First Screening of Entomopathogenic Nematodes and Fungus as Biocontrol Agents against an Emerging Pest of Sugarcane, Cacosceles newmannii (Coleoptera: Cerambycidae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Source of Insects
2.2. Entomopathogenic Nematode
2.2.1. Experiment 1: EPN Virulence
2.2.2. Experiment 2: Nematode Penetration
2.2.3. Experiment 3: Nematode Development in Haemolymph
2.2.4. Experiment 4: Virulence of Mutualistic Bacteria
2.3. Experiment 5: Virulence of EPF
3. Results
3.1. Nematodes
3.1.1. Experiment 1: EPN Virulence
3.1.2. Experiment 2: Nematode Penetration
3.1.3. Experiment 3: Nematode Development in Haemolymph
3.1.4. Experiment 4: Virulence of Mutualistic Bacteria
3.2. Experiment 5: Virulence of EPF
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferreira, G.W.S. The Parandrinae and the Prioninae of Southern Africa (Cerambycidae, Coleoptera); Memoir nr 13, Memoirs van die Nasionale Museum, Posbus 266; National Museum: Bloemfontein, South Africa, 1980; p. 334. [Google Scholar]
- Way, M.J.; Conlong, D.E.; Rutherford, R.S.; Sweby, D.L.; Gillespie, D.Y.; Stranack, R.A.; Lagerwall, G.; Grobbelaar, E.; Perissinotto, R. Cacosceles (Zelogenes) newmannii (Thomson) (Cerambycidae:Prioninae), a new pest in the South African sugarcane industry. In Proceedings of the 90th Annual Congress of the South African Sugar Technologists Association, Durban, South Africa, 15–17 August 2017; Volume 90, pp. 62–65. [Google Scholar]
- Javal, M.; Thomas, S.; Barton, M.G.; Gillespie, D.; Conlong, D.E.; Terblanche, J.S. Understanding the recent invasion of Cacosceles newmannii (Coleoptera: Cerambycidae) into sugarcane from a thermal perspective. In Proceedings of the 91st Annual Congress of the South African Sugar Technologists Association, Durban, South Africa, 14–16 August 2018; Volume 91, pp. 66–69. [Google Scholar]
- Oyafuso, A.; Arakaki, N.; Sadoyama, Y.; Kishita, M.; Kawamura, F.; Ishimine, M.; Kinjo, M.; Hirai, Y. Life history of the white grub Dasylepida sp. (Coleoptera: Scarabaeidae), a new and severe pest on sugarcane on the Miyako Islands, Okinawa. Appl. Entomol. Zool. 2002, 37, 595–601. [Google Scholar] [CrossRef]
