Tolerance of High Oral Doses of Nonradioactive and Radioactive Caesium Chloride in the Pale Grass Blue Butterfly Zizeeria maha
Abstract
1. Introduction
2. Materials and Methods
2.1. Butterfly and Its Host Plant
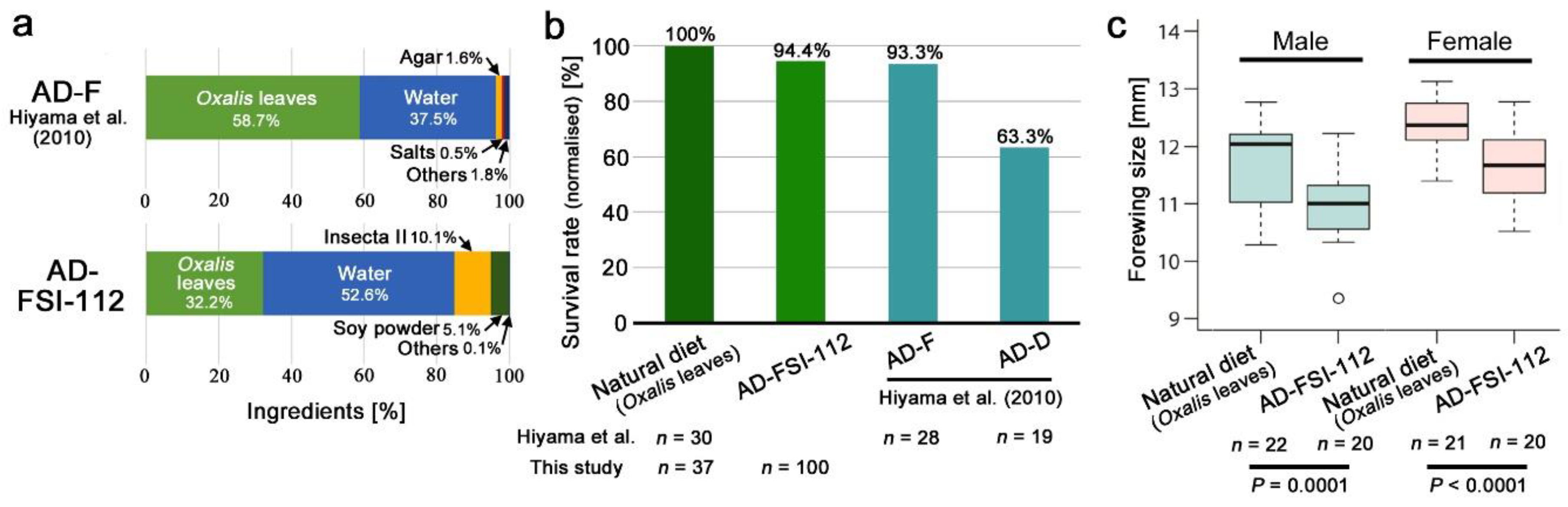
2.2. Preparation of the Artificial Diet
2.3. Egg Harvesting and Larval Rearing
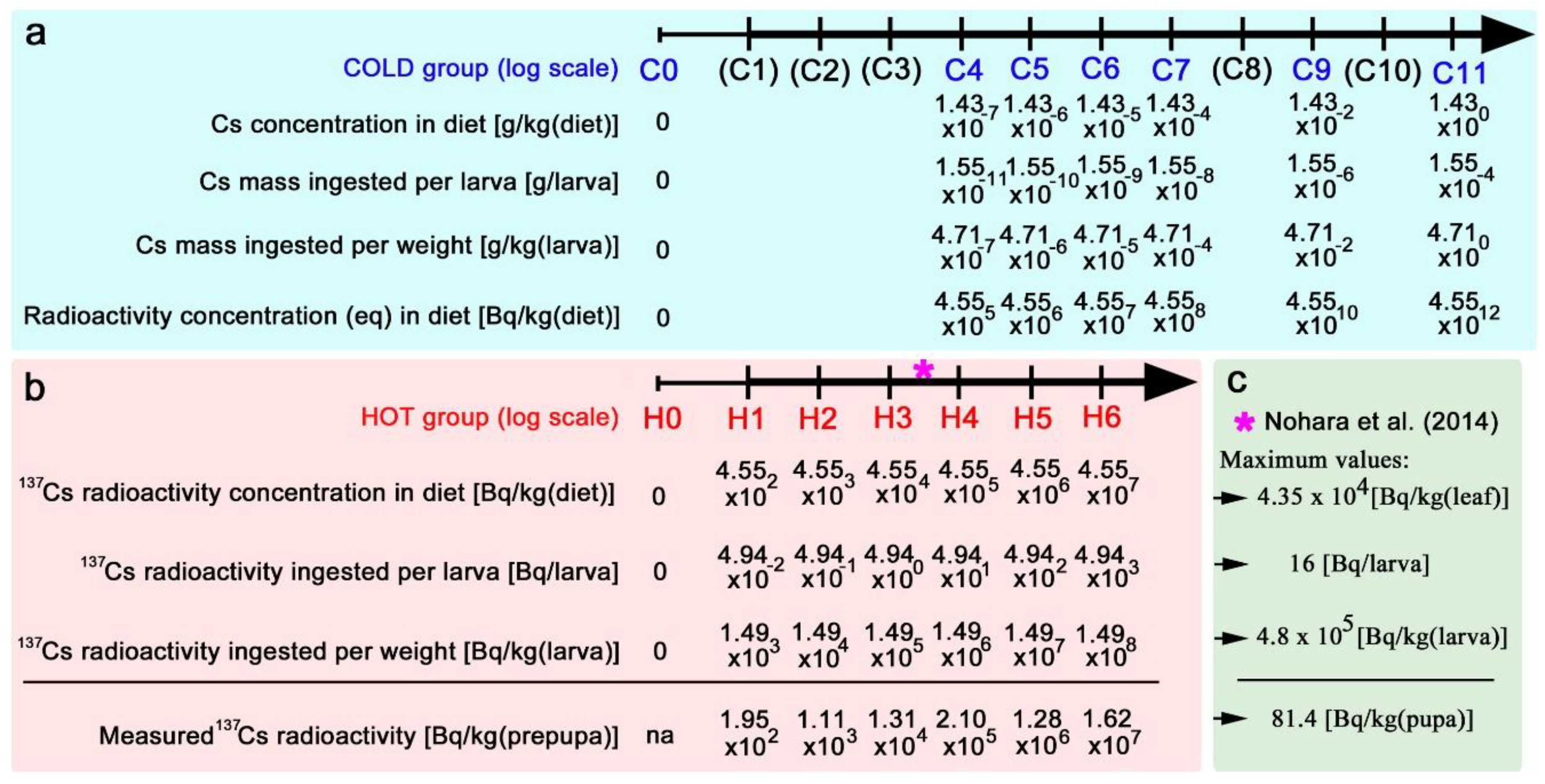
2.4. Nonradioactive and Radioactive Caesium Chloride Preparation
2.5. Toxicological Outputs
2.6. Image Analysis of the Artificial Diet Consumed
2.7. Radioactivity Measurements of Prepupae
2.8. Statistical Analysis
3. Results
3.1. Quality Check of the Artificial Diet
3.2. The Caesium Levels Tested
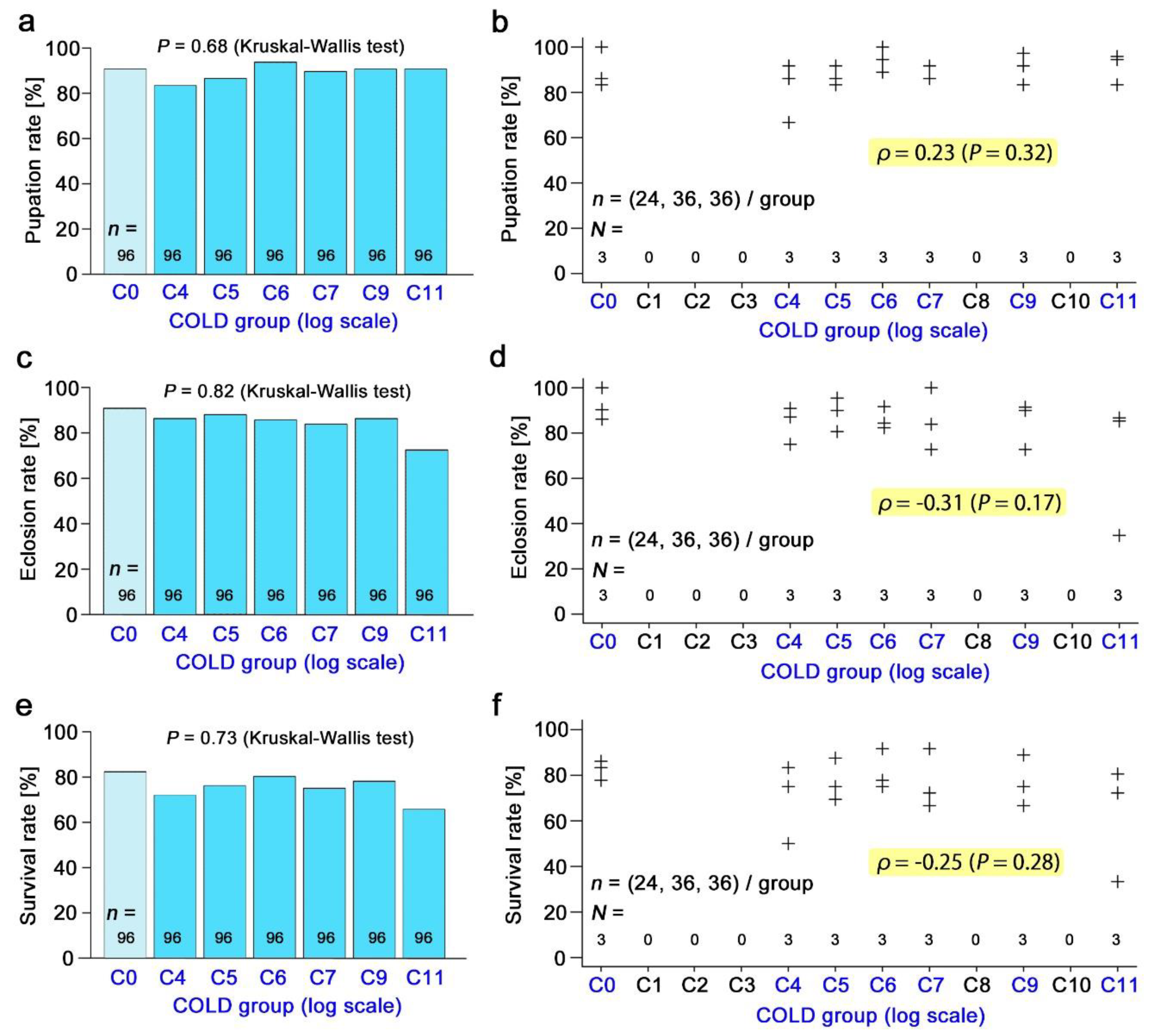
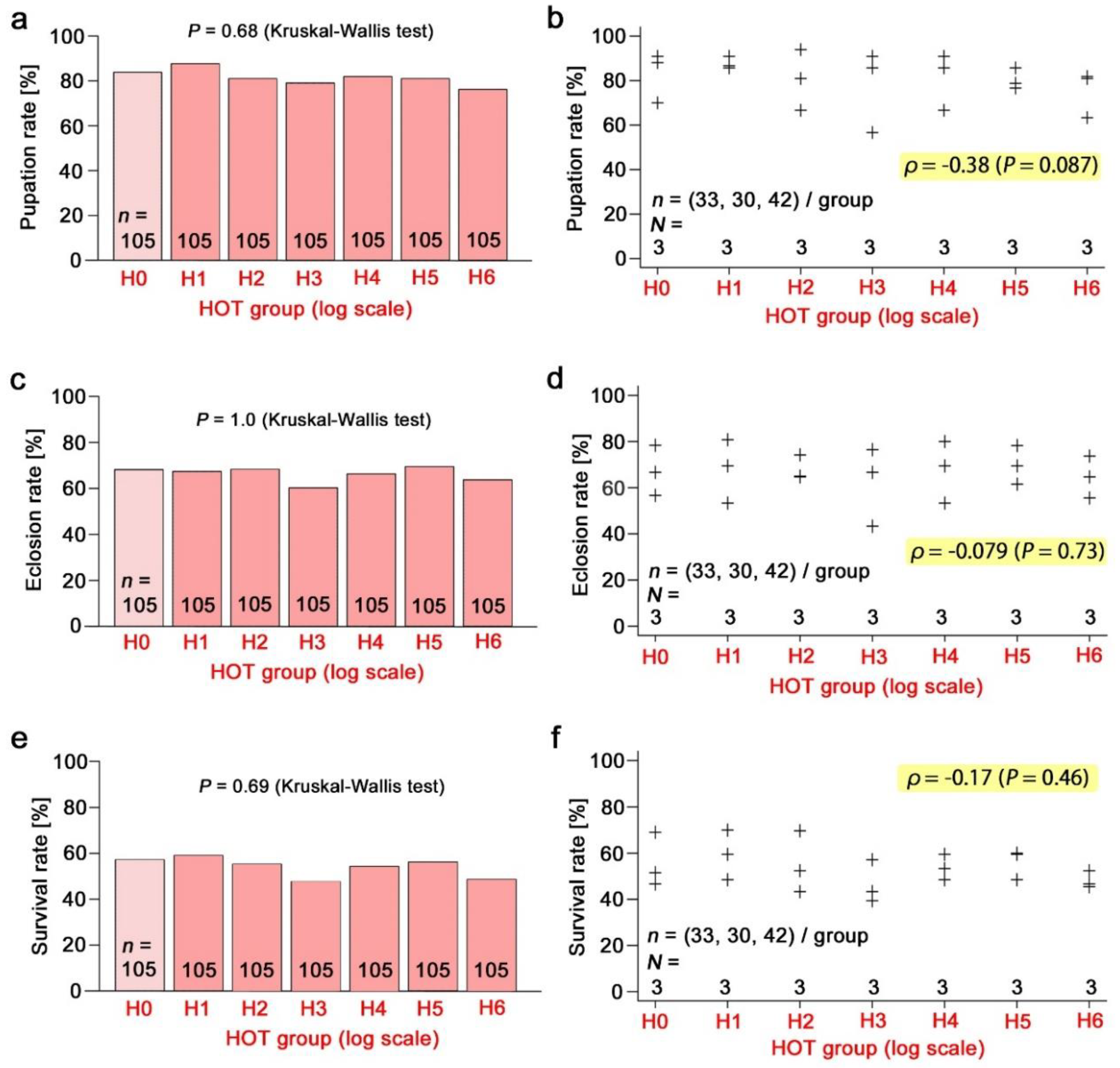
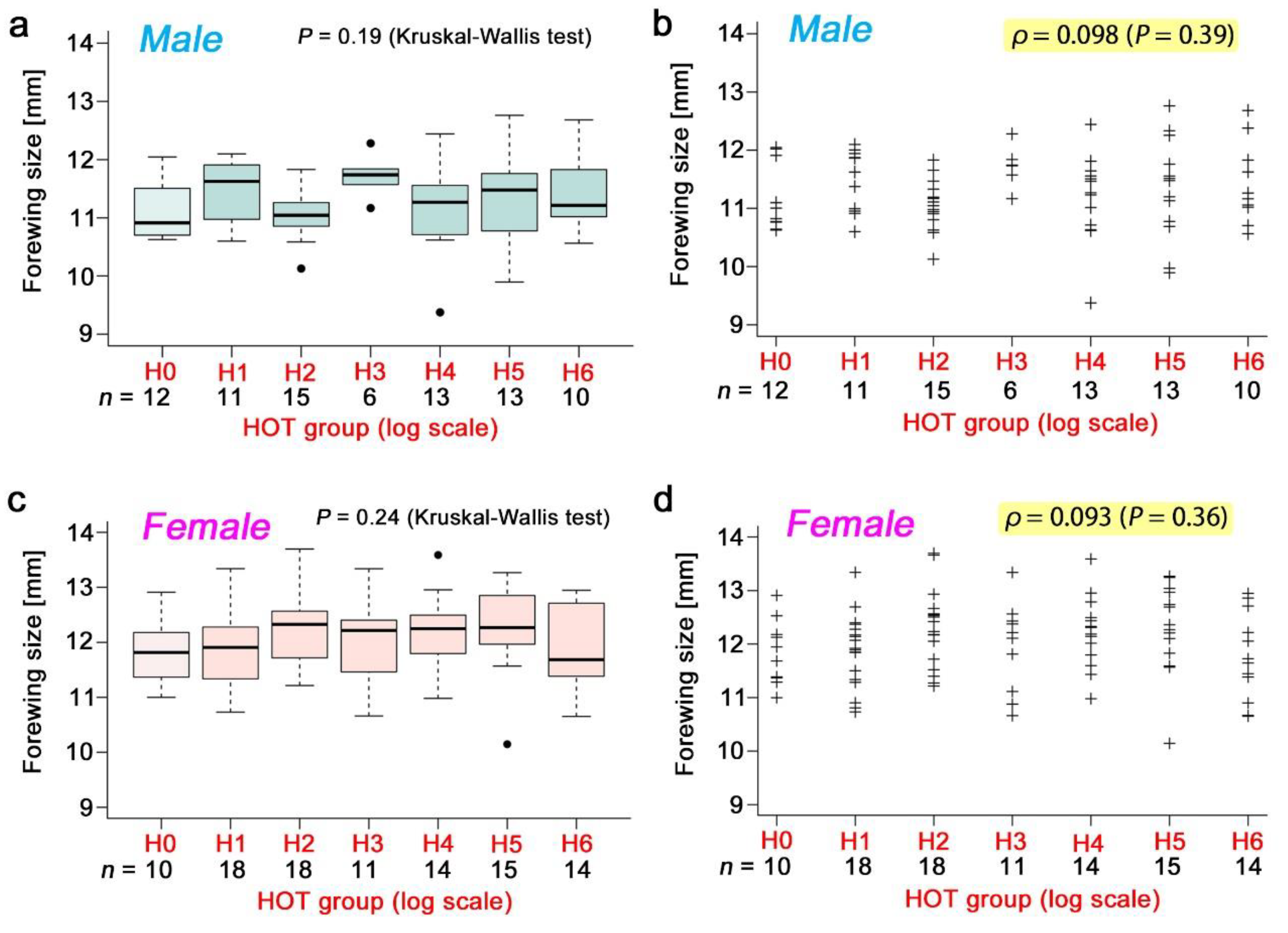
3.3. Nonradioactive Caesium Chloride
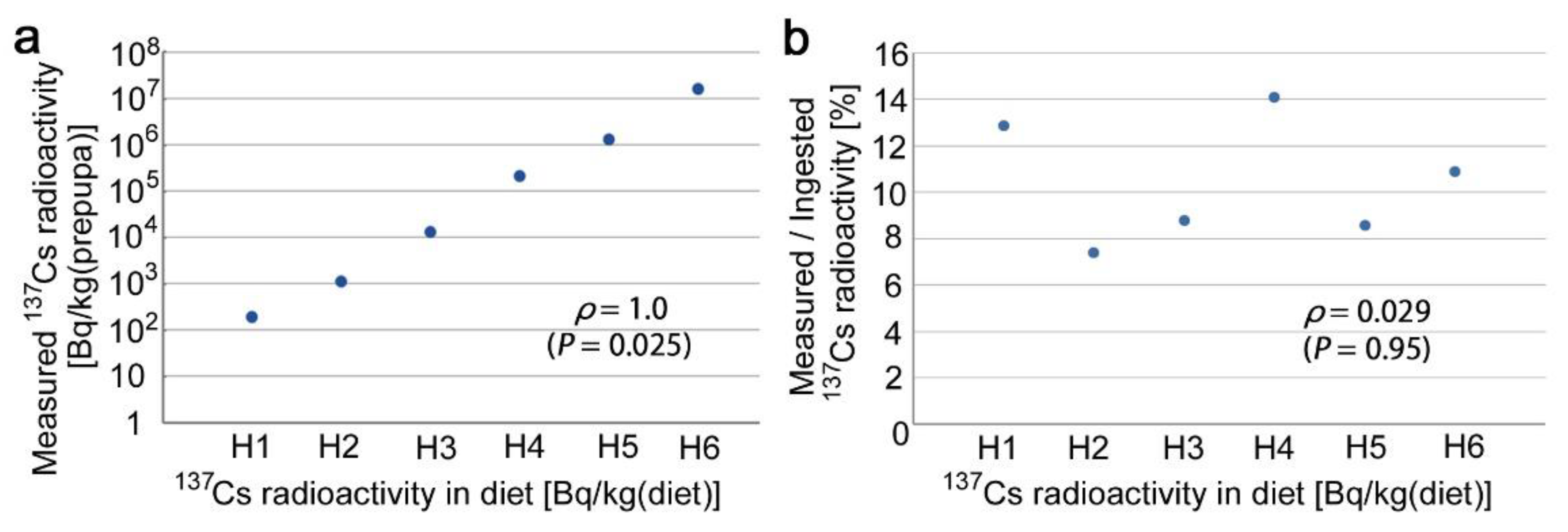
3.4. Radioactive Caesium Chloride
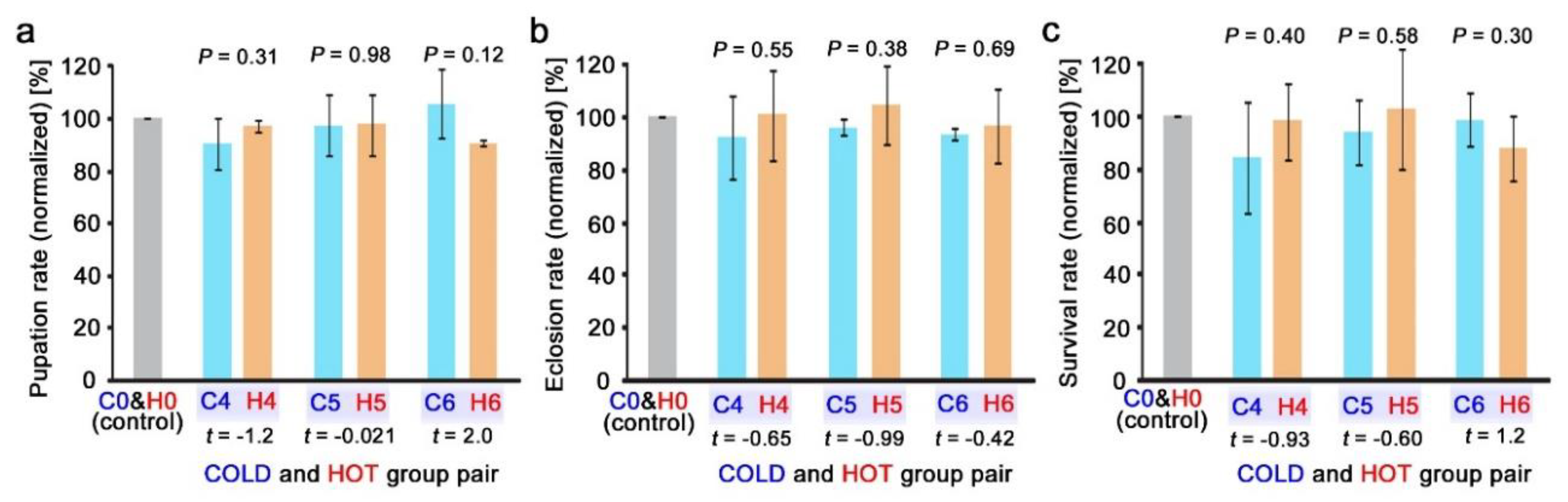
3.5. Direct Comparison of Equivalent Doses
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

References
- Møller, A.P.; Hagiwara, A.; Matsui, S.; Kasahara, S.; Kawatsu, K.; Nishiumi, I.; Suzuki, H.; Mousseau, T.A. Abundance of birds in Fukushima as judges from Chernobyl. Environ. Pollut. 2012, 164, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Møller, A.P.; Nishiumi, I.; Suzuki, H.; Ueda, K.; Mousseau, T.A. Differences in effects of radiation on abundance of animals in Fukushima and Chernobyl. Ecol. Indic. 2013, 24, 75–81. [Google Scholar] [CrossRef]
- Akimoto, S. Morphological abnormalities in gall-forming aphids in a radiation-contaminated area near Fukushima Daiichi: Selective impact of fallout? Ecol. Evol. 2014, 4, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, K.; Hayama, S.; Nakiri, S.; Nakanishi, S.; Ishii, N.; Uno, T.; Kato, T.; Konno, F.; Kawamoto, Y.; Tsuchida, S.; et al. Low blood cell counts in wild Japanese monkeys after the Fukushima Daiichi nuclear disaster. Sci. Rep. 2014, 4, 5793. [Google Scholar] [CrossRef] [PubMed]
- Hayama, S.; Tsuchiya, M.; Ochiaki, K.; Nakiri, S.; Nakanishi, S.; Ishii, N.; Kato, T.; Tanaka, A.; Konno, F.; Kawamoto, Y.; et al. Small head size and delayed body weight growth in wild Japanese monkey fetuses after the Fukushima Daiichi nuclear disaster. Sci. Rep. 2017, 7, 3528. [Google Scholar] [CrossRef] [PubMed]
