Spatial Vision and Visually Guided Behavior in Apidae
Abstract
1. Introduction
2. Visual Fields, Sensitivity, and Resolution of the Apposition Compound Eyes of Bees
2.1. Sensitivity
2.2. Resolution
2.3. Sensitivity, Resolution and Visual Field Size Depend on Body Size
2.4. Sexual Dimorphism of Eyes
3. Ocelli and Their Function
4. Visually Guided Behaviors in Bees
4.1. Nest Defense
4.2. Use of Landmarks for Navigation and Homing
4.3. Foraging: Flower Detection
4.4. Flight Ranges and Flight Control
4.5. Male Mating Flights: Detection of Females
5. Light Intensity and the Sensitivity of the Eyes
6. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Michener, C.D. The Bees of the World; The Johns Hopkins University Press: Baltimore, MD, USA; Londres, Argentina, 2007. [Google Scholar]
- Ascher, J.S.; Pickering, J. Discover Life Bee Species Guide and World Checklist (Hymenoptera: Apoidea: Anthophila). Available online: http://www.discoverlife.org/mp/20q?guide=Apoidea_species (accessed on 29 October 2019).
- Chittka, L.; Vorobyev, M.; Shmida, A.; Menzel, M. Bee colour vision—The optimal system for the discrimination of flower colours with three types of photoreceptor types? In Sensory Systems of Arthropods; Wiese, K., Gribakin, F.G., Popov, A.V., Renninger, G., Eds.; Birkhäuser: Basel, Switzerland, 1993; pp. 211–218. [Google Scholar]
- Hempel de Ibarra, N.; Vorobyev, M.R.; Menzel, R. Mechanisms, functions and ecology of colour vision in the honeybee. J. Comp. Physiol. B 2014, 200, 411–433. [Google Scholar] [CrossRef] [PubMed]
- Ribi, W.A.; Warrant, E.J.; Zeil, J. The organization of honeybee ocelli: Regional specialization and rhabdom arrangements. Arthropod Struct. Dev. 2011, 40, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Ribi, W.A.; Zeil, J. Diversity and common themes in the organization of ocelli in Hymenoptera, Odonata and Diptera. J. Comp. Physiol. A 2018, 204, 505–517. [Google Scholar] [CrossRef]
- Zeil, J.; Ribi, W.; Narendra, A. Polarisation vision in ants, bees and wasps. In Polarized Light and Polarization Vision in Animal Sciences; Horvath, G., Ed.; Springer: Heidelberg, Germany, 2014; pp. 41–60. [Google Scholar]
- Land, M.F. Visual acuity in insects. Annu. Rev. Entomol. 1997, 42, 147–177. [Google Scholar] [CrossRef]
- Mizunami, M. Functional diversity of neural organization in insect ocellar systems. Vis. Res. 1995, 35, 443–452. [Google Scholar] [CrossRef]
- Ribi, W.A.; Engels, E.; Engels, W. Sex and caste specific eye structures in stingless bees and honey bees (Hymenoptera: Trigonidae, Apidae). Entomol. Gener. 1989, 14, 233–242. [Google Scholar] [CrossRef]
- Menzel, J.G.; Wunderer, H.; Stavenga, D.G. Functional morphology of the divided compound eye of the honeybee drone (Apis mellifera). Tissue Cell Res. 1991, 23, 525–535. [Google Scholar] [CrossRef]
- Streinzer, M.; Brockmann, A.; Nagaraja, N.; Spaethe, J. Sex and caste-specific variation in compound eye morphology of five honeybee species. PLoS ONE 2013, 8, e57702. [Google Scholar] [CrossRef]
- Streinzer, M.; Spaethe, J. Functional morphology of the visual system and mating strategies in bumblebees (Hymenoptera, Apidae, Bombus). Zool. J. Linn. Soc. 2014, 170, 735–747. [Google Scholar] [CrossRef]
- Somanathan, H.; Borges, R.M.; Warrant, E.J.; Kelber, A. Visual adaptations for mate detection in the male carpenter bee Xylocopa tenuiscapa. PLoS ONE 2017, 12, e0168452. [Google Scholar] [CrossRef] [PubMed]