- Mukunthen, N.; Nirmala, R. New insect pests of sugarcane in India. SugarTech 2002, 4, 157–159. [Google Scholar] [CrossRef]
- Dolinski, C.; Del Valle, E.; Stuart, R.J. Virulence of entomopathogenic nematodes to larvae of the guava weevil, Conotrachelus psidii (Coleoptera: Curculionidae), in laboratory and greenhouse experiments. Biol. Control 2006, 38, 422–427. [Google Scholar] [CrossRef]
- Pliansinchai, U.; Jarnkoon, V.; Siengsri, S.; Kaenkong, C.; Pangma, S.; Weerathaworn, P. Ecology and destructive behaviour of cane boring grub (Dorysthenes buqueti Guerin) in North Eastern Thailand. In Proceedings of the XXVI Congress of the International Society of Sugar Cane Technologists, Durban, South Africa, 29 July–2 August 2007; pp. 863–867. [Google Scholar]
- Fravel, D.R. Commercialization and implementation of biocontrol. Annu. Rev. Phytopathol. 2005, 43, 337–359. [Google Scholar] [CrossRef] [PubMed]
- Lacey, L.A.; Frutos, R.; Kaya, H.K.; Vail, P. Insect pathogens as biological control agents: Do they have a future? Biol. Control 2001, 21, 230–248. [Google Scholar] [CrossRef]
- Bourguet, D.; Guillemaud, T. The hidden and external costs of pesticide use. Sustain. Agric. Rev. 2016, 19, 35–120. [Google Scholar] [CrossRef]
- Peters, A. The natural host range of Steinernema and Heterorhabditis spp. and their impact on insect populations. Biocontrol Sci. Technol. 1996, 6, 389–402. [Google Scholar] [CrossRef]
- Labaude, S.; Griffin, C.T. Transmission success of entomopathogenic nematodes used in pest control. Insects 2018, 9, 72. [Google Scholar] [CrossRef]
- Campos-Herrera, R. Nematode Pathogenesis of Insects and Other Pests: Ecology and Applied Technologies for Sustainable Plant and Crop Protection; Springer International Publishing: Basel, Switzerland, 2015; 531p. [Google Scholar]
- Poinar, G. Entomopathogenic nematodes in biological control. In Taxonomy and Biology of Steinernematidae and Heterorhabditidae; Gaugler, R., Kaya, H.K., Eds.; CRC Press: Boca Raton, FL, USA, 1990; pp. 23–74. [Google Scholar]
