Inter-Individual Nectar Chemistry Changes of Field Scabious, Knautia arvensis
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Field Site
2.2. Nectar Sampling
2.3. Sample Preparation
2.4. Amino Acid and Carbohydrate Analysis
2.5. Observations of Flower Visitors
2.6. Statistical Analyses
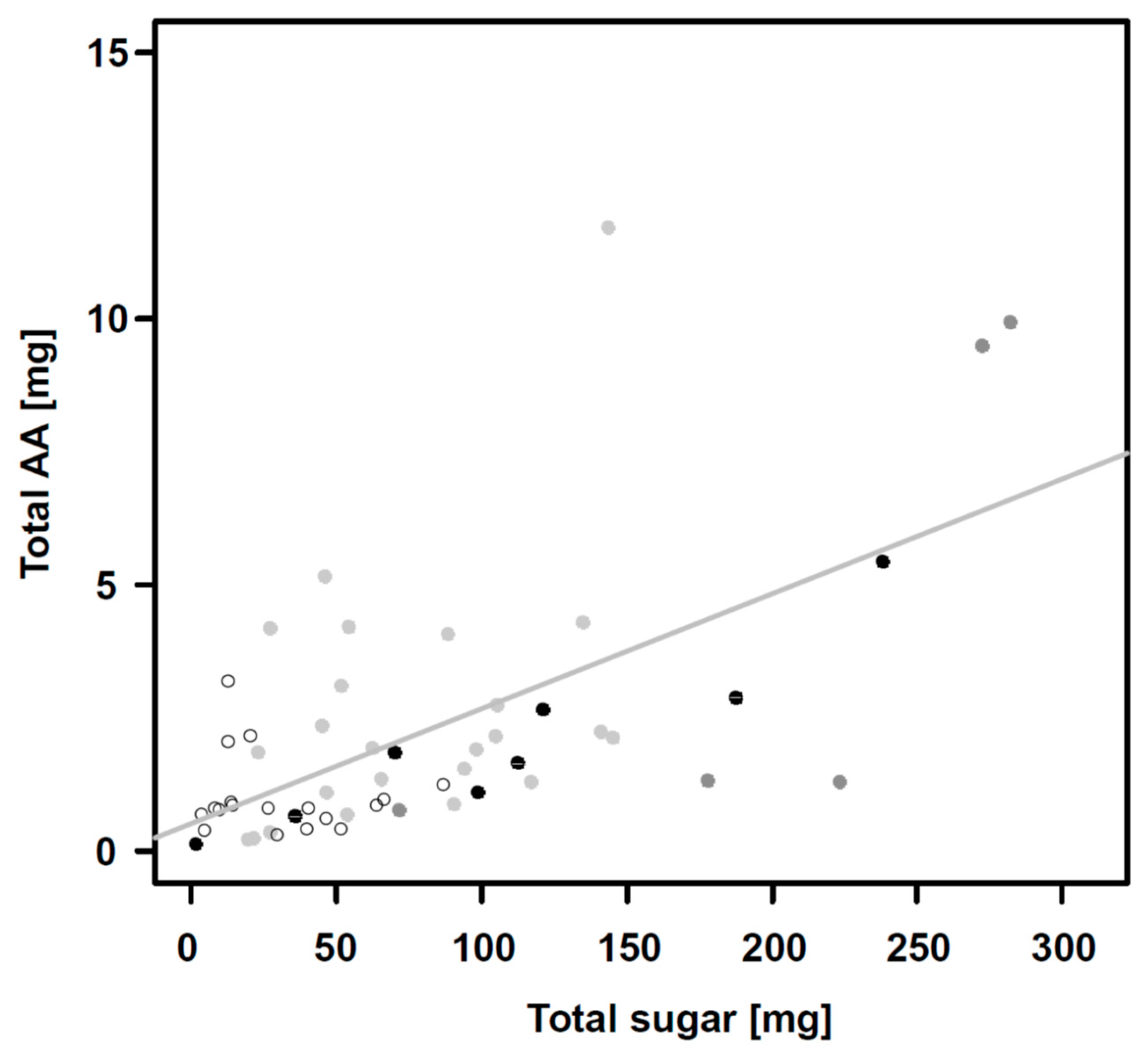
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Scherber, C.; Eisenhauer, N.; Weisser, W.W.; Schmid, B.; Voigt, W.; Fischer, M.; Schulze, E.-D.; Roscher, C.; Weigelt, A.; Allan, E.; et al. Bottom-up effects of plant diversity on multitrophic interactions in a biodiversity experiment. Nature 2010, 468, 553–556. [Google Scholar] [CrossRef] [PubMed]
- Balvanera, P.; Siddique, I.; Dee, L.; Paquette, A.; Isbell, F.; Gonzalez, A.; Byrnes, J.; O’Connor, M.I.; Hungate, B.A.; Griffin, J.N. Linking biodiversity and ecosystem services: Current uncertainties and the necessary next steps. Bioscience 2014, 64, 49–57. [Google Scholar] [CrossRef]
- Cardinale, B.J.; Matulich, K.L.; Hooper, D.U.; Byrnes, J.E.; Duffy, E.; Gamfeldt, L.; Balvanera, P.; O’Connor, M.I.; Gonzalez, A. The functional role of producer diversity in ecosystems. Am. J. Bot. 2011, 98, 572–592. [Google Scholar] [CrossRef] [PubMed]
- Ebeling, A.; Klein, A.-M.; Weisser, W.W.; Tscharntke, T. Multitrophic effects of experimental changes in plant diversity on cavity-nesting bees, wasps, and their parasitoids. Oecologia 2012, 169, 453–465. [Google Scholar] [CrossRef]
- Dudenhöffer, J.-H.; Pufal, G.; Roscher, C.; Klein, A.-M. Plant density can increase invertebrate postdispersal seed predation in an experimental grassland community. Ecol. Evol. 2016, 6, 3796–3807. [Google Scholar] [CrossRef]
- Neuschulz, E.L.; Mueller, T.; Schleuning, M.; Böhning-Gaese, K. Pollination and seed dispersal are the most threatened processes of plant regeneration. Sci. Rep. 2016, 6, 29839. [Google Scholar] [CrossRef]
- Michener, C.D. The Bees of the World, 2nd ed.; The Johns Hopkins University Press: Baltimore, MD, USA, 2007. [Google Scholar]
- Nicholls, C.I.; Altieri, M.A. Plant biodiversity enhances bees and other insect pollinators in agroecosystems. A review. Agron. Sustain. Dev. 2012, 33, 257–274. [Google Scholar] [CrossRef]
- Willmer, P. Rewards 2: The Biology of Nectar. In Pollination and Floral Ecology; Willmer, P., Ed.; Princeton University Press: Princeton, NJ, USA, 2011; pp. 190–220. [Google Scholar]
- Biesmeijer, J.C.; Roberts, S.P.M.; Reemer, M.; Ohlemüller, R.; Edwards, M.; Peeters, T.; Schaffers, A.P.; Potts, S.G.; Kleukers, R.; Thomas, C.D.; et al. Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science 2006, 313, 351–354. [Google Scholar] [CrossRef]
- Ebeling, A.; Klein, A.-M.; Schumacher, J.; Weisser, W.W.; Tscharntke, T. How does plant richness affect pollinator richness and temporal stability of flower visits? Oikos 2008, 117, 1808–1815. [Google Scholar] [CrossRef]
- Fründ, J.; Linsenmair, K.E.; Blüthgen, N. Pollinator diversity and specialization in relation to flower diversity. Oikos 2010, 119, 1581–1590. [Google Scholar] [CrossRef]
