Acetylcholine and Its Receptors in Honeybees: Involvement in Development and Impairments by Neonicotinoids
Abstract
1. Introduction
2. Acetylcholine Receptors in the Honeybee
3. Acetylcholine in Bee Development
4. Neonicotinoids Affect Larval and Adult Development
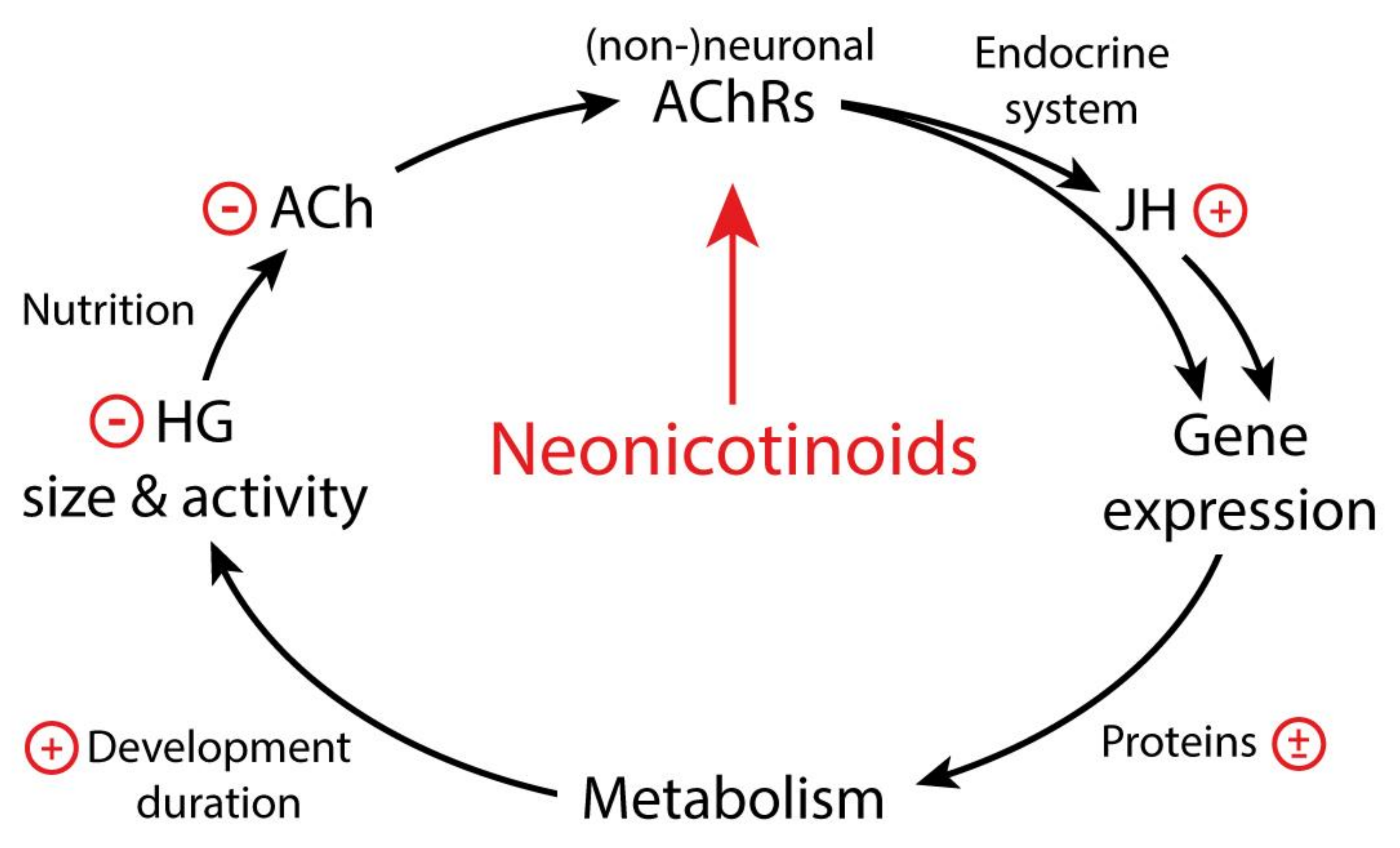
5. How Do Neonicotinoids Affect Honeybee Development?
6. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Sastry, B.V.; Sadavongvivad, C. Cholinergic systems in non-nervous tissues. Pharmacol. Rev. 1978, 30, 65–132. [Google Scholar]
- Wessler, I.; Kirkpatrick, C.J. Acetylcholine beyond neurons: the non-neuronal cholinergic system in humans. Br. J. Pharmacol. 2009, 154, 1558–1571. [Google Scholar] [CrossRef]
- Wessler, I.K.; Kirkpatrick, C.J. Non-neuronal acetylcholine involved in reproduction in mammals and honeybees. J. Neurochem. 2017, 142, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Wessler, I.; Kilbinger, H.; Bittinger, F.; Kirkpatrick, C.J. The biological role of non-neuronal acetylcholine in plants and Humans. Jpn. J. Pharmacol. 2001, 85, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Smallman, B.N.; Mansingh, A. The cholinergic system in insect development. Annu. Rev. Entomol. 1969, 14, 387–408. [Google Scholar] [CrossRef] [PubMed]
- Russell, R.J.; Claudianos, C.; Campbell, P.M.; Horne, I.; Sutherland, T.D.; Oakeshott, J.G. Two major classes of target site insensitivity mutations confer resistance to organophosphate and carbamate insecticides. Pestic. Biochem. Physiol. 2004, 79, 84–93. [Google Scholar] [CrossRef]
- Kim, Y.H.; Cha, D.J.; Jung, J.W.; Kwon, H.W.; Lee, S.H. Molecular and kinetic properties of two acetylcholinesterases from the western honey bee, Apis mellifera. PLoS One 2012, 7, e48838. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Kim, J.H.; Kim, K.; Lee, S.H. Expression of acetylcholinesterase 1 is associated with brood rearing status in the honey bee, Apis mellifera. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef]
- Lu, Y.; Park, Y.; Gao, X.; Zhang, X.; Yao, J.; Pang, Y.-P.; Jiang, H.; Zhu, K.Y. Cholinergic and non-cholinergic functions of two acetylcholinesterase genes revealed by gene-silencing in Tribolium castaneum. Sci. Rep. 2012, 2, 288. [Google Scholar] [CrossRef]
- Malloy, C.A.; Ritter, K.; Robinson, J.; English, C.; Cooper, R.L. Pharmacological identification of cholinergic receptor subtypes on Drosophila melanogaster larval heart. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2016, 186, 45–57. [Google Scholar] [CrossRef]