- Urushihara, Y.; Suzuki, T.; Shimizu, Y.; Ohtaki, M.; Kuwahara, Y.; Suzuki, M.; Uno, T.; Fujita, S.; Saito, A.; Yamashiro, H.; et al. Haematological analysis of Japanese macaques (Macaca fuscata) in the area affected by the Fukushima Daiichi Nuclear Power Plant accident. Sci. Rep. 2018, 8, 16748. [Google Scholar] [CrossRef] [PubMed]
- Bonisoli-Alquati, A.; Koyama, K.; Tedeschi, D.J.; Kitamura, W.; Sukuzi, H.; Ostermiller, S.; Arai, E.; Møller, A.P.; Mousseau, T.A. Abundance and genetic damage of barn swallows from Fukushima. Sci. Rep. 2015, 5, 9432. [Google Scholar] [CrossRef] [PubMed]
- Murase, K.; Murase, J.; Horie, R.; Endo, K. Effects of the Fukushima Daiichi nuclear accident on goshawk reproduction. Sci. Rep. 2015, 5, 9405. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, G.; Shibato, J.; Imanaka, T.; Cho, K.; Kubo, A.; Kikuchi, S.; Satoh, K.; Kimura, S.; Ozawa, S.; Fukutani, S.; et al. Unraveling low-level gamma radiation-responsive changes in expression of early and late genes in leaves of rice seedlings at Iitate Village, Fukushima. J. Hered. 2014, 105, 723–738. [Google Scholar] [CrossRef]
- Rakwal, R.; Hayashi, G.; Shibato, J.; Deepak, S.A.; Gundimeda, S.; Simha, U.; Padmanaban, A.; Gupta, R.; Han, S.I.; Kim, S.T.; et al. Progress toward rice seed OMICS in low-level gamma radiation environment in Iitate Village, Fukushima. J. Hered. 2017, 109, 206–211. [Google Scholar] [CrossRef]
- Watanabe, Y.; Ichikawa, S.; Kubota, M.; Hoshino, J.; Kubota, Y.; Maruyama, K.; Fuma, S.; Kawaguchi, I.; Yoschenko, V.I.; Yoshida, S. Morphological defects in native Japanese fir trees around the Fukushima Daiichi Nuclear Power Plant. Sci. Rep. 2015, 5, 13232. [Google Scholar] [CrossRef] [PubMed]
- Yoschenko, V.; Nanba, K.; Yoshida, S.; Watanabe, Y.; Takase, T.; Sato, N.; Keitoku, K. Morphological abnormalities in Japanese red pine (Pinus densiflora) at the territories contaminated as a result of the accident at Fukushima Dai-ichi Nuclear Power Plant. J. Environ. Radioact. 2016, 165, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, T.; Yoshii, H.; Mizuno, S.; Shiraishi, H. Decline in intertidal biota after the 2011 Great East Japan Earthquake and Tsunami and the Fukushima nuclear disaster: Field observations. Sci. Rep. 2011, 6, 20416. [Google Scholar] [CrossRef] [PubMed]
