Codling Moth Wing Morphology Changes Due to Insecticide Resistance
Abstract
1. Introduction
2. Materials and Methods
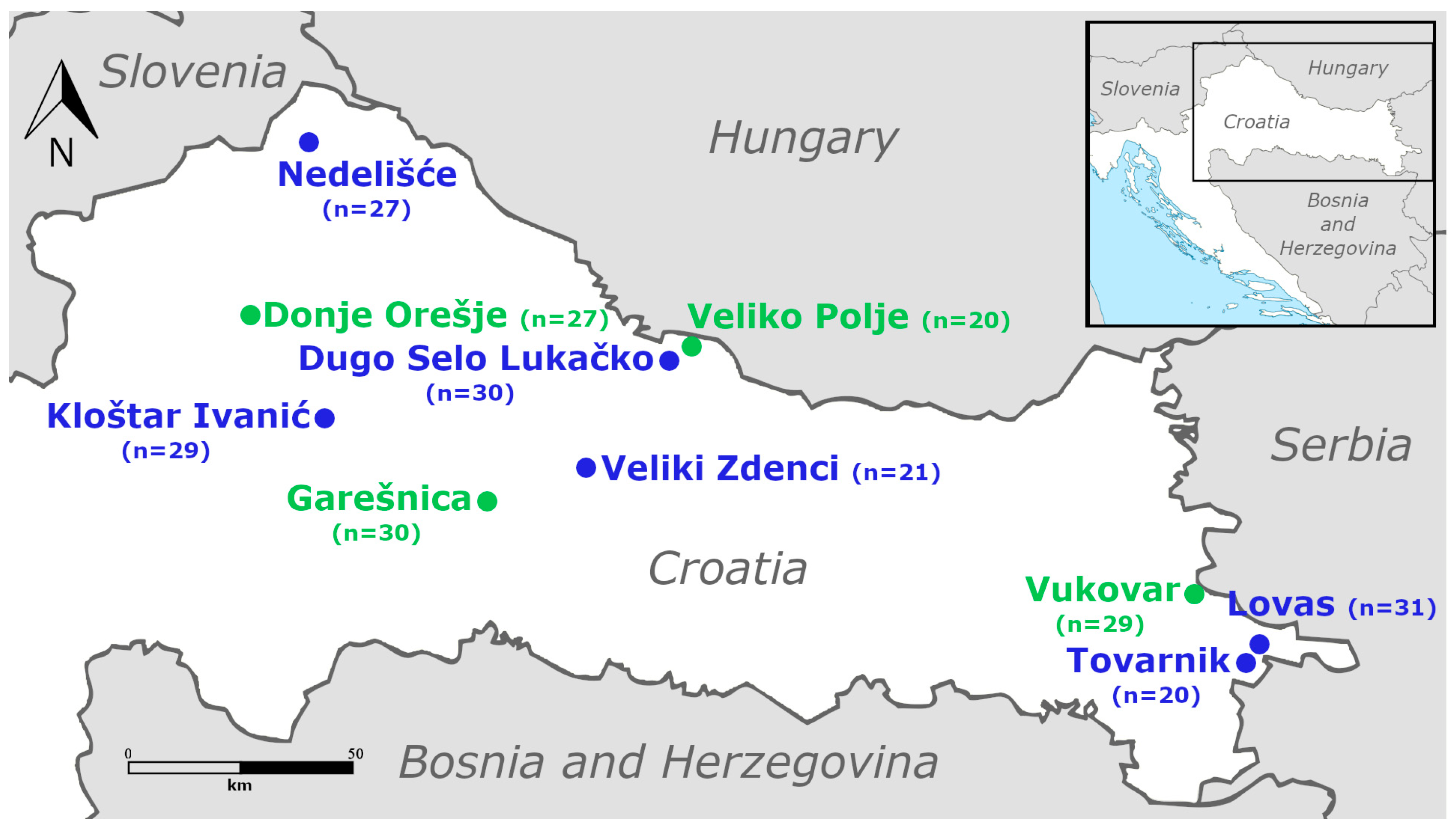
2.1. Collection Sites and Sampling
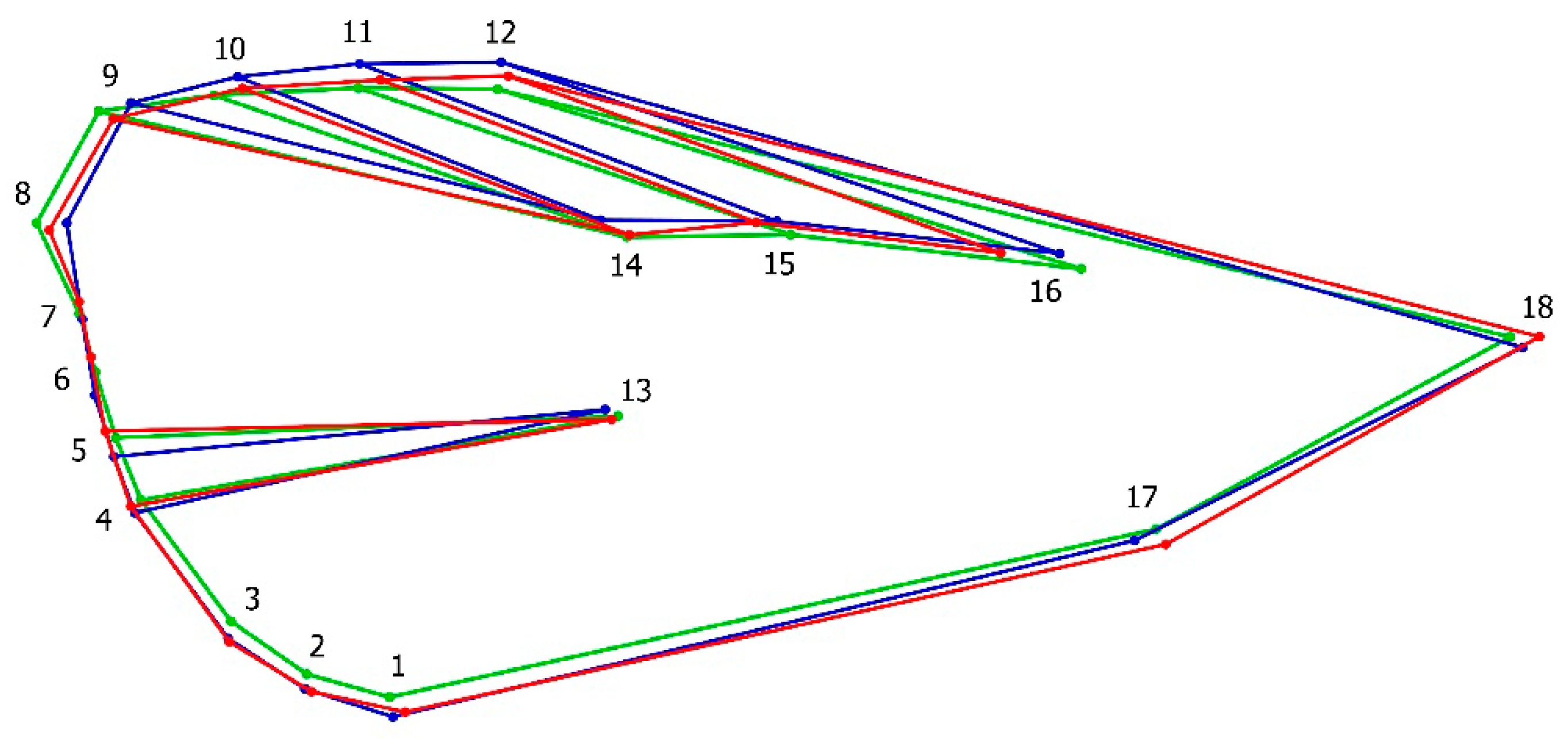
2.2. Geometric Morphometric Analysis
2.3. Finite Element Method (FEM)
3. Results
3.1. Geometric Morphometrics
3.2. Finite Element Method
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ciglar, I. Integrirana Zaštita Voćnjaka i Vinograda; Zrinski: Čakovec, Croatia, 1998. [Google Scholar]
- Ciglar, I.; Barić, B.; Tomšić, T.; Šubić, M. Suzbijanje jabukovog savijača (Cydia pomonella) metodom konfuzije. Glasilo Hrvatskog Agronomskog Društva 2000, 1–2, 85–93. [Google Scholar]
- Franck, P.; Reyes, M.; Olivares, J.; Sauphanor, B. Genetic architecture in codling moth populations: Comparison between microsatellite and insecticide resistance markers. Mol. Ecol. 2007, 16, 3554–3564. [Google Scholar] [CrossRef] [PubMed]
- Voudouris, C.C.; Franck, P.; Olivares, J.; Sauphanor, B.; Mamuris, Z.; Tsitsipis, J.A.; Margaritopoulos, J.T. Comparing the genetic structure of codling moth Cydia pomonella (L.) from Greece and France: Long distance gene-flow in a sedentary pest species. Bull. Entomol. Res. 2012, 102, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Meraner, A.; Brandstätter, A.; Thaler, R.; Array, B.; Unterlechner, M.; Niederstätter, H.; Parson, W.; Zelger, R.; Dalla Via, J.; Dallinger, R. Molecular phylogeny and population structure of the codling moth (Cydia pomonella) in Central Europe: I. Ancient clade splitting revealed by mitochondrial haplotype markers. Mol. Phylogenet. Evol. 2008, 48, 825–837. [Google Scholar] [CrossRef] [PubMed]
- Thaler, R.; Brandstätter, A.; Meraner, A.; Chabicovski, M.; Parson, W.; Zelger, R.; Dalla Via, J.; Dallinger, R. Molecular phylogeny and population structure of the codling moth (Cydia pomonella) in Central Europe: II. AFLP analysis reflects human-aided local adaptation of a global pest species. Mol. Phylogenet. Evol. 2008, 48, 838–849. [Google Scholar] [CrossRef]