- Baker, H.G. Non-sugar chemical constituents of nectar. Apidologie 1977, 8, 349–356. [Google Scholar] [CrossRef]
- Gardener, M.C.; Gillman, M.P. The taste of nectar - a neglected area of pollination ecology. Oikos 2002, 98, 552–557. [Google Scholar] [CrossRef]
- Nepi, M.; Soligo, C.; Nocentini, D.; Abate, M.; Guarnieri, M.; Cai, G.; Bini, L.; Puglia, M.; Bianchi, L.; Pacini, E. Amino acids and protein profile in floral nectar: Much more than a simple reward. Flora - Morphol. Distrib. Funct. Ecol. Plants 2012, 207, 475–481. [Google Scholar] [CrossRef]
- Herrera, C.M.; Pérez, R.; Alonso, C. Extreme intraplant variation in nectar sugar composition in an insect-pollinated perennial herb. Am. J. Bot. 2006, 93, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Gijbels, P.; Van den Ende, W.; Honnay, O. Landscape scale variation in nectar amino acid and sugar composition in a Lepidoptera pollinated orchid species and its relation with fruit set. J. Ecol. 2014, 102, 136–144. [Google Scholar] [CrossRef]
- Behmer, S.T. Insect Herbivore Nutrient Regulation. Annu. Rev. Entomol. 2009, 54, 165–187. [Google Scholar] [CrossRef]
- Carmona, D.; Lajeunesse, M.J.; Johnson, M.T.J. Plant traits that predict resistance to herbivores. Funct. Ecol. 2011, 25, 358–367. [Google Scholar] [CrossRef]
- Couture, J.J.; Mason, C.J.; Habeck, C.W.; Lindroth, R.L. Behavioral and morphological responses of an insect herbivore to low nutrient quality are inhibited by plant chemical defenses. Arthropod. Plant. Interact. 2016, 10, 341–349. [Google Scholar] [CrossRef]
- Li, L.; Li, C.; Lee, G.I.; Howe, G.A. Distinct roles for jasmonate synthesis and action in the systemic wound response of tomato. Proc. Natl. Acad. Sci. USA 2002, 99, 6416–6421. [Google Scholar] [CrossRef]
- Price, P.W. Resource-driven terrestrial interaction webs. Ecol. Res. 2002, 17, 241–247. [Google Scholar] [CrossRef]
- Heinrich, B. The energetics of pollination. Ann. Missouri Bot. Gard. 1981, 68, 370–378. [Google Scholar] [CrossRef]
- Nicolson, S.W. Bee food: the chemistry and nutritional value of nectar, pollen and mixtures of the two: review article. African Zool. 2011, 46, 197–204. [Google Scholar] [CrossRef]
- Pacini, E.; Nepi, M.; Vesprini, J. Nectar biodiversity: a short review. Plant Syst. Evol. 2003, 238, 7–21. [Google Scholar] [CrossRef]
- Pyke, G.H. What does it cost a plant to produce floral nectar? Nature 1991, 350, 58–59. [Google Scholar] [CrossRef]
- Nicolson, S.W. Pollination by passerine birds: why are the nectars so dilute? Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2002, 131, 645–652. [Google Scholar] [CrossRef]
- Nicolson, S.W.; Thornburg, R.W. Nectar chemistry. In Nectaries and Nectar; Nicolson, S.W., Nepi, M., Pacini, E., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 215–264. [Google Scholar]
- Buchmann, S.L. The ecology of oil flowers and their bees. Annu. Rev. Ecol. Syst. 1987, 18, 343–369. [Google Scholar] [CrossRef]