- Urushihara, Y.; Kawasumi, K.; Endo, S.; Tanaka, K.; Hirakawa, Y.; Hayashi, G.; Sekine, T.; Kino, Y.; Kuwahara, Y.; Suzuki, M.; et al. Analysis of plasma protein concentrations and enzyme activities in cattle within the ex-evacuation zone of the Fukushima Daiichi nuclear plant accident. PLoS ONE 2016, 11, e0155069. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, M.; Kato, A.; Kobayashi, J.; Okuda, K.; Kuwahara, Y.; Kino, Y.; Abe, Y.; Sekine, T.; Fukuda, T.; Isogai, E.; et al. Gene expression analyses of the small intestine of pigs in the ex-evacuation zone of the Fukushima Daiichi Nuclear Power Plant. BMC Vet. Res. 2017, 13, 337. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.J.; Suzuki, M.; Redon, C.E.; Kuwahara, Y.; Yamashiro, H.; Abe, Y.; Takahashi, S.; Fukuda, T.; Isogai, E.; Bonner, W.M.; et al. The causal relationship between DNA damage induction in bovine lymphocytes and the Fukushima Nuclear Power Plant Accident. Radiat. Res. 2017, 187, 630–636. [Google Scholar] [CrossRef]
- Takino, S.; Yamashiro, H.; Sugano, Y.; Fujishima, Y.; Nakata, A.; Kasai, K.; Hayashi, G.; Urushihara, Y.; Suzuki, M.; Shinoda, H.; et al. Analysis of the effect of chronic and low-dose radiation exposure on spermatogenic cells of male large Japanese field mice (Apodemus speciosus) after the Fukushima Daiichi Nuclear Power Plant Accident. Radiat. Res. 2017, 187, 161–168. [Google Scholar] [CrossRef]
- Kubota, Y.; Tsuji, H.; Kawagoshi, T.; Shiomi, N.; Takahashi, H.; Watanabe, Y.; Fuma, S.; Doi, K.; Kawaguchi, I.; Aoki, M.; et al. Chromosomal aberrations in wild mice captured in areas differentially contaminated by the Fukushima Dai-ichi Nuclear Power Plant accident. Environ. Sci. Technol. 2015, 49, 10074–10083. [Google Scholar] [CrossRef]
- Kawagoshi, T.; Shiomi, N.; Takahashi, H.; Watanabe, Y.; Fuma, S.; Doi, K.; Kawaguchi, I.; Aoki, M.; Kubota, M.; Furuhata, Y.; et al. Chromosomal aberrations in large Japanese field mice (Apodemus speciosus) captured near Fukushima Dai-ichi Nuclear Power Plant. Environ. Sci. Technol. 2017, 51, 4632–4641. [Google Scholar] [CrossRef]
- Korblein, A.; Küchenhoff, H. Perinatal mortality after the Fukushima accident: A spatiotemporal analysis. J. Radiol. Prot. 2019. [Google Scholar] [CrossRef]
