Bioaccumulation of Cadmium Affects Development, Mating Behavior, and Fecundity in the Asian Corn Borer, Ostrinia furnacalis
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects and Rearing Conditions
2.2. Chemical and Reagent Doses
2.3. Measurement of Cd Content
2.4. Developmental and Reproductive Evaluation
2.5. Mating Behavior Observation (Wind Tunnel Bioassay)
2.6. Statistical Analysis
3. Results
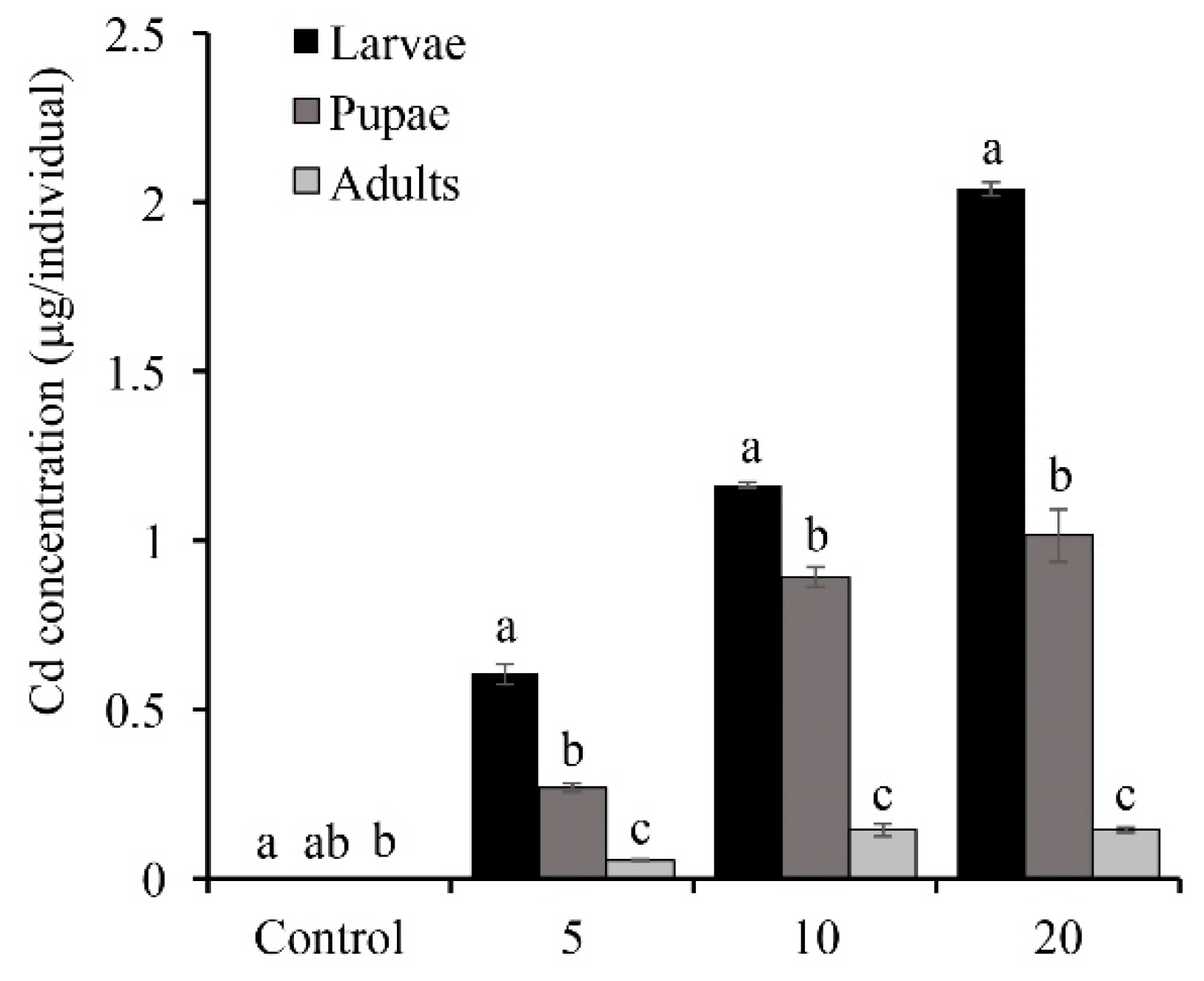
3.1. Multiple Routes of Excretion during the Life Cycle Result in Low Levels of Cd Residue in the Adult
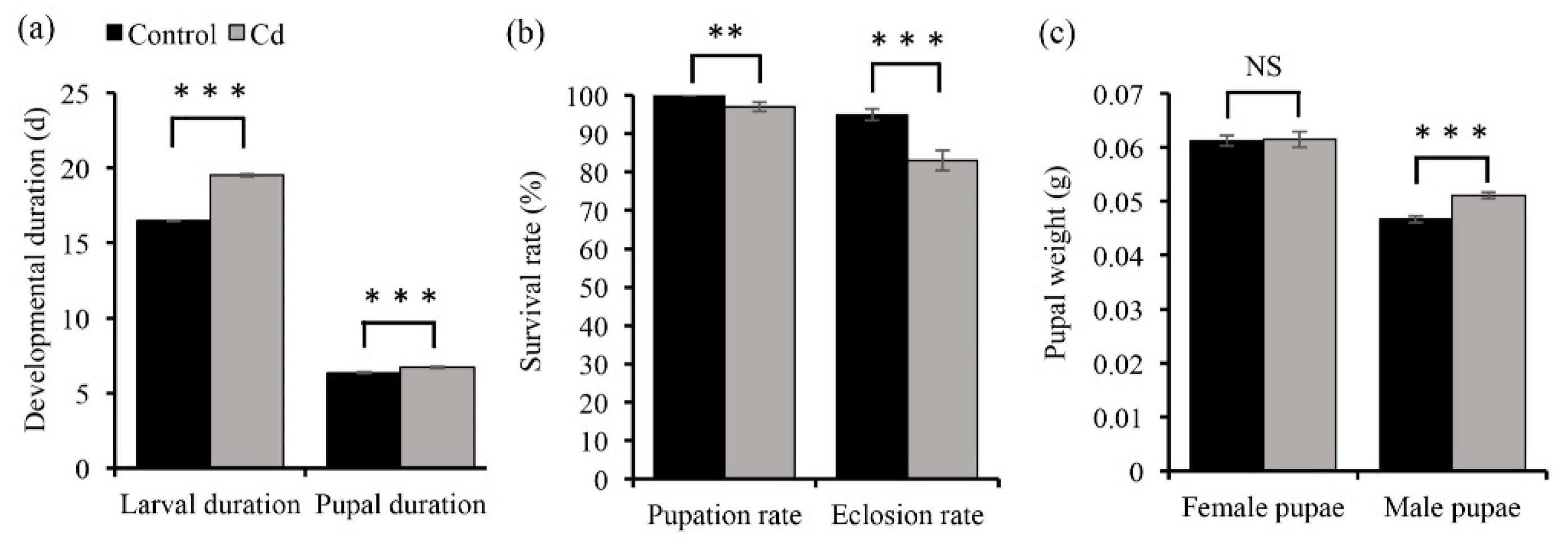
3.2. Cd Exposure in the Larval Stage Causes Detrimental Effects on the Development of O. furnacalis
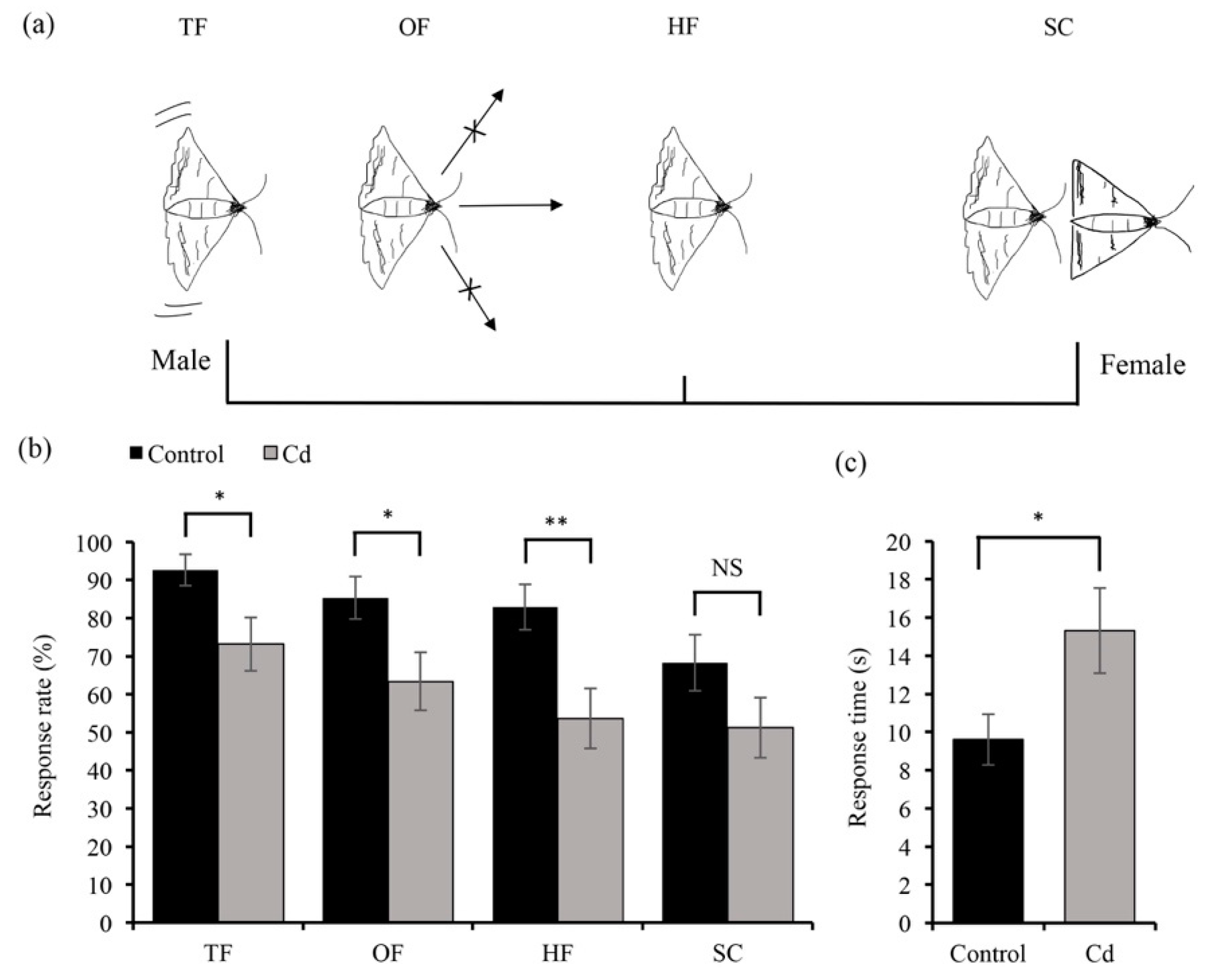
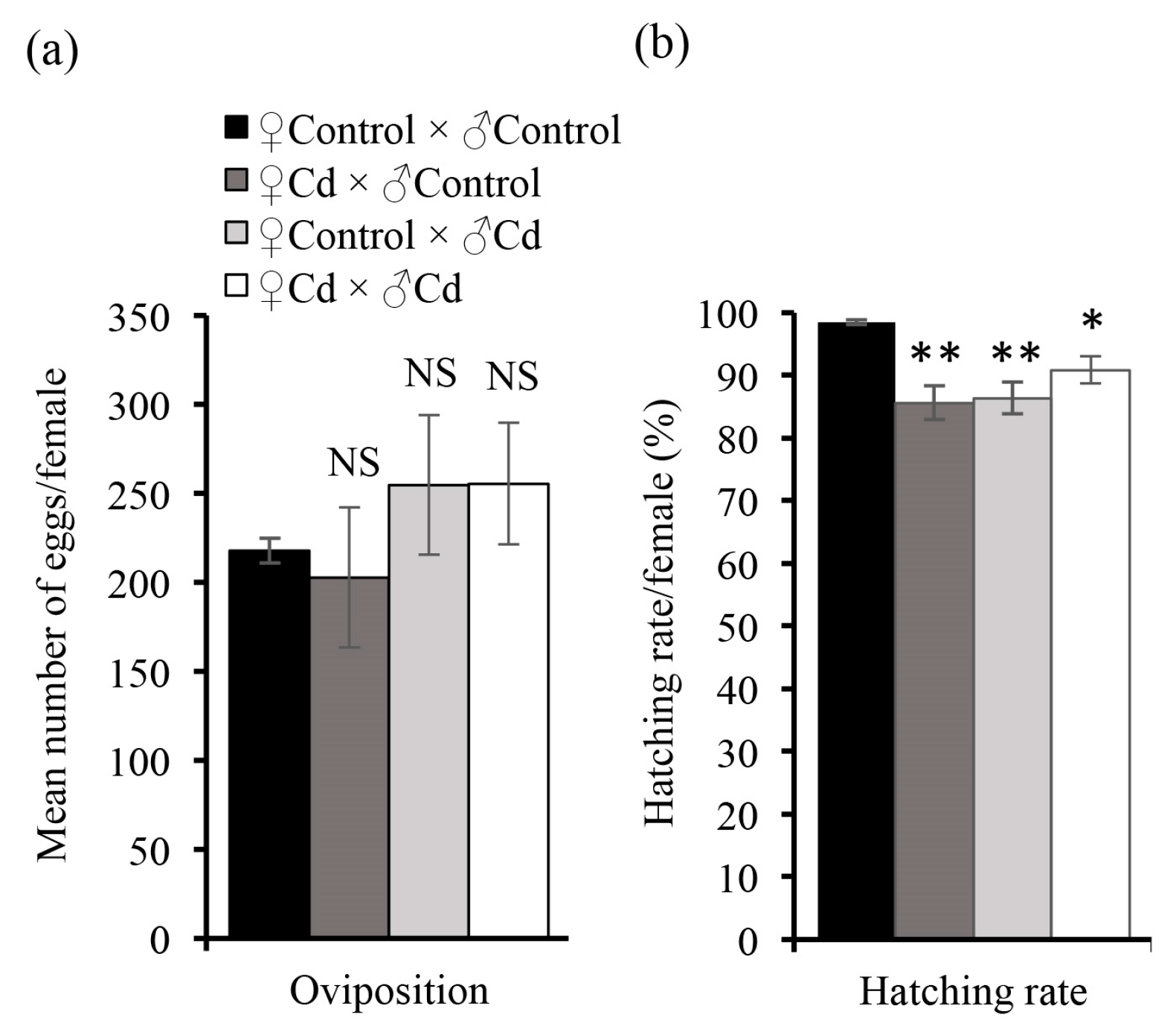
3.3. Cd Exposure to Larvae Affects Adult Mating Behavior and Fecundity in O. furnacalis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sharma, R.K.; Agrawal, M. Biological effects of heavy metals: An overview. J. Environ. Biol. 2005, 26, 301–313. [Google Scholar]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. EXS 2012, 101, 133–164. [Google Scholar]
- Stolpe, C.; Giehren, F.; Krämer, U.; Müller, C. Both heavy metal-amendment of soil and aphid-infestation increase Cd and Zn concentrations in phloem exudates of a metal-hyperaccumulating plant. Phytochemistry 2017, 139, 109–117. [Google Scholar] [CrossRef]
- Sang, W.; Xu, J.; Bashir, M.H.; Ali, S. Developmental responses of Cryptolaemus montrouzieri to heavy metals transferred across multi-trophic food chain. Chemosphere 2018, 205, 690–697. [Google Scholar] [CrossRef]
- Duruibe, J.O.; Ogwuegbu, M.O.C.; Egwurugwu, J.N. Heavy metal pollution and human biotoxic effects. Int. J. Phys. Sci. 2007, 2, 112–118. [Google Scholar]