- Brandt, A.; Gorenflo, A.; Siede, R.; Meixner, M.; Büchler, R. The neonicotinoids thiacloprid, imidacloprid, and clothianidin affect the immunocompetence of honey bees (Apis mellifera L.). J. Insect Physiol. 2016, 86, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Brandt, A.; Grikscheit, K.; Siede, R.; Grosse, R.; Meixner, M.D.; Büchler, R. Immunosuppression in honeybee queens by the neonicotinoids thiacloprid and clothianidin. Sci. Rep. 2017, 7, 4673. [Google Scholar] [CrossRef] [PubMed]
- Pamminger, T.; Basley, K.; Goulson, D.; Hughes, W. First indication of acetylcholine-based communication in honeybee haemocytes and its modulation by a neonicotinoid insecticide. bioRxiv 2017, 105700. [Google Scholar] [CrossRef]
- Thany, S.H. Advances in Experimental Medicine and Biology. In Insect Nicotinic Acetylcholine Receptors; Thany, S.H., Ed.; Springer: New York, NY, USA, 2010; Volume 683, ISBN 978-1-4419-6444-1. [Google Scholar]
- Goldberg, F.; Grünewald, B.; Rosenboom, H.; Menzel, R. Nicotinic acetylcholine currents of cultured Kenyon cells from the mushroom bodies of the honey bee Apis mellifera. J. Physiol. 1999, 514, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Oertner, T.G.; Single, S.; Borst, A. Separation of voltage- and ligand-gated calcium influx in locust neurons by optical imaging. Neurosci. Lett. 1999, 274, 95–98. [Google Scholar] [CrossRef]
- Grünewald, B. Cellular Mechanisms of Neuronal Plasticity in the Honeybee Brain. Handb. Behav. Neurosci. 2013, 22, 467–477. [Google Scholar]
- Gauthier, M. State of the art on insect nicotinic acetylcholine receptor function in learning and memory. In Insect Nicotinic Acetylcholine Receptors; Thany, S.H., Ed.; Springer: New York, NY, USA, 2010; Volume 683, pp. 97–115. [Google Scholar]
- Gauthier, M.; Grünewald, B. Neurotransmitter systems in the honeybee brain: functions in learning and memory. In Honeybee Neurobiology and Behavior; Galizia, C.G., Eisenhardt, D., Giurfa, M., Eds.; Springer: Dordrecht, Holland; Heidelberg, Germany; London, UK; New York, NY, USA, 2012; pp. 155–169. [Google Scholar]
- Kreissl, S.; Bicker, G. Histochemistry of acetylcholinesterase and immunocytochemistry of an acetylcholine receptor-like antigen in the brain of the honeybee. J. Comp. Neurol. 1989, 286, 71–84. [Google Scholar] [CrossRef]
- Wüstenberg, D.G.; Grünewald, B. Pharmacology of the neuronal nicotinic acetylcholine receptor of cultured Kenyon cells of the honeybee, Apis mellifera. J. Comp. Physiol. A 2004, 190, 807–821. [Google Scholar] [CrossRef]
- Barbara, G.S.; Zube, C.; Rybak, J.; Gauthier, M.; Grünewald, B. Acetylcholine, GABA and glutamate induce ionic currents in cultured antennal lobe neurons of the honeybee, Apis mellifera. J. Comp. Physiol. A 2005, 191, 823–836. [Google Scholar] [CrossRef]