- Horemans, N.; Nauts, R.; Vives i Batlle, J.; van Hees, M.; Jacobs, G.; Voorspoels, S.; Gaschak, S.; Nanba, K.; Saenen, E. Genome-wide DNA methylation changes in two Brassicaceae species sampled alongside a radiation gradient in Chernobyl and Fukushima. J. Environ. Radioact. 2018, 192, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Yamashiro, H.; Abe, Y.; Fukuda, T.; Kino, Y.; Kawaguchi, I.; Kuwahara, Y.; Fukumoto, M.; Takahashi, S.; Suzuki, M.; Kobayashi, J.; et al. Effects of radioactive caesium on bull testes after the Fukushima nuclear accident. Sci. Rep. 2013, 3, 2850. [Google Scholar] [CrossRef] [PubMed]
- Yamashiro, H.; Abe, Y.; Hayashi, G.; Urushihara, Y.; Kuwahara, Y.; Suzuki, M.; Kobayashi, J.; Kino, Y.; Fukuda, T.; Tong, B.; et al. Electron probe X-ray microanalysis of boar and inobuta testes after the Fukushima accident. J. Radiat. Res. 2015, 56, i42–i47. [Google Scholar] [CrossRef] [PubMed]
- Okano, T.; Ishiniwa, H.; Onuma, M.; Shindo, J.; Yokohata, Y.; Tamaoki, M. Effects of environmental radiation on testes and spermatogenesis in wild large Japanese field mice (Apodemus speciosus) from Fukushima. Sci. Rep. 2016, 6, 23601. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, H.; Yamaguchi, Y.; Yoshimura, T.; Fukumoto, M.; Todo, T. Fukushima simulation experiment: Assessing the effects of chronic low-dose-rate internal 137Cs radiation exposure on litter size, sex ratio, and biokinetics in mice. J. Radiat. Res. 2015, 56, i29–i35. [Google Scholar] [CrossRef]
- Sato, I.; Sasaki, J.; Satoh, H.; Deguchi, Y.; Chida, H.; Natsuhori, M.; Otani, K.; Okada, K. Decreased blood cell counts were not observed in cattle living in the “difficult-to-return zone” of the Fukushima nuclear accident. Anim. Sci. J. 2019, 90, 128–134. [Google Scholar] [CrossRef]
- Sasaki, J.; Uehara, M.; Sato, I.; Satoh, H.; Deguchi, Y.; Chida, H.; Natsuhori, M.; Murata, T.; Ochiai, K.; Otani, K.; et al. Pathological characteristics of thyroid glands from Japanese Black Cattle living in the restricted area of the Fukushima Daiichi Nuclear Power Plant accident. Anim. Sci. J. 2019. [Google Scholar] [CrossRef]
- Akimoto, S.I.; Li, Y.; Imanaka, T.; Sato, H.; Ishida, K. Effects of radiation from contaminated soil and moss in Fukushima on embryogenesis and egg hatching of the aphid Prociphilus oriens. J. Hered. 2018, 109, 199–205. [Google Scholar] [CrossRef]