- Wilby, D.; Aarts, T.; Tichit, P.; Bodey, A.; Rau, C.; Taylor, G.; Baird, E. Using micro-CT techniques to explore the role of sex and hair in the functional morphology of bumblebee (Bombus terrestris) ocelli. Vis. Res. 2019, 158, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Baumgärtner, H. Der Formensinn und die Sehschärfe der Bienen. Z. vgl. Physiol. 1928, 7, 56–143. [Google Scholar] [CrossRef]
- Rigosi, E.; Wiederman, S.D.; O’Carroll, D.C. Visual acuity of the honey bee retina and the limits for feature detection. Sci. Rep. 2017, 7, 45972. [Google Scholar] [CrossRef] [PubMed]
- Somanathan, H.; Warrant, E.J.; Borges, R.M.; Wallén, R.; Kelber, A. Resolution and sensitivity of the eyes of the Asian honeybees Apis florea, Apis cerana and Apis dorsata. J. Exp. Biol. 2009, 212, 2448–2453. [Google Scholar] [CrossRef] [PubMed]
- Spaethe, J.; Chittka, L. Interindividual variation of eye optics and single object resolution in bumblebees. J. Exp. Biol. 2003, 206, 3447–3453. [Google Scholar] [CrossRef] [PubMed]
- Dyer, A.G.; Streinzer, M.; Garcia, J. Flower detection and acuity of the Australian native stingless bee Tetragonula carbonaria Sm. J. Comp. Physiol. A 2016, 202, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Somanathan, H.; Kelber, A.; Borges, R.M.; Wallén, R.; Warrant, E.J. Visual ecology of Indian carpenter bees II: Adaptations of eyes and ocelli to nocturnal and diurnal lifestyles. J. Comp. Physiol. A 2009, 195, 571–583. [Google Scholar] [CrossRef]
- Greiner, B.; Ribi, W.A.; Wcislo, W.T.; Warrant, E.J. Neural organisation in the first optic ganglion of the nocturnal bee Megalopta genalis. Cell Tiss. Res. 2004, 318, 429–437. [Google Scholar] [CrossRef]
- Warrant, E.J.; Nilsson, D.-E. Absorption of white light in photoreceptors. Vis. Res. 1998, 38, 195–207. [Google Scholar] [CrossRef]
- Warrant, E.; Porombka, T.; Kirchner, W.H. Neural image enhancement allows honeybees to see at night. Proc. R. Soc. B 1996, 263, 1521–1526. [Google Scholar] [CrossRef]
- Seidl, R. Die Sehfelder und Ommatidien-Divergenzwinkel der drei Kasten der Honigbiene. Verh. Dtsch. Zool. Ges. 1980, 367. [Google Scholar]
- Taylor, G.J.; Tichit, P.; Schmidt, M.D.; Bodey, A.J.; Rau, C.; Baird, E. Bumblebee visual allometry results in locally improved resolution and globally improved sensitivity. eLife 2019, 8, e40613. [Google Scholar] [CrossRef] [PubMed]
- Stavenga, D.G. Pseudopupils of compound eyes. In Handbook of Sensory Physiology; Autrum, H., Ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1979; Volume VII/6A, pp. 357–439. [Google Scholar]
- Giurfa, M.; Vorobyev, M.; Kevan, P.; Menzel, R. Detection of coloured stimuli by honeybees: Minimum visual angles and receptor specific contrasts. J. Comp. Physiol. A 1996, 178, 699–709. [Google Scholar] [CrossRef]
- Theobald, J.C.; Greiner, B.; Wcislo, W.T.; Warrant, E.J. Visual summation in night-flying sweat bees: A theoretical study. Vision Res. 2006, 46, 2298–2309. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Warrant, E.J. Seeing in the dark: Vision and visual behaviour in nocturnal bees and wasps. J. Exp. Biol. 2008, 22, 1737–1746. [Google Scholar] [CrossRef]
- Kelber, A.; Warrant, E.J.; Pfaff, M.; Wallén, R.; Theobald, J.C.; Wcislo, W.; Raguso, R. Light intensity limits foraging activity in nocturnal and crepuscular bees. Behav. Ecol. 2006, 17, 63–72. [Google Scholar] [CrossRef]
- Kapustjanskij, A.; Streinzer, M.; Paulus, H.F.; Spaethe, J. Bigger is better: Implications of body size for flight ability under different light conditions and the evolution of alloethism in bumblebees. Funct. Ecol. 2007, 21, 1130–1136. [Google Scholar] [CrossRef]