- Dupuis, J.P.; Gauthier, M.; Raymond-Delpech, V. Expression patterns of nicotinic subunits α2, α7, α8, and β1 affect the kinetics and pharmacology of ACh-induced currents in adult bee olfactory neuropiles. J. Neurophysiol. 2011, 106, 1604–1613. [Google Scholar] [CrossRef]
- Thany, S.H.; Lenaers, G.; Crozatier, M.; Armengaud, C.; Gauthier, M. Identification and localization of the nicotinic acetylcholine receptor alpha3 mRNA in the brain of the honeybee, Apis mellifera. Insect Mol. Biol. 2003, 12, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Thany, S.H.; Crozatier, M.; Raymond-Delpech, V.; Gauthier, M.; Lenaers, G. Apisα2, Apisα7-1 and Apisα7-2: three new neuronal nicotinic acetylcholine receptor α-subunits in the honeybee brain. Gene 2005, 344, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.K.; Sattelle, D.B. Diversity of insect nicotinic acetylcholine receptor subunits. Adv. Exp. Med. Biol. 2010, 683, 25–43. [Google Scholar] [PubMed]
- Gundelfinger, E.D.; Schulz, R. Insect nicotinic acetylcholine receptors: genes, structure, physiological and pharmacological properties. In Neuronal Nicotinic Receptors; Clementi, F., Fornasari, D., Gotti, C., Eds.; Springer-Verlag: Heidelberg, Germany, 2000; pp. 497–521. [Google Scholar]
- Thany, S.H. Electrophysiological studies and pharmacological properties of insect native nicotinic acetylcholine receptors. Adv. Exp. Med. Biol. 2010, 683, 53–63. [Google Scholar]
- Casida, J.E. Neonicotinoids and other insect nicotinic receptor competitive modulators: progress and prospects. Annu. Rev. Entomol. 2018, 63, 125–144. [Google Scholar] [CrossRef]
- Tomizawa, M.; Casida, J.E. Selective toxicity of neonicotinoids attributable to specificity of insect and mammalian nicotinic receptors. Annu. Rev. Entomol. 2003, 48, 339–364. [Google Scholar] [CrossRef]
- Cabirol, A.; Haase, A. The neurophysiological bases of the impact of neonicotinoid pesticides on the behaviour of honeybees. Insects 2019, 10, 344. [Google Scholar] [CrossRef]
- Nauen, R.; Ebbinghaus-Kintscher, U.; Schmuck, R. Toxicity and nicotinic acetylcholine receptor interaction of imidacloprid and its metabolites in Apis mellifera (Hymenoptera: Apidae). Pest Manag. Sci. 2001, 57, 577–586. [Google Scholar] [CrossRef]
- Déglise, P.; Grünewald, B.; Gauthier, M. The insecticide imidacloprid is a partial agonist of the nicotinic receptor of honeybee Kenyon cells. Neurosci. Lett. 2002, 321, 13–16. [Google Scholar] [CrossRef]
- Palmer, M.J.; Moffat, C.; Saranzewa, N.; Harvey, J.; Wright, G.A.; Connolly, C.N. Cholinergic pesticides cause mushroom body neuronal inactivation in honeybees. Nat. Commun. 2013, 4, 1634. [Google Scholar] [CrossRef]