- Kaya, H.K.; Aguillera, M.M.; Alumai, A.; Choo, H.Y.; de la Torre, M.; Fodor, A.; Ganguly, S.; Hazır, S.; Lakatos, T.; Pye, A.; et al. Status of entomopathogenic nematodes and their symbiotic bacteria from selected countries or regions of the world. Biol. Control 2006, 38, 134–155. [Google Scholar] [CrossRef]
- Schroeder, W.J.; Beavers, J.B. Movement of the Entomogenous Nematodes of the Families Heterorhabditidae and Steinernematidae in Soil. J. Nematol. 1987, 19, 257–259. [Google Scholar] [PubMed]
- Poinar, G.O. Nematodes for Biological Control of Insects; CRC Press, Inc.: Boca Raton, FL, USA, 1979; 277p. [Google Scholar]
- Simões, N.; Rosa, J.S. Pathogenicity and host specificity of entomopathogenic nematodes. Biocontrol Sci. Technol. 1996, 6, 403–411. [Google Scholar] [CrossRef]
- Ehlers, R.-U. Mass production of entomopathogenic nematodes for plant protection. Appl. Microbiol. Biotechnol. 2001, 56, 623–633. [Google Scholar] [CrossRef]
- Suasa-ard, W.; Suksen, K.; Kernasa, O. Utilisation of the green muscardine, Metarhizium anisopliae, to controle the sugarcane longhorne stem borer Dorysthenes buqueti Guerin (Coleoptera: Cerambycidae). Int. Sugar J. 2012, 114, 37–40. [Google Scholar]
- Suasa-ard, W.; Sommartya, P.; Buchatian, P.; Puntongcum, A.; Chiangsin, R. Effect of Metarhizium anisopliae on infection of sugarcane stems borer, Dorysthenes buqueti Guerin (Coleoptera: Cerambycidae) in laboratory. In Proceedings of the 46th Kasetsart University Annual Conference, Kasetsart, Thailand, 29 January–1 February 2008; pp. 155–160. [Google Scholar]
- Jackson, M.A.; Jaronskib, S.T. Production of microsclerotia of the fungal entomopathogen Metarhizium anisopliae and their potential for use as a biocontrol agent for soil-inhabiting insects. Mycol. Res. 2009, 113, 842–850. [Google Scholar] [CrossRef]
- Hajek, A.E.; McManus, M.L.; Delalibera, I. A review of introductions of pathogens and nematodes for classical biological control of insects and mites. Biol. Control 2007, 41, 1–13. [Google Scholar] [CrossRef]