- Streinzer, M.; Huber, W.; Spaethe, J. Body size limits dim-light foraging activity in stingless bees (Apidae: Meliponini). J. Comp. Physiol. A 2016, 202, 643–655. [Google Scholar] [CrossRef]
- Roulston, T.H.; Cane, J.H. The effect of diet breadth and nesting ecology on body size variation in bees (Apiformes). J. Kans. Entomol. Soc. 2000, 73, 129–142. [Google Scholar]
- Seidl, R.; Kaiser, W. Visual field size, binocular domain and the ommatidial array of the compound eyes in worker honey bees. J. Comp. Physiol. A 1981, 143, 17–26. [Google Scholar] [CrossRef]
- Leys, R.; Hogendoorn, K. Correlated evolution of mating behaviour and morphology in large carpenter bees (Xylocopa). Apidologie 2008, 39, 119–132. [Google Scholar] [CrossRef][Green Version]
- Schricker, B. Die Orientierung der Biene in der Dämmerung, zugleich ein Beitrag zur Frage der Ocellenfunktion bei Bienen. Z. Vgl. Physiol. 1965, 49, 420–458. [Google Scholar] [CrossRef]
- Wellington, W.G. Bumblebee ocelli and navigation at dusk. Science 1974, 183, 550–551. [Google Scholar] [CrossRef]
- Hung, Y.-S.; Ibbotson, M.R. Ocellar structure and neural innervation in the honeybee. Front. Neuroanat. 2014, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Ribi, W.; Zeil, J.; Hemmi, J.M. Regional differences in the preferred e-vector orientation of honeybee ocellar photoreceptors. J. Exp. Biol. 2017, 220, 1701–1708. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G.J.; Ribi, W.; Bech, M.; Bodey, A.J.; Rau, C.; Steuwer, A.; Warrant, E.J.; Baird, E. The dual function of orchid bee ocelli as revealed by X-ray microtomography. Curr. Biol. 2016, 26, 1319–1324. [Google Scholar] [CrossRef]
- Möller, R. Insects could exploit UV-green contrast for landmark navigation. J. Theor. Biol. 2002, 214, 619–631. [Google Scholar] [CrossRef]
- Berry, R.P.; Wcislo, W.T.; Warrant, E.J. Ocellar adaptations for dim light vision in a nocturnal bee. J. Exp. Biol. 2011, 214, 1283–1293. [Google Scholar] [CrossRef]
- Kerfoot, W.B. Correlation between ocellar size and foraging activities of bees (Hymenoptera; Apoidea). Am. Nat. 1967, 101, 65–70. [Google Scholar] [CrossRef]
- Kelber, A.; Zeil, J. A robust procedure for visual stabilisation of hovering flight position in guard bees of Trigona (Tetragonisca) angustula (Apidae: Meliponini). J. Comp. Physiol. A 1990, 167, 569–577. [Google Scholar] [CrossRef]
- Kelber, A.; Zeil, J. Tetragonisca guard bees take expanding and contracting patterns as unintended displacement in space. J. Comp. Physiol. A 1997, 181, 257–265. [Google Scholar] [CrossRef]
- Wittmann, D.; Radtke, R.; Zeil, J.; Lübke, G.; Francke, W. Robber bees (Lestrimelitta limao) and their host chemical and visual cues in nest defense by Trigona (Tetragonisca) angustula (Apidae: Meliponinae). J. Chem. Ecol. 1990, 16, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Koeniger, N.; Kurze, C.; Phiancharoen, M.; Koeniger, G. “Up” or “down” that makes the difference. How giant honeybees (Apis dorsata) see the world. PLoS ONE 2017, 12, e0185325. [Google Scholar] [CrossRef] [PubMed]
- Wagner, W. Psychobiologische Untersuchungen an Hummeln. Zoologica 1907, 19, 1–239. [Google Scholar]
- Becker, L. Untersuchungen über das Heimfindevermögen der Bienen. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 1958, 41, 1–25. [Google Scholar]
- Vollbehr, J. Zur Orientierung junger Honigbienen bei ihrem ersten Orientierungsflug. Zool. Jb. Allg. Zool. Physiol. 1975, 79, 33–69. [Google Scholar]