- Grillone, G.; Laurino, D.; Manino, A.; Porporato, M. Toxicity of thiametoxam on in vitro reared honey bee brood. Apidologie 2017, 48, 635–643. [Google Scholar] [CrossRef]
- Aupinel, P.; Fortini, D.; Michaud, B.; Marolleau, F.; Tasei, J.-N.; Odoux, J.-F. Toxicity of dimethoate and fenoxycarb to honey bee brood (Apis mellifera), using a newin vitro standardized feeding method. Pest Manag. Sci. 2007, 63, 1090–1094. [Google Scholar] [CrossRef] [PubMed]
- Hendriksma, H.P.; Härtel, S.; Steffan-Dewenter, I. Honey bee risk assessment: new approaches for in vitro larvae rearing and data analyses. Methods Ecol. Evol. 2011, 2, 509–517. [Google Scholar] [CrossRef]
- Tasei, J.-N.; Lerin, J.; Ripault, G. Sub-lethal effects of imidacloprid on bumblebees, Bombus terrestris (Hymenoptera: Apidae), during a laboratory feeding test. Pest Manag. Sci. 2000, 56, 784–788. [Google Scholar] [CrossRef]
- De Souza Rosa, A.; Teixeira, J.S.G.; Vollet-Neto, A.; Queiroz, E.P.; Blochtein, B.; Pires, C.S.S.; Imperatriz-Fonseca, V.L. Consumption of the neonicotinoid thiamethoxam during the larval stage affects the survival and development of the stingless bee, Scaptotrigona aff. depilis. Apidologie 2016, 47, 729–738. [Google Scholar] [CrossRef]
- Dai, P.; Jack, C.J.; Mortensen, A.N.; Bustamante, T.A.; Bloomquist, J.R.; Ellis, J.D. Chronic toxicity of clothianidin, imidacloprid, chlorpyrifos, and dimethoate to Apis mellifera L. larvae reared in vitro. Pest Manag. Sci. 2019, 75, 29–36. [Google Scholar] [CrossRef]
- Rondeau, G.; Sánchez-Bayo, F.; Tennekes, H.A.; Decourtye, A.; Ramírez-Romero, R.; Desneux, N. Delayed and time-cumulative toxicity of imidacloprid in bees, ants and termites. Sci. Rep. 2015, 4, 5566. [Google Scholar] [CrossRef]
- Sandrock, C.; Tanadini, L.G.; Pettis, J.S.; Biesmeijer, J.C.; Potts, S.G.; Neumann, P. Sublethal neonicotinoid insecticide exposure reduces solitary bee reproductive success. Agric. For. Entomol. 2014, 16, 119–128. [Google Scholar] [CrossRef]
- Woodcock, B.A.; Bullock, J.M.; Shore, R.F.; Heard, M.S.; Pereira, M.G.; Redhead, J.; Ridding, L.; Dean, H.; Sleep, D.; Henrys, P.; et al. Country-specific effects of neonicotinoid pesticides on honey bees and wild bees. Science 2017, 356, 1393–1395. [Google Scholar] [CrossRef]
- Rundlöf, M.; Andersson, G.K.S.; Bommarco, R.; Fries, I.; Hederström, V.; Herbertsson, L.; Jonsson, O.; Klatt, B.K.; Pedersen, T.R.; Yourstone, J.; et al. Seed coating with a neonicotinoid insecticide negatively affects wild bees. Nature 2015, 521, 77–80. [Google Scholar] [CrossRef]
- Rolke, D.; Fuchs, S.; Grünewald, B.; Gao, Z.; Blenau, W. Large-scale monitoring of effects of clothianidin-dressed oilseed rape seeds on pollinating insects in Northern Germany: effects on honey bees (Apis mellifera). Ecotoxicology 2016, 25, 1648–1665. [Google Scholar] [CrossRef] [PubMed]