- Marannino, P.; Santiago-Álvarez, C.; de Lillo, E.; Quesada-Moraga, E. Evaluation of Metarhizium anisopliae (Metsch) Sorok. to target larvae and adults of Capnodis tenebrionis (L.) (Coleoptera: Buprestidae) in soil and fiber band applications. J. Invertebr. Pathol. 2008, 97, 237–244. [Google Scholar] [CrossRef]
- Shah, P.A.; Pell, J.K. Entomopathogenic fungi as biological control agents. Appl. Microbiol. Biotechnol. 2003, 61, 413–423. [Google Scholar] [CrossRef]
- Malan, A.P.; Ferreira, T. Entomopathogenic nematodes. In Nematology in South Africa: A View from the 21st Century; Fourie, H., Spaull, V.W., Jones, R.K., Daneel, M.S., De Waele, D., Eds.; Springer International: Cham, Switzerland, 2017; pp. 459–480. [Google Scholar]
- Ehlers, R.-U. Current and future use of nematodes in biocontrol: Practice and commercial aspects with regard to regulatory policy issues. Biocontrol Sci. Technol. 1996, 6, 303–316. [Google Scholar] [CrossRef]
- Hatting, J.L.; Moore, S.D.; Malan, A.P. Microbial control of phytophagous invertebrate pests in South Africa: Current status and future prospects. J. Invertebr. Pathol. 2018. [Google Scholar] [CrossRef]
- Ehler, L.E. Integrated pest management (IPM): Definition, historical development and implementation, and the other IPM. Pest Manag. Sci. 2006, 62, 787–789. [Google Scholar] [CrossRef]
- Van Zyl, C.; Malan, A.P. Cost-effective culturing of Galleria mellonella and Tenebrio molitor and nematode production in various hosts. Afr. Entomol. 2015, 23, 361–375. [Google Scholar] [CrossRef]
- Abaajeh, A.R.; Nchu, F. Isolation and pathogenicity of some South African entomopathogenic fungi (Ascomycota) against eggs and larvae of Cydia pomonella (Lepidoptera: Tortricidae). Biocontrol Sci. Technol. 2015, 25, 828–842. [Google Scholar] [CrossRef]
- Malan, A.P.; Nguyen, K.B.; Addison, M.F. Entomopathogenic nematodes (Steinernematidae and Heterorhabditidae) from the southwestern parts of South Africa. Afr. Plant Prot. 2006, 12, 65–69. [Google Scholar]
