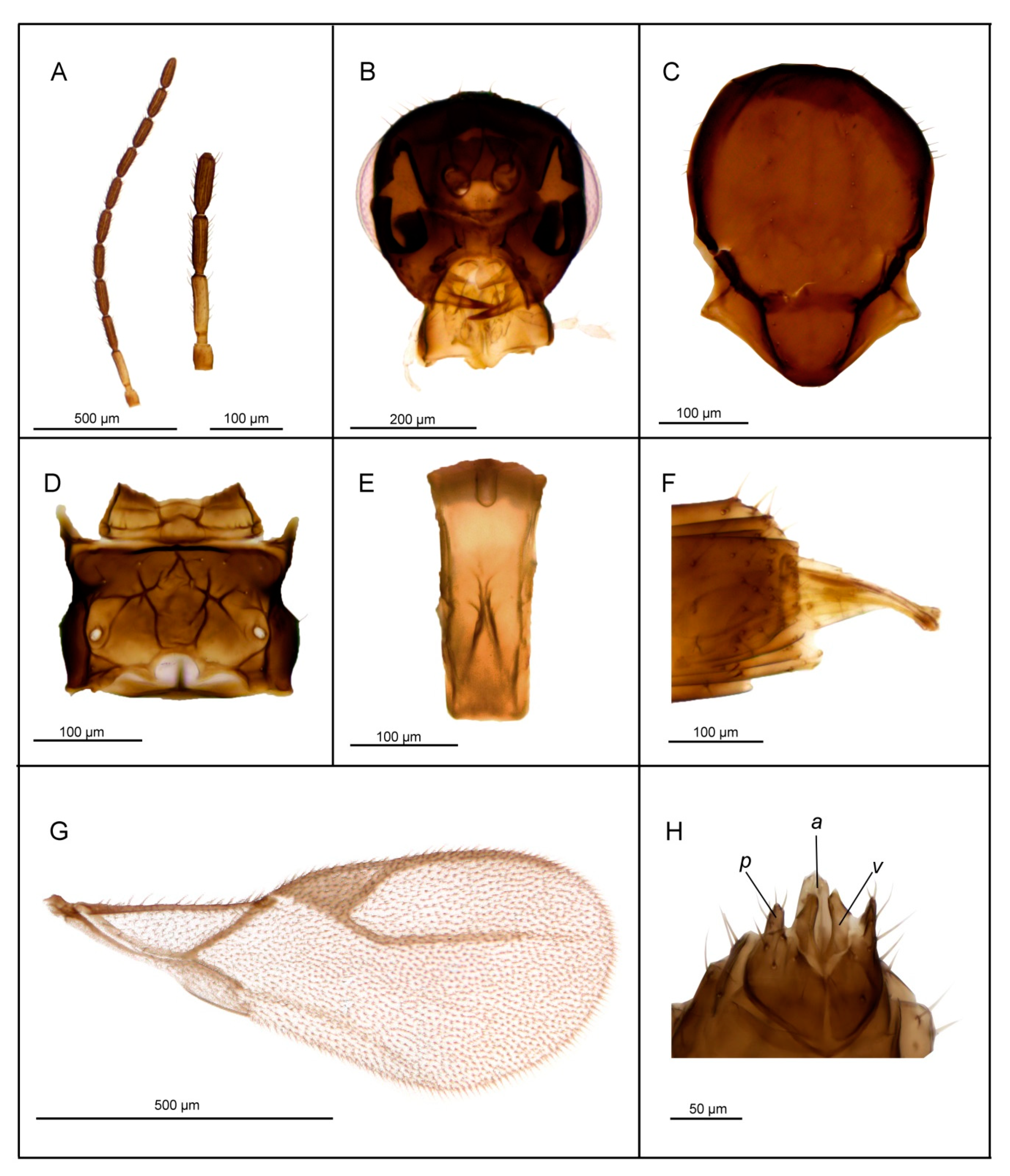
3.2.1. Lipolexis bengalensis sp. n. Tomanović and Kocić
Female
Male
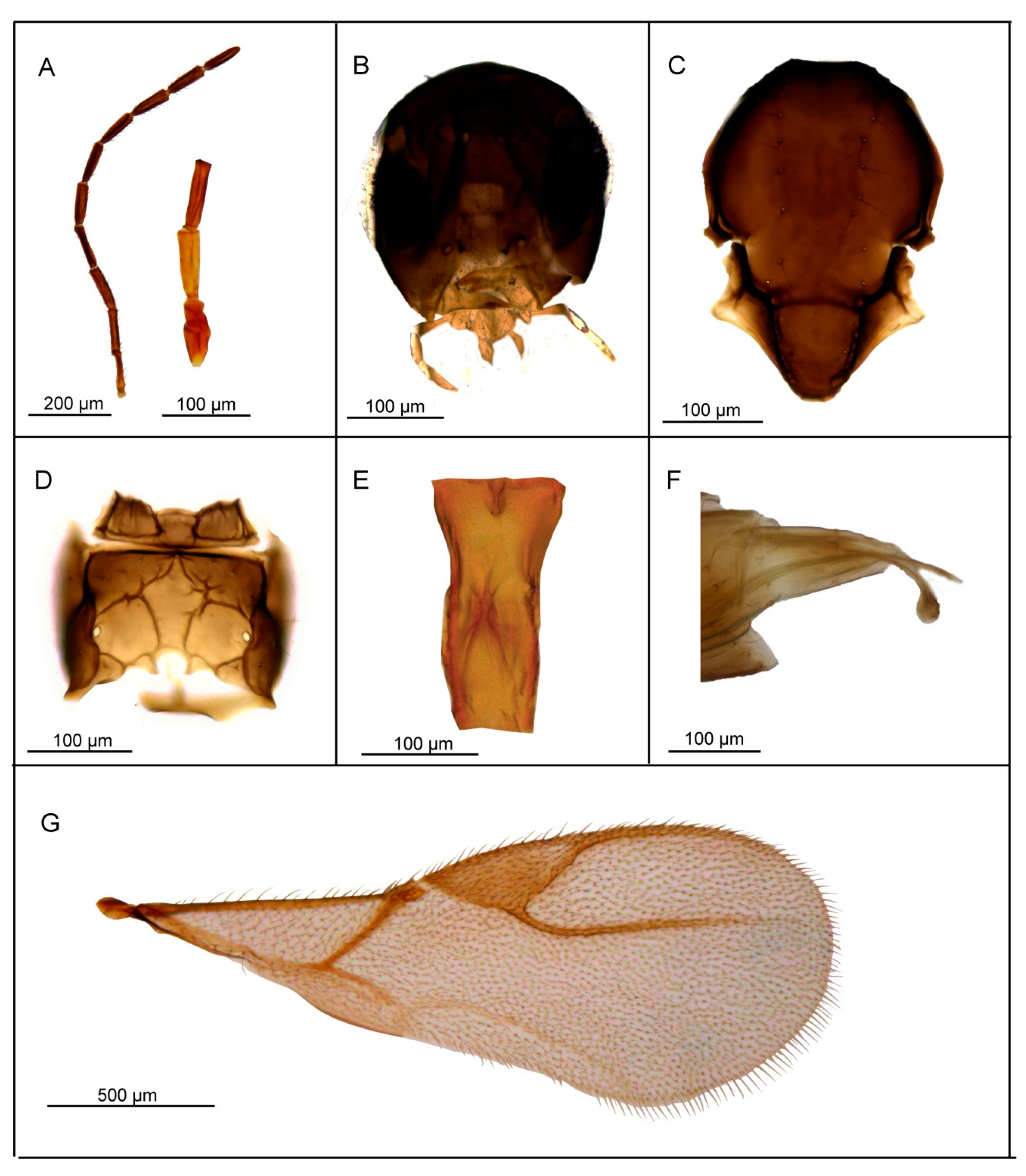
3.2.2. Lipolexis labialis sp. n. Tomanović and Kocić
Female
Male
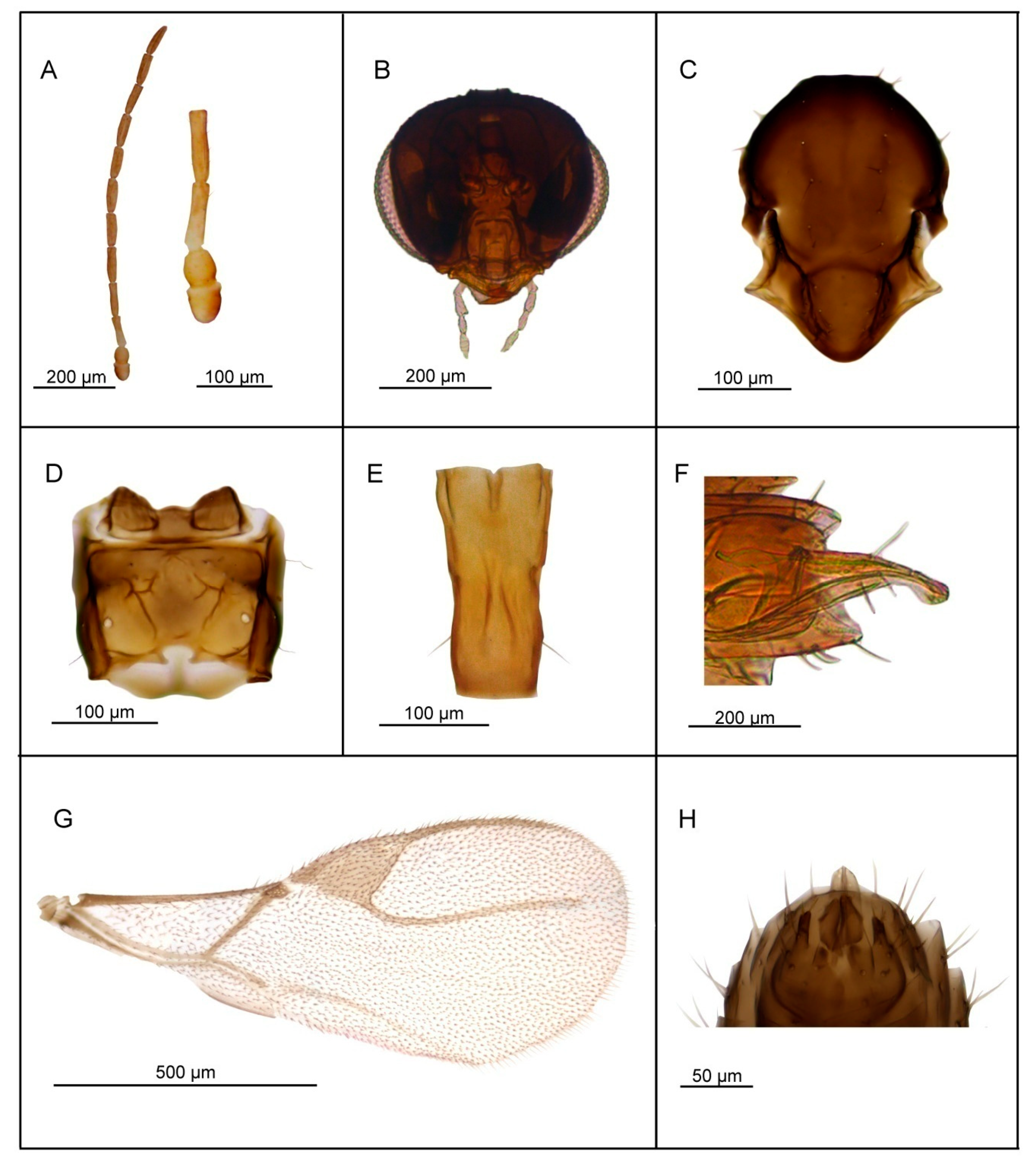
3.2.3. Lipolexis takadai sp. n. Tomanović and Kocić
Female
Male—Unknown
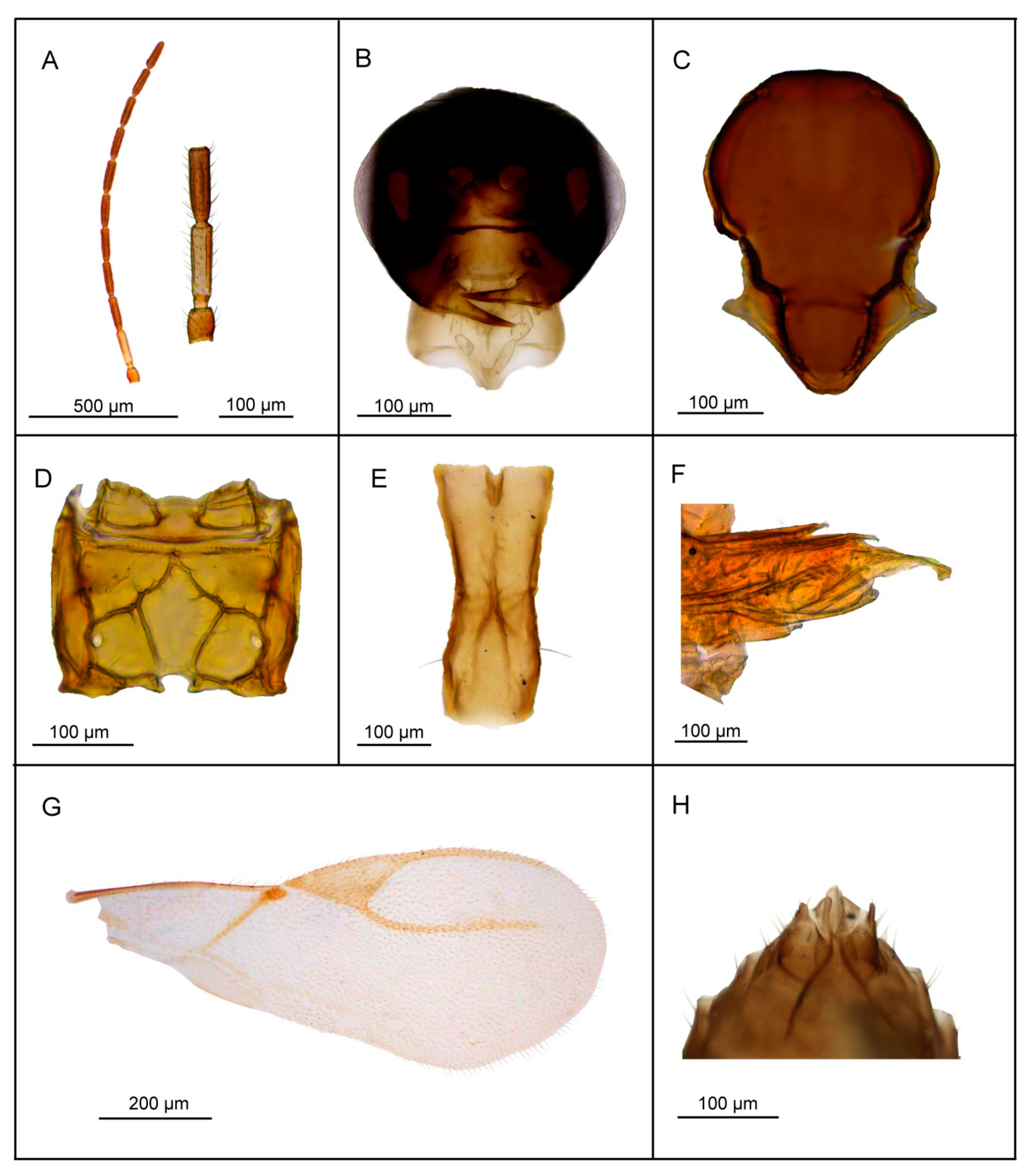
3.2.4. Lipolexis pelopsi sp. n. Tomanović and Kavallieratos
Female
Male
3.2.5. Lipolexis pakistanicus sp. n. Tomanović and Kocić
Female
Male
3.2.6. Lipolexis peregrinus sp. n. Tomanović and Kocić
Female
Male
3.3. Re-Description of L. gracilis and L. oregmae
3.3.1. Lipolexis gracilis Förster, 1862, re-description
Female
Male
3.3.2. Lipolexis oregmae (Gahan 1932)
Female
Male
3.4. Identification Key to the World Species of Genus Lipolexis
| 1. | Petiole dorsally smooth, bearing crenulated lateral longitudinal carinae (Figure 4E and Figure 11E). | ………2 oregmae group |
| - | Petiole with prominent bifurcating central carina dorsally, without crenulated lateral longitudinal carinae (Figure 5E, Figure 6E, Figure 7E, Figure 8E, Figure 9E, and Figure 10E). | …......3 gracilis group |