- Reyes, M.; Franck, P.; Charmillot, P.J.; Ioriatti, C.; Olivares, J.; Pasqualini, E.; Sauphanor, B. Diversity of insecticide resistance mechanisms and spectrum in European populations of the Codling moth, Cydia Pomonella. Pest Manag. Sci. 2007, 63, 890–902. [Google Scholar] [CrossRef] [PubMed]
- Reyes, M.; Franck, P.; Olivares, J.; Margaritopoulos, J.; Knight, A.; Sauphanor, B. Worldwide variability of insecticide resistance mechanisms in the codling moth, Cydia pomonella L. (Lepidoptera: Tortricidae). Bull. Entomol. Res. 2009, 99, 359–369. [Google Scholar] [CrossRef]
- Pajač Živković, I.; Barić, B. Rezistentnost jabukova savijača na insekticidne pripravke. Glasilo Biljne Zaštite 2017, 5, 469–479. [Google Scholar]
- Hough, W.S. Relative resistance to arsenical poisoning of two codling moth strains. J. Econ. Entomol. 1928, 21, 325–329. [Google Scholar] [CrossRef]
- Ioriatti, C.; Saphanor, B.; Cainelli, R.; Rizzi, C.; Tasin, M. Cydia pomonella L.: Primo caso di resistenza a diflubenzuron in Trentino. Atti Giornate Fitopatologiche 2000, 1, 319–325. [Google Scholar]
- Sauphanor, B.; Brosse, V.; Bouvier, J.C.; Speich, P.; Micoud, A.; Martinet, C. Monitoring resistance to diflubenzuron and deltamethrin in French codling moth populations (Cydia pomonella). Pest Manag. Sci. 2000, 56, 74–82. [Google Scholar] [CrossRef]
- Sauphanor, B.; Cuany, A.; Bouvier, J.C.; Brose, V.; Berge, J.B. Mechanism of resistance to deltamethrin in field populations of Cydia pomonella L. (Lepidoptera: Tortricidae). Pest Biochem. Physiol. 1997, 58, 109–117. [Google Scholar] [CrossRef]
- Sauphanor, B.; Brosse, V.; Monier, C.; Bouvier, J.C. Differencial ovicidal and larvicidal resistance to benzoylureas in the codling moth, Cydia pomonella. Entomol. Exp. Appl. 1998, 88, 247–253. [Google Scholar] [CrossRef]
- Dunley, J.E.; Welter, S.C. Correlated insecticide cross-resistance in azinphosmetyl resistant codling moth (Lepidoptera: Tortricidae). J. Econ. Entomol. 2000, 93, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Franck, P.; Guérin, F.; Loiseau, A.; Sauphanor, B. Isolation and characterization of microsatellite loci in the codling moth Cydia pomonella L. (Lepidoptera, Tortricidae). Mol. Ecol. Notes 2005, 5, 99–102. [Google Scholar] [CrossRef]
