Ecosystem Services, Global Diversity, and Rate of Stonefly Species Descriptions (Insecta: Plecoptera)
Abstract
1. Introduction
- Summarize existing ecosystem services of Plecoptera
- Summarize global and regional species richness across large geographic regions
- Examine the rate of species description globally and within large geographic regions
- Predict the number of new species described globally and for two fast-growing regions for the years 2050 and 2100.
2. Ecosystem Services Provided by Plecoptera
3. Compilation of Data on Global Diversity of Plecoptera
4. Global and Regional Species Richness
5. Distribution, Diversity, and Classification
6. Species Diversity
7. Rate of Species Description
8. Predicting Species Description in the 21st Century
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Béthoux, O.; Cui, Y.; Kondratieff, B.; Stark, B.; Ren, D. At last, a Pennsylvanian stem-stonefly (Plecoptera) discovered. BMC Evol. Biol. 2011, 11, 1–12. Available online: https://doi.org/10.1186/1471-2148-11-248 (accessed on 17 March 2019). [CrossRef] [PubMed][Green Version]
- DeWalt, R.E.; Kondratieff, B.C.; Sandberg, J.B. Order Plecoptera. In Ecology and General Biology: Thorp and Covich’s Freshwater Invertebrates; Thorp, J., Rogers, D.C., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 933–949. ISBN 9780123850263. [Google Scholar]
- Bybee, S.M.; Hansen, Q.; Bűsse, S.; Cahill, S.; Haley, M.; Branham, M.A. For consistency’s sake: The precise use of larva, nymph and naiad within Insecta. Syst. Ent. 2015, 40, 67–670. Available online: https://doi.org/10.1111/syen.12136 (accessed on 17 March 2019). [CrossRef]
- Miyasaka, H.; Genkai-Kato, M. Shift between carnivory and omnivory in stream stonefly predators. Ecol. Res. 2009, 24, 11–19. Available online: https://doi.org/10.1007/s11284-008-0475-3 (accessed on 17 March 2019). [CrossRef]
- Tierno de Figueroa, J.M.; Sánchez-Ortega, A. Imaginal feeding of certain Systellognathan stonefly species (Insecta: Plecoptera). Ann. Entomol. Soc. Am. 1999, 92, 218–221. Available online: https://doi.org/10.1093/aesa/92.2.218 (accessed on 17 March 2019). [CrossRef]
- Smith, B.J.; Collier, K.J. Interactions of adult stoneflies (Plecoptera) with riparian zones II. diet. Aquat. Insects 2000, 22, 285–296. Available online: https://doi.org/10.1076/0165-0424(200010)22:4;1-Y;FT285 (accessed on 17 March 2019). [CrossRef]
- McLellan, I.D. Antarctoperlaria (Insecta: Plecoptera). Fauna N.Z. 1993, 27, 1–70. [Google Scholar]
- Caires, A.M.; Chandra, S.; Nelson, C.R. Unique reproductive characteristics of Lake Tahoe’s Capnia lacustra (Plecoptera: Capniidae), a stonefly in decline. Freshw. Sci. 2016, 35, 1291–1299. Available online: https://doi.org/10.1086/688485 (accessed on 17 March 2019). [CrossRef]
- DeWalt, R.E.; Maehr, M.D.; Neu-Becker, U.; Stueber, G. Plecoptera Species File Online. Version 5.0/5.0. Available online: http://Plecoptera.SpeciesFile.org (accessed on 1 January 2019).
- Biodiversity Information System for Europe (BISE). Available online: https://biodiversity.europa.eu/ (accessed on 26 March 2019).
- Williams, D.; Williams, S. Aquatic insects and their potential to contribute to the diet of the globally expanding human population. Insects 2017, 8, 72. Available online: https://doi.org/10.3390/insects8030072 (accessed on 17 March 2019). [CrossRef]
- Stewart, K.W.; Stark, B.P. Nymphs of North American Stonefly Genera, 2nd ed.; Caddis Press: Columbus, OH, USA, 2002. [Google Scholar]
- Barbour, M.T.; Gerritsen, J.; Snyder, B.D.; Stribling, J.B. Rapid Bioassessment Protocols for Use in Streams and Wadeable Rivers: Periphyton, Benthic Macroinvertebrates and Fish, 2nd ed.; U.S. Environmental Protection Agency; Office of Water: Washington, DC, USA, 1999.
