Using Nutritional Geometry to Explore How Social Insects Navigate Nutritional Landscapes
Abstract
1. Introduction
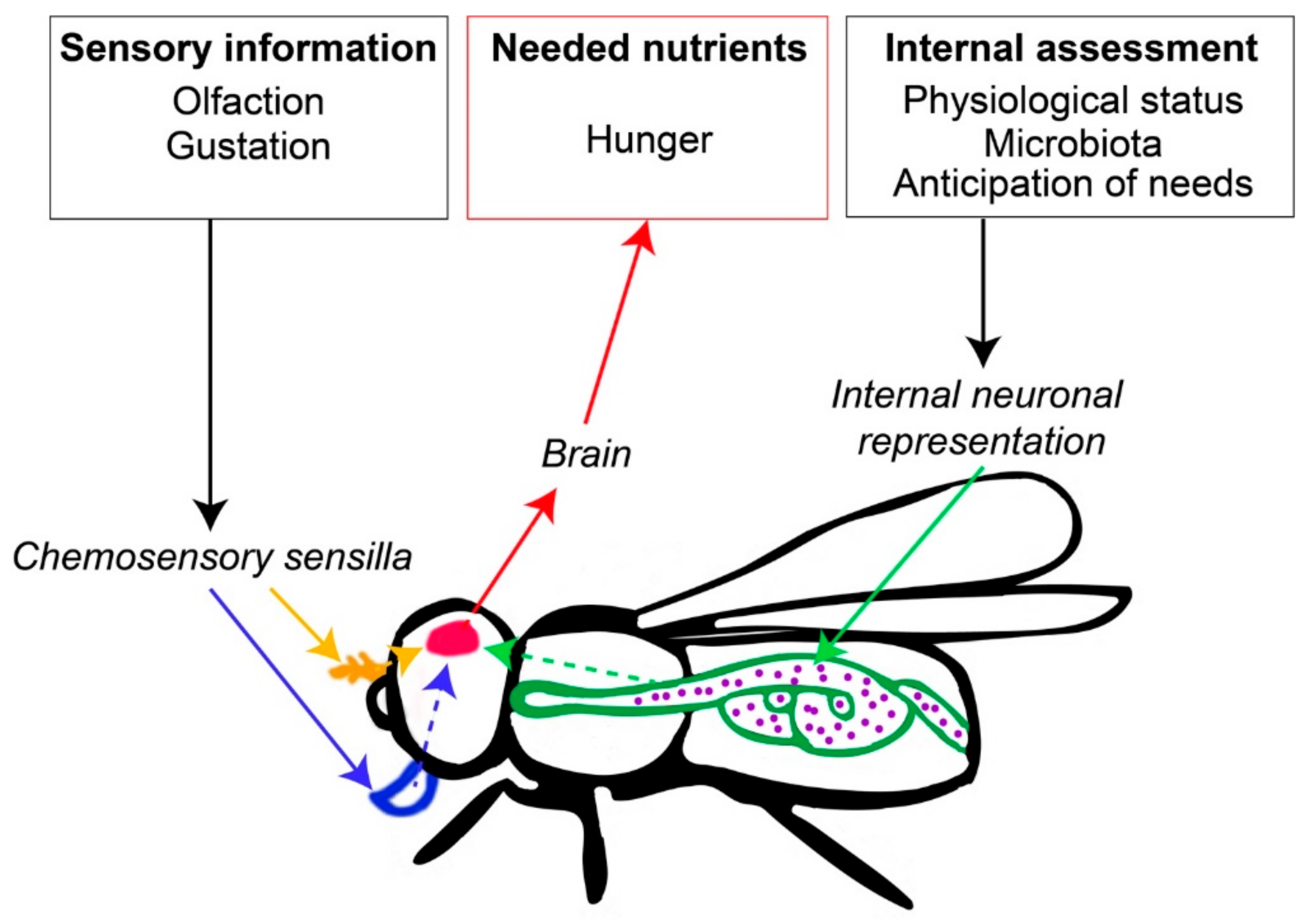
2. Cognitive Mechanisms Governing Nutritional Decisions
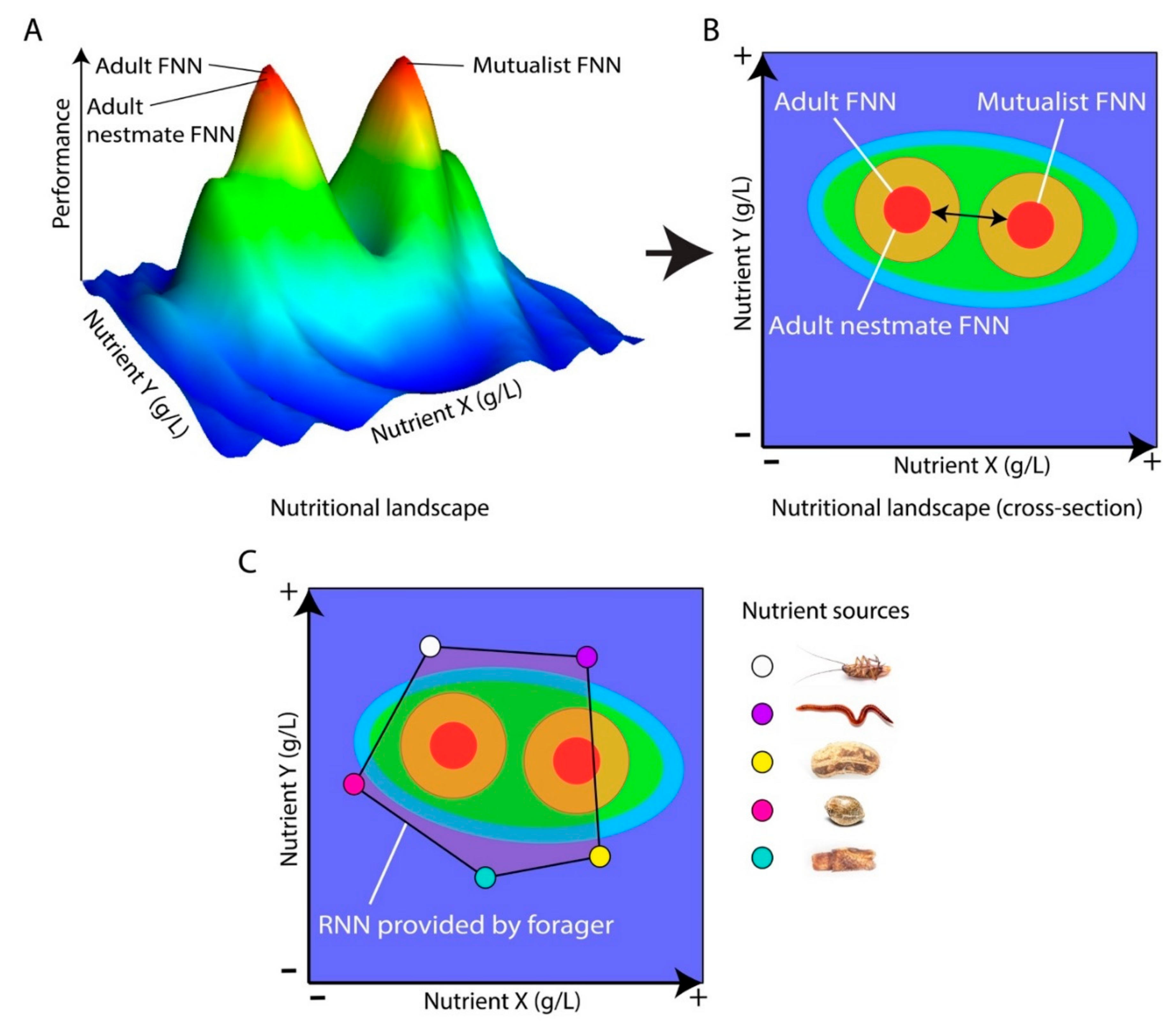
3. Using Ants to Apply NG to Cognition Research
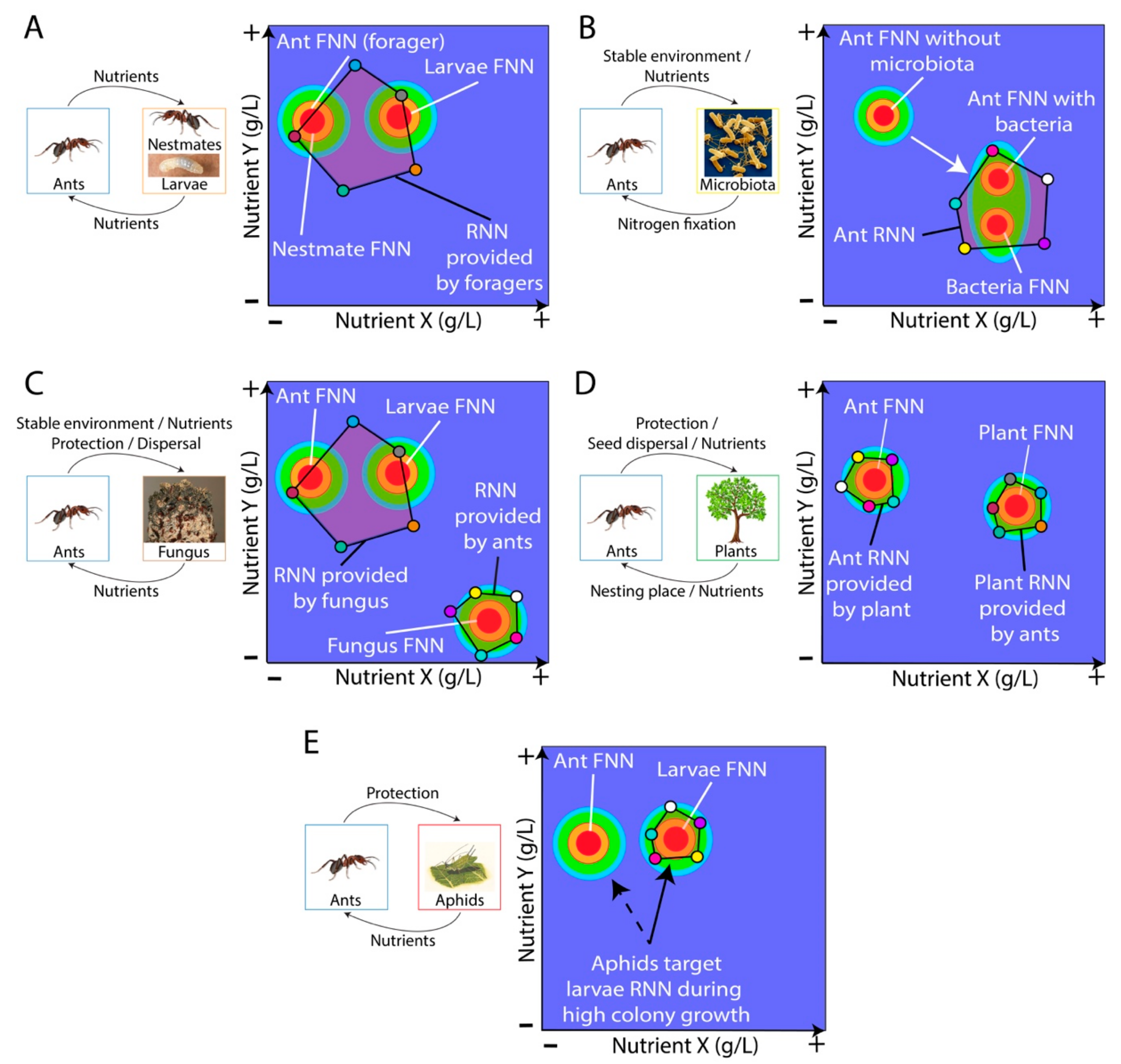
4. Mutualistic Relationships Involving Ants and an Unrelated Partner
4.1. Ant-Bacteria Mutualisms
4.2. Ant-Fungus Mutualisms
4.3. Ant-Plant Mutualisms
4.4. Ant-Insect Mutualisms
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Watanabe, K.; Kanaoka, Y.; Mizutani, S.; Uchiyama, H.; Yajima, S.; Watada, M.; Uemura, T.; Hattori, Y. Interspecies Comparative Analyses Reveal Distinct Carbohydrate-Responsive Systems among Drosophila Species. Cell Rep. 2019, 28, 2594. [Google Scholar] [CrossRef]
- Lee, K.P.; Simpson, S.J.; Clissold, F.J.; Brooks, R.; Ballard, J.W.O.; Taylor, P.W.; Soran, N.; Raubenheimer, D. Lifespan and reproduction in Drosophila: New insights from nutritional geometry. Proc. Natl. Acad. Sci. USA 2008, 105, 2498–2503. [Google Scholar] [CrossRef] [PubMed]
- Toshima, N.; Tanimura, T. Taste preference for amino acids is dependent on internal nutritional state in Drosophila melanogaster. J. Exp. Biol. 2012, 215, 2827–2832. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, J.G.; Shepherd, G.M. Mechanisms of olfactory discrimination: Converging evidence for common principles across phyla. Annu. Rev. Neurosci. 1997, 20, 595–631. [Google Scholar] [CrossRef] [PubMed]
- Amrein, H.; Thorne, N. Gustatory perception and behavior in Drosophila melanogaster. Curr. Biol. 2005, 15, R673–R684. [Google Scholar] [CrossRef]
- Yarmolinsky, D.A.; Zuker, C.S.; Ryba, N.J.P. Common sense about taste: From mammals to insects. Cell 2009, 139, 234–244. [Google Scholar] [CrossRef]
- Fujita, M.; Tanimura, T. Drosophila Evaluates and Learns the Nutritional Value of Sugars. Curr. Biol. 2011, 21, 751–755. [Google Scholar] [CrossRef]
- Itskov, P.M.; Ribeiro, C. The dilemmas of the gourmet fly: The molecular and neuronal mechanisms of feeding and nutrient decision making in Drosophila. Front. Neurosci. 2013, 7, 12. [Google Scholar] [CrossRef]
- Moreira, J.M.; Itskov, P.M.; Goldschmidt, D.; Baltazar, C.; Steck, K.; Tastekin, I.; Walker, S.J.; Ribeiro, C. optoPAD, a closed-loop optogenetics system to study the circuit basis of feeding behaviors. Elife 2019, 8, e43924. [Google Scholar] [CrossRef]
- Simpson, S.J.; Raubenheimer, D. The Nature of Nutrition: A Unifying Framework from Animal Adaptation to Human Obesity; Princeton University Press: Princeton, NJ, USA, 2012. [Google Scholar]
- Dussutour, A.; Nicolis, S.C.; Despland, E.; Simpson, S.J. Individual differences influence collective behaviour in social caterpillars. Anim. Behav. 2008, 76, 5–16. [Google Scholar] [CrossRef]