- Ellis, C.; Park, K.J.; Whitehorn, P.; David, A.; Goulson, D. The neonicotinoid insecticide thiacloprid impacts upon bumblebee colony development under field conditions. Environ. Sci. Technol. 2017, 51, 1727–1732. [Google Scholar] [CrossRef] [PubMed]
- Siviter, H.; Brown, M.J.F.; Leadbeater, E. Sulfoxaflor exposure reduces bumblebee reproductive success. Nature 2018, 561, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Havstad, L.T.; Øverland, J.I.; Valand, S.; Aamlid, T.S. Repellency of insecticides and the effect of thiacloprid on bumble bee colony development in red clover (Trifolium pratense L.) seed crops. Acta Agric. Scand. Sect. B Soil Plant Sci. 2019, 69, 439–451. [Google Scholar] [CrossRef]
- Henry, M.; Beguin, M.; Requier, F.; Rollin, O.; Odoux, J.-F.; Aupinel, P.; Aptel, J.; Tchamitchian, S.; Decourtye, A. A common pesticide decreases foraging success and survival in honey bees. Science 2012, 336, 348–350. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; Müller, T.; Spatz, A.-K.; Greggers, U.; Grünewald, B.; Menzel, R. Neonicotinoids interfere with specific components of naviagtion in honeybees. PLoS One 2014, 9, 1–10. [Google Scholar] [CrossRef]
- Tison, L.; Hahn, M.-L.; Holtz, S.; Rößner, A.; Greggers, U.; Bischoff, G.; Menzel, R. Honey bees’ behavior is impaired by chronic exposure to the neonicotinoid thiacloprid in the field. Environ. Sci. Technol. 2016, 50, 7218–7227. [Google Scholar] [CrossRef]
- Alkassab, A.T.; Kirchner, W.H. Assessment of acute sublethal effects of clothianidin on motor function of honeybee workers using video-tracking analysis. Ecotoxicol. Environ. Saf. 2018, 147, 200–205. [Google Scholar] [CrossRef]
- Decourtye, A.; Armengaud, C.; Renou, M.; Devillers, J.; Cluzeau, S.; Gauthier, M.; Pham-Delegue, M.H. Imidacloprid impairs memory and brain metabolism in the honeybee (Apis mellifera L.). Elsevier 2004, 78, 83–92. [Google Scholar] [CrossRef]
- Tison, L.; Rößner, A.; Gerschewski, S.; Menzel, R. The neonicotinoid clothianidin impairs memory processing in honey bees. Ecotoxicol. Environ. Saf. 2019, 180, 139–145. [Google Scholar] [CrossRef]
- Schneider, C.W.; Tautz, J.; Grünewald, B.; Fuchs, S. RFID tracking of sublethal effects of two neonicotinoid insecticides on the foraging behavior of Apis mellifera. PLoS One 2012, 7, e30023. [Google Scholar] [CrossRef] [PubMed]
- Siefert, P.; Hota, R.; Ramesh, V.; Grünewald, B. Chronic within-hive video registrations detect altered nursing behaviour and retarded larval development of neonicotinoid treated honey bees. Sci. Rep. under review.