- Baker, H.G.; Baker, I. Studies of nectar constitution and pollinator-plant coevolution. In Coevolution of Animals and Plants; Gilbert, L.E., Raven, P.H., Eds.; University of Texas Press: Austin, TX, USA, 1975; pp. 100–140. [Google Scholar]
- Nicolson, S.W. Nectar consumers. In Nectaries and Nectar; Nicolson, S.W., Nepi, M., Pacini, E., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 289–342. [Google Scholar]
- De Groot, A.P. Protein and amino acid requirements of the honeybee (Apis mellifica L.). Physiol. Comp. Oecologia 1953, 197–285. [Google Scholar]
- Bertazzini, M.; Forlani, G. Intraspecific variability of floral nectar volume and composition in rapeseed (Brassica napus L. var. oleifera). Front. Plant Sci. 2016, 7, 1–13. [Google Scholar] [CrossRef]
- Simpson, S.J.; Raubenheimer, D. The Nature of Nutrition: A Unifying Framework from Animal Adaptation to Human Obesity; Simpson, S.J., Raubenheimer, D., Eds.; Princeton University Press: Princeton, NJ, USA, 2012. [Google Scholar]
- Abbas, M.; Klein, A.-M.; Ebeling, A.; Oelmann, Y.; Ptacnik, R.; Weisser, W.W.; Hillebrand, H. Plant diversity effects on pollinating and herbivorous insects can be linked to plant stoichiometry. Basic Appl. Ecol. 2014, 15, 169–178. [Google Scholar] [CrossRef]
- Paoli, P.P.; Donley, D.; Stabler, D.; Saseendranath, A.; Nicolson, S.W.; Simpson, S.J.; Wright, G.A. Nutritional balance of essential amino acids and carbohydrates of the adult worker honeybee depends on age. Amino Acids 2014, 46, 1449–1458. [Google Scholar] [CrossRef]
- Vaudo, A.D.; Tooker, J.F.; Grozinger, C.M.; Patch, H.M. Bee nutrition and floral resource restoration. Curr. Opin. Insect Sci. 2015, 10, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Vaudo, A.D.; Patch, H.M.; Mortensen, D.A.; Tooker, J.F.; Grozinger, C.M. Macronutrient ratios in pollen shape bumble bee (Bombus impatiens) foraging strategies and floral preferences. Proc. Natl. Acad. Sci. USA 2016, 113, E4035–E4042. [Google Scholar] [CrossRef] [PubMed]
- Vaudo, A.D.; Stabler, D.; Patch, H.M.; Tooker, J.F.; Grozinger, C.M.; Wright, G.A. Bumble bees regulate their intake of essential protein and lipid pollen macronutrients. J. Exp. Biol. 2016, 219, 3962–3970. [Google Scholar] [CrossRef] [PubMed]
- Garratt, M.P.D.; Breeze, T.D.; Boreux, V.; Fountain, M.T.; McKerchar, M.; Webber, S.M.; Coston, D.J.; Jenner, N.; Dean, R.; Westbury, D.B.; et al. Apple pollination: Demand depends on variety and supply depends on pollinator identity. PLoS ONE 2016, 11, e0153889. [Google Scholar] [CrossRef]
- Knuth, P.E.O.W.; Appel, O.; Loew, E.; Müller, H. Handbuch der Blütenbiologie Band 2, Teil 1; Engelmann: Leipzig, Germany, 1898; pp. 557–562. [Google Scholar]
- Larsson, M. Higher pollinator effectiveness by specialist than generalist flower-visitors of unspecialized Knautia arvensis (Dipsacaceae). Oecologia 2005, 146, 394–403. [Google Scholar] [CrossRef]
- Ebeling, A.; Klein, A.-M.; Tscharntke, T. Plant–flower visitor interaction webs: Temporal stability and pollinator specialization increases along an experimental plant diversity gradient. Basic Appl. Ecol. 2011, 12, 300–309. [Google Scholar] [CrossRef]