- Csata, E.; Dussutour, A. Nutrient regulation in ants (Hymenoptera: Formicidae): A review. Myrmecol. News 2019, 29, 111–124. [Google Scholar] [CrossRef]
- Lihoreau, M.; Buhl, J.; Charleston, M.A.; Sword, G.A.; Raubenheimer, D.; Simpson, S.J. Nutritional ecology beyond the individual: A conceptual framework for integrating nutrition and social interactions. Ecol. Lett. 2015, 18, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Shik, J.Z.; Gomez, E.B.; Kooij, P.W.; Santos, J.C.; Wcislo, W.T.; Boomsma, J.J. Nutrition mediates the expression of cultivar-farmer conflict in a fungus-growing ant. Proc. Natl. Acad. Sci. USA 2016, 113, 10121–10126. [Google Scholar] [CrossRef] [PubMed]
- Behmer, S.T. Insect Herbivore Nutrient Regulation. Annu. Rev. Entomol. 2009, 54, 165–187. [Google Scholar] [CrossRef]
- Farina, W.M.; Grüter, C. Trophallaxis: A mechanism of information transfer. In Food Exploitation by Social Insects: Ecological, Behavioral, and Theoretical Approaches; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Nielsen, C.; Agrawal, A.A.; Hajek, A.E. Ants defend aphids against lethal disease. Biol. Lett. 2010, 6, 205–208. [Google Scholar] [CrossRef]
- Blatrix, R.; Mayer, V. Communication in Ant–Plant Symbioses. In Plant Communication from an Ecological Perspective; Baluška, F., Ninkovic, V., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 127–158. [Google Scholar] [CrossRef]
- Boucias, D.G.; Lietze, V.-U.; Teal, P. Chemical Signals That Mediate Insect-Fungal Interactions. In Biocommunication of Fungi; Witzany, G., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 305–336. [Google Scholar] [CrossRef]
- Brandão, C.R.F.; Diniz, J.L.M.; Tomotake, E.M.J.I.S. Thaumatomyrmex strips millipedes for prey: A novel predatory behaviour in ants, and the first case of sympatry in the genus (Hymenoptera: Formicidae). Insectes Sociaux 1991, 38, 335–344. [Google Scholar] [CrossRef]
- Riveros, A.J.; Seid, M.A.; Wcislo, W.T. Evolution of brain size in class-based societies of fungus-growing ants (Attini). Anim. Behav. 2012, 83, 1043–1049. [Google Scholar] [CrossRef]
- Machovsky-Capuska, G.E.; Senior, A.M.; Simpson, S.J.; Raubenheimer, D. The Multidimensional Nutritional Niche. Trends Ecol. Evol. 2016, 31, 355–365. [Google Scholar] [CrossRef]
- Hawley, J.; Simpson, S.J.; Wilder, S.M. Flesh flies regulate the consumption of 3 macronutrients to maximize lifespan and egg production. Behav. Ecol. 2016, 27, 245–251. [Google Scholar] [CrossRef]
- Krabbe, B.A.; Arnan, X.; Lannes, P.; Bergstedt, C.E.; Larsen, R.S.; Pedersen, J.S.; Shik, J.Z. Using nutritional geometry to define the fundamental macronutrient niche of the widespread invasive ant Monomorium pharaonis. PLoS ONE 2019, 14, e0218764. [Google Scholar] [CrossRef]