- Hiyama, A.; Nohara, C.; Kinjo, S.; Taira, W.; Gima, S.; Tanahara, A.; Otaki, J.M. The biological impacts of the Fukushima nuclear accident on the pale grass blue butterfly. Sci. Rep. 2012, 2, 570. [Google Scholar] [CrossRef]
- Hiyama, A.; Nohara, C.; Taira, W.; Kinjo, S.; Iwata, M.; Otaki, J.M. The Fukushima nuclear accident and the pale grass blue butterfly: Evaluating biological effects of long-term low-dose exposures. BMC Evol. Biol. 2013, 13, 168. [Google Scholar] [CrossRef]
- Taira, W.; Nohara, C.; Hiyama, A.; Otaki, J.M. Fukushima’s biological impacts: The case of the pale grass blue butterfly. J. Hered. 2014, 105, 710–722. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hiyama, A.; Taira, W.; Nohara, C.; Iwasaki, M.; Kinjo, S.; Iwata, M.; Otaki, J.M. Spatiotemporal abnormality dynamics of the pale grass blue butterfly: Three years of monitoring (2011–2013) after the Fukushima nuclear accident. BMC Evol. Biol. 2015, 15, 15. [Google Scholar] [CrossRef] [PubMed]
- Nohara, C.; Hiyama, A.; Taira, W.; Tanahara, A.; Otaki, J.M. The biological impacts of ingested radioactive materials on the pale grass blue butterfly. Sci. Rep. 2014, 4, 4946. [Google Scholar] [CrossRef] [PubMed]
- Nohara, C.; Taira, W.; Hiyama, A.; Tanahara, A.; Takatsuji, T.; Otaki, J.M. Ingestion of radioactively contaminated diets for two generations in the pale grass blue butterfly. BMC Evol. Biol. 2014, 14, 193. [Google Scholar] [CrossRef] [PubMed]
- Taira, W.; Hiyama, A.; Nohara, C.; Sakauchi, K.; Otaki, J.M. Ingestional and transgenerational effects of the Fukushima nuclear accident on the pale grass blue butterfly. J. Radiat. Res. 2015, 56, i2–i18. [Google Scholar] [CrossRef]
- UNSCEAR; United Nations Scientific Committee on the Effects of Atomic Radiation. Developments Since the 2013 UNSCEAR Report on the Levels and Effects of Radiation Exposure Due to the Nuclear Accident Following the Great East-Japan Earthquake and Tsunami: A 2017 White Paper to Guide the Scientific Committee’s Future Programme of Work; United Nations: New York, NY, USA, 2017. [Google Scholar]