- Lehrer, M. Bees which turn back and look. Naturwissenschaften 1991, 78, 274–276. [Google Scholar] [CrossRef]
- Capaldi, E.A.; Smith, A.D.; Osborne, J.L.; Fahrbach, S.E.; Farris, S.M.; Reynolds, D.R.; Edwards, A.S.; Martin, A.; Robinson, G.E.; Poppy, G.M.; et al. Ontogeny of orientation flight in the honeybee revealed by harmonic radar. Nature 2000, 403, 537–540. [Google Scholar] [CrossRef]
- Robert, T.; Frasnelli, E.; Hempel de Ibarra, N.; Collett, T.S. Variations on a theme: Bumblebee learning flights from the nest and from flowers. J. Exp. Biol. 2018, 221, jeb172601. [Google Scholar] [CrossRef]
- Zeil, J.; Kelber, A.; Voss, R. Structure and function of learning flights in ground-nesting bees and wasps. J. Exp. Biol. 1996, 199, 245–252. [Google Scholar]
- Philippides, A.; Hempel de Ibarra, N.; Riabinina, O.; Collett, T.S. Bumblebee calligraphy: The design and control of flight motifs in the learning and return flights of Bombus terrestris. J. Exp. Biol. 2013, 216, 1093–1104. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Robert, T.; Frasnelli, E.; Collett, T.S.; Hempel de Ibarra, N. Male bumblebees perform learning flights on leaving a flower but not when leaving their nest. J. Exp. Biol. 2017, 220, 930–937. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, M.V.; Lehrer, M. Spatial acuity of honeybee vision and its spectral properties. J. Comp. Physiol. A 1988, 162, 159–172. [Google Scholar] [CrossRef]
- Lehrer, M.; Bischof, S. Detection of model flowers by honeybees: The role of chromatic and achromatic contrast. Naturwissenschaften 1995, 82, 145–147. [Google Scholar] [CrossRef]
- Hempel de Ibarra, N.; Giurfa, M.; Vorobyev, M. Detection of coloured patterns by honeybees through chromatic and achromatic cues. J. Comp. Physiol. A 2001, 187, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-Z.; Zhang, S.-W.; Wang, Z.-L.; Yan, W.-Y.; Zeng, Z.-J. Cross-modal interaction between visual and olfactory learning in Apis cerana. J. Comp. Physiol. A 2014, 200, 899–909. [Google Scholar] [CrossRef]
- Wertlen, A.M.; Niggebrügge, C.; Vorobyev, M.; Hempel de Ibarra, N. Detection of patches of coloured discs by bees. J. Exp. Biol. 2008, 211, 2101–2104. [Google Scholar] [CrossRef]
- Somanathan, H.; Saryan, P.; Balamurali, G.S. Foraging strategies and physiological adaptations in large carpenter bees. J. Comp. Physiol. A 2019, 205, 387–398. [Google Scholar] [CrossRef]
- Chakravarthi., A.; Rajus, S.; Kelber, A.; Dacke, M.; Baird, E. Differences in spatial resolution and contrast sensitivity of flight control in the honeybees Apis cerana and Apis mellifera. J. Exp. Biol. 2018, 221, 184267. [Google Scholar] [CrossRef]
- Hecht, S.; Wolf, E. The visual acuity of the honey bee. J. Gen. Physiol. 1929, 12, 727–760. [Google Scholar] [CrossRef]
- Chakravarthi, A.; Baird, E.; Dacke, M.; Kelber, A. Spatial vision in Bombus terrestris. Front. Behav. Neurosci. 2016, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Macuda, T.; Gegear, R.J.; Laverty, T.M.; Timney, B. Behavioural assessment of visual acuity in bumblebees (Bombus impatiens). J. Exp. Biol. 2001, 204, 559–564. [Google Scholar]
- Dyhr, J.P.; Higgins, C.M. The spatial frequency tuning of optic-flow dependent behaviors in the bumblebee Bombus impatiens. J. Exp. Biol. 2010, 213, 1643–1650. [Google Scholar] [CrossRef] [PubMed]
- Riley, J.R.; Smith, A.D.; Reynolds, D.R.; Edwards, A.S.; Osborne, J.L.; Williams, I.H.; Poppy, G.M. Tracking bees with harmonic radar. Nature 1996, 379, 29. [Google Scholar] [CrossRef]