- Lenat, D.R. A biotic index for the southeastern United States: derivation and list of tolerance values, with criteria for assigning water quality ratings. J. N. Am. Benthol. Soc. 1993, 12, 279–290. Available online: https://doi.org/10.2307/1467463 (accessed on 17 March 2019). [CrossRef]
- DeWalt, R.E.; Favret, C.; Webb, D.W. Just how imperiled are aquatic insects? A case study of stoneflies (Plecoptera) in Illinois. Ann. Entomol. Soc. Am. 2005, 98, 941–950. Available online: http://doi.org/10.1603/0013-8746(2005)098%5B0941%3AJHIAAI%5D2.0.CO%3B2 (accessed on 17 March 2019). [CrossRef]
- Sánchez-Bayoa, F.; Wyckhuys, K.A.G. Worldwide decline of the entomofauna: A review of its drivers. Biol. Conserv. 2019, 232, 8–27. Available online: http://dx.doi.org/10.1016/j.biocon.2019.01.020 (accessed on 17 March 2019). [CrossRef]
- Global Biodiversity Information Facility. Available online: https://www.gbif.org (accessed on 17 March 2019).
- Kosterin, O.E.; Akimbekova, N.; Dubatolov, V.V.; Sivec, I. A stonefly species extinct in Europe (Taeniopteryx araneoides Klapalek, 1902, Taeniopterygidae, Plecoptera) is thriving in the Irtysh River in West Siberia and North Kazakhstan. Zootaxa 2017, 4247, 141. Available online: https://doi.org/10.11646/zootaxa.4247.2.5 (accessed on 17 March 2019). [CrossRef]
- Bojkova, J.; Komprdova, K.; Soldan, T.; Zahradkova, S. Species loss of stoneflies (Plecoptera) in the Czech Republic during the 20th century. Freshw. Biol. 2012, 57, 2550–2567. Available online: https://doi.org/10.1111/fwb.12027 (accessed on 17 March 2019). [CrossRef]
- Zwick, P. Stream habitat fragmentation–A threat to biodiversity. Biod. Cons. 1992, 1, 80–97. Available online: https://doi.org/10.1007/BF00731036 (accessed on 17 March 2019). [CrossRef]
- Soga, M.; Gaston, K.J. Shifting baseline syndrome: causes, consequences, and implications. Front. Ecol. Environ. 2018, 16, 222–230. Available online: https://doi.org/10.1002/fee.1794 (accessed on 17 March 2019). [CrossRef]
- Giersch, J.J.; Hotaling, S.; Kovach, R.P.; Jones, L.A.; Muhlfeld, C.C. Climate-induced glacier and snow loss imperils alpine stream insects. Glob. Chang. Biol. 2017, 23, 2577–2589. Available online: https://doi.org/10.1111/gcb.13565 (accessed on 17 March 2019). [CrossRef] [PubMed]
- Sheldon, A.L. Possible climate-induced shift of stoneflies in a southern Appalachian catchment. Freshw. Sci. 2012, 31, 765–774. Available online: https://doi.org/10.1899/11-135.1 (accessed on 17 March 2019). [CrossRef]
- Shah, D.N.; Domisch, S.; Pauls, S.U.; Haase, P.; Jähnig, S.C. Current and future latitudinal gradients in stream macroinvertebrate richness across North America. Freshw. Sci. 2014, 33, 1136–1147. Available online: https://doi.org/10.1086/678492 (accessed on 17 March 2019). [CrossRef]
- Lundquist, M.J.; Zhu, W. Aquatic insect functional diversity and nutrient content in urban streams in a medium-sized city. Ecosphere 2018, 9, e02284. Available online: https://doi.org/10.1002/ecs2.2284 (accessed on 17 March 2019). [CrossRef]
- Cuffney, T.F.; Wallace, J.B.; Webster, J.R. Pesticide manipulation of a headwater stream: Invertebrate responses and their significance for ecosystem processes. Freshw. Invertebr. Biol. 1984, 3, 153–171. Available online: http://doi.org/10.2307/1467120 (accessed on 17 March 2019). [CrossRef]