- Malan, A.P.; Knoetze, R.; Moore, S.D. Isolation and identification of entomopathogenic nematodes from citrus orchards and their biocontrol potential against false codling moth. J. Invertebr. Patholol. 2011, 108, 115–125. [Google Scholar] [CrossRef]
- Malan, A.P.; Knoetze, R.; Tiedt, L.R. Steinernema jeffreyense n. sp. (Rhabditida: Steinernematidae), new entomopathogenic nematode from South Africa. J. Helminthol. 2016, 90, 262–278. [Google Scholar] [CrossRef]
- Poinar, G.O., Jr.; Karunakar, G.K.; David, H. Heterorhabditis indicus n. sp. (Rhabditida: Nematoda) fom India: Separation of Heterorhabditis spp. by infective juveniles. Fundam. Appl. Nematol. 1992, 15, 467–472. [Google Scholar]
- Glazer, I.; Lewis, E.E. Bioassays for entomopathogenic nematodes. In Bioassays of Entomopathogenic Microbes and Nematodes; Navon, A., Ascher, K.R.S., Eds.; CABI Publishing: Wallingford, UK, 2000; pp. 229–248. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Dreyer, J.; Malan, A.P.; Dicks, L.M.T. Three novel Xenorhabdus–Steinernema associations and evidence of strains of X. khoisanae switching between different clades. Curr. Microbiol. 2017, 74, 938–942. [Google Scholar] [CrossRef]
- Ferreira, T.; Malan, A.P. In vitro Liquid Culture of a South African Isolate of Heterorhabditis zealandica for the Control of Insect Pests. Afr. Entomol. 2014, 22, 80–92. [Google Scholar] [CrossRef]
- Ekesi, S.; Chabi-Olaye, A.; Subramanian, S.; Borgemeister, C. Horticultural pest management and the African economy: Successes, challenges and opportunities in a changing global environment. Acta Hortic. 2011, 911, 165–183. [Google Scholar] [CrossRef]
- Brunner-Mendoza, C.; Del Rocío Reyes-Montes, M.; Moonjely, S.; Bidochka, M.J.; Toriello, C. A review on the genus Metarhizium as an entomopathogenic microbial biocontrol agent with emphasis on its use and utility in Mexico. Biocontrol Sci. Technol. 2019, 29, 83–102. [Google Scholar] [CrossRef]
- Mathulwe, L.L. Control of the Woolly Apple Aphid, Eriosoma lanigerum (Hausmann) (Hemiptera: Aphididae), Using Entomopathogenic Fungi. Master’s Thesis, University of Stellenbosch, Stellenbosch, South Africa, 2019. [Google Scholar]