- Roscher, C.; Schumacher, J.; Baade, J.; Wilcke, W.; Gleixner, G.; Weisser, W.W.; Schmid, B.; Schulze, E.-D. The role of biodiversity for element cycling and trophic interactions: an experimental approach in a grassland community. Basic Appl. Ecol. 2004, 5, 107–121. [Google Scholar] [CrossRef]
- Fornoff, F.; Klein, A.M.; Hartig, F.; Benadi, G.; Venjakob, C.; Schaefer, H.M.; Ebeling, A. Functional flower traits and their diversity drive pollinator visitation. Oikos 2017, 126, 1020–1030. [Google Scholar] [CrossRef]
- Canto, A.; Herrera, C.M.; Medrano, M.; Pérez, R.; García, I.M. Pollinator foraging modifies nectar sugar composition in Helleborus foetidus (Ranunculaceae): An experimental test. Am. J. Bot. 2008, 95, 315–320. [Google Scholar] [CrossRef]
- Venjakob, C.; Klein, A.-M.; Leonhardt, S.D. Nectar amino acid and carbohydrate composition are driven by flower morphology and phylogeny. Planta. Publishing status: major revision.
- Weisser, W.W.; Roscher, C.; Meyer, S.T.; Ebeling, A.; Luo, G.; Allan, E.; Beßler, H.; Barnard, R.L.; Buchmann, N.; Buscot, F.; et al. Biodiversity effects on ecosystem functioning in a 15-year grassland experiment: Patterns, mechanisms, and open questions. Basic Appl. Ecol. 2017, 23, 1–73. [Google Scholar] [CrossRef]
- Vange, V. Breeding system and inbreeding depression in the clonal plant species Knautia arvensis (Dipsacaceae): Implications for survival in abandoned grassland. Biol. Conserv. 2002, 108, 59–67. [Google Scholar] [CrossRef]
- Gottsberger, G.; Arnold, T.; Linskens, H.F. Variation in floral nectar amino acids with aging of flowers, pollen contamination, and flower damage. Isr. J. Bot. 1990, 39, 167–176. [Google Scholar]
- Pacini, E.; Nepi, M. Nectar production and presentation. In Nectaries and Nectar; Springer: Dordrecht, The Netherlands, 2007; pp. 167–214. [Google Scholar]
- Klein, A.-M.; Steffan-Dewenter, I.; Tscharntke, T. Pollination of Coffea canephora in relation to local and regional agroforestry management. J. Appl. Ecol. 2003, 40, 837–845. [Google Scholar] [CrossRef]
- Corbet, S. Nectar sugar content: estimating standing crop and secretion rate in the field. Apidologie 2003, 34, 1–10. [Google Scholar] [CrossRef]
- Henderson, J.W.; Brooks, A. Improved amino acid methods using Agilent ZORBAX Eclipse Plus C18 columns for a variety of Agilent LC instrumentation and separation goals. Agil. Technol. 2010, 5990–4547E, 1–16. [Google Scholar]
- Matsushita, K. Automatic precolumn derivatization of amino acids and analysis by fast LC using the Agilent 1290 Infinity LC system. Agil. Tech. Note 2010, 5990–5599E, 1–4. [Google Scholar]
- Henderson, J.W.; Ricker, R.D.; Bidlingmeyer, B.A.; Woodward, C. Rapid, accurate, sensitive, and reproducible HPLC analysis of amino acids and the Agilent 1100 HPLC. Agil. Tech. Note 2000, 5980–1193E, 1–10. [Google Scholar]
- DIN-10758 Analysis of honey - Determination of the content of saccharides fructose, glucose, saccharose, turanose and maitose - HPLC method; 1997.