- Raubenheimer, D. Toward a quantitative nutritional ecology: The right-angled mixture triangle. Ecol. Monogr. 2011, 81, 407–427. [Google Scholar] [CrossRef]
- Loewe, L. A framework for evolutionary systems biology. BMC Syst. Biol. 2009, 3, 27. [Google Scholar] [CrossRef] [PubMed]
- Dussutour, A.; Simpson, S.J. Communal Nutrition in Ants. Curr. Biol. 2009, 19, 740–744. [Google Scholar] [CrossRef] [PubMed]
- Hölldobler, B.; Wilson, E.O. The Superorganism: The beauty, Elegance, and Strangeness of Insect Societies; W.W. Norton: New York, NY, USA, 2009; p. 544. [Google Scholar]
- Martin, S.; Drijfhout, F. A Review of Ant Cuticular Hydrocarbons. J. Chem. Ecol. 2009, 35, 1151–1161. [Google Scholar] [CrossRef]
- Shik, J.Z.; Santos, J.C.; Seal, J.N.; Kay, A.; Mueller, U.G.; Kaspari, M. Metabolism and the Rise of Fungus Cultivation by Ants. Am. Nat. 2014, 184, 364–373. [Google Scholar] [CrossRef]
- Sorensen, A.A.; Vinson, S.B. Quantitative Food Distribution Studies within Laboratory Colonies of the Imported Fire Ant, Solenopsis-Invicta Buren. Insectes Sociaux 1981, 28, 129–160. [Google Scholar] [CrossRef]
- Weeks, R.D.; Wilson, L.T.; Vinson, S.B. Resource partitioning among colonies of polygyne red imported fire ants (Hymenoptera: Formicidae). Environ. Entomol. 2004, 33, 1602–1608. [Google Scholar] [CrossRef]
- Beattie, A.J. The Evolutionary ecology of Ant-Plant Mutualisms; Cambridge University Press: Cambridge, UK, 1985. [Google Scholar]
- Mueller, U.G.; Schultz, T.R.; Currie, C.R.; Adams, R.M.M.; Malloch, D. The origin of the attine ant-fungus mutualism. Q. Rev. Biol. 2001, 76, 169–197. [Google Scholar] [CrossRef]
- Russell, J.A.; Moreau, C.S.; Goldman-Huertas, B.; Fujiwara, M.; Lohman, D.J.; Pierce, N.E. Bacterial gut symbionts are tightly linked with the evolution of herbivory in ants. Proc. Natl. Acad. Sci. USA 2009, 106, 21236–21241. [Google Scholar] [CrossRef]
- Stadler, B.; Dixon, A.F.G. Mutualism: Ants and Their Insect Partners; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Shanbhag, S.R.; Vazhappilly, A.T.; Sane, A.; D’Silva, N.M.; Tripathi, S. Electrolyte transport pathways induced in the midgut epithelium of Drosophila melanogaster larvae by commensal gut microbiota and pathogens. J. Physiol. Lond. 2017, 595, 523–539. [Google Scholar] [CrossRef]
- Feldhaar, H.; Gross, R. Insects as hosts for mutualistic bacteria. Int. J. Med. Microbiol. 2009, 299, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.A.; Sanders, J.G.; Moreau, C.S. Hotspots for symbiosis: Function, evolution, and specificity of ant-microbe associations from trunk to tips of the ant phylogeny (Hymenoptera: Formicidae). Myrmecol. News 2017, 24, 43–69. [Google Scholar]