- Wikelski, M.; Moxley, J.; Eaton-Mordas, A.; Lopez-Uribe, M.M.; Holland, R.; Moskowitz, D.; Roubik, D.W.; Kays, R. Large-range movements of neotropical orchid bees observed via radio telemetry. PLoS ONE 2010, 5, e10738. [Google Scholar] [CrossRef] [PubMed]
- Van Nieuwstadt, M.G.L.; Iraheta, C.R. Relation between size and foraging range in stingless bees (Apidae, Meliponinae). Apidologie 1996, 27, 219–228. [Google Scholar] [CrossRef]
- Dyer, F.C.; Seeley, T.D. Dance dialects and foraging range in three Asian honey bee species. Behav. Ecol. Sociobiol. 1991, 28, 227–233. [Google Scholar] [CrossRef]
- Steffan-Dewenter, I.; Kuhn, A. Honeybee foraging in differentially structured landscapes. Proc. R. Soc. B 2003, 270, 569–575. [Google Scholar] [CrossRef]
- Abrol, D.P. Foraging range of subtropical bees, Megachile flavipes, Megachile nana (Hymenoptera: Megachilidae) and Apis florea (Hymenoptera: Apidae). J. Indian Inst. Sci. 1988, 68, 43–47. [Google Scholar]
- Dhaliwal, H.S.; Sharma, P.L. Foraging range of the Indian honeybee. J. Apicult. Res. 1974, 13, 137–141. [Google Scholar] [CrossRef]
- Visscher, P.K.; Seeley, T.D. Foraging strategy of honeybee colonies in a temperate deciduous forest. Ecology 1982, 63, 1790–1801. [Google Scholar] [CrossRef]
- Beekman, M.; Ratnieks, F.L.W. Long-range foraging by the honey-bee, Apis mellifera L. Funct. Ecol. 2000, 14, 490–496. [Google Scholar] [CrossRef]
- Walther-Hellwig, K.; Frankl, R. Foraging distances of Bombus muscorum, Bombus lapidarius, and Bombus terrestris (Hymenoptera, Apidae). J. Insect Behav. 2000, 13, 239–246. [Google Scholar] [CrossRef]
- Osborne, J.L.; Martin, A.P.; Carreck, N.L.; Swain, J.L.; Knight, M.E.; Goulson, D.; Hale, R.J.; Sanderson, R.A. Bumblebee flight distances in relation to the forage landscape. J. Anim. Ecol. 2008, 77, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Osborne, J.L.; Clark, S.J.; Morris, R.J.; Williams, I.H.; Riley, J.R.; Smith, A.D.; Reynolds, D.R.; Edwards, A.S. A landscape-scale study of bumble bee foraging range and constancy, using harmonic radar. J. Appl. Ecol. 1999, 36, 519–533. [Google Scholar] [CrossRef]
- Pasquet, R.S.; Peltier, A.; Hufford, M.B.; Oudin, E.; Saulnier, J.; Paul, L.; Knudsen, J.T.; Herren, H.R.; Gepts, P. Long-distance pollen flow assessment through evaluation of pollinator foraging range suggests transgene escape distances. Proc. Natl. Acad. Sci. USA 2008, 105, 13456–13461. [Google Scholar] [CrossRef]
- Molitor, A. Zur vergleichenden Psychobiologie der akuleaten Hymenopteren auf experimenteller Grundlage. Biol. Gen. 1937, 13, 294–333. [Google Scholar]
- Araújo, E.D.; Costa, M.; Chaud-Netto, J.; Fowler, H.G. Body size and flight distance in stingless bees (Hymenoptera: Meliponini): Inference of flight range and possible ecological implications. Braz. J. Biol. 2004, 64, 563–568. [Google Scholar] [CrossRef]
- Roubik, D.W.; Aluja, M. Flight ranges of Melipona and Trigona in tropical forest. J. Kansas Entom. Soc. 1983, 56, 217–222. [Google Scholar]
- Greenleaf, S.S.; Williams, N.M.; Winfree, R.; Kremen, C. Bee foraging ranges and their relationship to body size. Oecologia 2007, 153, 589–596. [Google Scholar] [CrossRef]
- Punchihewa, R.W.K.; Koeniger, N.; Kevan, P.G.; Gadawski, R.M. Observations on the dance communication and natural foraging ranges of Apis cerana, Apis dorsata and Apis florea in Sri Lanka. J. Apicul. Res. 1985, 24, 168–175. [Google Scholar] [CrossRef]
- Esch, H.E.; Zhang, S.; Srinivasan, M.V.; Tautz, J. Honeybee dances communicate distances measured by optic flow. Nature 2001, 411, 581. [Google Scholar] [CrossRef] [PubMed]