- Crawford, L.A.; Lepp, N.W.; Hodkinson, I.D. Accumulation and egestion of dietary copper and cadmium by the grasshopper Locusta migratoria R & F (Orthoptera: Acrididae). Environ. Pollut. 1996, 92, 241–246. [Google Scholar]
- Devkota, B.; Schmidt, G. Accumulation of heavy metals in food plants and grasshoppers from the Taigetos Mountains, Greece. Agric. Ecosyst. Environ. 2000, 78, 85–91. [Google Scholar] [CrossRef]
- Liu, J.; Liang, J.; Li, K.; Zhang, Z.; Yu, B.; Lu, X.; Yang, J.; Zhu, Q. Correlations between cadmium and mineral nutrients in absorption and accumulation in various genotypes of rice under cadmium stress. Chemosphere 2003, 52, 1467–1473. [Google Scholar] [CrossRef]
- Thompson, J.; Bannigan, J. Cadmium: Toxic effects on the reproductive system and the embryo. Reprod. Toxicol. 2008, 25, 304–315. [Google Scholar] [CrossRef]
- Borowiak, K.; Kanclerz, J.; Mleczek, M.; Lisiak, M.; Drzewiecka, K. Accumulation of Cd and Pb in water, sediment and two littoral plants (Phragmites australis, Typha angustifolia) of freshwater ecosystem. Arch. Environ. Prot. 2016, 42, 47–57. [Google Scholar] [CrossRef]
- Chen, W.; Lu, S.; Peng, C.; Jiao, W.; Wang, M. Accumulation of Cd in agricultural soil under long-term reclaimed water irrigation. Environ. Pollut. 2013, 178, 294–299. [Google Scholar] [CrossRef]
- Li, Z.; Ma, Z.; van der Kuijp, T.J.; Yuan, Z.; Huang, L. A review of soil heavy metal pollution from mines in China: Pollution and health risk assessment. Sci. Total Environ. 2014, 468, 843–853. [Google Scholar] [CrossRef]
- Li, H.; Luo, N.; Li, Y.W.; Cai, Q.Y.; Li, H.Y.; Mo, C.H.; Wong, M.H. Cadmium in rice: Transport mechanisms, influencing factors, and minimizing measures. Environ. Pollut. 2017, 224, 622–630. [Google Scholar] [CrossRef]