- Mehdiabadi, N.J.; Schultz, T.R. Natural history and phylogeny of the fungus-farming ants (Hymenoptera: Formicidae: Myrmicinae: Attini). Myrmecol. News 2010, 13, 37–55. [Google Scholar]
- Poulsen, M.; Currie, C.R. On ants, plants and fungi. N. Phytol. 2009, 182, 785–788. [Google Scholar] [CrossRef]
- Rico-Gray, V.; Oliveira, P.S. The Ecology and Evolution of Ant-Plant Interactions; University of Chicago Press: Chicago, IL, USA, 2007; p. 331. [Google Scholar]
- Kay, A.D.; Bruning, A.J.; van Alst, A.; Abrahamson, T.T.; Hughes, W.O.H.; Kaspari, M. A carbohydrate-rich diet increases social immunity in ants. Proc. Biol. Sci 2014, 281, 20132374. [Google Scholar] [CrossRef]
- Shik, J.Z.; Concilio, A.; Kaae, T.; Adams, R.M.M. The farming ant Sericomyrmex amabilis nutritionally manages its fungal symbiont and its social parasite. Ecol. Entomol. 2018, 43, 440–446. [Google Scholar] [CrossRef]
- Feldhaar, H.; Straka, J.; Krischke, M.; Berthold, K.; Stoll, S.; Mueller, M.J.; Gross, R. Nutritional upgrading for omnivorous carpenter ants by the endosymbiont Blochmannia. BMC Biol. 2007, 5, 48. [Google Scholar] [CrossRef]
- Feldhaar, H. Bacterial symbionts as mediators of ecologically important traits of insect hosts. Ecol. Entomol. 2011, 36, 533–543. [Google Scholar] [CrossRef]
- Sapountzis, P.; Zhukova, M.; Hansen, L.H.; Sorensen, S.J.; Schiott, M.; Boomsma, J.J. Acromyrmex Leaf-Cutting Ants Have Simple Gut Microbiota with Nitrogen-Fixing Potential. Appl. Environ. Microb. 2015, 81, 5527–5537. [Google Scholar] [CrossRef]
- Hu, Y.; Sanders, J.G.; Lukasik, P.; D’Amelio, C.L.; Millar, J.S.; Vann, D.R.; Lan, Y.M.; Newton, J.A.; Schotanus, M.; Kronauer, D.J.C.; et al. Herbivorous turtle ants obtain essential nutrients from a conserved nitrogen-recycling gut microbiome. Nat. Commun. 2018, 9, 964. [Google Scholar] [CrossRef]
- Sapountzis, P.; Zhukova, M.; Shik, J.Z.; Schiott, M.; Boomsma, J.J. Reconstructing the functions of endosymbiotic Mollicutes in fungus-growing ants. Elife 2018, 7, e39209. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.; Schrader, L.; Gil, R.; Manzano-Marin, A.; Florez, L.; Wheeler, D.; Werren, J.H.; Latorre, A.; Heinze, J.; Kaltenpoth, M.; et al. A novel intracellular mutualistic bacterium in the invasive ant Cardiocondyla obscurior. ISME J. 2016, 10, 376–388. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.C.N.; Wang, Q.P.; Morimoto, J.; Senior, A.M.; Lihoreau, M.; Neely, G.G.; Simpson, S.J.; Ponton, F. Gut Microbiota Modifies Olfactory-Guided Microbial Preferences and Foraging Decisions in Drosophila. Curr. Biol. 2017, 27, 2397. [Google Scholar] [CrossRef] [PubMed]