- Tautz, J.; Zhang, S.; Spaethe, J.; Brockmann, A.; Si, A.; Srinivasan, M. Honeybee odometry: Performance in varying natural terrain. PLoS Biol. 2004, 2, e211. [Google Scholar] [CrossRef]
- Srinivasan, M.V.; Zhang, S.; Lehrer, M.; Collett, T. Honeybee navigation en route to the goal: Visual flight control and odometry. J. Exp. Biol. 1996, 199, 237–244. [Google Scholar] [PubMed]
- Srinivasan, M.V. Going with the flow: A brief history of the study of the honeybee’s navigational ‘odometer’. J. Comp. Physiol. A 2014, 200, 563–573. [Google Scholar] [CrossRef]
- Chittka, L.; Tautz, J. The spectral input to honeybee visual odometry. J. Exp. Biol. 2003, 206, 2393–2397. [Google Scholar] [CrossRef]
- Linander, N.; Baird, E.; Dacke, M. Bumblebee flight performance in environments of different proximity. J. Comp. Physiol. A 2016, 202, 97–103. [Google Scholar] [CrossRef]
- Baird, E.; Srinivasan, M.V.; Zhang, S.-W.; Lamont, R.; Cowling, A. Visual control of flight speed and height in the honeybee. Anim. Animat. 2006, 9, 40–51. [Google Scholar]
- Eckles, M.A.; Roubik, D.W.; Nieh, J.C. A stingless bee can use visual odometry to estimate both height and distance. J. Exp. Biol. 2012, 215, 3155–3160. [Google Scholar] [CrossRef]
- Baird, E.; Kreiss, E.; Wcislo, W.; Warrant, E.; Dacke, M. Nocturnal insects use optic flow for flight control. Biol. Lett. 2011, 7, 499–501. [Google Scholar] [CrossRef]
- Paxton, R.J. Male mating behaviour and mating systems of bees: An overview. Apidologie 2005, 36, 145–156. [Google Scholar] [CrossRef]
- Vallet, A.M.; Coles, J.A. The perception of small objects by the drone honeybee. J. Comp. Physiol. A 1993, 172, 183–188. [Google Scholar] [CrossRef]
- Praagh, J.V.; Ribi, W.; Wehrhahn, C.; Wittmann, D. Drone bees fixate the queen with the dorsal frontal part of their compound eyes. J. Comp. Physiol. A 1980, 136, 263–266. [Google Scholar] [CrossRef]
- Rinderer, T.E.; Oldroyd, B.P.; Wongsiri, S.; Sylvester, H.A.; de Guzman, L.I.; Potichot, S.; Sheppard, W.S.; Buchmann, S.L. Time of drone flight in 4 honey bee species in south-eastern Thailand. J. Apicult. Res. 1993, 32, 27–33. [Google Scholar] [CrossRef]
- De Figueiredo-Mecca, G.; Bego, L.R.; do Nascimento, F.S. Foraging behavior of Scaptotrigona depilis (Hymenoptera, Apidae, Meliponini) and its relationship with temporal and abiotic factors. Sociobiology 2013, 60, 267–282. [Google Scholar] [CrossRef]
- Heard, T.A.; Hendrikz, J.K. Factors influencing flight activity of colonies of the stingless bee Trigona carbonaria (Hymenoptera, Apidae). Aust. J. Zool. 1993, 41, 343–353. [Google Scholar] [CrossRef]
- Dyer, F.C. Nocturnal orientation by the Asian honey bee, Apis dorsata. Anim. Behav. 1985, 33, 769–774. [Google Scholar] [CrossRef]
- Borges, R.M.; Somanathan, H.; Kelber, A. Patterns and processes in nocturnal and crepuscular pollination services. Quart. Rev. Biol. 2016, 91, 389–418. [Google Scholar] [CrossRef]
- Kirchner, W.H.; Dreller, C.; Grasser, A.; Baidya, D. The silent dances of the Himalayan honeybee, Apis laboriosa. Apidologie 1996, 27, 331–339. [Google Scholar] [CrossRef]
- Somanathan, H.; Borges, R.M.; Warrant, E.J.; Kelber, A. Visual ecology of Indian carpenter bees I: Light intensities and flight activity. J. Comp. Physiol. A 2008, 194, 97–107. [Google Scholar] [CrossRef]
- Somanathan, H.; Borges, R.M.; Warrant, E.J.; Kelber, A. Nocturnal bees learn landmark colours in starlight. Curr. Biol. 2008, 18, R996–R997. [Google Scholar] [CrossRef] [PubMed]