- Malan, A.P.; Hatting, J. Entomopathogenic Nematode Exploitation: Case Studies in Laboratory and Field Applications from South Africa. In Sustainability in Plant and Crop Protection: Ecology and Applied Technologies for Sustainable Plant and Crop Protection; Campos-Herrera, R., Ed.; Springer: Cham, Switzerland, 2015; pp. 475–506. [Google Scholar]
- Narayanan, K. Insect defence: Its impact on microbial control of insect pests. Curr. Sci. 2006, 86, 800–814. [Google Scholar]
- Baiocchi, T.; Lee, G.; Chloe, D.-H.; Dillman, A.R. Host seeking parasitic nematodes use specific odors to assess host resources. Sci. Rep. 2017, 7, 6270. [Google Scholar] [CrossRef]
- Banu, J.G.; Gannayane, I.; Meena, K.S. Entomopathogenic nematodes: General biology and behaviour. In Biocontrol Agents Entomopathogenic and Slug Parasitic Nematodes; Abd-Elgawad, M., Askary, T.H., Coupland, J., Eds.; CABI Publishing: Wallingford, UK, 2017; pp. 63–87. [Google Scholar]
- Lomer, C.J.; Bateman, R.P.; Johnson, D.L.; Langewald, J.; Thomas, M. Biological control of locusts and grasshoppers. Annu. Rev. Entomol. 2001, 46, 667–702. [Google Scholar] [CrossRef]
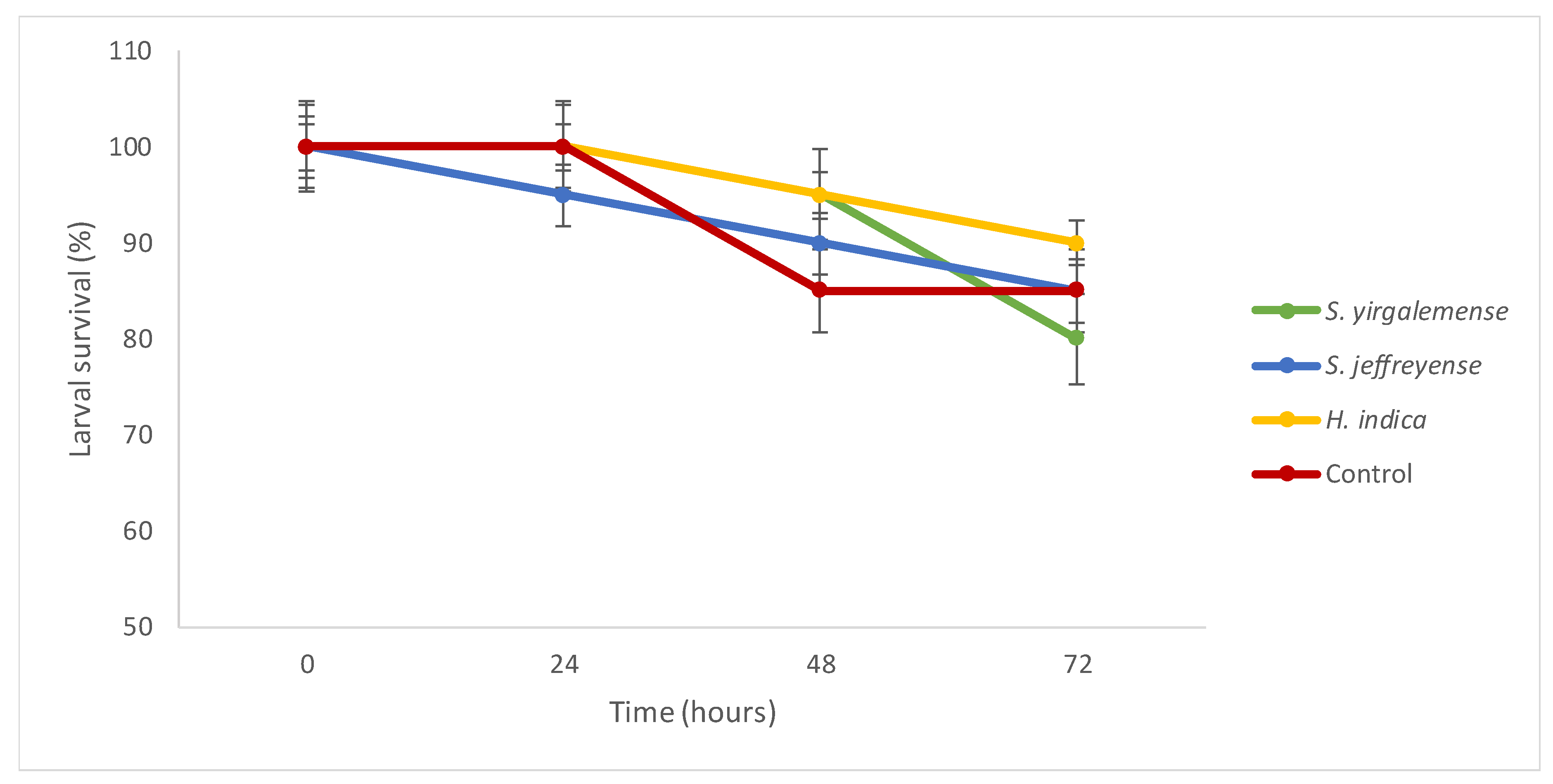
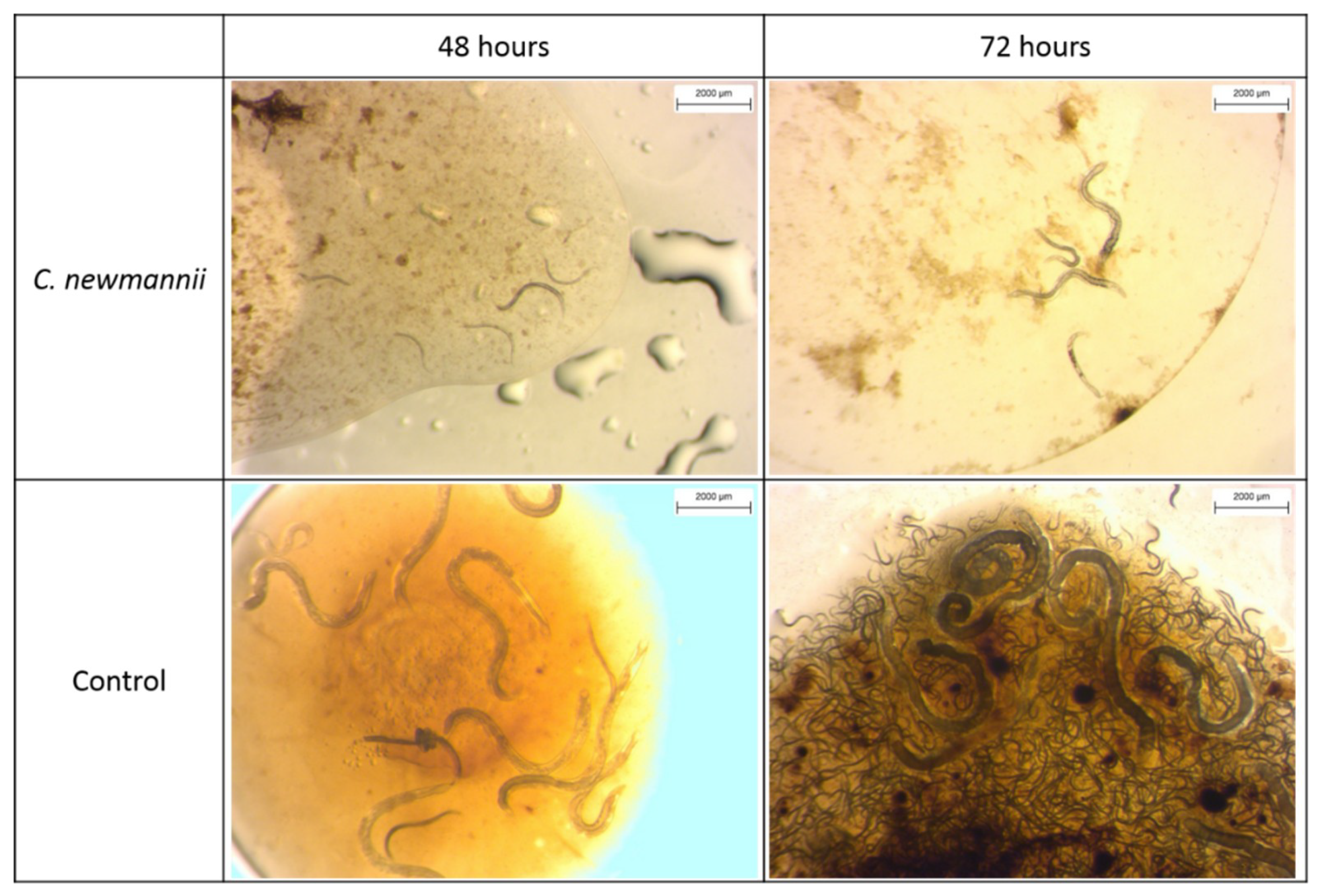
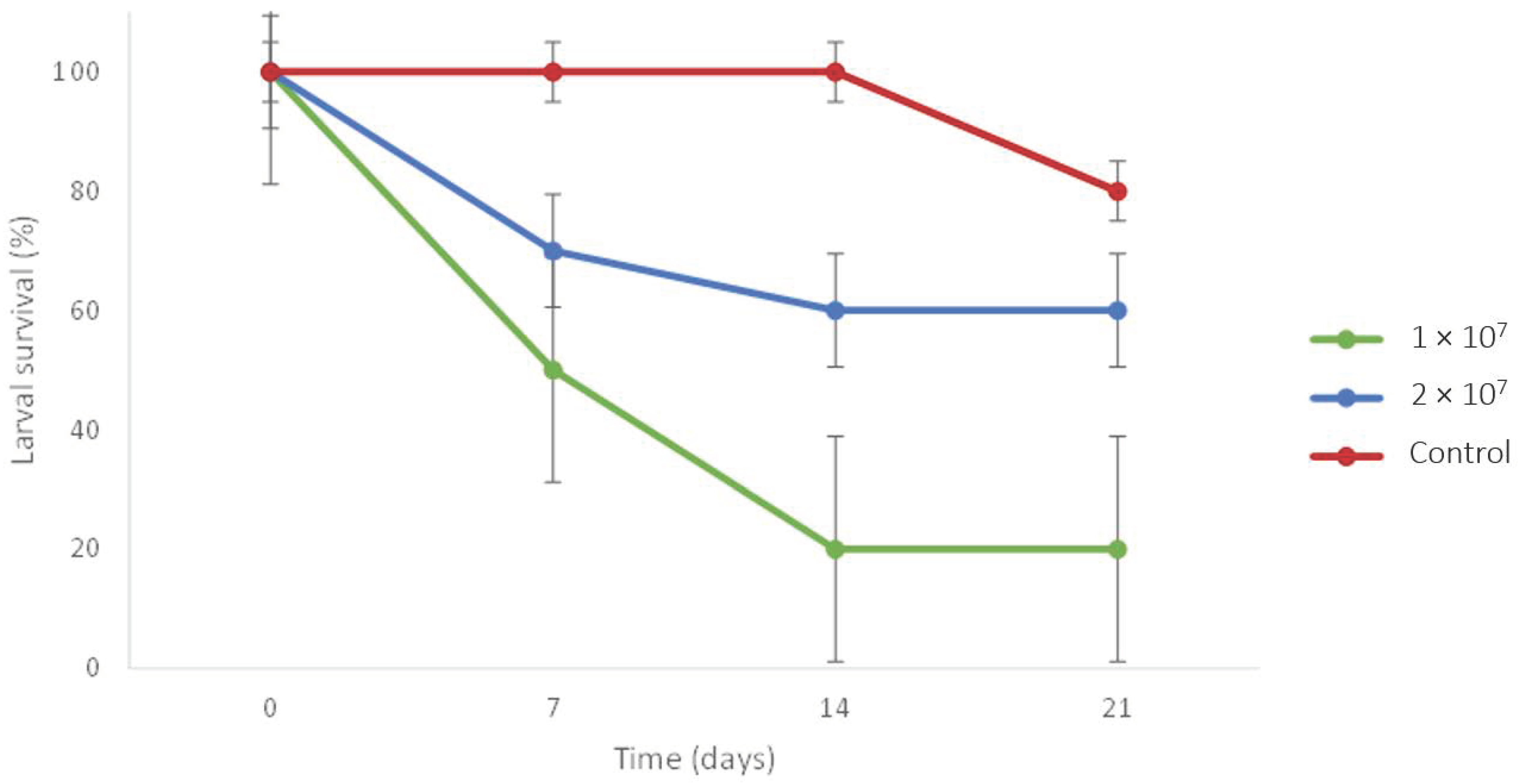
| Experiment/Trial | Treatment | Specimen Number/Weight (g) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||
| Experiment 1 trial 1 | S. yirgalemense | 0.5092 | 0.6696 | 1.1424 | 0.5743 | 1.0989 | 0.133 | 1.9325 | 0.9046 | 1.1363 | 0.1565 |
| S. jeffreyense | 1.5117 | 1.1034 | 1.1784 | 0.718 | 0.4332 | 1.5098 | 0.4571 | 0.3876 | 0.219 | 0.8709 | |
| H. indica | 1.1406 | 1.2646 | 0.4083 | 0.2439 | 0.8227 | 0.3296 | 1.8647 | 1.0868 | 0.9111 | 2.0262 | |
| Control | 0.6808 | 1.5231 | 1.1688 | 0.2032 | 0.7458 | 0.2797 | 0.2515 | 0.516 | 1.401 | 0.8454 | |
| Experiment 1 trial 2 | S. yirgalemense | 2.2417 | 1.8483 | 1.4638 | 1.2731 | 1.2127 | 1.4574 | 1.5666 | 1.2842 | 2.0365 | 1.1792 |
| S. jeffreyense | 1.0811 | 1.2079 | 1.0275 | 1.9138 | 0.7602 | 1.0263 | 1.754 | 1.8591 | 0.1342 | 1.1917 | |