- Garnier-Laplace, J.; Geras’kin, S.; Della-Vedova, C.; Beaugelin-Seiller, K.; Hinton, T.G.; Real, A.; Oudalova, A. Are radiosensitivity data derived from natural field conditions consistent with data from controlled exposures? A case study of Chernobyl wildlife chronically exposed to low dose rates. J. Environ. Radioact. 2013, 121, 12–21. [Google Scholar] [CrossRef]
- Strand, P.; Sundell-Bergman, S.; Brown, J.E.; Dowall, M. On the divergences in assessment of environmental impacts from ionising radiation following the Fukushima accident. J. Environ. Radioact. 2017, 169–170, 159–173. [Google Scholar] [CrossRef]
- Beaugelin-Seiller, K.; Della-Vedova, C.; Garnier-Laplace, J. Is non-human species radiosensitivity in the lab a good indicator of that in the field? Making the comparison more robust. J. Environ. Radioact. 2019. [Google Scholar] [CrossRef]
- Otaki, J.M. Fukushima’s lessons from the blue butterfly: A risk assessment of the human living environment in the post-Fukushima era. Integr. Environ. Assess. Manag. 2016, 12, 667–672. [Google Scholar] [CrossRef]
- Otaki, J.M.; Taira, W. Current status of the blue butterfly in Fukushima research. J. Hered. 2017, 109, 178–187. [Google Scholar] [CrossRef]
- Otaki, J.M. Understanding low-dose exposure and field effects to resolve the field-laboratory paradox: Multifaceted biological effects from the Fukushima nuclear accident. In New Trends in Nuclear Science; Awwad, N.S., AlFaify, S.A., Eds.; InTechOpen: London, UK, 2018; pp. 49–71. [Google Scholar]
- Hiyama, A.; Iwata, M.; Otaki, J.M. Rearing the pale grass blue Zizeeria maha (Lepidoptera, Lycaenidae): Toward the establishment of a lycaenid model system for butterfly physiology and genetics. Entomol. Sci. 2010, 13, 293–302. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Matsuyama, S.; Yamaji, K. Oxalic acid as a larval feeding stimulant for the pale grass blue butterfly Zizeeria maha (Lepidoptera: Lycaenidae). Appl. Entomol. Zool. 2016, 51, 91–98. [Google Scholar] [CrossRef]
- Shozugawa, K. Advances in γ-ray analysis. Kagaku 2013, 83, 723–726. [Google Scholar]
- Johnson, G.T.; Lewis, T.R.; Wagner, W.D. Acute toxicity of caesium and rubidium compounds. Toxicol. Appl. Pharmacol. 1975, 32, 239–245. [Google Scholar] [CrossRef]
- Cochran, K.W.; Doull, J.; Mazur, M.; DuBois, K.P. Acute toxicity of zirconium, columbium, strontium, lanthanum, caesium, tantalum and yttrium. Arch. Indusl. Hyg. Occup. Med. 1950, 1, 637–650. [Google Scholar]