| 2. | Maxillary palps with 3 palpomeres, labial palps with 1 palpomere. F1 4.45× as long as wide, number of longitudinal placodes on F1 and F2, 2–3 and 4–5, respectively (Figure 11A). | Lipolexis oregmae |
| - | Maxillary palps with 2 palpomeres, labial palps with 1 palpomere. F1 4× as long as wide, F2 with 2–3 number of longitudinal placodes on F1 and F2, 1–2 and 2–3, respectively (Figure 4A). | Lipolexis bengalensis sp. n. |
| 3. | Maxillary palps with 4 palpomeres. | ………4 |
| - | Maxillary palps with 3 palpomeres. | ………6 |
| 4. | Labial palps with 2 palpomeres, F1 3.7–4.0× as long as wide, number of longitudinal placodes on F1 and F2, 1–2 and 2–4, respectively (Figure 5A). | Lipolexis labialis sp. n. |
| - | Labial palps with one palpomere, F1 3.0–3.6× as long as wide, number of longitudinal placodes on F1 and F2, 2–4 and 4–6, respectively (Figure 7A and Figure 10A). | ………5 |
| 5. | Petiole 2.4–2.6× as long as wide (Figure 7E). R1 slightly longer than pterostigma (Figure 7G). | Lipolexis pelopsi sp. n. |
| - | Petiole 2.7–3.2× as long as wide (Figure 10E). R1 equal or slightly shorter than pterostigma (Figure 10G). | Lipolexis gracilis |
