Neo Sex Chromosomes, Colour Polymorphism and Male-Killing in the African Queen Butterfly, Danaus chrysippus (L.)
Abstract
1. Introduction
2. The Record
2.1. Host Plants
2.2. Cardenolide Glycosides
2.3. Pyrrolizidine Alkaloids
2.4. Courtship and Copulation
2.5. Defence
2.6. ‘Polymorphism’
2.7. Genetic Control of Colour Patterns
2.8. All-Female Broods
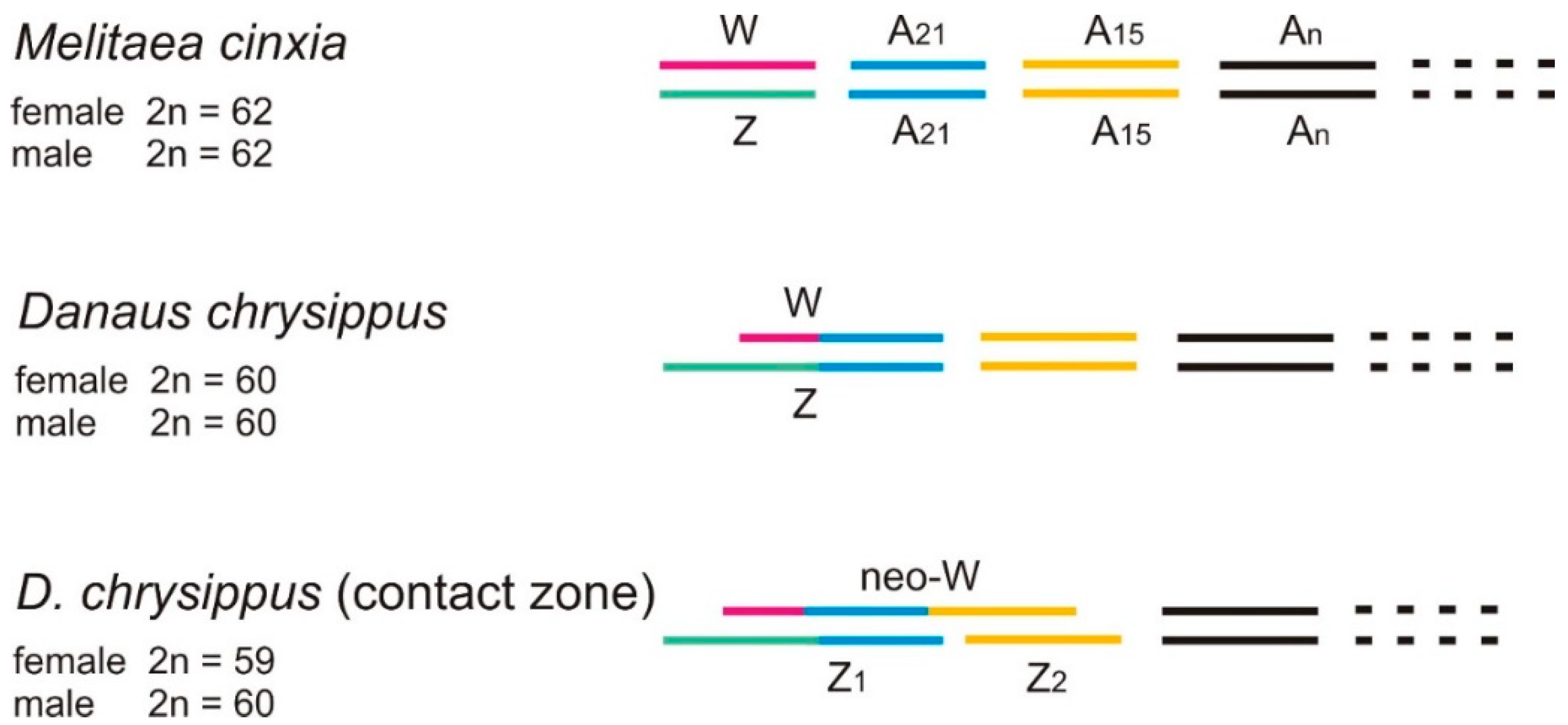
2.9. The neoW Chromosome
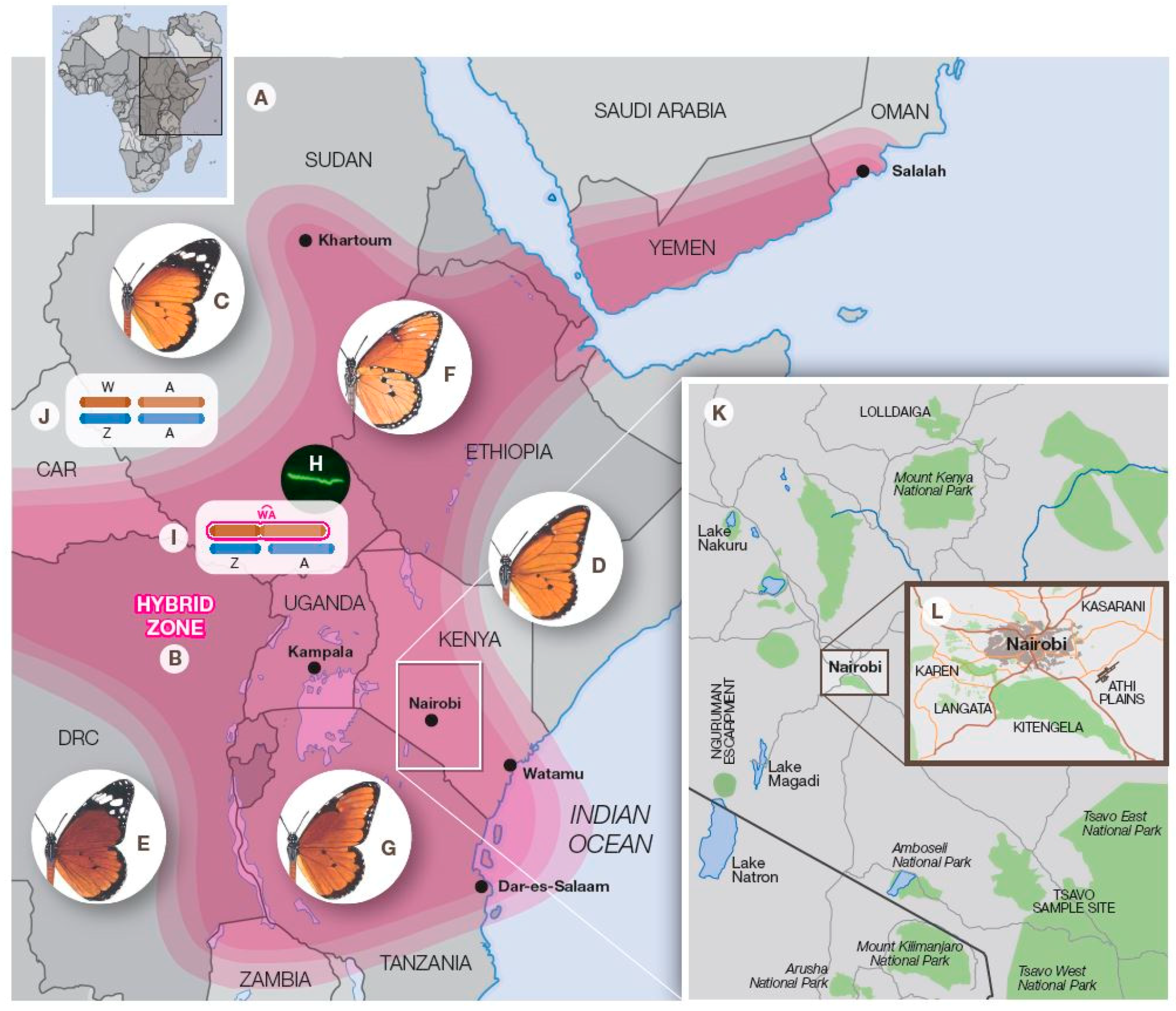
3. On-Going Hybrid Studies in the Nairobi Region, Kenya
3.1. Introduction
3.2. The Athi Plains
3.3. Kasarani
3.4. Kitengela
- (1)
- The population is ‘polymorphic’ for ssp. Dorippus (CC) and ssp. Chrysippus (cc). However, from the data in Table 4 it is clear, first, that the genotype arrays in two sexes differ sharply and indicate that they come predominantly from different source populations; secondly, much the most frequent female genotype is the hybrid form transiens (Cc).
- (2)
- The occasional arrival of males in numbers following substantial rains, unaccompanied by females of the same genotype, suggests that the migratory behaviour of the two sexes differs sharply [96].
- (3)
- Mating preference for C locus genotype was absent in both sexes, probably because the majority of males were CC and females Cc; thus, choice was severely curtailed.
- (4)
- It follows from (1) and (3) that pairing is disassortative (negatively non-random) for genotype (Table S2, p < 0.00001) since shortage of males effectively eliminates female choice, while male choice is restricted because 83% of females are transiens (Cc). Thus, ‘choice’ is in practice denied to both sexes.
- (5)
- Spermatophores per female averaged 1.7 (n = 260), while only 7.3% of females were unmated. These data mean that all females eventually find a mate. In a 1:1 population from Ghana, free from MK, the spermatophore/female average was 3.5 (n = 20) [8].
- (6)
- Through a mark-recapture study in May–July 2015, 63.4% of all males caught were in copula compared to only 13.4% of females. The implication of the sex difference is that, if all females are eventually mated (see 5), the average male must mate 5 times, but to achieve 1.7 spermatophores per female, males must mate 8.5 times, hence, neoW females are likely to receive under-sized spermatophores from over-taxed males.
- (7)
- The butterfly is a permanent resident varying in density between a minimum of 5.4/ha in October 2014 after a long drought and 68.8/ha in May 2015 following heavy rain.
- (8)
- The mean sex ratio over three years was 84.1% female, varying between extremes of 100% female in the driest periods, up to six months duration, down to 72.3% female in May 2015. The mean sex ratio at Kitengela is the highest recorded for D. chrysippus anywhere and exceeds the long-term (30-year) average of 74.5% for the Nairobi region which includes places such as Athi Plains and Kasarani.
- (9)
- The Spiroplasma infection rate of females at Kitengela, assessed by PCR, was 87.9% (n = 72), an estimate that tallies well with sex ratios and male-killing frequencies throughout the Nairobi region and over the years.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smith, D.A.S.; Lushai, G.; Allen, J.A. A classification of Danaus butterflies (Lepidoptera: Nymphalidae) based upon data from morphology and DNA. Zool. J. Linn. Soc. 2005, 144, 191–212. [Google Scholar] [CrossRef][Green Version]
- Poulton, E.B. Essays on Evolution 1889–1907; Clarendon Press: Oxford, UK, 1908. [Google Scholar]
- Larson, T.B. Il y a trios millénaires et demi que Danaus chrysippus (L.) est connu en haute-Egypte (Lepidoptera: Danaidae). Linn. Belg. 1977, 7, 55–58. [Google Scholar]