- Ghosh, A.; Sharma, A.; Talukder, G. Clastogenic effects of caesium chloride on mouse bone marrow cells in vivo. Mut. Res. 1990, 244, 295–298. [Google Scholar] [CrossRef]
- Ghosh, A.; Sharma, A.; Talukder, G.; Oleson, F.B. Cytogenetic damage induced in vivo to mice by single exposure to caesium. Environ. Mol. Mutagen. 1991, 18, 87–91. [Google Scholar] [CrossRef]
- Ghosh, A.; Sharma, A.; Talukder, G. Effects of caesium on cellular systems. Biol. Trace Element Res. 1993, 38, 165–203. [Google Scholar] [CrossRef]
- Yamagata, N.; Yamagata, T.; Matsuda, S. The different distribution of rubidium and caesium in natural plants. Bull. Chem. Soc. Jpn. 1959, 32, 407–414. [Google Scholar] [CrossRef]
- Arthur, V.; Machi, A.; Mastrangelo, T. Ionizing radiations in entomology. In Evolution of Ionizing Radiation Research; Nenoi, M., Ed.; InTechOpen: London, UK, 2015; pp. 213–234. [Google Scholar]
- Takada, N.; Yamauchi, E.; Fujimoto, H.; Banno, Y.; Tsuchida, K.; Hashido, K.; Nakajima, Y.; Tu, Z.; Takahashi, M.; Fujii, H.; et al. A novel indicator for radiation sensitivity using the wing size reduction of Bombyx mori pupae caused by γ-ray irradiation. J. Insect Biotechnol. Sericol. 2006, 75, 161–165. [Google Scholar] [CrossRef]
- Otaki, J.M. Fukushima Nuclear Accident: Potential health effects inferred from butterfly and human cases. In A Handbook of Environmental Toxicology: Human Disorders and Ecotoxicology; D’Mello, J.P.F., Ed.; CAB International: Wallingford, UK, 2019; pp. 497–514. [Google Scholar]
- Taira, W.; Toki, M.; Kakinohana, K.; Sakauchi, K.; Otaki, J.M. Developmental and hemocytological effects of ingesting Fukushima’s radiocesium on the cabbage white butterfly Pieris Rapae. Sci. Rep. 2019, 9, 2625. [Google Scholar] [CrossRef] [PubMed]
- Bréchignac, F.; Oughton, D.; Mays, C.; Barnthouse, L.; Beasley, J.C.; Bonisoli-Alguati, A.; Bradshaw, C.; Brown, J.; Dray, S.; Geras’kin, S.; et al. Addressing ecological effects of radiation on populations and ecosystems to improve protection of the environment against radiation: Agreed statements from a Consensus Symposium. J. Environ. Radioact. 2016, 158–159, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Bréchignac, F. The need to integrate laboratory- and ecosystem-level research for assessment of the ecological impact of radiation. Integr. Environ. Assess. Manag. 2016, 12, 673–676. [Google Scholar] [CrossRef] [PubMed]