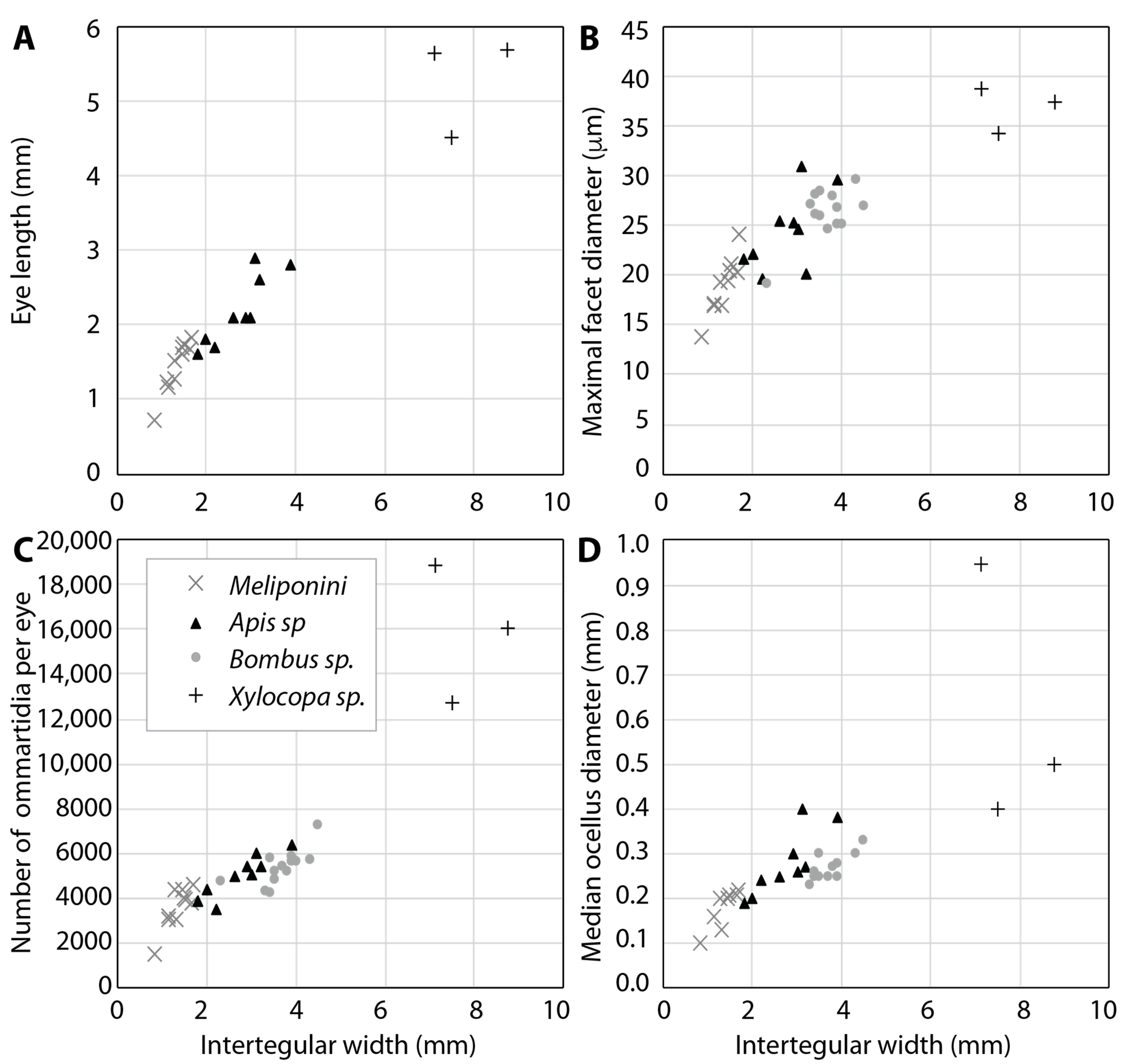
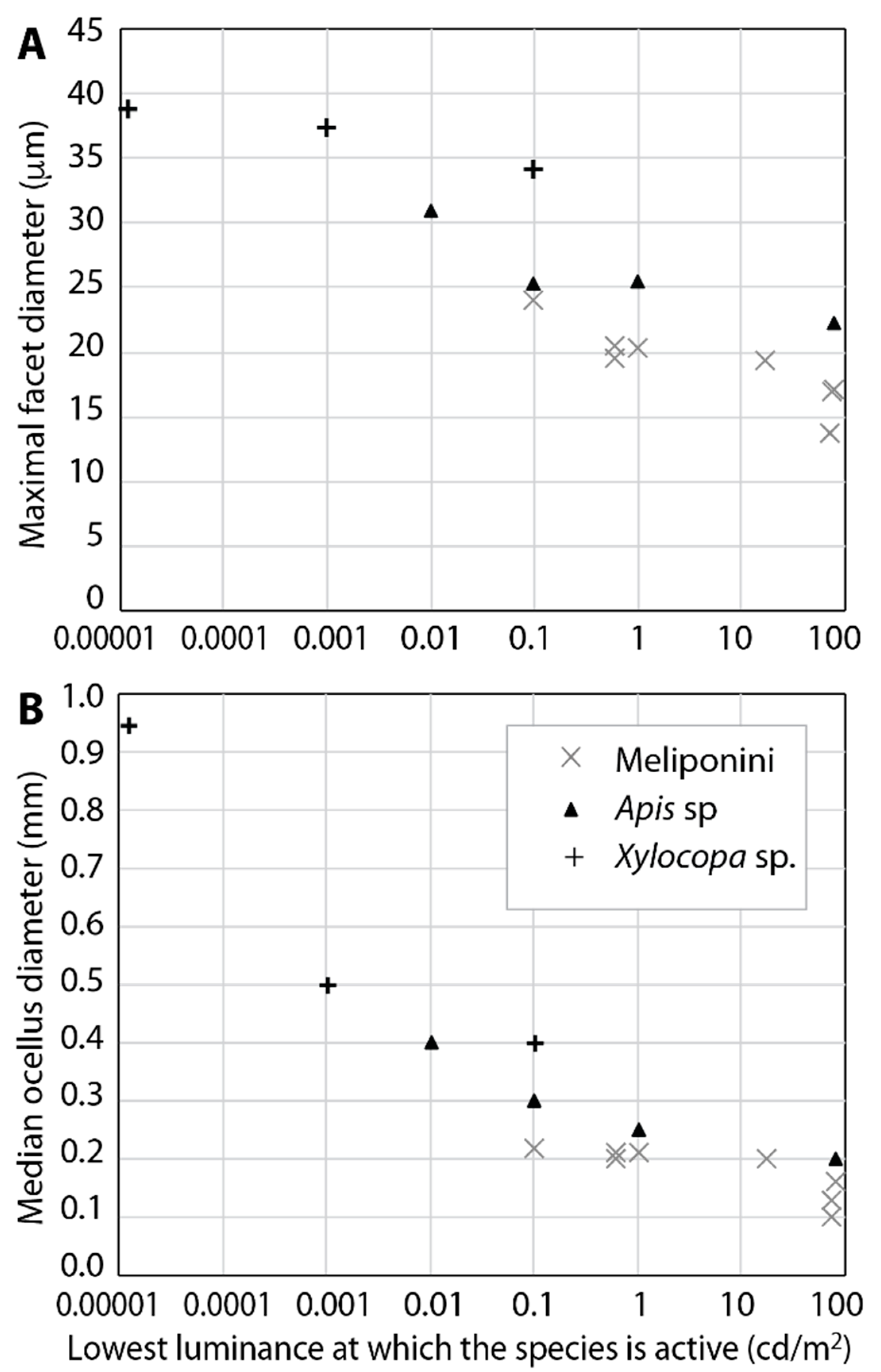
| Species | Intertegular Width (mm) | Interommatial Angle | Acceptance Angle (°) | Sensitivity S (m2 sr) | References |
|---|---|---|---|---|---|
| Apis mellifera | 3.2 | 0.9° v/1.6° h (c) | 1.7 (a), 1.6 (e) | 0.11 | [16,17,18] |
| Apis cerana | 3.0 | 1.2 (a) | 0.07 | [18] | |
| Apis florea | 2.2 | 1.1 (a) | 0.03 | [18] | |
| Apis dorsata | 3.9 | 1.8 (a) | 0.21 | [18] | |
| Bombus terrestris | small 3.0 large 4.2 | 1.2 v/2.9 h (pa) 0.9 v/2.1 h (pa) | [19] | ||
| Tetragonula carbonaria | 1.3 v/2.3 h (pa) | [20] | |||
| Xylocopa leucothorax | 7.5 | 0.9 v/1.5 h (po) | 0.8 (a) | 0.1 | [21] |
| Xylocopa tenuiscapa | 8.8 | 0.8 v/1.5 h (po) | 1.1 (a) | 0.3 | [21] |
| Xylocopa tranquebarica | 7.1 | 0.7 v/1.0 h (po) | 2.7 (a) | 2.7 | [21] |
| Megalopta genalis | 2.8 | 1.4 (po) | 5.6 | 2.7 | [22] |
| Species | Sex/Caste | Maximal Facet Diameter (m) | Number of Facets/Eye | Minimal Interommatial Angle | Median Ocellus Diameter (mm) | References |
|---|---|---|---|---|---|---|
| Apis mellifera | queen | 26.1 | 4460 | 0.30 | [10,11,12,16,25] | |
| worker | 25.2 | 5375 | 1.6 | 0.28 | ||
| drone | 40.1 | 9993 | 1.0 | 0.34 | ||
| Apis cerana | queen | 25.9 | 3582 | 0.27 | [12] | |
| worker | 25.4 | 4921 | 0.25 | |||
| drone | 35.8 | 7994 | 0.30 | |||
| Apis florea | queen | 24.9 | 4036 | 0.27 | [12] | |
| worker | 22.1 | 4394 | 0.20 | |||
| drone | 38.0 | 9434 | 0.32 | |||
| Apis dorsata | queen | 34.7 | 4479 | 0.38 | [12] | |
| worker | 30.8 | 5974 | 0.40 | |||
| drone | 46.3 | 8383 | 0.40 | |||
| Bombus pratorum | queen | 30.1 | 5805 | 0.30 | [13] | |
| worker | 27.1 | 4301 | 0.23 | |||
| male | 28.5 | 4492 | 0.25 | |||
| Bombus terrestris | queen | 29.3 | 7691 | 0.38 | [13] | |
| worker | 25.1 | 5656 | 0.28 | |||
| male | 27.4 | 5624 | 0.31 | |||
| Bombus melaleucus | queen | 36.9 | 8528 | 0.39 | [13] | |
| worker | 29.5 | 5659 | 0.30 | |||
| male | 39.3 | 8299 | 0.36 | |||
| Bombus niveatus | queen | 28.8 | 8617 | 0.42 | [13] | |
| worker | 26.8 | 7230 | 0.33 | |||
| male | 36.4 | 8051 | 0.34 | |||
| Bombus wurflenii | queen | 32.6 | 6960 | 0.34 | [13] | |
| worker | 27.9 | 5213 | 0.27 | |||
| male | 28.6 | 5604 | 0.30 | |||
| Bombus lapidarius | queen | 29.9 | 6765 | 0.38 | [13] | |
| worker | 25.9 | 4800 | 0.30 | |||
| male | 29.3 | 5214 | 0.30 | |||
| Bombus hortorum | queen | 30.2 | 7010 | 0.31 | [13] | |
| worker | 28.4 | 5170 | 0.25 | |||
| male | 28.0 | 5232 | 0.25 | |||
| Bombus pascuorum | queen | 32.2 | 6426 | 0.33 | [13] | |
| worker | 28.0 | 5803 | 0.25 | |||
| male | 29.1 | 5666 | 0.27 | |||
| Bombus soroeensis | queen | 28.8 | 6042 | 0.31 | [13] | |
| worker | 26.0 | 4250 | 0.24 | |||
| male | 27.8 | 4968 | 0.26 | |||
| Bombus confusus | queen | 29.7 | 7569 | 0.35 | [13] | |
| worker | 26.7 | 5870 | 0.25 | |||
| male | 39.2 | 7821 | 0.33 | |||
| Bombus mendax | queen | 28.1 | 6868 | 0.35 | [13] | |
| worker | 24.5 | 5375 | 0.25 | |||
| male | 34.0 | 7032 | 0.28 | |||
| Scaptotrigona postica | queen | 19 | 3800 | 0.24 | [10] | |
| worker | 21 | 3900 | 0.22 | |||
| drone | 21 | 4500 | 0.29 | |||
| queen | 19 | 3500 | 0.24 | |||
| Xylocopa tenuiscapa | female | 37.3 | 15,994 | 1.0 | 0.50 | [14,21] |
| male | 48.0 | 15,751 | 0.7 | 0.60 | ||
| Xylocopa leucothorax | female | 34.2 | 12,716 | 0.40 | [14,21] | |
| male | 35.0 | 11,331 | 0.40 | |||
| Xylocopa tranquebarica | female | 38.7 | 18,804 | 0.95 | [14,21] | |
| male | 40.0 | 15,511 | 0.90 |