- Venjakob, C.; Klein, A.-M.; Ebeling, A.; Tscharntke, T.; Scherber, C. Plant diversity increases spatio-temporal niche complementarity in plant-pollinator interactions. Ecol. Evol. 2016, 6, 2249–2261. [Google Scholar] [CrossRef]
- Brittain, C.; Kremen, C.; Klein, A.-M. Biodiversity buffers pollination from changes in environmental conditions. Glob. Chang. Biol. 2013, 19, 540–547. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N.; Walker, N.; Saveliev, A.A.; Smith, G.M. Mixed effects models and extensions in ecology with R; Statistics for Biology and Health; Springer New York: New York, NY, USA, 2009. [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing; R Foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
- Magurran, A.E. Measuring Biological Diversity; Blackwell Science: Oxford, UK, 2004. [Google Scholar]
- Vannette, R.L.; Fukami, T. Nectar microbes can reduce secondary metabolites in nectar and alter effects on nectar consumption by pollinators. Ecology 2016, 97, 1410–1419. [Google Scholar] [CrossRef]
- Brose, U.; Tielbörger, K. Subtle differences in environmental stress along a flooding gradient affect the importance of inter-specific competition in an annual plant community. Plant Ecol. 2005, 178, 51–59. [Google Scholar] [CrossRef]
- Flacher, F.; Raynaud, X.; Hansart, A.; Motard, E.; Dajoz, I. Competition with wind-pollinated plant species alters floral traits of insect-pollinated plant species. Sci. Rep. 2015, 5, 13345. [Google Scholar] [CrossRef] [PubMed]
- Von Arx, M.; Goyret, J.; Davidowitz, G.; Raguso, R.A. Floral humidity as a reliable sensory cue for profitability assessment by nectar-foraging hawkmoths. Proc. Natl. Acad. Sci. USA 2012, 109, 9471–9476. [Google Scholar] [CrossRef] [PubMed]
- Hendriksma, H.P.; Oxman, K.L.; Shafir, S. Amino acid and carbohydrate tradeoffs by honey bee nectar foragers and their implications for plant–pollinator interactions. J. Insect Physiol. 2014, 69, 56–64. [Google Scholar] [CrossRef]
- Bertazzini, M.; Medrzycki, P.; Bortolotti, L.; Maistrello, L.; Forlani, G. Amino acid content and nectar choice by forager honeybees (Apis mellifera L.). Amino Acids 2010, 39, 315–318. [Google Scholar] [CrossRef]