- Vannote, R.L.; Minshall, G.W.; Cummins, K.W.; Sedell, J.R.; Cushing, C.E. The river continuum concept. Can. J. Fish. Aquat. Sci. 1980, 37, 130–137. Available online: https://doi.org/10.1139/f80-017 (accessed on 17 March 2019). [CrossRef]
- Leiser, E.; Boyle, R.H. Stoneflies for the Angler; Knopf: New York, NY, USA, 1982. [Google Scholar]
- Claassen, P.W. A catalogue of the Plecoptera of the world. Mem. Cornell Univ. Ag. Exp. Sta. 1940, 232, 1–235. [Google Scholar]
- Illies, J. Katalog der rezenten Plecoptera. Das Tierreich—Eine Zusammenstellung und Kennzeichnung der rezenten Tierformen 1966, 82, 1–631. [Google Scholar]
- Zwick, P. Insecta: Plecoptera Phylogenetisches System und Katalog. Das Tierreich—Eine Zusammenstellung und Kennzeichnung der rezenten Tierformen 1973, 94, 1–465. [Google Scholar]
- Fochetti, R.; Tierno de Figueroa, J.M.; Hallan, J. World checklist of freshwater Plecoptera species. Available online: http://fada.biodiversity.be (accessed on 1 January 2019).
- Fochetti, R.; Tierno de Figueroa, J.M. Global diversity of stoneflies (Plecoptera; Insecta) in freshwater. Hydrobiologia 2008, 595, 365–377. Available online: https://doi.org/10.1007/s10750-007-9031-3 (accessed on 17 March 2019). [CrossRef]
- Species File Software: A Foundation for Taxonomic Database Development. Available online: http://software.speciesfile.org (accessed on 17 March 2019).
- International Commission on Zoological Nomenclature. Available online: http://iczn.org (accessed on 17 March 2019).
- Catalogue of Life. Available online: http://www.catalogueoflife.org (accessed on 1 January 2019).
- Brummitt, R.K.; Pando, F.; Hollis, S.; Brummitt, N.A. Plant Taxonomic Database Standards Ver. 2.2. World Geographical Scheme for Recording Plant Distributions. Available online: https://github.com/tdwg/wgsrpd (accessed on 31 March 2019).
- Taxonomic Databases Working Group. Available online: https://www.tdwg.org (accessed on 17 March 2019).
- Barcode of Life Data System. Available online: http://www.boldsystems.org (accessed on 17 March 2019).
- Encyclopedia of Life. Available online: https://eol.org (accessed on 17 March 2019).
- Illiesia, The International Journal for Stonefly Research. Available online: http://illiesia.speciesfile.org (accessed on 17 March 2019).
- McCulloch, G.A.; Wallis, G.P.; Waters, J.M. A time-calibrated phylogeny of southern hemisphere stoneflies: testing for Gondwanan origins. Mol. Phyl. Evol. 2016, 150–160. Available online: https://doi.org/10.1016/j.ympev.2015.10.028 (accessed on 17 March 2019). [CrossRef]
- Zwick, P. Notes on Madagascan Stoneflies (Plecoptera: Notonemouridae). Zootaxa 2015, 4059, 169–180. Available online: http://dx.doi.org/10.11646/zootaxa.4059.1.9 (accessed on 17 March 2019). [CrossRef]
- Yang, D.; Li, W.; Fang, Z. Plecoptera Nemouroidea; Fauna Sinica Insect, Chinese Academy of Science, Science Press: Beijing, China, 2014. [Google Scholar]
- Yang, D.; Li, W. Species Catalogue of China. Vol. 2. Animals, Insecta (III), Plecoptera; Science Press: Beijing, China, 2018. [Google Scholar]
- Zwick, P. (Schwarzer Stock 9, Schlitz, Germany). Personal communication, 2018.