| 6. | F1 4.2–4.7× as long as wide (Figure 6A and Figure 8A), petiole 2.7–2.8× as long as wide (Figure 6E and Figure 8E). | ………..7 |
| - | F1 3.4–3.8× as long as wide (Figure 9A), petiole 3.1–3.3× as long as wide (Figure 9E) | Lipolexis peregrinus sp. n. |
| 7. | F1 and F2 4.7 and 4.8–5.0× as long as wide, respectively (Figure 6A), scutellum laterally crenulated along sides (Figure 6C), number of longitudinal placodes on F1 and F2 2 and 3, respectively (Figure 6A) | Lipolexis takadai sp. n. |
| - | Both F1 and F2 4.2× as long as wide (Figure 8A), scutellum laterally not bearing crenulations (Figure 8C), F1 and F2 with 2–3 and 4–5 longitudinal placodes, respectively | Lipolexis pakistanicus sp. n. |
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Forster, A. Synopsis der familien und gattungen der Braconiden. Verhandlungen des Naturhistorischen Vereins der Preussischen Rheinlande und Westfalens 1862, 19, 225–288. [Google Scholar]
- Smith, C.F. The Aphidiinae of North America (Hymenoptera: Braconidae); The Ohio State University: Columbus, OH, USA, 1944; Volume 6, pp. 1–154. [Google Scholar]