- Zhou, Y.; Gu, H.; Dorn, S. Isolation of microsatellite loci in the codling moth, Cydia pomonella (Lepidoptera: Tortricidae). Mol. Ecol. Notes 2005, 5, 226–227. [Google Scholar] [CrossRef]
- Franck, P.; Timm, A.E. Population genetic structure of Cydia pomonella: A review and case study comparing spatiotemporal variation. J. Appl. Entomol. 2010, 134, 191–200. [Google Scholar] [CrossRef]
- Fuentes-Contreras, E.; Espinoza, J.L.; Lavandero, B.; Ramírez, C.C. Population genetic structure of codling moth (Lepidoptera: Tortricidae) from apple orchards in central Chile. J. Econ. Entomol. 2008, 101, 190–198. [Google Scholar] [CrossRef]
- Pajač, I.; Barić, B.; Šimon, S.; Mikac, M.K.; Pejić, I. An initial examination of the population genetic structure of Cydia pomonella (Lepidoptera: Tortricidae) in Croatian apple orchards. J. Food Agric. Environ. 2011, 9, 459–464. [Google Scholar]
- Mikac, K.M.; Douglas, J.; Spencer, J.L. Wing shape and size of the western corn rootworm (Coleoptera: Chrysomelidae) is related to sex and resistance to soybean-maize crop rotation. J. Econ. Entomol 2013, 106, 1517–1524. [Google Scholar] [CrossRef]
- Mikac, K.M.; Lemic, D.; Bažok, R.; Benítez, H.A. Wing shape changes: A morphological view of the Diabrotica virgifera virgifera European invasion. Biol. Invasions 2016, 18, 3401–3407. [Google Scholar] [CrossRef]
- Mikac, K.M.; Lemic, D.; Benitez, H.A.; Bažok, R. Changes in corn rootworm wing morphology are related to resistance development. J. Pest Sci. 2019, 92, 443–451. [Google Scholar] [CrossRef]
- Pajač, I.; Barić, B. The behaviour of codling moth (Lepidoptera: Tortricidae) in the Croatian apple orchards. IOBC WPRS Bull. 2012, 74, 79–82. [Google Scholar]
- Pajač, I.; Barić, B.; Mikac, M.K.; Pejić, I. New insights into the biology and ecology of Cydia pomonella from apple orchards in Croatia. Bull. Insectology 2012, 65, 185–193. [Google Scholar]
- Bouyer, J.; Ravel, S.; Dujardin, J.P.; De Meeus, T.; Via, L.; Thevenon, S.; Guerrini, L.; Sidibe, I.; Solano, P. Population structuring of Glossina palpalis gambiensis (Diptera: Glossinidae) according to landscape fragmentation in the Mouhoun river Burkina Faso. J. Med. Entomol. 2007, 44, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Lemic, D.; Benítez, H.A.; Bazok, R. Intercontinental effect on sexual shape dimorphism and allometric relationships in the beetle pest Diabrotica virgifera virgifera LeConte (Coleoptera: Chrysomelidae). Zool. Anz. 2014, 253, 203–206. [Google Scholar] [CrossRef]