- Pasquaretta, C.; Gomez-Moracho, T.; Heeb, P.; Lihoreau, M. Exploring Interactions between the Gut Microbiota and Social Behavior through Nutrition. Genes 2018, 9, 534. [Google Scholar] [CrossRef]
- Holmes, A.J.; Chew, Y.V.; Colakoglu, F.; Cliff, J.B.; Klaassens, E.; Read, M.N.; Solon-Biet, S.M.; McMahon, A.C.; Cogger, V.C.; Ruohonen, K.; et al. Diet-Microbiome Interactions in Health Are Controlled by Intestinal Nitrogen Source Constraints. Cell Metab. 2017, 25, 140–151. [Google Scholar] [CrossRef]
- Defossez, E.; Selosse, M.A.; Dubois, M.P.; Mondolot, L.; Faccio, A.; Djieto-Lordon, C.; McKey, D.; Blatrix, R. Ant-plants and fungi: A new threeway symbiosis. N. Phytol. 2009, 182, 942–949. [Google Scholar] [CrossRef]
- Nepel, M.; Voglmayr, H.; Schonenberger, J.; Mayer, V.E. High Diversity and Low Specificity of Chaetothyrialean Fungi in Carton Galleries in a Neotropical Ant-Plant Association. PLoS ONE 2014, 9, e112756. [Google Scholar] [CrossRef]
- Maschwitz, U.; Hölldobler, B. Der Kartonnestbau bei Lasius fuliginosus Latr. (Hym. Formicidae). Z. Vgl. Physiol. 1970, 66, 176–189. [Google Scholar] [CrossRef]
- Blatrix, R.; Djieto-Lordon, C.; Mondolot, L.; La Fisca, P.; Voglmayr, H.; McKey, D. Plant-ants use symbiotic fungi as a food source: New insight into the nutritional ecology of ant-plant interactions. Proc. R. Soc. B Biol. Sci. 2012, 279, 3940–3947. [Google Scholar] [CrossRef]
- Shik, J.Z.; Rytter, W.; Arnan, X.; Michelsen, A. Disentangling nutritional pathways linking leafcutter ants and their co-evolved fungal symbionts using stable isotopes. Ecology 2018, 99, 1999–2009. [Google Scholar] [CrossRef]
- Nygaard, S.; Hu, H.F.; Li, C.; Schiott, M.; Chen, Z.S.; Yang, Z.K.; Xie, Q.L.; Ma, C.Y.; Deng, Y.; Dikow, R.B.; et al. Reciprocal genomic evolution in the ant-fungus agricultural symbiosis. Nat. Commun. 2016, 7, 12233. [Google Scholar] [CrossRef] [PubMed]
- Mueller, U.G.; Scott, J.J.; Ishak, H.D.; Cooper, M.; Rodrigues, A. Monoculture of Leafcutter Ant Gardens. PLoS ONE 2010, 5, e12668. [Google Scholar] [CrossRef] [PubMed]
- De Fine Licht, H.H.; Boomsma, J.J. Forage collection, substrate preparation, and diet composition in fungus-growing ants. Ecol. Entomol. 2010, 35, 259–269. [Google Scholar] [CrossRef]
- De Fine Licht, H.H.; Boomsma, J.J.; Tunlid, A. Symbiotic adaptations in the fungal cultivar of leaf-cutting ants. Nat. Commun. 2014, 5, 5675. [Google Scholar] [CrossRef]
- Quinlan, R.J.; Cherrett, J.M. Role of Fungus in the Diet of the Leaf-Cutting Ant Atta cephalotes (L.). Ecol. Entomol. 1979, 4, 151–160. [Google Scholar] [CrossRef]
- Martin, M.M.; Macconnell, J.G.; Gale, G.R. The Chemical Basis for the Attine Ant-Fungus Symbiosis. Absence of Antibiotics. Ann. Entomol. Soc. Am. 1969, 62, 386–388. [Google Scholar] [CrossRef]