- Wilson, S.P.; Costello, M.J. Predicting future discoveries of European marine species by using a non-homogeneous renewal process. J. Roy. Stat. Soc. Ser. C Appl. Stat. 2005, 54, 897–918. Available online: https://doi.org/10.1111/j.1467-9876.2005.00513.x (accessed on 17 March 2019). [CrossRef]
- Costello, M.J.; Wilson, S.P. Predicting the number of known and unknown species in the European seas using rates of description. Glob. Ecol. Biog. 2011, 20, 319–330. Available online: https://doi.org/10.1111/j.1466-8238.2010.00603.x (accessed on 17 March 2019). [CrossRef]
- Bebber, D.P.; Marriott, F.H.C.; Gaston, K.J.; Harris, S.A.; Scotland, R.W. Predicting unknown species numbers using discovery curves. Proc. R. Soc. B Biol. Sci. 2007, 274, 1651–1658. Available online: http://dx.doi.org/10.1098/rspb.2007.0464 (accessed on 17 March 2019). [CrossRef]
- Zhou, X.; Robinson, J.L.; Geraci, C.J.; Parker, C.R.; Flint, O.S.J.; Etnier, D.A.; Ruiter, D.; DeWalt, R.E.; Jacobus, L.M.; Herbert, P.D.N. Accelerated construction of a regional DNA-barcode reference library: Caddisflies (Trichoptera) in the Great Smoky Mountains National Park. J. N. Am. Benth. Soc. 2011, 30, 131–162. Available online: http://dx.doi.org/10.1899/10-010.1 (accessed on 17 March 2019). [CrossRef]
- Gattolliat, J.; Vincon, G.; Wyler, S.; Pawlowski, J.; Sartori, M. Toward a comprehensive COI DNA barcode library for Swiss stoneflies (Insecta: Plecoptera) with special emphasis on the genus Leuctra. Zoosymposia 2016, 11, 135–155. Available online: http://dx.doi.org/10.11646/zoosymposia.11.1.15 (accessed on 17 March 2019). [CrossRef]