- Starý, P. Redescription of the aphidiine genus Lipolexis Förster, 1862 (Hym., Braconidae). Acta Soc. Entomol. Čechoslov. 1959, 59, 93. [Google Scholar]
- Gahan, A.B. Miscellaneous descriptions and notes on parasitic Hymenoptera. Ann. Entomol. Soc. Am. 1932, 25, 736–757. [Google Scholar] [CrossRef]
- Starý, P. The generic classification of the family Aphidiidae (Hymenoptera). Acta Soc. Entomol. Čechoslov. 1960, 57, 238–252. [Google Scholar]
- Starý, P. The Systematic Position of “Diaeretus oregmae Gahan” (Hymenoptera: Aphidiidae). Insecta Matsumurana 1960, 23, 109–111. [Google Scholar]
- Mackauer, M. Drei neue blattlaus-parasiten aus Hongkong. Entomophaga 1962, 34, 87–103. [Google Scholar] [CrossRef]
- Miller, R.H.; Pike, K.S.; Starý, P. Aphid Parasitoids (Hymenoptera: Aphidiidae) on Guam. Micronesica 2002, 34, 87–103. [Google Scholar]
- Chen, J. Description of a new species of the genus Lipolexis Forster (Hymenoptera: Aphidiidae). Entomotaxonomia 1980, 2, 169–172. [Google Scholar]
- Starý, P.; Ghosh, A.K. Aphid Parasitoids of India and Adjacent Countries (Hymenoptera: Aphidiidae); India, G., Ed.; Zoological Survey of India: Calcuta, India, 1983.