- Ackery, P.R.; Vane-Wright, R.I. Milkweed Butterflies: Their Cladistics and Biology; British Museum (Natural History): London, UK, 1984. [Google Scholar]
- Lushai, G.; Zalucki, M.P.; Smith, D.A.S.; Goulson, D.; Daniels, G. The lesser wanderer butterfly, Danaus petilia (Stoll 1790) stat. rev. (Lepidoptera: Danainae), reinstated as a species. Aust. J. Entomol. 2005, 44, 6–14. [Google Scholar] [CrossRef]
- Braby, M.F.; Farias-Quipildor, G.E.; Vane-Wright, R.I.; Lohman, D.J. Morphological and molecular evidence supports the recognition of Danaus petilia (Stoll, 1790) (Lepidoptera: Nymphalidae) as a species distinct from Danaus chrysippus (Linnaeus, 1758). Syst. Biodivers. 2015, 13, 386–402. [Google Scholar] [CrossRef]
- Moore, F. A monograph of the Limnaina and Euploina, two groups of diurnal Lepidoptera belonging to the subfamily Euploeinae, with descriptions of new genera and species. Proc. Zool. Soc. Lond. 1883, 201–324. [Google Scholar] [CrossRef]
- Smith, D.A.S.; Gordon, I.J.; Traut, W.; Herren, J.; Collins, S.; Martins, D.J.; Saitoti, K.; Ireri, P.; Ffrench-Constant, R. A neo-W chromosome in a tropical butterfly links colour pattern, male-killing and speciation. Proc. R. Soc. B 2016, 283, 20160821. [Google Scholar] [CrossRef]
- Smith, D.A.S. African Queens and Their Kin: A Darwinian Odyssey; Brambleby Books: Taunton, UK, 2014. [Google Scholar]
- Owen, D.F.; Chanter, D.O. Population biology of tropical African butterflies. 2. Sex ratio and polymorphism in Danaus chrysippus L. Rev. Zool. Bot. Afr. 1968, 78, 81–97. [Google Scholar]
- Smith, D.A.S. Genetics of some polymorphic forms of the African butterfly Danaus chrysippus L. (Lepidoptera: Danaidae). Insect Syst. Evol. 1975, 6, 134–144. [Google Scholar] [CrossRef]
- Ford, E.B. Polymorphism and taxonomy. In The New Systematics; Huxley, J., Ed.; Clarendon Press: Oxford, UK, 1940; pp. 493–513. [Google Scholar]
- Smith, D.A.S.; Owen, D.F.; Gordon, I.J.; Lowis, N.K. The butterfly Danaus chrysippus (L.) in East Africa: Polymorphism and morph-ratio clines within a complex, extensive and dynamic hybrid zone. Zool. J. Linn. Soc. 1997, 120, 51–78. [Google Scholar] [CrossRef]
- Smith, D.A.S.; Gordon, I.J.; Depew, L.A.; Owen, D.F. Genetics of the butterfly Danaus chrysippus (L.) in a broad hybrid zone, with special reference to sex ratio, polymorphism and intragenomic conflict. Biol. J. Linn. Soc. 1998, 65, 1–40. [Google Scholar] [CrossRef]
- Bates, H.W. Contributions to an insect fauna of the Amazon Valley. Lepidoptera-Nymphalinae. Trans. Linn. Soc. Lond. 1862, 23, 495–566. [Google Scholar] [CrossRef]
- Müller, F. Notes on Brazilian Entomology. Trans. Entomol. Soc. Lond. 1878, 26, 211–233. [Google Scholar] [CrossRef]
- Swailem, S.M.; Ishmail, I.I. Biological studies on Danaus chrysippus L. (Lepidoptera: Danaidae). Bull. Société Entomol. d’Egypte 1971, 55, 211–218. [Google Scholar]
- Mebs, D.; Reuss, E.; Schneider, M. Studies on the cardenolide sequestration in African milkweed butterflies (Danaidae). Toxicon 2005, 45, 581–584. [Google Scholar] [CrossRef] [PubMed]
- Brower, L.P. Ecological chemistry. Sci. Am. 1969, 220, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, M.; Kellet, D.N. Reactions of various predators to insects storing heart poisons (cardiac glycosides) in their tissues. J. Entomol. A 1972, 46, 103–110. [Google Scholar] [CrossRef]
- Dobler, S.; Dalla, S.; Wagschal, V.; Agrawal, A.A. Community-wide convergent evolution in insect adaptation to toxic cardenolides by substitutions in the Na, K-ATPase. Proc. Natl. Acad. Sci. USA 2012, 109, 13040–13045. [Google Scholar] [CrossRef]