- Lemic, D.; Mikac, M.K.; Kozina, A.; Benitez, A.H.; McLean, M.C.; Bažok, R. Monitoring techniques of the western corn rootworm are the precursor to effective IPM strategies. Pest Manag. Sci. 2016, 72, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Lemic, D.; Benítez, H.A.; Püschel, T.; Virić Gašparić, H.; Šatvar, M.; Bažok, R. Ecological morphology of the sugar beet weevil Croatian populations: Evaluating the role of environmental conditions on body shape. Zool. Anz. 2016, 260, 25–32. [Google Scholar] [CrossRef]
- Benítez, A.H.; Lemic, D.; Bažok, R.; Gallardo-Araya, M.C.; Mikac, M.K. Evolutionary directional asymmetry and shape variation in Diabrotica v. virgifera (Coleoptera: Chrysomelidae): An example using hind wings. Biol. J. Linn. Soc. 2014, 111, 110–118. [Google Scholar]
- Benítez, H.A.; Lemic, D.; Bažok, R.; Bravi, R.; Buketa, M.; Püschel, T. Morphological integration and modularity in Diabrotica virgifera virgifera LeConte (Coleoptera: Chrysomelidae) hind wings. Zool. Anz. 2014, 253, 461–468. [Google Scholar] [CrossRef]
- Levine, E.; Oloumi-Sadeghi, H. Western corn rootworm (Coleoptera: Chrysomelidae) larval injury to corn grown for seed production following soybeans grown for seed production. J. Econ. Entomol. 1996, 89, 1010–1016. [Google Scholar] [CrossRef]
- Khaghaninia, S.; Mohammadi, S.A.; Srafrazi, A.M.; Irani Nejad, K.H.; Zahiri, R. Geometric morphometric study on geographic dimorphism of coding moth Cydia pomonella (Lepidoptera, Tortricidae) from north west of Iran. Vestn. Zool. 2011, 45, e20–e28. [Google Scholar] [CrossRef][Green Version]
- Khaghaninia, S.; Mohammadi, S.A.; Sarafrazi, A.M.; Iraninejad, K.H.; Ebrahimi, E.; Zahiri, R. An analysis of seasonal dimorphism in codling moths, Cydia pomonella, from Iran using geometric morphometrics. Bull. Insectology 2014, 67, 43–50. [Google Scholar]