- Wang, M.; Chen, W.; Peng, C. Risk assessment of Cd polluted paddy soils in the industrial and township areas in Hunan, Southern China. Chemosphere 2016, 144, 346–351. [Google Scholar] [CrossRef]
- Van Nguyen, N.; Ferrero, A. Meeting the Challenges of Global Rice Production; Springer: New York, NY, USA, 2006. [Google Scholar]
- Inaba, T.; Kobayashi, E.; Suwazono, Y.; Uetani, M.; Oishi, M.; Nakagawa, H.; Nogawa, K. Estimation of cumulative cadmium intake causing Itai–itai disease. Toxicol. Lett. 2005, 159, 192–201. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, C.; Qiu, B.; Ashraf, U.; Azad, R.; Wu, J.; Ali, S. Biotransfer of Cd along a soil-plant-mealybug-ladybird food chain: A comparison with host plants. Chemosphere 2017, 168, 699–706. [Google Scholar] [CrossRef]
- Zhuang, P.; Zou, H.; Shu, W. Biotransfer of heavy metals along a soil-plant-insect-chicken food chain: Field study. J. Environ. Sci. 2009, 21, 849–853. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, G.; Yang, M.; Wu, H.; Zhang, J.; Song, S.; Ma, E.; Guo, Y. Chronic accumulation of cadmium and its effects on antioxidant enzymes and malondialdehyde in Oxya chinensis (Orthoptera: Acridoidea). Ecotoxicol. Environ. Saf. 2011, 74, 1355–1362. [Google Scholar] [CrossRef]
- Cervera, A.; Maymó, A.C.; Sendra, M.; Martínezpardo, R.; Garcerá, M.D. Cadmium effects on development and reproduction of Oncopeltus fasciatus (Heteroptera: Lygaeidae). J. Insect Physiol. 2004, 50, 737–749. [Google Scholar] [CrossRef]
- Hu, X.; Fu, W.; Yang, X.; Mu, Y.; Gu, W.; Zhang, M. Effects of cadmium on fecundity and defence ability of Drosophila melanogaster. Ecotoxicol. Environ. Saf. 2019, 171, 871–877. [Google Scholar] [CrossRef]