- Aardema, M.L.; Zhen, Y.; Andolfatto, P. The evolution of cardenolide-resistant forms of NA+, K+-ATPase in Danainae butterflies. Mol. Ecol. 2011, 21, 340–349. [Google Scholar] [CrossRef]
- Rothschild, M.; von Euw, J.; Reichstein, J.; Smith, D.A.S.; Pierre, J. Cardenolide storage in Danaus chrysippus with additional notes on D. plexippus. Proc. R. Soc. B 1975, 190, 1–31. [Google Scholar]
- Bernays, E.A.; Singer, M.S.; Rodrigues, D. Trenching behaviour by caterpillars of the Euphorbia specialist, Pygarctia roseicapitis: A field study. J. Insect Behav. 2004, 17, 41–52. [Google Scholar] [CrossRef]
- Singer, M.S.; Stireman, J.O., III. Does anti-parasitoid defense explain host-plant selection by a polyphagous caterpillar? Oikos 2003, 100, 554–562. [Google Scholar] [CrossRef]
- Singer, M.S.; Mace, K.C.; Bernays, E.A. Self-medication as adaptive plasticity: Increased ingestion of plant toxins by parasitized caterpillars. PLoS ONE 2009, 4, e4796. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, M.; Reichstein, R.T.; Von Euw, J.; Apin, R.T.; Harmann, R.R.M. Toxic Lepidoptera. Toxicon 1970, 8, 293–299. [Google Scholar] [CrossRef]
- Pliske, T.E. Attraction of Lepidoptera to plants containing pyrrolizidine alkaloids. Environ. Entomol. 1975, 4, 474–479. [Google Scholar] [CrossRef]
- Owen, D.F. Tropical Butterflies; Clarendon Press: Oxford, UK, 1971. [Google Scholar]
- Edgar, J.A.; Culvenor, C.C.J. Pyrrolizidine ester alkaloid in danaid butterflies. Nature 1974, 248, 614–616. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.S., Jr. Adult-obtained pyrrolizidine alkaloids defend ithomiine butterflies against a spider predator. Nature 1984, 309, 707–709. [Google Scholar] [CrossRef]
- Brown, K.S., Jr. Chemical ecology of dehydropyrrolizidine alkaloids in adult Ithomiinae (Lepidoptera: Nymphalidae). Rev. Bras. Biol. 1984, 44, 435–460. [Google Scholar]
- Edgar, J.A.; Culvenor, C.C.J.; Smith, L.W. Dihydropyrrolizidine derivatives in the hair pencil secretion of danaid butterflies. Experientia 1971, 27, 761–762. [Google Scholar] [CrossRef]
- Schulz, S.; Boppré, M.; Vane-Wright, R.I. Specific mixtures of secretions from male scent organs of African milkweed butterflies (Danainae). Philos. Trans. R. Soc. B 1993, 342, 161–181. [Google Scholar]
- Edgar, J.A.; Boppré, M.; Schneider, D. Pyrrolizidine alkaloid storage in African and Australian danaid butterflies. Experientia 1979, 35, 1447–1448. [Google Scholar] [CrossRef]
- Dussourd, D.E.; Harvis, C.A.; Meinwald, J.; Eisner, T. Paternal allocation of sequestered plant pyrrolizidine alkaloid to eggs in the danaine butterfly Danaus gilippus. Cell. Mol. Life Sci. 1989, 45, 896–898. [Google Scholar] [CrossRef] [PubMed]
- Eisner, T.; Eisner, M. Unpalatability of the pyrrolizidine-containing moth Utetheisa ornatrix and its larva to wolf spiders. Psyche 1991, 98, 111–118. [Google Scholar] [CrossRef]
- Bull, L.B.; Culvenor, C.C.G.; Dick, A.T. The Pyrrolizidine Alkaloids, Their Chemistry, Pathogenicity and Other Biological Properties; Frontiers of Biology: Amsterdam, The Netherlands, 1968. [Google Scholar]
- Bate-Smith, E.C. Attractants and repellents in higher animals. In Phytochemical Ecology; Harborne, J.B., Ed.; Academic Press: London, UK, 1972; pp. 45–56. [Google Scholar]
- Kassarov, L. Are birds able to taste and reject butterflies based on ‘beak mark tasting’? A different point of view. Behaviour 1999, 136, 965–981. [Google Scholar] [CrossRef]
- Birkhead, T. Bird Sense: What It’s Like to Be a Bird; Bloomsbury: London, UK, 2012. [Google Scholar]