- Green, P.W.C.; Kooij, P.W. The role of chemical signalling in maintenance of the fungus garden by leaf-cutting ants. Chemoecology 2018, 28, 101–107. [Google Scholar] [CrossRef]
- Moreau, C.S.; Bell, C.D. Testing the Museum Versus Cradle Tropical Biological Diversity Hypothesis: Phylogeny, Diversification, and Ancestral Biogeographic Range Evolution of the Ants. Evolution 2013, 67, 2240–2257. [Google Scholar] [CrossRef]
- Chomicki, G.; Staedler, Y.M.; Schonenberger, J.; Renner, S.S. Partner choice through concealed floral sugar rewards evolved with the specialization of ant-plant mutualisms. N. Phytol. 2016, 211, 1358–1370. [Google Scholar] [CrossRef]
- Koptur, S. Extrafloral nectary-mediated interactions between insects and plants. Insect Plant Interact. 1992, IV, 81–129. [Google Scholar]
- Fiala, B.; Maschwitz, U. Food Bodies and Their Significance for Obligate Ant-Association in the Tree Genus Macaranga (Euphorbiaceae). Bot. J. Linn. Soc. 1992, 110, 61–75. [Google Scholar] [CrossRef]
- Galetto, L.; Bernardello, L. Nectar Secretion Pattern and Removal Effects in Six Argentinean Pitcairnioideae (Bromeliaceae). Bot. Acta 1992, 105, 292–299. [Google Scholar] [CrossRef]
- Heil, M.; Baumann, B.; Kruger, R.; Linsenmair, K.E. Main nutrient compounds in food bodies of Mexican Acacia ant-plants. Chemoecology 2004, 14, 45–52. [Google Scholar] [CrossRef]
- Heil, M.; Hilpert, A.; Fiala, B.; Bin Hashim, R.; Strohm, E.; Zotz, G.; Linsenmair, K.E. Nutrient allocation of Macaranga triloba ant plants to growth, photosynthesis and indirect defence. Funct. Ecol. 2002, 16, 475–483. [Google Scholar] [CrossRef]
- O’Dowd, D.J. Pearl Bodies as Ant Food: An Ecological Role for Some Leaf Emergences of Tropical Plants. Biotropica 1982, 14, 40–49. [Google Scholar] [CrossRef]
- Hatada, A.; Itioka, T.; Yamaoka, R.; Itino, T. Carbon and nitrogen contents of food bodies in three myrmecophytic species of Macaranga: Implications for antiherbivore defense mechanisms. J. Plant Res. 2002, 115, 179–184. [Google Scholar] [CrossRef]
- Sagers, C.L.; Ginger, S.M.; Evans, R.D. Carbon and nitrogen isotopes trace nutrient exchange in an ant-plant mutualism. Oecologia 2000, 123, 582–586. [Google Scholar] [CrossRef]
- Culver, D.C.; Beattie, A.J. Myrmecochory in Viola—Dynamics of Seed-Ant Interactions in Some West-Virginia Species. J. Ecol. 1978, 66, 53–72. [Google Scholar] [CrossRef]
- Youngsteadt, E.; Nojima, S.; Haberlein, C.; Schulz, S.; Schal, C. Seed odor mediates an obligate ant-plant mutualism in Amazonian rainforests. Proc. Natl. Acad. Sci. USA 2008, 105, 4571–4575. [Google Scholar] [CrossRef]
- Bond, W.; Slingsby, P. Collapse of an Ant-Plant Mutalism: The Argentine Ant (Iridomyrmex Humilis) and Myrmecochorous Proteaceae. Ecology 1984, 65, 1031–1037. [Google Scholar] [CrossRef]