- Plachetka-Bozek, A.; Kafel, A.; Augustyniak, M. Reproduction and development of Spodoptera exigua from cadmium and control strains under differentiated cadmium stress. Ecotoxicol. Environ. Saf. 2018, 166, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Chen, J.; Jin, P.; Li, J.; Wang, J.; Shu, Y. Effects of Cd accumulation on cutworm Spodoptera litura larvae via Cd treated Chinese flowering cabbage Brassica campestris and artificial diets. Chemosphere 2018, 200, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Rivero, A.; Giron, D.; Casas, J. Lifetime allocation of juvenile and adult nutritional resources to egg production in a holometabolous insect. Proc. R. Soc. B Biol. Sci. 2001, 268, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Balduf, W. The rise of entomophagy among Lepidoptera. Am. Nat. 1938, 72, 358–379. [Google Scholar] [CrossRef]
- Holland, R.A.; Wikelski, M.; Wilcove, D.S. How and why do insects migrate? Science 2006, 313, 794–796. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, X.; He, K.; Zhou, D. Review of history, present situation and prospect of the Asian maize borer research in China. J. Shenyang Agric. Univ. Chin. J. 2000, 31, 402–412. [Google Scholar]
- Afidchao, M.M.; Cjm, M.; De, S.G.R. Asian corn borer (ACB) and non-ACB pests in GM corn (Zea mays L.) in the Philippines. Pest Manag. Sci. 2013, 69, 792–801. [Google Scholar] [CrossRef]
- Wang, S.; Wu, W.; Liu, F.; Liao, R.; Hu, Y. Accumulation of heavy metals in soil-crop systems: A review for wheat and corn. Environ. Sci. Pollut. Res. Int. 2017, 24, 15209–15225. [Google Scholar] [CrossRef]
- Cui, J.; Wang, W.; Peng, Y.; Zhou, F.; He, D.; Wang, J.; Chang, Y.; Yang, J.; Zhou, J.; Wang, W.; et al. Effects of simulated Cd deposition on soil Cd availability, microbial response, and crop Cd uptake in the passivation-remediation process of Cd-contaminated purple soil. Sci. Total Environ. 2019, 683, 782–792. [Google Scholar] [CrossRef]