- Gould, S.J.; Lewontin, R.C. The spandrels of San Marco and the Panglossian paradigm: A critique of the adaptationist programme. Proc. R. Soc. B 1979, 205, 581–598. [Google Scholar]
- Koonin, E.V. Splendor and misery of adaptation, or the importance of neutral null for understanding evolution. BMC Biol. 2016, 14, 114. [Google Scholar] [CrossRef] [PubMed]
- Boppré, M. Leaf scratching—A specialized behaviour of danaine butterflies for gathering secondary plant substances. Oecologia 1983, 59, 414–416. [Google Scholar] [CrossRef]
- Seibt, U.; Schneider, D.; Eisner, T. Duftpinsel, Flügeltaschen und Balz das Tagfalters Danaus chrysippus. Beih. Z. Tierppsychol. 1972, 31, 513–530. [Google Scholar] [CrossRef]
- Brower, L.P.; Brower, J.V.Z.; Cranston, F.P. Courtship behaviour of the queen butterfly Danaus gilippus berenice. Zool. N. Y. 1965, 50, 1–39. [Google Scholar]
- Smith, D.A.S.; Gordon, I.J.; Lushai, G.; Goulson, D.; Allen, J.A.; Maclean, N. Hybrid queen butterflies from a cross Danaus chrysippus (L.) × D. gilippus (Cramer): Confirmation of species status for the parents and further support for Haldane’s Rule. Biol. J. Linn. Soc. 2002, 76, 535–544. [Google Scholar] [CrossRef][Green Version]
- Edgar, J.A.; Cockrum, C.C.J.; Frahn, J.L. Pyrrolizidine alkaloids in Danaus plexippus L. and Danaus chrysippus L. Experientia 1976, 32, 1535–1537. [Google Scholar] [CrossRef]
- Sakai, H.; Oshima, H.; Yuri, H.; Gotoh, H.; Daimon, T.; Yaginuma, T.; Sahara, K.; Niimi, T. Dimorphic sperm formation by Sex-lethal. Proc. Natl. Acad. Sci. USA 2019, 116, 10412–10417. [Google Scholar] [CrossRef]
- Trimen, R. On some remarkable mimetic analogies among African butterflies. Trans. Linn. Soc. 1869, 26, 497–522. [Google Scholar] [CrossRef]
- Wallace, A.R. The Malay Archipelago: The Land of the Orang-Utan and the Bird of Paradise. A Narrative of Travel with Studies of Man and Nature; 2 vols; Macmillan: London, UK, 1869. [Google Scholar]
- Darwin, C. The Descent of Man and Selection in Relation to Sex, 2nd ed.; John Murray: London, UK, 1874. [Google Scholar]
- Swynnerton, C.F.M. Experiments and observations bearing on the explanation of form and colouring, 1908–1913. J. Linn. Soc. Zool. 1919, 33, 203–385. [Google Scholar] [CrossRef]
- Parsons, J.A. A digitalis-like toxin in the monarch butterfly, Danaus plexippus. J. Physiol. 1965, 178, 290–303. [Google Scholar] [CrossRef]
- Poulton, E.B. The Colours of Animals: Their Meaning and Use, Especially Considered in the Case of Insects; Kegan Paul: London, UK, 1890. [Google Scholar]
- Brower, L.P.; Brower, J.V.Z. Birds, butterflies and plant poisons: A study in ecological chemistry. Zool. N.Y. 1964, 49, 137–149. [Google Scholar]
- Carpenter, G.D.H. The relative frequency of beak marks on butterflies of different edibility to birds. Proc. Zool. Soc. Lond. 1941, 111, 223–231. [Google Scholar] [CrossRef]
- Smith, D.A.S. The significance of beak marks on the wings of an aposematic and distasteful butterfly. Nature 1979, 281, 215–216. [Google Scholar] [CrossRef]
- Wright, S. Isolation by distance. Genetics 1943, 28, 114–138. [Google Scholar]
- Smith, D.A.S. Mate selection in butterflies: Competition, coyness, choice and chauvinism. In The Biology of Butterflies; Vane-Wright, R.I., Ackery, P.R., Eds.; Academic Press: London, UK, 1984; pp. 225–244. [Google Scholar]
- Owen, D.F.; Smith, D.A.S. Danaus chrysippus and its polymorphic Müllerian mimics in tropical Africa. Trop. Lepid. 1993, 4, 77–81. [Google Scholar]
- Smith, D.A.S. Batesian mimicry between Danaus chrysippus and Hypolimnas misippus (Lepidoptera) in Tanzania. Nature 1973, 242, 129–131. [Google Scholar] [CrossRef]