- DeWalt, R.E. DNA barcoding: a taxonomic point of view. J. N. Am. Benth. Soc. 2011, 30, 174–181. Available online: http://dx.doi.org/10.1899/10-021.1 (accessed on 17 March 2019). [CrossRef]
- Costello, M.J.; May, R.M.; Stork, N.E. Can we name Earth’s species before they go extinct? Science 2013, 339, 413–416. Available online: http://dx.doi.org/10.1126/science.1230318 (accessed on 17 March 2019). [CrossRef] [PubMed]
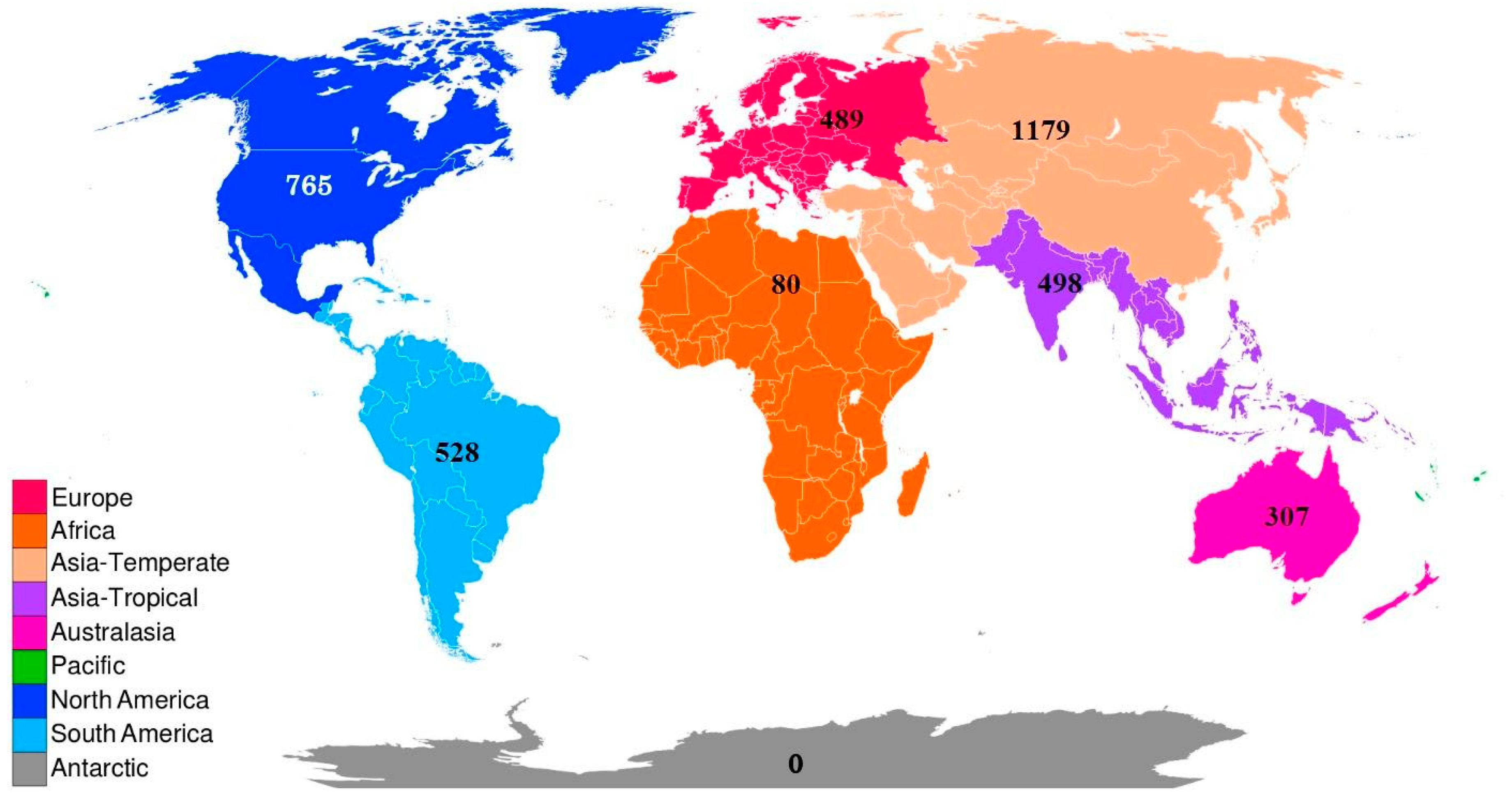
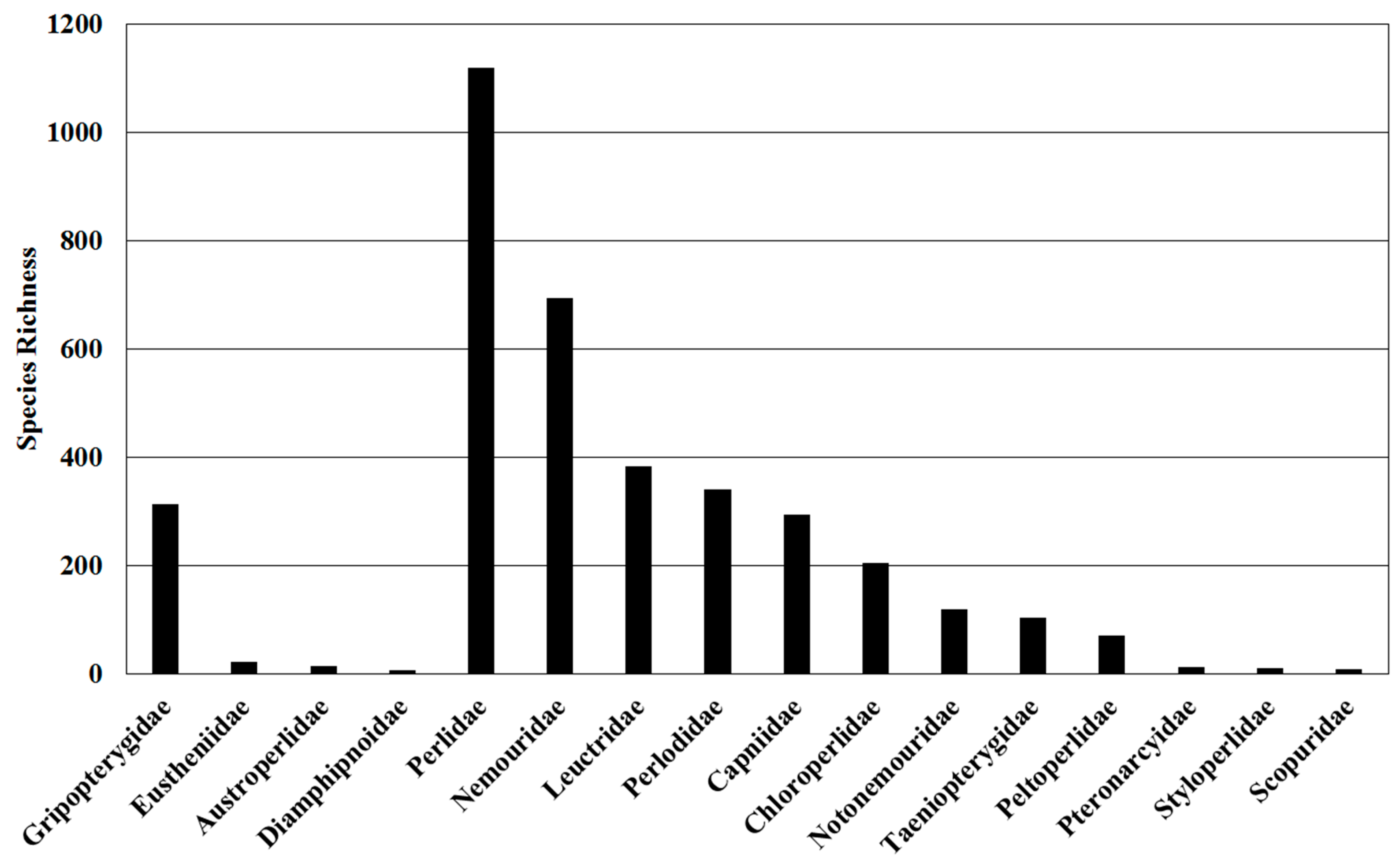
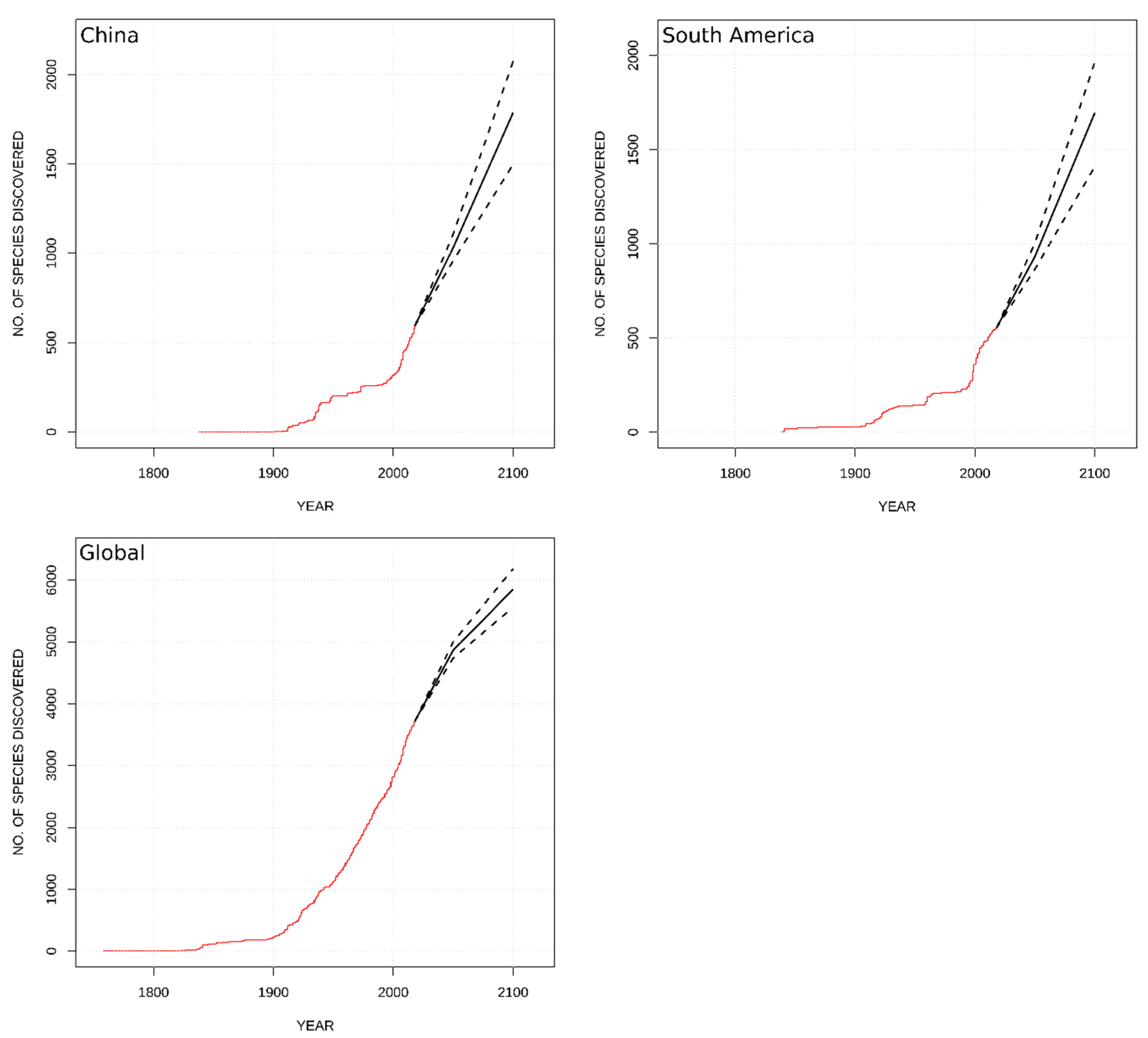
| Classification | Global | Europe | Africa | Asia- Temperate | Asia-Tropical | Austral-Asia | North America | South America |
|---|---|---|---|---|---|---|---|---|
| Antarctoperlaria | - | - | - | - | - | - | - | - |
| Eusthenioidea | - | - | - | - | - | - | - | - |
| Diamphipnoidae | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 6 |
| Eustheniidae | 22 | 0 | 0 | 0 | 0 | 20 | 0 | 2 |
| Gripopterygoidea | - | - | - | - | - | - | - | - |
| Austroperlidae | 15 | 0 | 0 | 0 | 0 | 11 | 0 | 4 |
| Gripopterygidae | 313 | 0 | 0 | 0 | 0 | 214 | 0 | 99 |
| Arctoperlaria | - | - | - | - | - | - | - | - |
| Euholognatha | - | - | - | - | - | - | - | - |
| Nemouroidea | - | - | - | - | - | - | - | - |
| Capniidae | 295 | 28 | 4 | 104 | 9 | 0 | 167 | 0 |