- Chen, J. Description of a new species on aphid parasites of Oregma lanigera (Zehntner) (Hymenoptera, Aphidiidae). J. Fuj. Agri. Coll. 1981, 6, 33–38. [Google Scholar]
- Pramanik, D.R.; Raychaudhuri, D. New aphid-parasitoids (Hymenoptera: Aphidiidae) from Nagaland, Northeast India. Akitu 1984, 61, 1–4. [Google Scholar]
- Kavallieratos, N.G.; Tomanović, Ž.; Starý, P.; Athanassiou, C.G.; Sarlis, G.P.; Petrović, O.; Niketić, M.; Veroniki, M.A. A survey of aphid parasitoids (Hymenoptera: Braconidae: Aphidiinae) of Southeastern Europe and their aphid-plant associations. Appl. Entomol. Zool. 2004, 39, 527–563. [Google Scholar] [CrossRef]
- Mifsud, D.; Starý, P. Lipolexis gracilis Forster, 1862—New record of an aphid parasitoid from Malta (Hymenoptera, Braconidae, Aphidiinae). Bull. Entomol. Soc. Malta 2012, 5, 175–177. [Google Scholar]
- Kaliuzhna, M.O. A review of the genus Lipolexis Förster, 1862 (Hymenoptera, Braconidae: Aphidiinae) in the Fauna of Ukraine. Ukr. Entomol. J. 2018, 15, 22–27. [Google Scholar] [CrossRef]
- Persad, A.B.; Hoy, M.A.; Nguyen, R. Establishment of Lipolexis oregmae (Hymenoptera: Aphidiidae) in a lassical biological control program directed against the brown citrus aphid (Homoptera: Aphididae) in Florida. Fla. Entomol. 2007, 90, 204–213. [Google Scholar] [CrossRef]
- Hoy, M.A.; Jeyaprakash, A.; Clarke-Harris, D.; Rhodes, L. Molecular and field analyses of the fortuitous establishment of Lipolexis oregmae (Hymenoptera: Aphidiidae) in Jamaica as a natural enemy of the brown citrus aphid. Biocontrol Sci. Technol. 2007, 17, 473–482. [Google Scholar] [CrossRef]
- Cocco, A.; Jeyaprakash, A.; Hoy, M.A. Parasitism of the brown citrus aphid in Dominica by Lysiphlebus testaceipes and Lipolexis oregmae (Hymenoptera: Aphidiinae). Fla. Entomol. 2009, 92, 497. [Google Scholar] [CrossRef]
- Zamora Mejías, D.; Hanson, P.E.; Starý, P.; Rakhshani, E. Parasitoid (Hym., Braconidae, Aphidiinae) complex of the black citrus aphid, Toxoptera citricidus (Kirkaldy) (Hem., Aphididae) in Costa Rica and its relationships to nearby areas. J. Entomol. Res. Soc. 2011, 13, 107–115. [Google Scholar]
- Stanković, S.; Ilić Milošević, M.; Žikić, V. Potential candidates for biological control of the black bean aphid Aphis fabae in Serbia. Biol. Nyssana 2015, 6, 49–54. [Google Scholar]
- Heimpel, G.E.; Ragsdale, D.W.; Venette, R.; Hopper, K.R.; O’Neil, R.J.; Rutledge, C.E.; Wu, Z. Prospects for importation biological control of the soybean aphid: Anticipating potential costs and benefits. Ann. Entomol. Soc. Am. 2004, 97, 249–258. [Google Scholar] [CrossRef]
- Knorr, L.C.; DuCharme, E.P. The relationship between Argentina’s lepra explosiva and Florida’s scaly bark, with implications for the Florida citrus grower. Plant Dis. Rpt. 1951, 35, 70–75. [Google Scholar]
- Mackauer, M. Hymenopterorum Catalogus. Pars 3 Aphidiidae; Dr. W. Junk: The Hague, The Netherland, 1968. [Google Scholar]
- Belshaw, R.; Quicke, D.L.J. A molecular phylogeny of the Aphidiinae (Hymenoptera: Braconidae). Mol. Phylogenet. Evol. 1997, 7, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Finlayson, T. The systematics and taxonomy of final-instar larvae of the family Aphidiidae (Hymenoptera). Mem. Entomol. Soc. Can. 1990, 122, 3–74. [Google Scholar] [CrossRef]
- Smith, P.T.; Kambhampati, S.; Volkl, W.; Mackauer, M. A phylogeny of aphid parasitoids (Hymenoptera: Braconidae: Aphidiinae) inferred from mitochondrial NADH 1 dehydrogenase gene sequence. Mol. Phylogenet. Evol. 1999, 11, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Kambhampati, S.; Volkl, W.; Mackauer, M. Phylogenetic relationship among genera of Aphidiinae (Hymenoptera: Braconidae) based on DNA sequence of the mitochrondrial 16S rDNA gene. Syst. Entomol. 2000, 25, 437–445. [Google Scholar] [CrossRef]
- Sanchis, A.; Latorre, A.; Gonzales-Candelas, F.; Michelena, J.M. An 18S rDNA-based molecular phylogeny of Aphidiinae (Hymenoptera: Braconidae). Mol. Phylogenet. Evol. 2000, 14, 180–194. [Google Scholar] [CrossRef]
- Shi, M.; Chen, X.X. Molecular phylogeny of the Aphidiinae (Hymenoptera: Braconidae) based on DNA sequences of 16s rRNA, 18S rDNA and ATPase 6 genes. Eur. J. Entomol. 2005, 102, 133–138. [Google Scholar] [CrossRef]
- Derocles, S.A.P.; Le Ralec, A.; Plantegenest, M.; Chaubet, B.; Cruaud, C.; Cruaud, A.; Rasplus, J.Y. Identification of molecular markers for DNA barcoding in the Aphidiinae (Hym. Braconidae). Mol. Ecol. Resour. 2012, 12, 197–208. [Google Scholar] [CrossRef]
- Schneider, C.; Rasband, W.; Eliceiri, K. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Sharkey, M.J.; Wharton, R.A. Morphology and terminology. In Manual of the New World Genera of the Family Braconidae; Wharton, R.A., Marsh, P.M., Sharkey, M.J., Eds.; International Society of Hymenopterists: Washington, DC, USA, 1997. [Google Scholar]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl. Acids. Symp. 1999, 95–98. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A. MEGA 6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Tamura, K. Estimation of the number of nucleotide substitutions when there are strong transition-transversion and G+C-content biases. Mol. Biol. Evol. 1992, 9, 678–687. [Google Scholar] [PubMed]
- Drummond, A.J.; Suchard, M.A.; Xie, D.; Rambaut, A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evol. 2012, 29, 1969–1973. [Google Scholar] [CrossRef]
- Rambaut, A.; Drummond, A.; Xie, D.; Baele, G.; Suchard, M. Posterior summarisation in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef] [PubMed]
- Xia, X. DAMBE7: New and improved tools for data analysis in molecular biology and evolution. Mol. Biol. Evol. 2018, 35, 1550–1552. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Xie, Z.; Salemi, M.; Chen, L.; Wang, Y. An index of substitution saturation and its application. Mol. Phylogenet. Evol. 2003, 26, 1–7. [Google Scholar] [CrossRef]
- Rambaut, A. FigTree Version 1.4.4. 2009. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 25 September 2020).