- Torres, F.; Rodríguez, M.A.; Lavandero, B.; Fuentes-Contreras, E. Body mass and wing geometric morphology of the codling moth (Lepidoptera: Tortricidae) according to sex, location and host plant in the region of Maule, Chile. Cienc. Investig. Agrar. 2015, 42, 397–406. [Google Scholar] [CrossRef]
- Varela, L.G.; Welter, S.C.; Jones, V.P.; Brunner, J.F.; Riedl, H. Monitoring and characterization of insecticide resistance in codling moth (Lepidoptera, Tortricidae) in four Western States. J. Econ. Entomol. 1993, 86, 1–10. [Google Scholar] [CrossRef]
- Reyes, M.; Barros-Parada, W.; Ramírez, C.C.; Fuentes-Contreras, E. Organophosphate resistance and its main mechanism in populations of Cydia pomonella (Lepidoptera: Tortricidae) from central Chile. J. Econ. Entomol. 2015, 108, 277–285. [Google Scholar] [CrossRef]
- Combes, S.A.; Daniel, T.L. Flexural stiffness in insect wings. II. Spatial distribution and dynamic wing bending. J. Exp. Biol. 2003, 206, 2989–2997. [Google Scholar] [CrossRef]
- Combes, S.A.; Daniel, T.L. Into thin air: Contributions of aerodynamic and inertial-elastic forces to wing bending in the hawkmoth Manduca sexta. J. Exp. Biol. 2003, 206, 2999–3006. [Google Scholar] [CrossRef]
- Ha, N.S.; Truong, Q.T.; Goo, N.S.; Park, H.C. Biomechanical Properties of Insect Wings: The Stress Stiffening Effects on the Asymmetric Bending of the Allomyrina dichotoma Beetle’s Hind Wing. PLoS ONE 2013, 8, e80689. [Google Scholar] [CrossRef]
- Schumacher, P.; Weyeneth, A.; Weber, D.C.; Dorn, S. Long flights in Cydia pomonella L. (Lepidoptera: Tortricidae) measured by a flight mill: Influence on sex, mated status and age. Physiol. Entomol. 1997, 22, 149–160. [Google Scholar] [CrossRef]
- Schumacher, P.; Weber, D.C.; Hagger, C.; Dorn, S. Heritability of flight distance for Cydia pomonella. Entomol. Exp. Appl. 1997, 85, 169–175. [Google Scholar] [CrossRef]
- Penzar, I.; Penzar, B. Agrometeorologija; Skolska knjiga: Zagreb, Croatia, 2000. [Google Scholar]
- EUR-Lex. Directive 2009/128/EC of the European Parliament and of the Council of 21 October 2009 establishing a framework for Community action to achieve the sustainable use of pesticides (Text with EEA relevance). Off. J. Eur. Union Spec. Ed. Croat. 2009, 15, 253–268.