- Smith, D.A.S. Phenotypic diversity, mimicry and natural selection in the African butterfly Hypolimnas misippus L. (Lepidoptera: Nymphalidae). Biol. J. Linn. Soc. 1976, 8, 183–204. [Google Scholar] [CrossRef]
- Gordon, I.J. Natural selection for rare and mimetic colour pattern combinations in wild populations of the diadem butterfly Hypolimnas misippus (L.). Biol. J. Linn. Soc. 1987, 31, 1–23. [Google Scholar] [CrossRef]
- Gordon, I.J.; Smith, D.A.S. Diversity in mimicry. Trends Ecol. Evol. 1999, 13, 150–151. [Google Scholar] [CrossRef]
- Gordon, I.J.; Edmunds, M.; Edgar, J.A.; Lawrence, J.; Smith, D.A.S. Linkage disequilibrium and natural selection for mimicry in the Batesian mimic Hypolimnas misippus (L.) in the Afrotropics. Biol. J. Linn. Soc. 2010, 100, 180–194. [Google Scholar] [CrossRef]
- Poulton, P.B. Danaida chrysippus L. and D. dorippus Klug, proved by breeding to be two forms of the same species. Proc. Entomol. Soc. Lond. 1924, 1925, cxix–cxxiii. [Google Scholar]
- Gordon, I.J. Polymorphism of the tropical butterfly Danaus chrysippus L. in Africa. Heredity 1984, 53, 583–593. [Google Scholar] [CrossRef]
- Clarke, C.A.; Sheppard, P.M.; Smith, A.G. The genetics of fore and hindwing colour in crosses between Danaus chrysippus from Australia and Sierra Leone (Danaidae). J. Lepid. Soc. 1973, 27, 73–77. [Google Scholar]
- Sturtevant, A.H. Linkage in the silkworm moth. Am. Nat. 1915, 28, 315–317. [Google Scholar] [CrossRef]
- Suomalainen, E.; Cook, L.M.; Turner, J.R.G. Achiasmate oogenesis in the Heliconiine butterflies. Hereditas 1973, 74, 302–304. [Google Scholar] [CrossRef]
- Turner, J.R.G.; Sheppard, P.M. Absence of crossing-over in female butterflies (Heliconius). Heredity 1975, 34, 265–269. [Google Scholar] [CrossRef]
- Martin, S.H.; Singh, K.S.; Gordon, I.J.; Omufwoko, K.S.; Collins, S.; Warren, I.A.; Munby, H.; Brattström, O.; Traut, W.; Martins, D.J.; et al. Whole-chromosome hitchhiking driven by a male-killing endosymbiont. BioRxiv 2019. [Google Scholar] [CrossRef]
- Hurst, G.G.D.; Hurst, L.D.; Majerus, M. Cytoplasmic sex ratio distorters. In Influential Passengers; O’Neil, S.L., Hoffman, A.A., Werren, J.H., Eds.; Oxford University Press: Oxford, UK, 1997; pp. 125–154. [Google Scholar]
- Hamilton, W.D. Extraordinary sex ratios. Science 1967, 156, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.A.S. All-female broods in Danaus chrysippus L. and their ecological significance. Heredity 1975, 34, 363–371. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jiggins, F.M.; Hurst, G.D.D.; Jiggins, C.D.; Schulenburg, J.H.G.V.D.; Majerus, M.E.N. The butterfly Danaus chrysippus is infected by a male-killing Spiroplasma bacterium. Parasitology 2000, 120, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Herren, J.; Gordon, I.J.; Holland, P.W.H.; Smith, D.A.S. The butterfly Danaus chrysippus (L.) in Kenya is variably infected with respect to genotype and body size by a maternally transmitted male-killing endosymbiont (Spiroplasma). Int. J. Trop. Insect Sci. 2007, 27, 62–69. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; Idris, E.; Majerus, M.E.N. Male-killer dynamics in Danaus chrysippus (L.) (Lepidoptera: Nymphalidae) in East Africa. Afr. J. Ecol. 2012, 50, 489–499. [Google Scholar] [CrossRef]
- Smith, D.A.S.; Gordon, I.J.; Allen, J.A. Reinforcement in hybrids among once isolated semispecies of Danaus chrysippus (L.) and evidence for sex chromosome evolution. Ecol. Entomol. 2010, 35, 77–89. [Google Scholar] [CrossRef]