- Rozas, J.; Ferrer-Mata, A.; Sanchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsis, S.E.; Sanchez-Gracia, A. DnaSP v6: DNA sequence polymorphism analysis of large datasets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef]
- Yu, D.S.; van Achterberg, C.; Horstmann, K. Taxapad 2012—World Ichneumonoidea 2011. Taxonomy, Biology, Morphology and Distribution; Taxapad: Vancouver, BC, Canada. Available online: www.taxapad.com (accessed on 29 March 2019).
- Tomanović, Ž.; Mitrović, M.; Petrović, A.; Kavallieratos, N.G.; Žikić, V.; Ivanović, A.; Rakhshani, E.; Starý, P.; Vorburger, C. Revision of the European Lysiphlebus species (Hymenoptera: Braconidae: Aphidiinae) on the basis of COI and 28SD2 molecular markers and morphology. Arthropod Syst. Phylogeny 2018, 76, 179–213. [Google Scholar]
- Ye, Z.; Vollhardt, I.M.G.; Tomanovic, Z.; Traugott, M. Evaluation of three molecular markers for identification of European primary parasitoids of cereal aphids and their hyperparasitoids. PLoS ONE 2017, 12, e0177376. [Google Scholar] [CrossRef]
- Derocles, S.A.P.; Plantegenest, M.; Rasplus, J.Y.; Marie, A.; Evans, D.M.; Lunt, D.H.; Le Ralec, A. Are generalist Aphidiinae (Hym. Braconidae) mostly cryptic species complexes? Syst. Entomol. 2016, 41, 379–391. [Google Scholar] [CrossRef]
- Kocić, K.; Petrović, A.; Čkrkic, J.; Mitrović, M.; Tomanović, Ž. Phylogenetic relationships and subgeneric classification of European Ephedrus species (Hymenoptera, Braconidae, Aphidiinae). Zookeys 2019, 878, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Mitrovski-Bogdanović, A.; Petrović, A.; Mitrović, M.; Ivanović, A.; Žikić, V.; Starý, P.; Vorburger, C.; Tomanović, Ž. Identification of two cryptic species within the Praon abjectum group (Hymenoptera: Braconidae: Aphidiinae) using molecular markers and geometric morphometrics. Ann. Entomol. Soc. Am. 2013, 106, 170–180. [Google Scholar] [CrossRef]