- Roelofs, W.L.; Rooney, A.P. Molecular genetics and evolution of pheromone biosynthesis in Lepidoptera. Proc. Natl. Acad. Sci. USA 2003, 100, 9179–9184. [Google Scholar] [CrossRef]
- Wei, H.Y.; Du, J.W. Sublethal effects of larval treatment with deltamethrin on moth sex pheromone communication system of the Asian corn borer. Ostrinia furnacalis. Pestic. Biochem. Physiol. 2004, 80, 12–20. [Google Scholar] [CrossRef]
- Cao, H.; Wei, H. Effect of the heavy metals Cd2+ and Ni2+ on the calling behavior of Ostrinia furnacalis (Lepidoptera: Crambidae). Chin. J. Appl. Entomol. 2016, 53, 793–801. [Google Scholar]
- Zhou, D.R.; Wang, Y.Y.; Liu, B.L.; Ju, Z.L. Study on large breeding of Ostrinia furnacalis I: An artificial diet and its improvement. Acta Pharm. Sin. 1980, 7, 113–122. [Google Scholar]
- Luo, M.; Zhou, X.C.; Wang, Z.; Chen, J.X.; Chung, H.; Wei, H.Y. Identification and Gene Expression Analysis of the Pheromone Biosynthesis Activating Neuropeptide Receptor (PBANR) From the Ostrinia furnacalis (Lepidoptera: Pyralidae). J. Insect Sci. 2019, 19, 25. [Google Scholar] [CrossRef]
- Lavado, R.S.; Rodríguez, M.; Alvarez, R.; Taboada, M.A.; Zubillaga, M.S. Transfer of potentially toxic elements from biosolid-treated soils to maize and wheat crops. Agric. Ecosyst. Environ. 2007, 118, 312–318. [Google Scholar] [CrossRef]
- Zarco-Fernandez, S.; Coto-García, A.; Munoz-Olivas, R.; Sanz-Landaluze, J.; Rainieri, S.; Cámara, C. Bioconcentration of ionic cadmium and cadmium selenide quantum dots in zebrafish larvae. Chemosphere 2016, 148, 328–335. [Google Scholar] [CrossRef]
- Baker, T.; Linn, C. Wind tunnels in pheromone research. In Techniques in Pheromone Research; Springer: New York, NY, USA, 1984; pp. 75–110. [Google Scholar]
