Warming Increases Pollen Lipid Concentration in an Invasive Thistle, with Minor Effects on the Associated Floral-Visitor Community
Abstract
1. Introduction
2. Methods
2.1. Study Species
2.2. Experimental Set-Up
2.3. Pollen Collection and Analysis
2.4. Observations
2.5. Collections
2.6. Data Analysis
3. Results
3.1. Treatment Effects on Plants
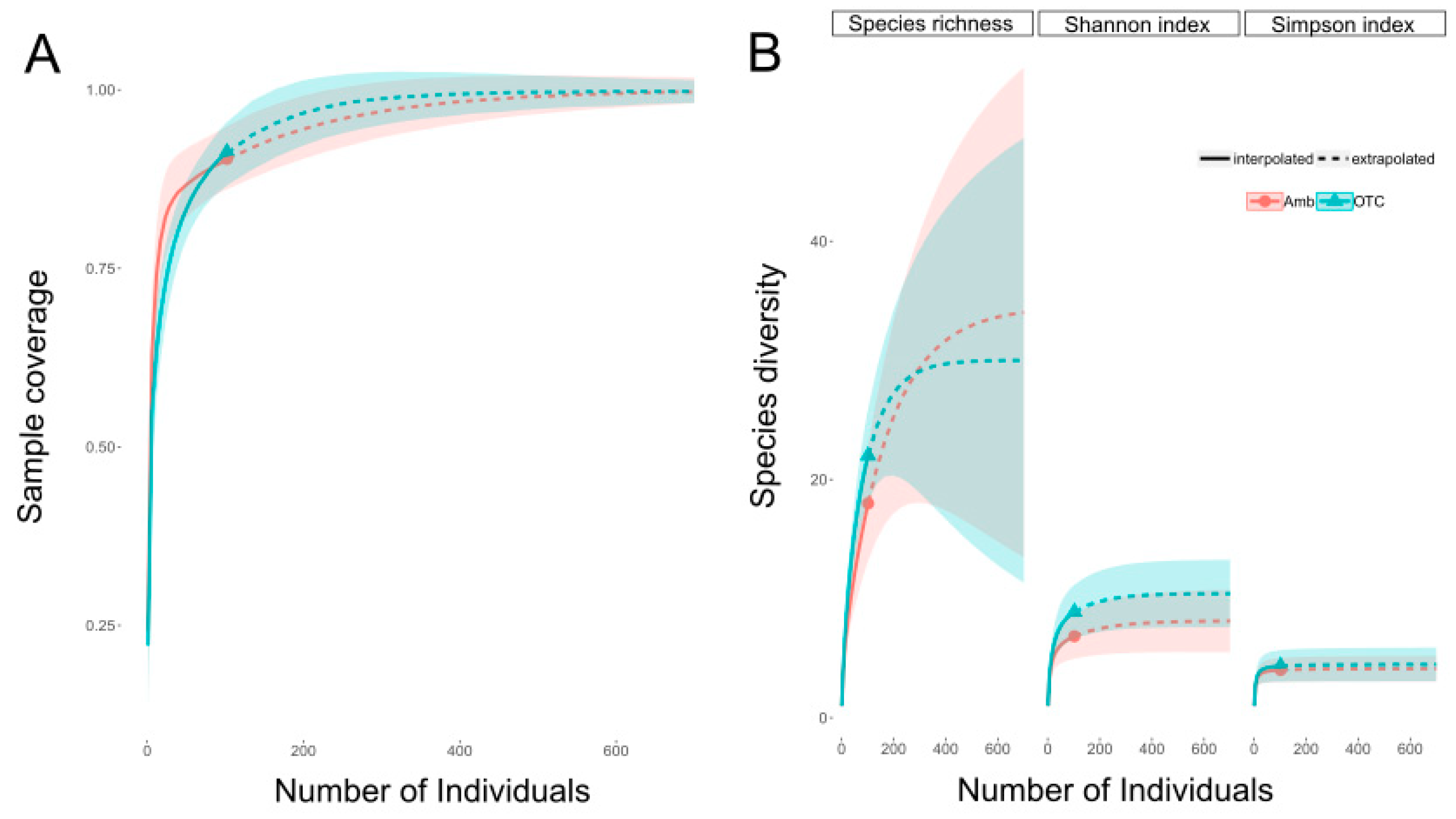
3.2. Observed Flower-Visiting Insects
3.3. Collected Bees
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, I.C.; Hill, J.K.; Ohlemüller, R.; Roy, D.B.; Thomas, C.D. Rapid range shifts of species associated with high levels of climate warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef]
- Zhang, R.; Post, E.; Shea, K. Warming leads to divergent responses but similarly improved performance of two invasive thistles. Popul. Ecol. 2012, 54, 583–589. [Google Scholar] [CrossRef]
- Teller, B.J.; Zhang, R.; Shea, K. Seed release in a changing climate: Initiation of movement increases spread of an invasive species under simulated climate warming. Divers. Distrib. 2016, 22, 708–716. [Google Scholar] [CrossRef]
- Zhang, R.; Jongejans, E.; Shea, K. Warming increases the spread of an invasive thistle. PLoS ONE 2011, 6, e21725. [Google Scholar] [CrossRef]
- Scaven, V.; Rafferty, N. Physiological effects of climate warming on flowering plants and insect pollinators and potential consequences for their interactions. Curr. Zool. 2013, 59, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.; Peng, Y.; Xi, X.; Wu, X.; Li, G.; Niklas, K.J.; Sun, S. Artificial asymmetric warming reduces nectar yield in a Tibetan alpine species of Asteraceae. Ann. Bot. 2015, 116, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Russo, L.; Vaudo, A.D.; Fisher, C.J.; Grozinger, C.M.; Shea, K. Bee community preference for an invasive thistle associated with higher pollen protein content. Oecologia 2019, 190, 901–912. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, M.A.; Burkle, L.A.; Manson, J.S.; Runyon, J.B.; Trowbridge, A.M.; Zientek, J. Global change effects on plant–insect interactions: The role of phytochemistry. Curr. Opin. Insect Sci. 2017, 23, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Leshak, A.; Shea, K. Decreased structural defence of an invasive thistle under warming. Plant Biol. 2012, 14, 249–252. [Google Scholar] [CrossRef]