| Leuctridae | 384 | 144 | 9 | 148 | 28 | 0 | 64 | 0 |
| Nemouridae | 694 | 150 | 13 | 338 | 148 | 0 | 80 | 0 |
| Notonemouridae | 120 | 0 | 39 | 0 | 0 | 62 | 0 | 20 |
| Taeniopterygidae | 104 | 48 | 2 | 27 | 1 | 0 | 36 | 0 |
| Superfamily not assigned | ||||||||
| Scopuridae | 8 | 0 | 0 | 8 | 0 | 0 | 0 | 0 |
| Systellognatha | - | - | - | - | - | - | - | - |
| Perloidea | - | - | - | - | - | - | - | - |
| Chloroperlidae | 204 | 23 | 1 | 71 | 6 | 0 | 107 | 0 |
| Perlidae | 1120 | 20 | 10 | 330 | 276 | 0 | 125 | 397 |
| Perlodidae | 340 | 76 | 2 | 118 | 6 | 0 | 152 | 0 |
| Pteronarcyoidea | - | - | - | - | - | - | - | - |
| Peltoperlidae | 71 | 0 | 0 | 24 | 23 | 0 | 24 | 0 |
| Pteronarcyidae | 12 | 0 | 0 | 2 | 0 | 0 | 10 | 0 |
| Styloperlidae | 10 | 0 | 0 | 9 | 1 | 0 | 0 | 0 |
| 3718 | 489 | 80 | 1179 | 498 | 307 | 765 | 528 | |
| Area | Number Described | Rate of Description |
|---|---|---|
| Global | 1657 | 43.6 |
| Asia-Temperate | 519 | 13.7 |
| South America | 343 | 9.0 |
| China | 335 | 8.8 |
| Asia-Tropical | 278 | 7.3 |
| North America | 250 | 6.6 |
| Australasia | 173 | 4.6 |
| Europe | 119 | 3.1 |
| Africa | 17 | 0.4 |
| Location | Predicted Number of Species Discovered | |
|---|---|---|
| 2019–2050 | 2019–2100 | |
| Globally | 1140 (1010–1280) | 2130 (1840–2470) |
| China | 440 (360–420) | 1190 (900–1480) |
| South America | 370 (310–450) | 1140 (855–1400) |
| Worker | Affiliation | Active in Regions |
|---|---|---|