- Ding, P.; Zhuang, P.; Li, Z.; Xia, H.; Lu, H. Accumulation and detoxification of cadmium by larvae of Prodenia litura (Lepidoptera: Noctuidae) feeding on Cd-enriched amaranth leaves. Chemosphere 2013, 91, 28–34. [Google Scholar] [CrossRef]
- Gao, Q.; Wang, X.; Wang, W.; Lei, C.; Zhu, F. Influences of chromium and cadmium on the development of black soldier fly larvae. Environ. Sci. Pollut. Res. 2017, 24, 8637–8644. [Google Scholar] [CrossRef]
- Kafel, A.; Zawisza-Raszka, A.; Szulińska, E. Effects of multigenerational cadmium exposure of insects (Spodoptera exigua larvae) on anti-oxidant response in haemolymph and developmental parameters. Environ. Pollut. 2012, 162, 8–14. [Google Scholar] [CrossRef]
- Bagatto, G.; Shorthouse, J.D. Accumulation of Cu and Ni in successive stages of Lymantria dispar L. (Lymantriidae, Lepidoptera) near ore smelters at Sudbury, Ontario, Canada. Environ. Pollut. 1996, 92, 7–12. [Google Scholar] [CrossRef]
- Jiang, D.; Yan, S. Effects of Cd, Zn or Pb stress in Populus alba berolinensis on the development and reproduction of Lymantria dispar. Ecotoxicology 2017, 26, 1305–1313. [Google Scholar] [CrossRef] [PubMed]
- Osman, W.; El-Samad, L.M.; Mokhamer, E.H.; El-Touhamy, A.; Shonouda, M. Ecological, morphological, and histological studies on Blaps polycresta (Coleoptera: Tenebrionidae) as biomonitors of cadmium soil pollution. Environ. Sci. Pollut. Res. 2015, 22, 14104–14115. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Huang, Y.; Du, J. Sex pheromones and reproductive behavior of Spodoptera litura (Fabricius) moths reared from larvae treated with four insecticides. J. Chem. Ecol. 2004, 30, 1457–1466. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wu, W.; Guo, J.; Xiao, R.; Jiang, F.; Zheng, L.; Zhang, G. Effects of nickel exposure on testicular function, oxidative stress, and male reproductive dysfunction in Spodoptera litura Fabricius. Chemosphere 2016, 148, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Telfer, W.H. Egg formation in Lepidoptera. J. Insect Sci. 2009, 9, 1–21. [Google Scholar] [CrossRef]