- Roy, B.A.; Güsewell, S.; Harte, J. Response of plant pathogens and herbivores to a warming experiment. Ecology 2004, 85, 2570–2581. [Google Scholar] [CrossRef]
- Petanidou, T.; Smets, E. Does temperature stress induce nectar secretion in Mediterranean plants? New Phytol. 1996, 133, 513–518. [Google Scholar] [CrossRef]
- Takkis, K.; Tscheulin, T.; Tsalkatis, P.; Petanidou, T. Climate change reduces nectar secretion in two common Mediterranean plants. AoB Plants 2015, 7. [Google Scholar] [CrossRef] [PubMed]
- Koti, S.; Reddy, K.R.; Reddy, V.R.; Kakani, V.G.; Zhao, D. Interactive effects of carbon dioxide, temperature, and ultraviolet-B radiation on soybean (Glycine max L.) flower and pollen morphology, pollen production, germination, and tube lengths. J. Exp. Bot. 2005, 56, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Hodge, S.; Prasad, A. Factors influencing the foraging activity of the allodapine bee Braunsapis puangensis on creeping daisy (Sphagneticola trilobata) in Fiji. J. Hymenopt. Res. 2013, 35, 56–69. [Google Scholar] [CrossRef]
- Cardoza, Y.J.; Harris, G.K.; Grozinger, C.M. Effects of soil quality enhancement on pollinator-plant interactions. Psyche J. Entomol. 2012, 2012, 8. [Google Scholar] [CrossRef]
- Jongejans, E.; Sheppard, A.W.; Shea, K. What controls the population dynamics of the invasive thistle Carduus nutans in its native range? J. Appl. Ecol. 2006, 43, 877–886. [Google Scholar] [CrossRef]
- Skinner, K.; Smith, L.; Rice, P. Using noxious weed lists to prioritize targets for developing weed management strategies. Weed Sci. 2000, 48, 640–644. [Google Scholar] [CrossRef]
- Yang, S.; Ferrari, M.J.; Shea, K. Pollinator behavior mediates negative interactions between two congeneric invasive plant species. Am. Nat. 2011, 177, 110–118. [Google Scholar] [CrossRef]
- Hollister, R.D.; Webber, P.J. Biotic validation of small open-top chambers in a tundra ecosystem. Glob. Chang. Biol. 2000, 6, 835–842. [Google Scholar] [CrossRef]