- Lohaus, G.; Schwerdtfeger, M. Comparison of sugars, iridoid glycosides and amino acids in nectar and phloem sap of Maurandya barclayana, Lophospermum erubescens, and Brassica napus. PLoS ONE 2014, 9, e87689. [Google Scholar] [CrossRef]
- Simcock, N.K.; Gray, H.E.; Wright, G.A. Single amino acids in sucrose rewards modulate feeding and associative learning in the honeybee. J. Insect Physiol. 2014, 69, 41–48. [Google Scholar] [CrossRef]
- Simpson, B.B.; Neff, J.L. Evolution and diversity of floral rewards. In Handbook of Experimental Pollination Biology; Scientific and Academic Editions: New York, NY, USA, 1983; pp. 142–159. [Google Scholar]
- Stabler, D.; Paoli, P.P.; Nicolson, S.W.; Wright, G.A. Nutrient balancing of the adult worker bumblebee (Bombus terrestris) depends on the dietary source of essential amino acids. J. Exp. Biol. 2015, 218, 793–802. [Google Scholar] [CrossRef]
- Alm, J.; Ohnmeiss, T.E.; Lanza, J.; Vriesenga, L. Preference of cabbage white butterflies and honey bees for nectar that contains amino acids. Oecologia 1990, 84, 53–57. [Google Scholar] [CrossRef]
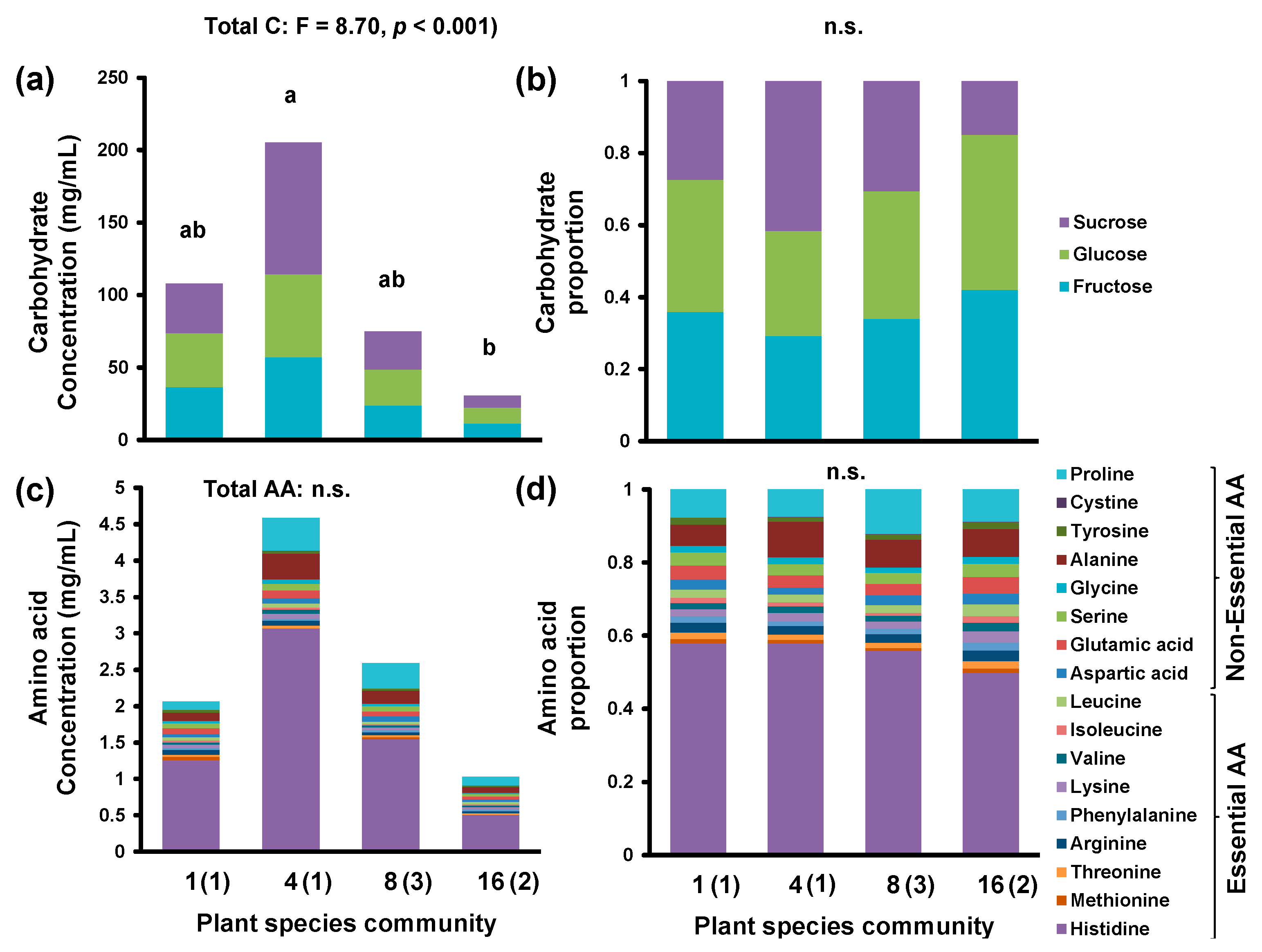

| Plant Species Community 1 | 1 | 4 | 8 | 16 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N Samples 2 | 8 | 5 | 24 | 18 | ||||||
| Mean | ±SD | Mean | ±SD | Mean | ±SD | Mean | ±SD | |||
| Volume Nectar 3 | µL | 3 | 4.11 | 3.31 | 2.71 | 2.73 | 2.73 | 4.35 | 2.76 | |
| Amino Acids | ||||||||||
| Asp 4 | C | mg/mL | 0.05 | 0.03 | 0.08 | 0.09 | 0.07 | 0.07 | 0.03 | 0.03 |
| Glu | O | 0.08 | 0.08 | 0.11 | 0.09 | 0.07 | 0.08 | 0.04 | 0.03 | |
| Ser | N | 0.07 | 0.07 | 0.09 | 0.07 | 0.07 | 0.08 | 0.03 | 0.02 | |
| His | C | 1.26 | 1 | 3.06 | 3.38 | 1.55 | 1.65 | 0.5 | 0.31 | |
| Gly | E | 0.04 | 0.04 | 0.06 | 0.04 | 0.04 | 0.05 | 0.02 | 0.01 | |
| Thr | N | 0.03 | 0.03 | 0.04 | 0.03 | 0.03 | 0.02 | 0.02 | 0.01 | |
| Arg | T | 0.06 | 0.09 | 0.06 | 0.04 | 0.04 | 0.03 | 0.03 | 0.02 | |
| Ala | R | 0.11 | 0.09 | 0.35 | 0.31 | 0.17 | 0.14 | 0.08 | 0.07 | |
| Tyr | A | 0.04 | 0.04 | 0.04 | 0.02 | 0.03 | 0.03 | 0.02 | 0.02 | |
| Cystine | T | 0 | 0 | 0.01 | 0.01 | 0 | 0 | 0 | 0 | |