| Species | Stimulus | Behavioral Response Tested | Minimum Spatial Wavelength (deg) | Minimum Spatial Frequency (Cycle deg−1) | Reference |
|---|---|---|---|---|---|
| Apis cerana | Sine wave stationary gratings | Object discrimination | 2.8–3.8 | 0.26–0.36 | [61] |
| Sine wave gratings | Flight control, centering response | 1.2–8.3 | 0.12–0.8 | [64] | |
| Apis mellifera | Gratings, bright light | Object discrimination | 4 | 0.25 | [24] |
| Gratings, dim light | Object discrimination | 8.3 | 0.12 | [24] | |
| Square object | Object discrimination | 5.7 (a) | 0.18 | [16] | |
| Square wave gratings | Optomotor response, walking bees | 2.1 | 0.48 | [65] | |
| Square wave grating | Object discrimination | 4 | 0.25 | [58] | |
| Point object | Object detection | 6–11 (a) | 0.09–0.17 | [28,59,60,62] | |
| Sine wave gratings | Flight control, centering response | 5.5–8 | 0.12–0.18 | [64] | |
| Bombus terrestris | Point object | Object discrimination | 3.6–14 (a) | 0.27–0.07 | [19,62] |
| Sine wave gratings | Object discrimination | 4.8 | 0.21 | [66] | |
| Sine wave gratings | Flight control, centering response | 4.8 | 0.21 | [64] | |
| Bombus impatiens | Sine wave gratings | Object discrimination | 2.8–2.9 | 0.35–0.36 | [67] |
| Sine wave gratings | Flight control, centering response | 7.1 | 0.14 | [68] | |
| Tetragonula carbonaria | Point object | Object detection | 18.8 (a) | 0.053 | [20] |
| Species | Method Used to Infer Foraging Ranges | Average Foraging Distance (m) | Maximal Distance Estimate (m) | Reference |
|---|---|---|---|---|
| Honeybees | ||||
| Apis florea | Mark-recapture | 150–250 | 750 | [74] |
| Dances | 268 | >800 | [72] | |
| Apis cerana | Dances | 195 | 1200 | [72] |
| Feeder | 650 | 1423 | [75] | |
| Apis dorsata | Dances | 863 | 1000 | [72] |
| Apis mellifera | Dances | 10,000 | [72] | |
| Dances | 2300 | approx. 8000 | [76] | |
| Dances | 1570 | 10,000 | [73] | |
| Dances | 5500 | >10,000 | [77] | |
| Bumblebees | ||||
| Bombus muscorum | Mark-recapture | 55 | 125 | [78] |
| Bombus lapidarius | Mark-recapture | 260 | 1500 | [78] |
| Bombus terrestris | Mark-recapture | 663 | 1750 | [78] |
| Mark-recapture | 1500 | [79] | ||
| Harmonic radar | 630 | [80] | ||
| Carpenter bees | ||||
| Xylocopa flavorufa | Radio-transmitter | 6040 | [81] | |
| Xylocopa violacea | Mark-recapture | 1200 | [82] | |
| Stingless bees | ||||
| Plebeia droryana | 540 | [83] | ||
| Melipona compressipes | 2470 | [83] | ||
| Trigona spinipes | 840 | [83] | ||
| Melipona quadrifasciata | 2000 | [83] | ||
| Melipona fasciata | Release | 2085 | [84] | |
| Trigona capitata | Release | 1547 | [84] | |
| Trigona corvina | release/feeder | 590/320 | [71] | |
| Tetragonisca angustula | release/feeder | 662/680 | [71] | |
| Nanotrigona testaceicornis | release/feeder | 484/120 | [71] | |
| Paramona cff cupira | release/feeder | 622/520 | [71] | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kelber, A.; Somanathan, H. Spatial Vision and Visually Guided Behavior in Apidae. Insects 2019, 10, 418. https://doi.org/10.3390/insects10120418
Kelber A, Somanathan H. Spatial Vision and Visually Guided Behavior in Apidae. Insects. 2019; 10(12):418. https://doi.org/10.3390/insects10120418
Chicago/Turabian StyleKelber, Almut, and Hema Somanathan. 2019. "Spatial Vision and Visually Guided Behavior in Apidae" Insects 10, no. 12: 418. https://doi.org/10.3390/insects10120418
APA StyleKelber, A., & Somanathan, H. (2019). Spatial Vision and Visually Guided Behavior in Apidae. Insects, 10(12), 418. https://doi.org/10.3390/insects10120418