- Yang, Y.; Halbritter, A.H.; Klanderud, K.; Telford, R.J.; Wang, G.; Vandvik, V. Transplants, open top chambers (OTCs) and gradient studies ask different questions in climate change effects studies. Front. Plant Sci. 2018, 9, 1574. [Google Scholar] [CrossRef]
- Van Handel, E.; Day, J.F. Assay of lipids, glycogen and sugars in individual mosquitoes: Correlations with wing length in field-collected Aedes vexans. J. Am. Mosq. Control Assoc. 1988, 4, 549–550. [Google Scholar] [PubMed]
- Vaudo, A.D.; Patch, H.M.; Mortensen, D.A.; Tooker, J.F.; Grozinger, C.M. Macronutrient ratios in pollen shape bumble bee (Bombus impatiens) foraging strategies and floral preferences. Proc. Natl. Acad. Sci. USA 2016, 113, E4035–E4042. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2014, 67, 1–48. [Google Scholar]
- Chao, A.; Gotelli, N.J.; Hsieh, T.C.; Sander, E.L.; Ma, K.H.; Colwell, R.K.; Ellison, A.M. Rarefaction and extrapolation with Hill numbers: A framework for sampling and estimation in species diversity studies. Ecol. Monogr. 2014, 84, 45–67. [Google Scholar] [CrossRef]
- Russo, L.; Shea, K. Experimentally increased network connectance is associated with increased bee species richness and abundance in a plant-pollinator community. J. Complex Netw. 2017, 473–485. [Google Scholar]
- Vaudo, A.D.; Stabler, D.; Patch, H.M.; Tooker, J.F.; Grozinger, C.M.; Wright, G.A. Bumble bees regulate their intake of the essential protein and lipid pollen macronutrients. J. Exp. Biol. 2016, 219, 3962–3970. [Google Scholar] [CrossRef]
- Di Pasquale, G.; Salignon, M.; Le Conte, Y.; Belzunces, L.P.; Decourtye, A.; Kretzschmar, A.; Suchail, S.; Brunet, J.L.; Alaux, C. Influence of pollen nutrition on honey bee health: Do pollen quality and diversity matter? PLoS ONE 2013, 8, e72016. [Google Scholar] [CrossRef]
- Brodschneider, R.; Crailsheim, K. Nutrition and health in honey bees. Apidologie 2010, 41, 278–294. [Google Scholar] [CrossRef]

| Group | Response Variable | Distribution | Comparison | Variable Type | Effect SIZE | z- or t-Value | p-Value |
|---|---|---|---|---|---|---|---|
| Observed flower-visitors | Inflorescences | poisson(link = “log”) | OTC-Ambient | categorical | −0.08 | −0.7 | 0.48 |
| log transformed Abundance | gaussian(link = “identity”) | Inflorescences | continuous | 0.05 | 7.68 | <<0.001 | |