| Val | I | 0.03 | 0.03 | 0.05 | 0.04 | 0.03 | 0.02 | 0.02 | 0.02 | |
| Met | O | 0.04 | 0.1 | 0.01 | 0.02 | 0.02 | 0.05 | 0.01 | 0.01 | |
| Phe | N | 0.03 | 0.03 | 0.03 | 0.02 | 0.03 | 0.03 | 0.02 | 0.02 | |
| Ile | 0.03 | 0.02 | 0.03 | 0.02 | 0.01 | 0.01 | 0.02 | 0.01 | ||
| Leu | 0.04 | 0.04 | 0.06 | 0.03 | 0.04 | 0.03 | 0.03 | 0.03 | ||
| Lys | 0.04 | 0.04 | 0.07 | 0.04 | 0.04 | 0.02 | 0.03 | 0.03 | ||
| Pro | 0.12 | 0.09 | 0.45 | 0.52 | 0.35 | 0.37 | 0.12 | 0.17 | ||
| Total AA 5 | 2.07 | 1.66 | 4.59 | 4.71 | 2.59 | 2.4 | 1.03 | 0.74 | ||
| EAA 6 | 1.57 | 1.31 | 3.41 | 3.59 | 1.79 | 1.75 | 0.69 | 0.43 | ||
| nEAA 7 | 0.49 | 0.4 | 1.18 | 1.12 | 0.8 | 0.69 | 0.34 | 0.33 | ||
| Asp 4 | P | 0.03 | 0.01 | 0.02 | 0.01 | 0.03 | 0.01 | 0.03 | 0.01 | |
| Glu | R | 0.04 | 0.01 | 0.03 | 0.02 | 0.03 | 0.02 | 0.05 | 0.02 | |
| Ser | O | 0.04 | 0.02 | 0.03 | 0.02 | 0.03 | 0.02 | 0.04 | 0.02 | |
| His | P | 0.58 | 0.13 | 0.58 | 0.12 | 0.56 | 0.09 | 0.5 | 0.13 | |
| Gly | O | 0.02 | 0.01 | 0.02 | 0.01 | 0.02 | 0.01 | 0.02 | 0.01 | |
| Thr | R | 0.02 | 0.01 | 0.01 | 0.01 | 0.02 | 0.01 | 0.02 | 0.01 | |
| Arg | T | 0.03 | 0.01 | 0.02 | 0.02 | 0.02 | 0.01 | 0.03 | 0.01 | |
| Ala | I | 0.06 | 0.02 | 0.1 | 0.03 | 0.08 | 0.03 | 0.08 | 0.02 | |
| Tyr | O | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | 0.01 | |
| Cystine | N | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Val | 0.02 | 0.01 | 0.02 | 0.01 | 0.02 | 0.01 | 0.02 | 0.01 | ||
| Met | 0.01 | 0.02 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | 0.02 | ||
| Phe | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | 0.01 | ||
| Ile | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | 0.01 | ||
| Leu | 0.02 | 0.01 | 0.02 | 0.01 | 0.02 | 0.01 | 0.03 | 0.01 | ||
| Lys | 0.02 | 0.01 | 0.02 | 0.01 | 0.02 | 0.01 | 0.03 | 0.01 | ||
| Pro | 0.08 | 0.06 | 0.08 | 0.03 | 0.12 | 0.07 | 0.09 | 0.05 | ||
| Total AA 5 | 1 | - | 1 | - | 1 | - | 1 | - | ||
| EAA 6 | 0.73 | 0.09 | 0.71 | 0.04 | 0.68 | 0.07 | 0.69 | 0.08 | ||
| nEAA7 | 0.27 | 0.09 | 0.29 | 0.04 | 0.32 | 0.07 | 0.31 | 0.08 | ||
| Carbohydrates | ||||||||||