- Čkrkic, J.; Petrović, A.; Kocić, K.; Mitrović, M.; Kavallieratos, N.G.; van Achterberg, C.; Hebert, P.D.N.; Tomanović, Ž. Phylogeny of the subtribe Monoctonina (Hymenoptera, Braconidae, Aphidiinae). Insects 2020, 11, 160. [Google Scholar] [CrossRef] [PubMed]
- Starý, P.; Schlinger, E.I. A Revision of the Far East Asian Aphidiidae (Hymenoptera); Dr W. Junk: The Hague, The Netherlands, 1967. [Google Scholar]
- Uesugi, R.; Nagasaka, K. Mitochondrial COI Sequences to identify species of primary and secondary parasitoid wasps of aphids in the agricultural environment in Japan. Annu. Rep. Kanto-Tosan Plant Prot. Soc. 2017, 2017, 143–145. [Google Scholar] [CrossRef]
- Dong, Z.; Liu, S.; Zhang, Z. Efficacy of using DNA barcoding to identify parasitoid wasps of the melon-cotton aphid (Aphis gossypii) in watermelon cropping system. BioControl 2018, 63, 677–685. [Google Scholar] [CrossRef]
- Starý, P. Biology of Aphid Parasites (Hymenoptera: Aphidiidae) with Respect to Integrated Control; Dr W. Junk: The Hague, The Netherlands, 1970. [Google Scholar]
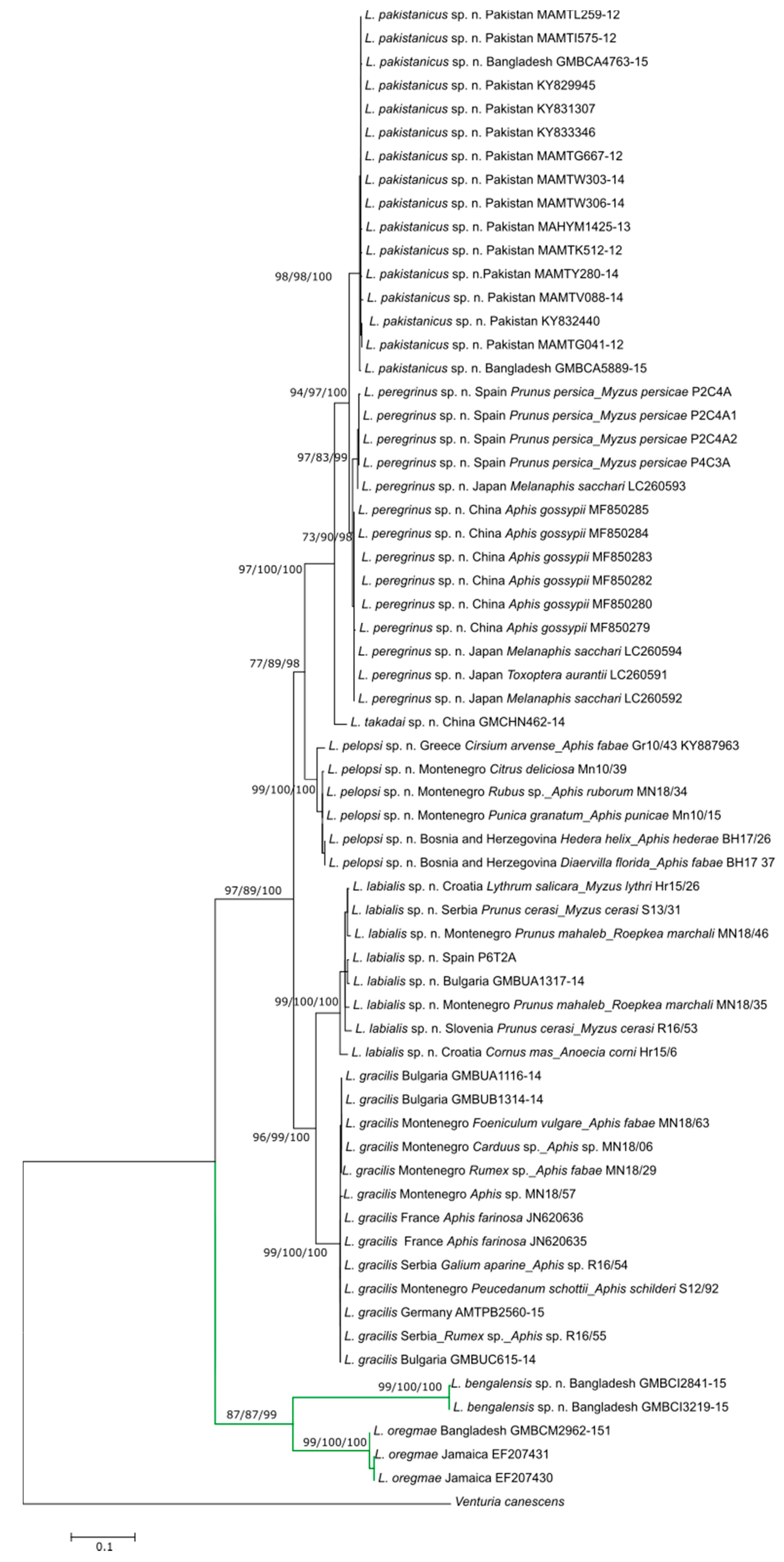
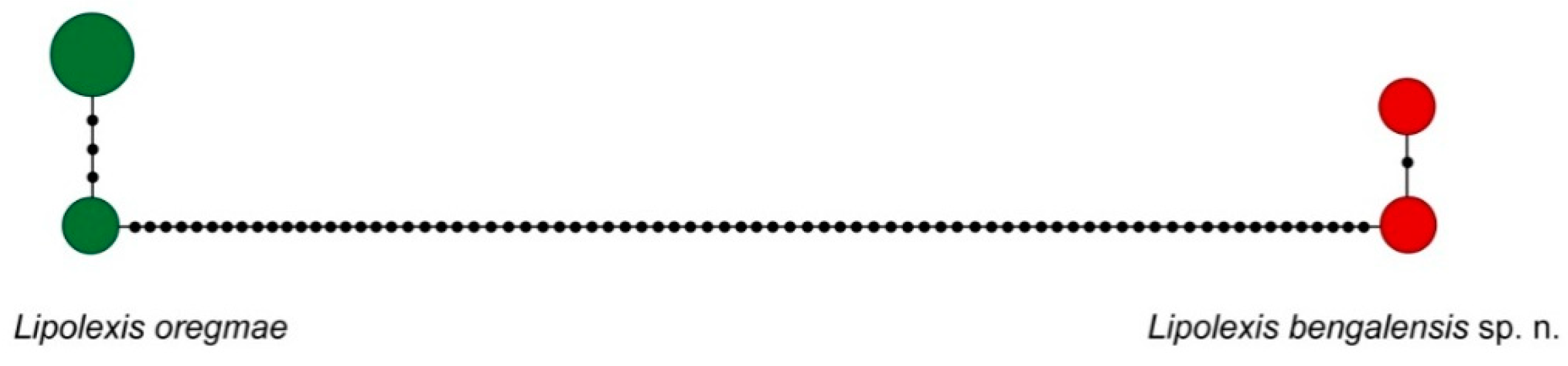
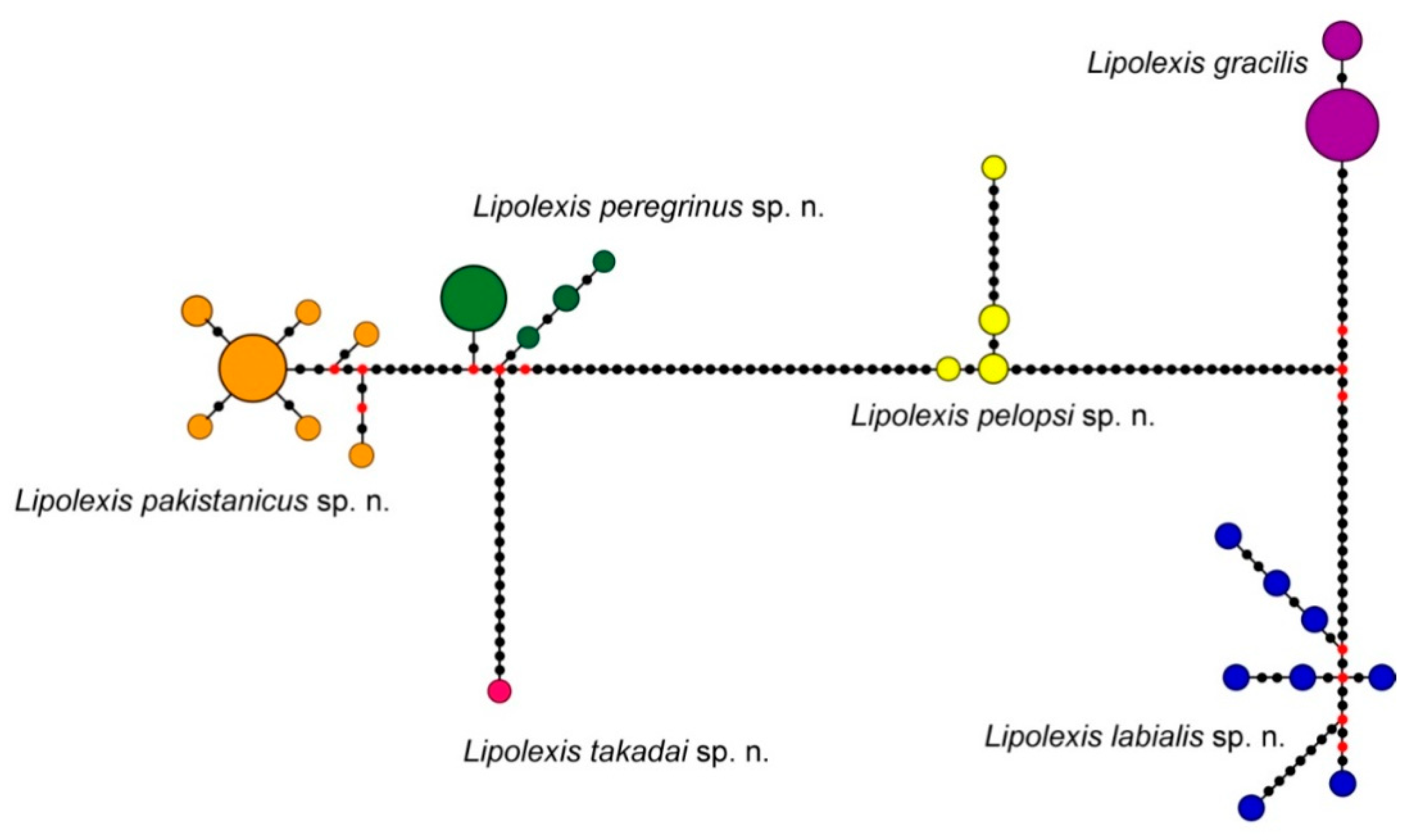


| Species | L. peregrinus sp. n. | L. gracilis | L. pelopsi sp. n. | L. labialis sp. n. | L. pakistanicus sp. n. | L. takadai sp. n. | L. bengalensis sp. n. |
|---|---|---|---|---|---|---|---|
| L. peregrinus sp. n. | |||||||
| L. gracilis | 0.111 | ||||||
| L. pelopsi sp. n. | 0.069 | 0.086 | |||||
| L. labialis sp. n. | 0.123 | 0.073 | 0.097 | ||||
| L. pakistanicus sp. n. | 0.022 | 0.110 | 0.086 | 0.132 | |||
| L. takadai sp. n. | 0.039 | 0.097 | 0.068 | 0.106 | 0.045 | ||