| H. indica | 0.9668 | 0.5257 | 1.1613 | 1.0642 | 0.4061 | 1.1529 | 1.1435 | 0.2527 | 0.6853 | 0.4843 | |
| Control | 0.9822 | 0.8035 | 1.0267 | 1.7146 | 0.6135 | 0.5404 | 0.6147 | 0.5446 | 3.4241 | 1.7076 | |
| Experiment 2 | S. yirgalemense | 0.9743 | 1.5439 | 1.5915 | 1.3206 | 1.6647 | |||||
| S. jeffreyense | 1.5141 | 0.7764 | 1.3605 | 1.3751 | 1.1906 | ||||||
| H. indica | 1.4426 | 0.9689 | 1.6716 | 0.8531 | 1.6249 | ||||||
| Control | 1.5206 | 1.3141 | 1.6471 | 0.8701 | 1.3683 | ||||||
| Experiment 4 | S. jeffreyense | 1.9546 | 1.3257 | 1.1025 | 1.3365 | 1.4679 | 0.9835 | 1.2546 | 0.8796 | 1.0257 | |
| control | 1.163 | 1.2869 | 0.8889 | 0.7858 | 2.6022 | ||||||
| Experiment 5 | 1 × 107 | 1.4064 | 1.4323 | 0.3762 | 1.9986 | 0.568 | 0.8674 | 0.6631 | 1.0008 | 0.5686 | 1.8468 |
| 2 × 107 | 1.9988 | 1.4221 | 1.4567 | 2.4397 | 1.0324 | 0.9704 | 0.3289 | 0.8421 | 1.3158 | 0.8968 | |
| Control | 1.778 | 2.2929 | 1.1989 | 1.1747 | 5.8577 | ||||||
| Species Name | Strain | Habitat | Locality | GenBank Accession Number | Length of IJ (µm) | Body Width of IJ (µm) | Reference |
|---|---|---|---|---|---|---|---|
| S. yirgalemense | 157-C | Citrus orchard | Friedenheim, Mpumalanga | EU625295 | 685 (570–740) | 29 (24–33) | [33] |
| S. jeffreyense | J194 | Guava tree | Jeffrey’s Bay, Eastern Cape | KC897093 | 924 (784–1043) | 35 (23–43) | [34] |
| H. indica | SGS | Grapevine | Bonnievale, Western Cape | GQ377411 | 528 (479–573) | 20 (19–22) | [35] |
| Specimen Number | 1 | 2 | 3 | 4 | 5 | Penetration Value |
|---|---|---|---|---|---|---|
| Steinernema yirgalemense | 8 | 17 | 25 | 4 | 11 | 1.3 |
| Steinernema jeffreyense | 10 | 5 | 13 | 10 | 4 | 4.2 |
| Heterorhabditis indica | 14 | 25 | 28 | 21 | 9 | 9.7 |
| 48 h | 72 h | 96 h | 120 h | ||||||
| Galleria mellonella | Females | Females with Eggs | Males | Infective Juveniles | Dead | Total Inoculated | Status | Status | Status |
| 1 | 7 | 6 | 7 | 0 | 0 | 14 | P | P | P |
| 2 | 2 | 1 | 6 | 2 | 0 | 10 | E | P | P |
| 3 | 3 | 2 | 1 | 2 | 1 | 7 | E | P | P |
| 4 | 10 | 10 | 3 | 1 | 0 | 14 | P | P | P |
| 5 | 2 | 2 | 2 | 0 | 4 | 8 | P | P | P |