- Upton, M.F.S.; Mantel, B.L. Methods for Collecting, Preserving and Studying Insects and Other Terrestrial Arthropods; The Australian Entomological Society Miscellaneous Pub: Sydney, Australia, 2010. [Google Scholar]
- Bookstein, F.L. Morphometric Tools for Landmark Data: Geometry and Biology; Cambridge University Press: Cambridge, UK, 1991. [Google Scholar]
- Rohlf, F.J. TPSdig, v. 2.17; NY State University at Stony Brook: New York, NY, USA, 2013. [Google Scholar]
- Rohlf, F.J.; Slice, D. Extensions of the Procustes methods for the optimal superimposition of landmarks. Syst. Zool. 1990, 39, 40–59. [Google Scholar] [CrossRef]
- Dryden, I.L.; Mardia, K.V. Statistical Shape Analysis; Wiley: Chichester, UK, 1998. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Adams, D.C.; Otárola-Castillo, E. Geomorph: An Package for the Collection and Analysis of Geometric Morphometric Shape Data. Methods Ecol. Evol. 2013, 4, 393–399. [Google Scholar] [CrossRef]
- Klingenberg, C.P. MorphoJ: An integrated software package for geometric morphometrics. Mol. Ecol. Resour. 2011, 11, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Klingenberg, C.P.; McIntyre, G.S. Geometric Morphometrics of Developmental Instability: Analyzing Patterns of Fluctuating Asymmetry with Procrustes Methods. Evolution 2006, 52, 1363. [Google Scholar] [CrossRef]
- Monteiro, L.R. Multivariate regression models and geometric morphometrics: The search for causal factors in the analysis of shape. Syst. Biol. 1999, 48, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Jolliffe, I.T. Principal Component Analysis, 2nd ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Schlager, S. Morpho and Rvcg—Shape analysis in R. In Statistical Shape and Deformation Analysis; Zheng, G., Li, S., Szekely, G., Eds.; Academic Press: Washington, DC, USA, 2017; pp. 217–256. [Google Scholar]
- Damos, P.; Escudero Colomar, L.-A.; Ioriatti, C. Integrated Fruit Production and Pest Management in Europe: The Apple Case Study and How Far We Are from the Original Concept? Insects 2015, 6, 626–657. [Google Scholar] [CrossRef]
- Cassanelli, S.; Reyes, M.; Rault, M.; Manicardi, G.C.; Sauphanor, B. Acetylcholinesterase mutation in an insecticide-resistant population of the codling moth Cydia pomonella (L.). Insect Biochem. Mol. Biol. 2006, 36, 642–653. [Google Scholar] [CrossRef]
- Coop, L.; Kogan, M.; Bajwa, W. Area-wide programme for suppression of codling moth: Summary of the effect of 5 years of control. Extending the principles and lessons learned outside the project and to other commodities. In Proceedings of the 95th Annual Meeting of the Washington State Horticultural Association, Wenatchee, WA, USA, 8–10 December 2000; pp. 176–183. [Google Scholar]
- Whalon, M.E.; Mota-Sanchez, D.; Hollingworth, R.M. Arthropods. Analysis of Global Pesticide Resistance; CABI Publishing, CAB International: Wallingford, UK, 2008; pp. 5–32. [Google Scholar]
- Benítez, H.A.; Lemic, D.; Püschel, T.A.; Viric Gasparic, H.; Kos, T.; Baric, B.; Bazok, R.; Pajac Zivkovic, I. Fluctuating asymmetry indicates levels of disturbance between agricultural productions: An example in Croatian population of Pterostichus melas melas (Coleptera: Carabidae). Zool. Anz. 2018, 276, 42–49. [Google Scholar] [CrossRef]