| Pablo Pessacq | Centro de Investigaciones Esquel de Montaña y Estepa Patagónicos, Argentina | Argentina |
| Julia H. Mynott | La Trobe University, Australia | Australasia |
| Gunther Theischinger | New South Wales Department Planning & Environment, Australia | Australasia |
| Lucas S. Lecci | Universidade de São Paulo, Brazil | Brazil |
| Marcos Carneiro Novaes | Universidade Estadual Paulista, Brazil | Brazil |
| Tacio Duarte | Universidade Federal da Bahia, Brazil | Brazil |
| Pitágoras da Conseição Bispo | Universidade de São Paulo, Brazil | Brazil |
| Leandro Silva Barbosa | Museu Nacional, Brazil | Brazil |
| Gutierrez-Fonseca | University of Puerto Rico Rio Piedras | Central America |
| Weihai Li | Henan Institute of Science and Technology, China | China, SE Asia |
| Yu-Zhou Du | Yangzhou University, China | China, SE Asia |
| Maria del Carmen Zúñiga | Universidad del Valle, Colombia | Columbia |
| Gilles Vinçon | Grenoble, France | Europe |
| Wolfram Graf | University of Natural Resources and Applied Life Sciences, Austria | Europe |
| Tierno de Figueroa | University of Granada, Spain | Europe |
| Romolo Fochetti | University of Viterbo, Italy | Europe |
| Bill P. Stark | Mississippi College, USA | Global |
| Dávid Murányi | Hungarian Natural History Museum, Budapest | Global |
| Satoko Hanada | Fukuoka City, Japan | Japan |
| Takao Shimizu | Aqua Restoration Research Center, Japan | Japan, Taiwan |