- Smith, D.A.S. Heterosis, epistasis and linkage disequilibrium in a wild population of the polymorphic butterfly Danaus chrysippus L. Zool. J. Linn. Soc. 1980, 69, 87–109. [Google Scholar] [CrossRef]
- Gordon, I.J.; Ireri, P.; Smith, D.A.S. Hologenomic speciation: Synergy between a male-killing bacterium and sex-linkage creates a ‘magic trait’ in a butterfly hybrid zone. Biol. J. Linn. Soc. 2014, 100, 180–194. [Google Scholar] [CrossRef]
- Traut, W.; Ahola, V.; Smith, D.A.S.; Gordon, I.J.; Ffrench-Constant, R.H. Karyotypes versus Genomes: The Nymphalid Butterflies Melitaea cinxia, Danaus plexippus and D. chrysippus. Cytogenet. Genome Res. 2017, 153, 46–53. [Google Scholar] [CrossRef]
- White, M.J.D. Modes of Speciation; Freeman & Co.: San Francisco, CA, USA, 1978. [Google Scholar]
- Gazarve, E.; Catalan, J.; da Graca Ramalino, M.; da Luz Mathias, M.; Nunes, A.C.; Brittan-Davidian, J.; Auffray, J.C. Do chromosomal hybrids necessarily suffer from developmental instability? Biol. J. Linn. Soc. 2006, 88, 33–43. [Google Scholar] [CrossRef]
- Smith, D.A.S. Negative non-random mating in the polymorphic butterfly Danaus chrysippus in Tanzania. Nature 1973, 242, 131–132. [Google Scholar] [CrossRef]
- Gupta, Y. Chromosome studies of some Indian Lepidoptera. Chromosoma 1964, 15, 540–561. [Google Scholar] [CrossRef]
- De Lesse, H.; Condamin, M. Formules chromosomiques de quelques Lépidoptères Rhopalocères du Sénégal. Bull. l’Institut Fondam. d’Afrique Noire Dakar 1962, 24, 464–473. [Google Scholar]
- Servedio, M.R.; Van Doorn, G.S.; Kopp, M.; Frame, A.M.; Nosil, P. Magic traits in speciation: ‘magic’ but not rare? Trends Ecol. Evol. 2011, 26, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Haller, B.C.; Luis, F.; Rolshausen, G.; Gotanda, K.M.; Hendry, A.P. Magic traits: Distinguishing the important from the trivial. Trends Ecol. Evol. 2013, 27, 4–5. [Google Scholar] [CrossRef][Green Version]
- Williams, C.B. Insect Migration; Oliver and Boyd: Edinburgh, UK, 1958. [Google Scholar]
- Smith, D.A.S.; Owen, D.F. Colour genes as markers for migratory activity: The butterfly Danaus chrysippus (L.) in Africa. Oikos 1997, 78, 127–135. [Google Scholar] [CrossRef]
- Lushai, G.; Smith, D.A.S.; Gordon, I.J.; Goulson, D.; Allen, J.A.; Maclean, N. Incomplete sexual isolation in sympatry between subspecies of the butterfly Danaus chrysippus (L.) and the creation of a hybrid zone. Heredity 2003, 90, 236–246. [Google Scholar] [CrossRef]
- Gordon, I.J.; Ireri, P.; Smith, D.A.S. Preference for isolated plants facilitates invasion of Danaus chrysippus (Linnaeus, 1758) (Lepidoptera: Nymphalidae) by a bacterial male-killer Spiroplasma. Austral Entomol. 2014, 54, 210–216. [Google Scholar] [CrossRef]
- Majerus, M.E.N. Sex Wars; Princeton University Press: Princeton, NJ, USA, 2003. [Google Scholar]
- Zalucki, M.P. Sex around the milkweed patch. In The Biology and Conservation of the Monarch Butterfly; Malcolm, S.B., Zalucki, M.P., Eds.; Natural History Museum of Los Angeles County: Los Angeles, CA, USA, 1993; pp. 69–76. [Google Scholar]
- Cane, M.A.; Molnar, P. Closing of the Indonesian seaway as a precursor to east African aridification around 3–4 million years ago. Nature 2001, 411, 157–162. [Google Scholar] [CrossRef]
- Fer, I.; Tietjen, B.; Jeltsch, F.; Trauth, M.H. Modelling vegetation change during Late Cenozoic uplift of the East African plateau. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2017, 467, 120–130. [Google Scholar] [CrossRef]
- Vrba, E.S. African Bovidae: Evolutionary events since the Miocene. S. Afr. J. Sci. 1985, 81, 263–266. [Google Scholar]