| Fructose | C | mg/mL | 36.44 | 25.51 | 57.25 | 22.67 | 23.95 | 12.76 | 11.21 | 7.39 |
| Glucose | O | 37.12 | 27.73 | 57.04 | 22.2 | 24.6 | 12.65 | 11.25 | 7.16 | |
| Sucrose | N | 34.41 | 27.43 | 91.14 | 44.25 | 26.54 | 23.9 | 8.12 | 11.42 | |
| Total C5 | C. | 107.98 | 77.02 | 205.43 | 85.82 | 75.09 | 41.62 | 30.58 | 24.39 | |
| P | ||||||||||
| Fructose | R | 0.36 | 0.08 | 0.29 | 0.06 | 0.34 | 0.1 | 0.42 | 0.09 | |
| Glucose | O | 0.37 | 0.1 | 0.29 | 0.06 | 0.35 | 0.11 | 0.43 | 0.1 | |
| Sucrose | P | 0.27 | 0.18 | 0.42 | 0.13 | 0.31 | 0.21 | 0.15 | 0.18 | |
| Carbohydrate and Amino Acid Ratios | R | |||||||||
| A | ||||||||||
| mean C:AA8 | T | 50.96 | 22.97 | 89.41 | 62.48 | 45.55 | 28.95 | 42.03 | 37.72 | |
| mean C:EAA9 | I | 61.64 | 47.66 | 100 | 89.38 | 89.37 | 145.87 | 103.17 | 137.14 | |
| mean C:nEAA10 | O | 422.54 | 761.96 | 286.99 | 282.55 | 732.98 | 1882.35 | 726.34 | 1120.32 | |
| Plant Species Community | 8 (B3A20) | 8 (B2A12) | 16 (B1A20) |
|---|---|---|---|
| Shannon Index | 0.72 | 0.97 | 0.96 |
| Total Numbers | 232 | 300 | 383 |
| Species Richness of Flower Visitors | 9 | 12 | 15 |
| Beetles | - | 3 | 9 |
| Bumblebees | 28 | 49 | 36 |
| Butterflies | - | 2 | 10 |
| Flies | - | 5 | 2 |
| Honeybees | 195 | 230 | 303 |
| Hoverflies | 6 | 8 | 17 |
| Solitary bees | 3 | 2 | 6 |
| Wasps | - | 1 | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venjakob, C.; Leonhardt, S.; Klein, A.-M. Inter-Individual Nectar Chemistry Changes of Field Scabious, Knautia arvensis. Insects 2020, 11, 75. https://doi.org/10.3390/insects11020075
Venjakob C, Leonhardt S, Klein A-M. Inter-Individual Nectar Chemistry Changes of Field Scabious, Knautia arvensis. Insects. 2020; 11(2):75. https://doi.org/10.3390/insects11020075
Chicago/Turabian StyleVenjakob, Christine, Sara Leonhardt, and Alexandra-Maria Klein. 2020. "Inter-Individual Nectar Chemistry Changes of Field Scabious, Knautia arvensis" Insects 11, no. 2: 75. https://doi.org/10.3390/insects11020075
APA StyleVenjakob, C., Leonhardt, S., & Klein, A.-M. (2020). Inter-Individual Nectar Chemistry Changes of Field Scabious, Knautia arvensis. Insects, 11(2), 75. https://doi.org/10.3390/insects11020075