| 6 | 1 | 0 | 4 | 0 | 3 | 8 | - | E | P |
| 7 | 2 | 2 | 5 | 1 | 0 | 8 | E | P | P |
| 8 | 7 | 7 | 4 | 0 | 0 | 11 | P | P | P |
| 9 | 6 | 1 | 2 | 0 | 0 | 8 | E | P | P |
| 10 | 1 | 0 | 5 | 4 | 0 | 10 | E | P | P |
| 48 h | 72h | 96h | 120h | ||||||
| Cacosceles newmannii | Females | Females with Eggs | Males | Infective Juveniles | Dead | Total Inoculated | Status | Status | Status |
| 1 | 0 | 0 | 1 | 9 | 0 | 10 | - | - | - |
| 2 | 0 | 0 | 6 | 3 | 0 | 9 | - | - | - |
| 3 | 4 | 0 | 5 | 4 | 0 | 13 | - | - | - |
| 4 | 0 | 0 | 2 | 5 | 0 | 7 | - | - | - |
| 5 | 4 | 0 | 2 | 2 | 0 | 8 | E | E | P* |
| 6 | 1 | 0 | 3 | 5 | 0 | 9 | - | - | - |
| 7 | 1 | 0 | 6 | 1 | 0 | 8 | E | E | E |
| 8 | 2 | 0 | 2 | 0 | 3 | 7 | - | P* | P* |
| 9 | 1 | 0 | 2 | 7 | 0 | 10 | E | P* | P* |
| 10 | 2 | 0 | 5 | 1 | 0 | 8 | - | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Javal, M.; Terblanche, J.S.; Conlong, D.E.; Malan, A.P. First Screening of Entomopathogenic Nematodes and Fungus as Biocontrol Agents against an Emerging Pest of Sugarcane, Cacosceles newmannii (Coleoptera: Cerambycidae). Insects 2019, 10, 117. https://doi.org/10.3390/insects10040117
Javal M, Terblanche JS, Conlong DE, Malan AP. First Screening of Entomopathogenic Nematodes and Fungus as Biocontrol Agents against an Emerging Pest of Sugarcane, Cacosceles newmannii (Coleoptera: Cerambycidae). Insects. 2019; 10(4):117. https://doi.org/10.3390/insects10040117
Chicago/Turabian StyleJaval, Marion, John S. Terblanche, Desmond E. Conlong, and Antoinette P. Malan. 2019. "First Screening of Entomopathogenic Nematodes and Fungus as Biocontrol Agents against an Emerging Pest of Sugarcane, Cacosceles newmannii (Coleoptera: Cerambycidae)" Insects 10, no. 4: 117. https://doi.org/10.3390/insects10040117
APA StyleJaval, M., Terblanche, J. S., Conlong, D. E., & Malan, A. P. (2019). First Screening of Entomopathogenic Nematodes and Fungus as Biocontrol Agents against an Emerging Pest of Sugarcane, Cacosceles newmannii (Coleoptera: Cerambycidae). Insects, 10(4), 117. https://doi.org/10.3390/insects10040117