- Leakey, M.G.; Spoor, F.; Brown, F.H.; Gathogo, P.N.; Kiairie, C.; Leakey, L.N.; McDougall, I. A new hominin genus from eastern Africa shows diverse middle Pliocene lineages. Nature 2001, 410, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Moreau, R.E. The Bird Faunas of Africa and Its Islands; Academic Press: London, UK, 1966. [Google Scholar]
- Dartnell, L. Origins: How the Earth Made Us; The Bodley Head: London, UK, 2019. [Google Scholar]
- Maslin, M.A.; Brierley, C.M.; Milner, A.M.; Shultz, S.; Trauth, M.H.; Wilson, K.E. East African climate pulses and early human evolution. Quat. Sci. Rev. 2014, 101, 1–17. [Google Scholar] [CrossRef]
- Jung, G.; Prange, M.; Schulz, M. Influence of topography on tropical African vegetation coverage. Clim. Dyn. 2016, 46, 2535–2549. [Google Scholar] [CrossRef]
- Moreau, R.E. Vicissitudes of the African biomes in the Late Pleistocene. Proc. Zool. Soc. Lond. 1963, 141, 395–421. [Google Scholar] [CrossRef]
- Williams, M.A.J.; Faure, H. The Sahara and The Nile: Quaternary Environments and Prehistoric Occupation in Northern Africa; A.A. Balkema: Rotterdam, The Netherlands, 1980; pp. 207–224. [Google Scholar]
- Lancaster, N. Evidence for a widespread late Pleistocene humid period in the Kalahari. Nature 1979, 279, 145–146. [Google Scholar] [CrossRef]
- Alin, S.R.; Cohen, A.S. Lake level history of Lake Tanganyika, East Africa, for the past 2500 years based on Ostracod inferred water depth reconstruction. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2003, 199, 31–49. [Google Scholar] [CrossRef]
- Flenley, J.R. The Equatorial Rain Forest: A Geological History; Butterworths: London, UK, 1979. [Google Scholar]
- Maslin, M.A.; Christensen, B. Tectonics, orbital forcing, global climate change and human evolution in Africa: Introduction to the African paleoclimate special volume. J. Hum. Evol. 2007, 53, 443–464. [Google Scholar] [CrossRef]
- Trauth, M.H.; Maslin, M.A.; Deino, A.L.; Junginger, A.; Lesoloyia, E.O.; Odada, D.O.; Olaka, L.A.; Strecker, M.R.; Tiedermann, R. Human evolution in a variable environment: The amplifier Lakes of East Africa. Quat. Sci. Rev. 2010, 29, 2981–2988. [Google Scholar] [CrossRef]
- DeMenocal, P.B.; Ortiz, J.; Guilderson, T.; Adkins, J.; Sarntheim, M.; Baker, L.; Yarusinsky, M. Abrupt onset and termination of the African Humid Period: Rapid climate responses to gradual insolation forcing. Quat. Sci. Rev. 2000, 19, 347–361. [Google Scholar] [CrossRef]
- Gasse, F. Hydrological changes in the African tropics since the last glacial maximum. Quat. Sci. Rev. 2000, 19, 189–211. [Google Scholar] [CrossRef]
- Russell, J.M.; Johnson, T.C. A high-resolution geochemical record from Lake Edward, Uganda-Congo, and the timing and causes of tropical African drought during the late Holocene. Quat. Sci. Rev. 2005, 24, 1375–1389. [Google Scholar] [CrossRef]
- Russell, J.M.; Johnson, T.C. Little Ice Age drought in Equatorial Africa: ITCZ migrations and ENSO variability. Geology 2007, 35, 21–24. [Google Scholar] [CrossRef]
- Smith, D.A.S.; Owen, D.F.; Gordon, I.J.; Owiny, A.M. Polymorphism and evolution in the butterfly Danaus chrysippus (L.) in Africa. Heredity 1993, 71, 127–135. [Google Scholar] [CrossRef]
- Haldane, J.B.S. Sex ratio and unisexual sterility in hybrid animals. J. Genet. 1922, 12, 101–109. [Google Scholar] [CrossRef]
- Smith, D.A.S. Evidence for autosomal meiotic drive in the butterfly Danaus chrysippus L. Heredity 1976, 36, 139–142. [Google Scholar] [CrossRef]
- Idris, E.; Hassan, S.S.M. The Queen Butterfly, Danaus chrysippus (L.) (Lepidoptera: Nymphalidae) at Khartoum, Sudan. Egypt. Acad. J. Biol. Sci. 2012, 5, 95–102. [Google Scholar] [CrossRef]