- Janzen, D.H. Interaction of the bull’s-horn acacia (Acacia cornigera L.) with an ant inhabitant (Pseudomyrmex ferrugineus F. Smith) in eastern Mexico. Kans. Univ. Sci. Bull. 1967, 47, 315–558. [Google Scholar]
- Kusmenoglu, S.; Rockwood, L.L.; Gretz, M.R. Fatty-Acids and Diacylglycerols from Elaiosomes of Some Ant-Dispersed Seeds. Phytochemistry 1989, 28, 2601–2602. [Google Scholar] [CrossRef]
- Lanza, J.; Schmitt, M.A.; Awad, A.B. Comparative chemistry of elaiosomes of three species of Trillium. J. Chem. Ecol. 1992, 18, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Hughes, L.; Westoby, M.; Jurado, E. Convergence of Elaiosomes and Insect Prey—Evidence from Ant Foraging Behavior and Fatty-Acid Composition. Funct. Ecol. 1994, 8, 358–365. [Google Scholar] [CrossRef]
- Fischer, R.C.; Olzant, S.M.; Wanek, W.; Mayer, V. The fate of Corydalis cava elaiosomes within an ant colony of Myrmica rubra: Elaiosomes are preferentially fed to larvae. Insectes Sociaux 2005, 52, 55–62. [Google Scholar] [CrossRef]
- Chomicki, G.; Renner, S.S. Partner abundance controls mutualism stability and the pace of morphological change over geologic time. Proc. Natl. Acad. Sci. USA 2017, 114, 3951–3956. [Google Scholar] [CrossRef]
- Youngsteadt, E.; Baca, J.A.; Osborne, J.; Schal, C. Species-Specific Seed Dispersal in an Obligate Ant-Plant Mutualism. PLoS ONE 2009, 4, e4335. [Google Scholar] [CrossRef]
- Blüthgen, N.; Schmit-Neuerburg, V.; Engwald, S.; Barthlott, W. Ants as epiphyte gardeners: Comparing the nutrient quality of ant and termite canopy substrates in a Venezuelan lowland rain forest. J. Trop. Ecol. 2001, 17, 887–894. [Google Scholar] [CrossRef]
- Hughes, L.; Westoby, M. Fate of Seeds Adapted for Dispersal by Ants in Australian Sclerophyll Vegetation. Ecology 1992, 73, 1285–1299. [Google Scholar] [CrossRef]
- Orona-Tamayo, D.; Wielsch, N.; Blanco-Labra, A.; Svatos, A.; Farias-Rodriguez, R.; Heil, M. Exclusive rewards in mutualisms: Ant proteases and plant protease inhibitors create a lock-key system to protect Acacia food bodies from exploitation. Mol. Ecol. 2013, 22, 4087–4100. [Google Scholar] [CrossRef]
- Stadler, B.; Dixon, A.F.G. Ecology and evolution of aphid-ant interactions. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 345–372. [Google Scholar] [CrossRef]
- Nash, D.R.; Als, T.D.; Boomsma, J.J. Survival and growth of parasitic Maculinea alcon caterpillars (Lepidoptera, Lycaenidae) in laboratory nests of three Myrmica ant species. Insectes Sociaux 2011, 58, 391–401. [Google Scholar] [CrossRef]
- Nixon, G.E.J. The Association of Ants with Aphids and Coccids; Commonwealth Institute of Entomology: London, UK, 1951; p. 36. [Google Scholar]