| gaussian(link = “identity”) | OTC-Ambient | categorical | 0.15 | 2.25 | 0.02 | ||
| Morphotypes | poisson(link = “log”) | Inflorescences | continuous | 0.03 | 5.07 | <<0.001 | |
| poisson(link = “log”) | OTC-Ambient | categorical | 0.07 | 0.97 | 0.33 | ||
| Collected bees | Inflorescences | poisson(link = “log”) | OTC-Ambient | categorical | −0.13 | −1.08 | 0.28 |
| Abundance | poisson(link = “log”) | Inflorescences | continuous | 0.02 | 2.18 | 0.03 | |
| poisson(link = “log”) | OTC-Ambient | categorical | 0.15 | 1.08 | 0.28 | ||
| Richness | gaussian(link = “identity”) | Inflorescences | continuous | 0.01 | 1.2 | 0.23 | |
| gaussian(link = “identity”) | OTC-Ambient | categorical | 0.15 | 0.96 | 0.34 | ||
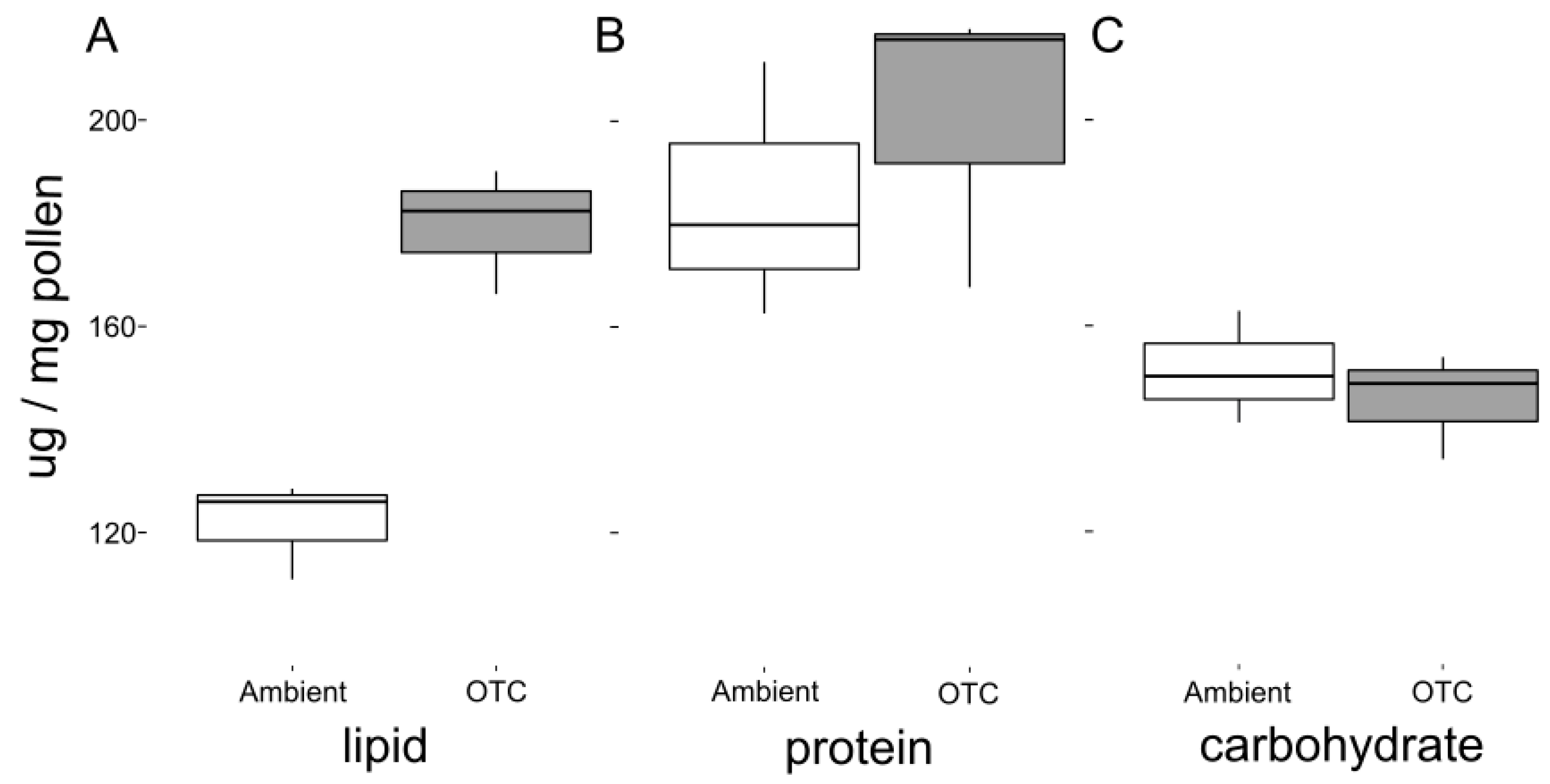
| Pollen nutrition | Lipid concentration | gaussian(link = “identity”) | OTC-Ambient | categorical | 57.76 | 6.48 | 0.003 |
| Carbohydrate concentration | gaussian(link = “identity”) | OTC-Ambient | categorical | −5.84 | −0.68 | 0.54 | |
| Protein concentration | gaussian(link = “identity”) | OTC-Ambient | categorical | 15.8 | 0.73 | 0.51 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, L.; Keller, J.; Vaudo, A.D.; Grozinger, C.M.; Shea, K. Warming Increases Pollen Lipid Concentration in an Invasive Thistle, with Minor Effects on the Associated Floral-Visitor Community. Insects 2020, 11, 20. https://doi.org/10.3390/insects11010020
Russo L, Keller J, Vaudo AD, Grozinger CM, Shea K. Warming Increases Pollen Lipid Concentration in an Invasive Thistle, with Minor Effects on the Associated Floral-Visitor Community. Insects. 2020; 11(1):20. https://doi.org/10.3390/insects11010020
Chicago/Turabian StyleRusso, Laura, Joseph Keller, Anthony D. Vaudo, Christina M. Grozinger, and Katriona Shea. 2020. "Warming Increases Pollen Lipid Concentration in an Invasive Thistle, with Minor Effects on the Associated Floral-Visitor Community" Insects 11, no. 1: 20. https://doi.org/10.3390/insects11010020
APA StyleRusso, L., Keller, J., Vaudo, A. D., Grozinger, C. M., & Shea, K. (2020). Warming Increases Pollen Lipid Concentration in an Invasive Thistle, with Minor Effects on the Associated Floral-Visitor Community. Insects, 11(1), 20. https://doi.org/10.3390/insects11010020