- Charlat, S.; Hornett, E.A.; Fullard, J.H.; Davies, N.; Roderick, G.K.; Wedell, N.; Hurst, G.D.D. Extraordinary flux in sex ratio. Science 2007, 317, 214. [Google Scholar] [CrossRef]
- Hornett, E.A.; Moran, B.; Reynolds, L.A.; Charlat, S.; Tazzyman, S.; Wedell, N.; Jiggins, C.D.; Hurst, G.D.D. The Evolution of Sex Ratio Distorter Suppression Affects a 25cM Genomic Region in the Butterfly Hypolimnas bolina. PLoS Genet. 2014, 10, e1004822. [Google Scholar] [CrossRef]

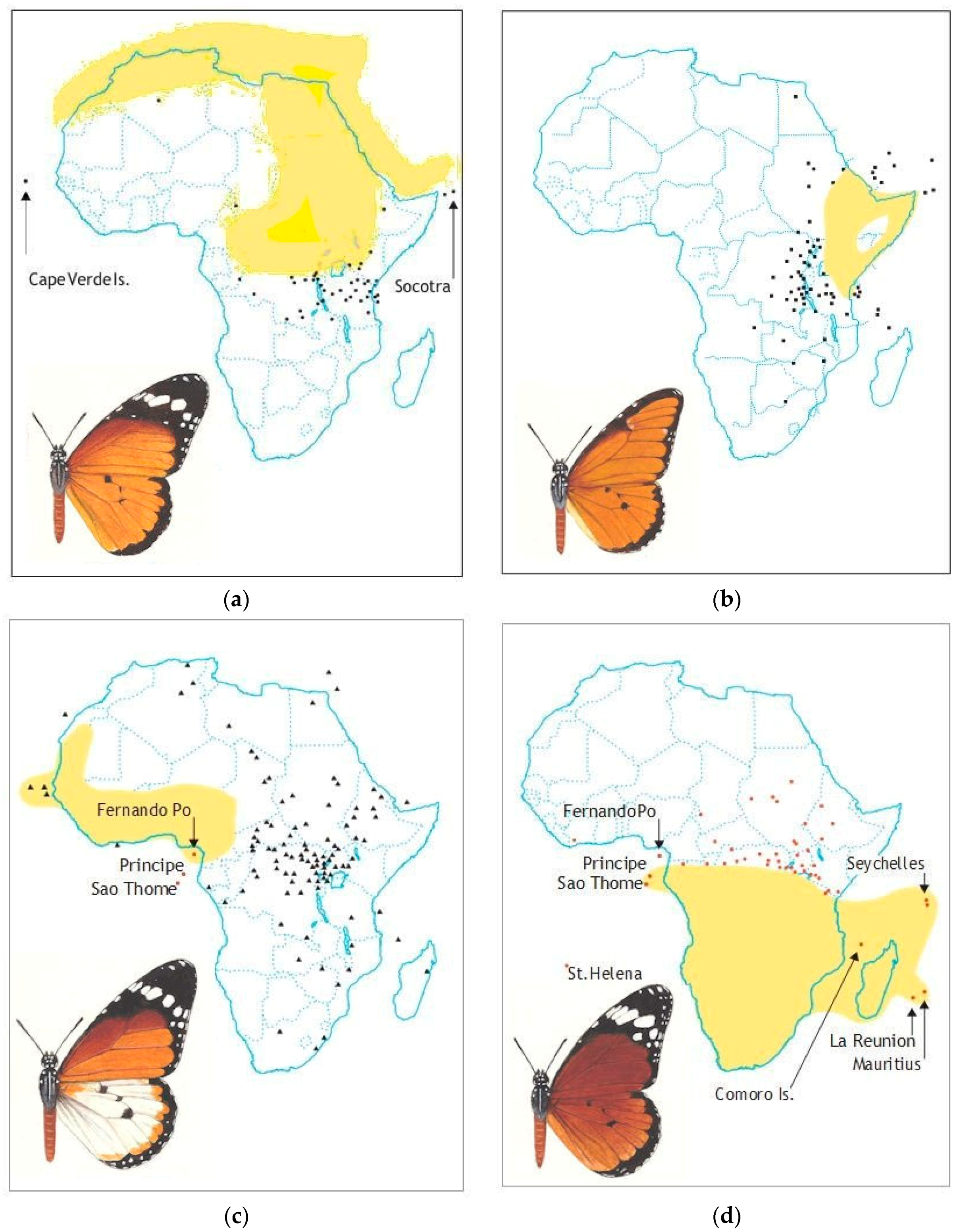

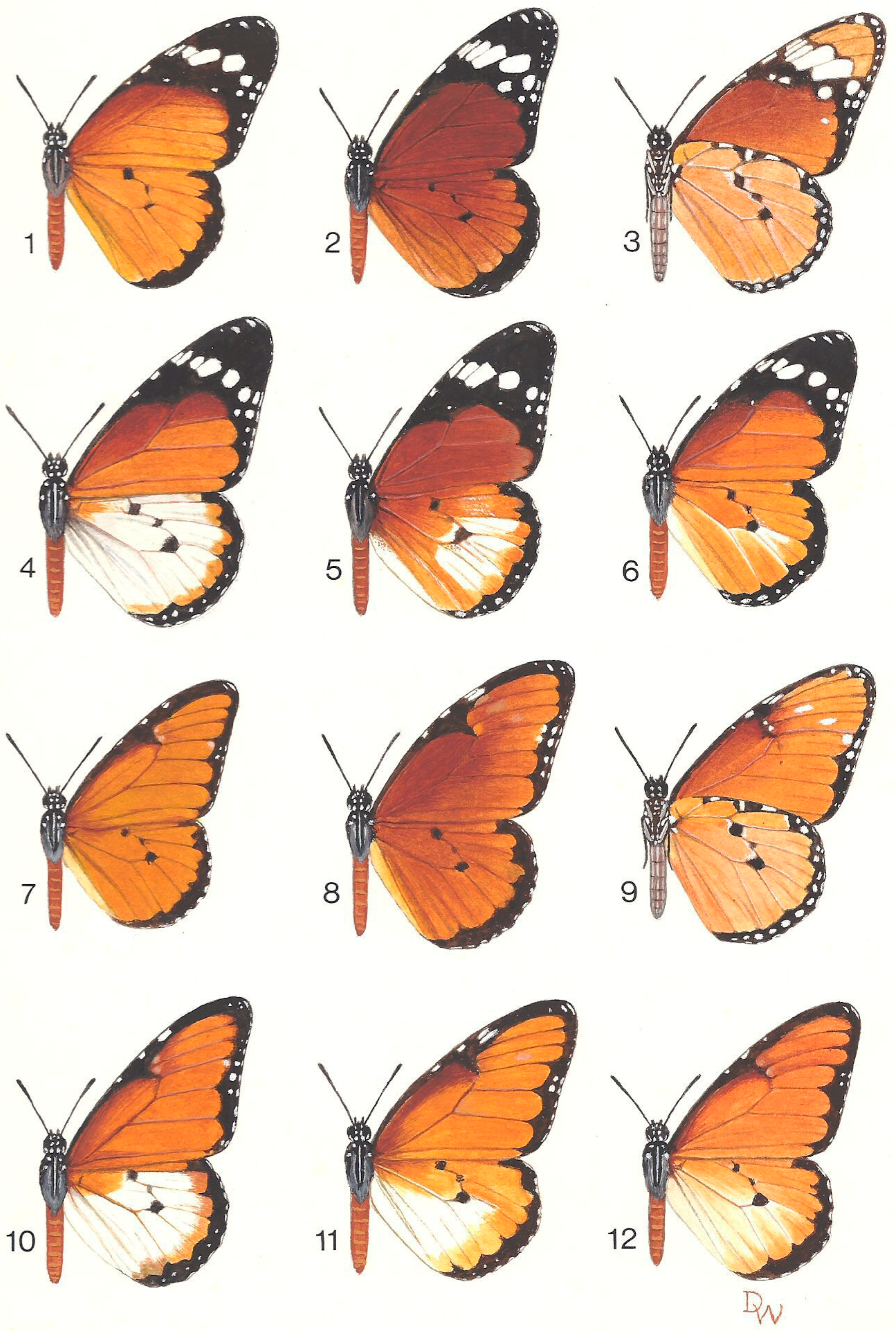
| Subspecies & Forms | Original Status & Authority | Present Status & Range |
|---|---|---|
| chrysippus | Papilio chrysippus, L. 1758 | ssp, S. Europe, N. Africa, Asia |
| alcippus | Papilio alcippus, Cramer 1777 | ssp, W. Africa |
| dorippus | Euploea dorippus, Klug 1845 | ssp, NE Africa, Arabia |
| orientis | f. of Danaida chrysippus, Aurivillius 1909 | ssp, S. Africa, Indian Ocean |
| klugii | Limnas klugii, Butler 1886 | orientis × dorippus F1 hybrid |
| infumata | f. of Danaida chrysippus, Aurivillius 1898 | chrysippus × orientis F1 hybrid |
| transiens | f. of Danaida dorippus, Suffert 1900 | chrysippus × dorippus F1 hybrid |
| alcippoides 1 | f. of Danaus chrysippus, Moore 1883 | chrysippus or orientis × alcippus F1 hybrid |
| albinus | f. of Danaida dorippus, Lanz 1896 | alcippus × dorippus F2 hybrid |
| semialbinus | f. of Danaida dorippus, Strand 1910 | alcippus × dorippus F1 hybrid |
| Genotype | AA | Aa | aa |
|---|---|---|---|
| bc/bc | chrysippus | orange alcippoides orange alcippus | orange alcippus |
| Bc/Bc | orientis | brown alcippoides brown alcippus | brown alcippus |
| bC/bC | dorippus | semialbinus albinus | albinus |
| bC/bc | transiens | semialbinus-transiens albinus-transiens | albinus-transiens |
| Bc/bC | klugii | semialbinus-klugii albinus-klugii | albinus-klugii |
| Bc/bc | infumata | alcippoides-infumata alcippus-infumata | alcippus-infumata |
| Study Areas | Spiroplasma | ||
|---|---|---|---|
| + | − | n | |
| East Africa | 30 1 | 0 | 30 |
| Elsewhere | 2 2 | 12 3 | 14 |
| Totals | 32 | 12 | 44 |
| Sex | Genotype Frequencies | |||
|---|---|---|---|---|
| CC | Cc | cc | n | |
| Females | 0 | 801 | 118 | 919 |
| Males | 130 | 37 | 7 | 174 |
| Totals | 130 | 838 | 125 | 1093 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, D.A.S.; Traut, W.; Martin, S.H.; Ireri, P.; Omufwoko, K.S.; ffrench-Constant, R.; Gordon, I.J. Neo Sex Chromosomes, Colour Polymorphism and Male-Killing in the African Queen Butterfly, Danaus chrysippus (L.). Insects 2019, 10, 291. https://doi.org/10.3390/insects10090291
Smith DAS, Traut W, Martin SH, Ireri P, Omufwoko KS, ffrench-Constant R, Gordon IJ. Neo Sex Chromosomes, Colour Polymorphism and Male-Killing in the African Queen Butterfly, Danaus chrysippus (L.). Insects. 2019; 10(9):291. https://doi.org/10.3390/insects10090291
Chicago/Turabian StyleSmith, David A.S., Walther Traut, Simon H. Martin, Piera Ireri, Kennedy S. Omufwoko, Richard ffrench-Constant, and Ian J. Gordon. 2019. "Neo Sex Chromosomes, Colour Polymorphism and Male-Killing in the African Queen Butterfly, Danaus chrysippus (L.)" Insects 10, no. 9: 291. https://doi.org/10.3390/insects10090291
APA StyleSmith, D. A. S., Traut, W., Martin, S. H., Ireri, P., Omufwoko, K. S., ffrench-Constant, R., & Gordon, I. J. (2019). Neo Sex Chromosomes, Colour Polymorphism and Male-Killing in the African Queen Butterfly, Danaus chrysippus (L.). Insects, 10(9), 291. https://doi.org/10.3390/insects10090291