- Pajač Živković, I.; Lemić, D.; Mešić, A.; Barić, B.; Órdenes, R.; Benítez, A.H. Effect of fruit host on wing morphology in Drosophila suzukii (Diptera: Drosophilidae): A first view using geometric morphometrics. Entomol. Res. 2018, 48, 262–268. [Google Scholar] [CrossRef]
- Gerard, M.; Michez, D.; Debat, V.; Fullgrabe, L.; Meeus, I.; Piot, N.; Sculfort, O.; Vastrade, M.; Smagghe, G.; Vanderplanck, M. Stressful conditions reveal decrease in size, modification of shape but relatively stable asymmetry in bumblebee wings. Sci. Rep. 2018, 8, 15169. [Google Scholar] [CrossRef] [PubMed]
- Abbott, W.S. A method of computing the effectiveness of insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Insecticide Resistance and Its Implications for Potato Production in the UK. British Potato Council. Available online: http://www.potato.org.uk (accessed on 1 September 2018).
- Liu, N. Pyrethroid resistance in insects: Genes, mechanisms, and regulation. In Insecticides-Advances in Integrated Pest Management; Perveen, F., Ed.; InTech: Shanghai, China, 2012; pp. 457–468. [Google Scholar]
- DeVries, P.J.; Penz, C.M.; Hill, R.I. Vertical distribution, flight behavior and evolution of wing morphology in Morpho butterflies. J. Anim. Ecol. 2010, 79, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Mani, E.; Wildbolz, T. The dispersal of male codling moths (Laspeyresia pomonella L.) in the Upper Rhine Valley. J. Appl. Entomol. 2009, 83, 161–168. [Google Scholar] [CrossRef]






| df | SS | MS | Rsq | F | Z | Pr (>F)* | |
|---|---|---|---|---|---|---|---|
| Resistant Type | 2 | 0.01 | 0.007 | 0.04 | 55.74 | 56.63 | 0.001 ** |
| Sex | 1 | 0.007 | 0.007 | 0.02 | 54.18 | 43.41 | 0.001 ** |
| Residuals | 235 | 0.31 | 0.001 | 0.92 | |||
| Total | 238 | 0.33 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pajač Živković, I.; Benitez, H.A.; Barić, B.; Drmić, Z.; Kadoić Balaško, M.; Lemic, D.; Dominguez Davila, J.H.; Mikac, K.M.; Bažok, R. Codling Moth Wing Morphology Changes Due to Insecticide Resistance. Insects 2019, 10, 310. https://doi.org/10.3390/insects10100310
Pajač Živković I, Benitez HA, Barić B, Drmić Z, Kadoić Balaško M, Lemic D, Dominguez Davila JH, Mikac KM, Bažok R. Codling Moth Wing Morphology Changes Due to Insecticide Resistance. Insects. 2019; 10(10):310. https://doi.org/10.3390/insects10100310
Chicago/Turabian StylePajač Živković, Ivana, Hugo Alejandro Benitez, Božena Barić, Zrinka Drmić, Martina Kadoić Balaško, Darija Lemic, Jose H. Dominguez Davila, Katarina Maryann Mikac, and Renata Bažok. 2019. "Codling Moth Wing Morphology Changes Due to Insecticide Resistance" Insects 10, no. 10: 310. https://doi.org/10.3390/insects10100310
APA StylePajač Živković, I., Benitez, H. A., Barić, B., Drmić, Z., Kadoić Balaško, M., Lemic, D., Dominguez Davila, J. H., Mikac, K. M., & Bažok, R. (2019). Codling Moth Wing Morphology Changes Due to Insecticide Resistance. Insects, 10(10), 310. https://doi.org/10.3390/insects10100310