- Volkl, W.; Woodring, J.; Fischer, M.; Lorenz, M.W.; Hoffmann, K.H. Ant-aphid mutualisms: The impact of honeydew production and honeydew sugar composition on ant preferences. Oecologia 1999, 118, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.K.; Shingleton, A.W. Host plant and ants influence the honeydew sugar composition of aphids. Funct. Ecol. 2001, 15, 544–550. [Google Scholar] [CrossRef]
- Shik, J.Z.; Silverman, J. Towards a nutritional ecology of invasive establishment: Aphid mutualists provide better fuel for incipient Argentine ant colonies than insect prey. Biol. Invasions 2013, 15, 829–836. [Google Scholar] [CrossRef]
- Helms, K.R.; Vinson, S.B. Plant resources and colony growth in an invasive ant: The importance of honeydew-producing Hemiptera in carbohydrate transfer across trophic levels. Environ. Entomol. 2008, 37, 487–493. [Google Scholar] [CrossRef]
- Kiss, A. Melezitose, Aphids and Ants. Oikos 1981, 37, 382. [Google Scholar] [CrossRef]
- Fischer, M.K.; Volkl, W.; Schopf, R.; Hoffmann, K.H. Age-specific patterns in honeydew production and honeydew composition in the aphid Metopeurum fuscoviride: Implications for ant-attendance. J. Insect Physiol. 2002, 48, 319–326. [Google Scholar] [CrossRef]
- Cloutier, C.; Mackauer, M. The effect of parasitism by Aphidius smithi (Hymenoptera: Aphidiidae) on the food budget of the pea aphid, Acyrthosiphon pisum (Homoptera: Aphididae). Can. J. Zool. 1979, 57, 1605–1611. [Google Scholar] [CrossRef]
- Volkl, W. Aphids or Their Parasitoids—Who Actually Benefits from Ant-Attendance. J. Anim. Ecol. 1992, 61, 273–281. [Google Scholar] [CrossRef]
- Sakata, H. How an Ant Decides to Prey on or to Attend Aphids. Popul. Ecol. 1994, 36, 45–51. [Google Scholar] [CrossRef]
- Becerra, J.X.I.; Venable, D.L. Extrafloral Nectaries—A Defense against Ant-Homoptera Mutualisms. Oikos 1989, 55, 276–280. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crumière, A.J.J.; Stephenson, C.J.; Nagel, M.; Shik, J.Z. Using Nutritional Geometry to Explore How Social Insects Navigate Nutritional Landscapes. Insects 2020, 11, 53. https://doi.org/10.3390/insects11010053
Crumière AJJ, Stephenson CJ, Nagel M, Shik JZ. Using Nutritional Geometry to Explore How Social Insects Navigate Nutritional Landscapes. Insects. 2020; 11(1):53. https://doi.org/10.3390/insects11010053
Chicago/Turabian StyleCrumière, Antonin J. J., Calum J. Stephenson, Manuel Nagel, and Jonathan Z. Shik. 2020. "Using Nutritional Geometry to Explore How Social Insects Navigate Nutritional Landscapes" Insects 11, no. 1: 53. https://doi.org/10.3390/insects11010053
APA StyleCrumière, A. J. J., Stephenson, C. J., Nagel, M., & Shik, J. Z. (2020). Using Nutritional Geometry to Explore How Social Insects Navigate Nutritional Landscapes. Insects, 11(1), 53. https://doi.org/10.3390/insects11010053