- Manti, L.; D’Arco, A. Cooperative biological effects between ionising radiation and other physical and chemical agents. Mut. Res. 2010, 704, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Mothersill, C.; Rusin, A.; Fernandez-Palomo, C.; Seymour, C. History of bystander effects research 1905-present; what is in a name? Int. J. Radiat. Biol. 2018, 94, 696–707. [Google Scholar] [CrossRef] [PubMed]
- Mothersill, C.; Rusin, A.; Seymour, C. Low doses and non-targeted effects in environmental radiation protection; where are we now and where should we go? Environ. Res. 2017, 159, 484–490. [Google Scholar] [CrossRef]
- Hancock, S.; Vo, N.T.K.; Omar-Nazir, L.; Batlle, J.V.I.; Otaki, J.M.; Hiyama, A.; Byun, S.H.; Seymour, C.B.; Mothersill, C. Transgenerational effects of historic radiation dose in pale grass blue butterflies around Fukushima following the Fukushima Dai-ichi Nuclear Power Plant meltdown accident. Environ. Res. 2019, 168, 230–240. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gurung, R.D.; Taira, W.; Sakauchi, K.; Iwata, M.; Hiyama, A.; Otaki, J.M. Tolerance of High Oral Doses of Nonradioactive and Radioactive Caesium Chloride in the Pale Grass Blue Butterfly Zizeeria maha. Insects 2019, 10, 290. https://doi.org/10.3390/insects10090290
Gurung RD, Taira W, Sakauchi K, Iwata M, Hiyama A, Otaki JM. Tolerance of High Oral Doses of Nonradioactive and Radioactive Caesium Chloride in the Pale Grass Blue Butterfly Zizeeria maha. Insects. 2019; 10(9):290. https://doi.org/10.3390/insects10090290
Chicago/Turabian StyleGurung, Raj D., Wataru Taira, Ko Sakauchi, Masaki Iwata, Atsuki Hiyama, and Joji M. Otaki. 2019. "Tolerance of High Oral Doses of Nonradioactive and Radioactive Caesium Chloride in the Pale Grass Blue Butterfly Zizeeria maha" Insects 10, no. 9: 290. https://doi.org/10.3390/insects10090290
APA StyleGurung, R. D., Taira, W., Sakauchi, K., Iwata, M., Hiyama, A., & Otaki, J. M. (2019). Tolerance of High Oral Doses of Nonradioactive and Radioactive Caesium Chloride in the Pale Grass Blue Butterfly Zizeeria maha. Insects, 10(9), 290. https://doi.org/10.3390/insects10090290