| Scott A. Grubbs | Western Kentucky University, USA | North America |
| Boris C. Kondratieff | Colorado State University, USA | North America |
| Richard W. Baumann | Brigham Young University, USA | North America |
| R. Edward DeWalt | University of Illinois, USA | North America |
| Luke W. Myers | State University of New York, USA | North America |
| Valentina Teslenko | Russian Academy of Sciences, Vladivostok | Russian |
| Claudio G. Froehlich | University of São Paulo, Brazil | South America |
| Fernanda Avelino-Capestrata | Faculdades São José, Brazil | South America |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
DeWalt, R.E.; Ower, G.D. Ecosystem Services, Global Diversity, and Rate of Stonefly Species Descriptions (Insecta: Plecoptera). Insects 2019, 10, 99. https://doi.org/10.3390/insects10040099
DeWalt RE, Ower GD. Ecosystem Services, Global Diversity, and Rate of Stonefly Species Descriptions (Insecta: Plecoptera). Insects. 2019; 10(4):99. https://doi.org/10.3390/insects10040099
Chicago/Turabian StyleDeWalt, R. Edward, and Geoffrey Donald Ower. 2019. "Ecosystem Services, Global Diversity, and Rate of Stonefly Species Descriptions (Insecta: Plecoptera)" Insects 10, no. 4: 99. https://doi.org/10.3390/insects10040099
APA StyleDeWalt, R. E., & Ower, G. D. (2019). Ecosystem Services, Global Diversity, and Rate of Stonefly Species Descriptions (Insecta: Plecoptera). Insects, 10(4), 99. https://doi.org/10.3390/insects10040099