| L. bengalensis sp. n. | 0.232 | 0.217 | 0.227 | 0.228 | 0.231 | 0.229 | |
| L. oregmae | 0.206 | 0.215 | 0.218 | 0.232 | 0.213 | 0.200 | 0.199 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kocić, K.; Petrović, A.; Čkrkić, J.; Kavallieratos, N.G.; Rakhshani, E.; Arnó, J.; Aparicio, Y.; Hebert, P.D.N.; Tomanović, Ž. Resolving the Taxonomic Status of Potential Biocontrol Agents Belonging to the Neglected Genus Lipolexis Förster (Hymenoptera, Braconidae, Aphidiinae) with Descriptions of Six New Species. Insects 2020, 11, 667. https://doi.org/10.3390/insects11100667
Kocić K, Petrović A, Čkrkić J, Kavallieratos NG, Rakhshani E, Arnó J, Aparicio Y, Hebert PDN, Tomanović Ž. Resolving the Taxonomic Status of Potential Biocontrol Agents Belonging to the Neglected Genus Lipolexis Förster (Hymenoptera, Braconidae, Aphidiinae) with Descriptions of Six New Species. Insects. 2020; 11(10):667. https://doi.org/10.3390/insects11100667
Chicago/Turabian StyleKocić, Korana, Andjeljko Petrović, Jelisaveta Čkrkić, Nickolas G. Kavallieratos, Ehsan Rakhshani, Judit Arnó, Yahana Aparicio, Paul D. N. Hebert, and Željko Tomanović. 2020. "Resolving the Taxonomic Status of Potential Biocontrol Agents Belonging to the Neglected Genus Lipolexis Förster (Hymenoptera, Braconidae, Aphidiinae) with Descriptions of Six New Species" Insects 11, no. 10: 667. https://doi.org/10.3390/insects11100667
APA StyleKocić, K., Petrović, A., Čkrkić, J., Kavallieratos, N. G., Rakhshani, E., Arnó, J., Aparicio, Y., Hebert, P. D. N., & Tomanović, Ž. (2020). Resolving the Taxonomic Status of Potential Biocontrol Agents Belonging to the Neglected Genus Lipolexis Förster (Hymenoptera, Braconidae, Aphidiinae) with Descriptions of Six New Species. Insects, 11(10), 667. https://doi.org/10.3390/insects11100667