- Braeckman, B.P. Heavy Metal Toxicity in an Insect Cell Line (Methyl-HgCl, HgCl2, CdCl2 and CuSO4). In Cellular Effects of Heavy Metals; Springer: New York, NY, USA, 2011; pp. 115–144. [Google Scholar]
- Braeckman, B.; Raes, H.; Van Hoye, D. Heavy-metal toxicity in an insect cell line. Effects of cadmium chloride, mercuric chloride and methylmercuric chloride on cell viability and proliferation in Aedes albopictus cells. Cell Biol. Toxicol. 1997, 13, 389–397. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, M.; Cao, H.-M.; Fan, Y.-Y.; Zhou, X.-C.; Chen, J.-X.; Chung, H.; Wei, H.-Y. Bioaccumulation of Cadmium Affects Development, Mating Behavior, and Fecundity in the Asian Corn Borer, Ostrinia furnacalis. Insects 2020, 11, 7. https://doi.org/10.3390/insects11010007
Luo M, Cao H-M, Fan Y-Y, Zhou X-C, Chen J-X, Chung H, Wei H-Y. Bioaccumulation of Cadmium Affects Development, Mating Behavior, and Fecundity in the Asian Corn Borer, Ostrinia furnacalis. Insects. 2020; 11(1):7. https://doi.org/10.3390/insects11010007
Chicago/Turabian StyleLuo, Mei, Hong-Mei Cao, Ying-Ying Fan, Xiao-Cao Zhou, Jun-Xian Chen, Henry Chung, and Hong-Yi Wei. 2020. "Bioaccumulation of Cadmium Affects Development, Mating Behavior, and Fecundity in the Asian Corn Borer, Ostrinia furnacalis" Insects 11, no. 1: 7. https://doi.org/10.3390/insects11010007
APA StyleLuo, M., Cao, H.-M., Fan, Y.-Y., Zhou, X.-C., Chen, J.-X., Chung, H., & Wei, H.-Y. (2020). Bioaccumulation of Cadmium Affects Development, Mating Behavior, and Fecundity in the Asian Corn Borer, Ostrinia furnacalis. Insects, 11(1), 7. https://doi.org/10.3390/insects11010007