- Blacquiere, T.; Smagghe, G.; van Gestel, C.A.; Mommaerts, V. Neonicotinoids in bees: a review on concentrations, side-effects and risk assessment. Ecotoxicology 2012, 21, 973–992. [Google Scholar] [CrossRef] [PubMed]
- Godfray, H.C.J.; Blacquière, T.; Field, L.M.; Hails, R.S.; Petrokofsky, G.; Potts, S.G.; Raine, N.E.; Vanbergen, A.J.; McLean, A.R. A restatement of the natural science evidence base concerning neonicotinoid insecticides and insect pollinators. Proc. R. Soc. B Biol. Sci. 2014, 281, 20140558. [Google Scholar] [CrossRef] [PubMed]
- Godfray, H.C.J.; Blacquière, T.; Field, L.M.; Hails, R.S.; Potts, S.G.; Raine, N.E.; Vanbergen, A.J.; McLean, A.R. A restatement of recent advances in the natural science evidence base concerning neonicotinoid insecticides and insect pollinators. Proc. R. Soc. B Biol. Sci. 2015, 282, 20151821. [Google Scholar] [CrossRef]
- Wood, T.J.; Goulson, D. The environmental risks of neonicotinoid pesticides: a review of the evidence post 2013. Environ. Sci. Pollut. Res. 2017, 24, 17285–17325. [Google Scholar] [CrossRef]
- Jones, A.K.; Raymond-Delpech, V.; Thany, S.H.; Gauthier, M.; Sattelle, D.B. The nicotinic acetylcholine receptor gene family of the honey bee, Apis mellifera. Genome Res. 2006, 16, 1422–1430. [Google Scholar] [CrossRef]
- Barbara, G.S.; Grünewald, B.; Paute, S.; Gauthier, M.; Raymond-Delpech, V. Study of nicotinic acetylcholine receptors on cultured antennal lobe neurones from adult honeybee brains. Invertebr. Neurosci. 2008, 8, 19–29. [Google Scholar] [CrossRef]
- Himmelreich, S.; Grünewald, B. Cellular physiology of olfactory learning in the honeybee brain. Apidologie 2012, 43, 308–321. [Google Scholar] [CrossRef]
- Bicker, G.; Kreissl, S. Calcium imaging reveals nicotinic acetylcholine receptors on cultured mushroom body neurons. J. Neurophysiol. 1994, 71, 808–810. [Google Scholar] [CrossRef][Green Version]
- Lapied, B.; Le Corronc, H.; Hue, B. Sensitive nicotinic and mixed nicotinic-muscarinic receptors in insect neurosecretory cells. Brain Res. 1990, 533, 132–136. [Google Scholar] [CrossRef]
- Tomizawa, M.; Casida, J.E. Neonicotinoid insecticide toxicology: mechanisms of selective action. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 247–268. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, P.; Nauen, R. Neonicotinoids-from zero to hero in insecticide chemistry. Pest Manag. Sci. 2008, 64, 1084–1098. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, P.; Nauen, R.; Beck, M.E. Nicotinic acetylcholine receptor agonists: a milestone for modern crop protection. Angew. Chemie Int. Ed. 2013, 52, 9464–9485. [Google Scholar] [CrossRef] [PubMed]
- Lozano, V.C.; Bonnard, E.; Gauthier, M.; Richard, D. Mecamylamine-induced impairment of acquisition and retrieval of olfactory conditioning in the honeybee. Behav. Brain Res. 1996, 81, 215–222. [Google Scholar] [CrossRef]
- Gauthier, M.; Dacher, M.; Thany, S.H.; Niggebrügge, C.; Déglise, P.; Kljucevic, P.; Armengaud, C.; Grünewald, B. Involvement of alpha-bungarotoxin-sensitive nicotinic receptors in long-term memory formation in the honeybee (Apis mellifera). Neurobiol. Learn. Mem. 2006, 86, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Andrione, M.; Vallortigara, G.; Antolini, R.; Haase, A. Neonicotinoid-induced impairment of odour coding in the honeybee. Sci. Rep. 2016, 6, 38110. [Google Scholar] [CrossRef] [PubMed]
- Collin, C.; Hauser, F.; de Valdivia, E.G.; Li, S.; Reisenberger, J.; Carlsen, E.M.M.; Khan, Z.; Hansen, N.Ø.; Puhm, F.; Søndergaard, L.; et al. Two types of muscarinic acetylcholine receptors in Drosophila and other arthropods. Cell. Mol. Life Sci. 2013, 70, 3231–3242. [Google Scholar] [CrossRef]
- Xia, R.-Y.; Li, M.-Q.; Wu, Y.-S.; Qi, Y.-X.; Ye, G.-Y.; Huang, J. A new family of insect muscarinic acetylcholine receptors. Insect Mol. Biol. 2016, 25, 362–369. [Google Scholar] [CrossRef]
- Henschler, D. Hoher Acetylcholingehalt von Bienenfuttersäften. Naturwissenschaften 1954, 41, 142. [Google Scholar] [CrossRef]
- Goldschmidt, S.; Burkert, H. Die Hydrolyse des cholinergischen Honigwirkstoffes und anderer Cholinester mittels Cholinesterasen und deren Hemmung im Honig. Hoppe Seyler´s Zeitschrift für Physiol. Chemie 1955, 301, 78–89. [Google Scholar] [CrossRef]
- Wessler, I.; Gärtner, H.-A.; Michel-Schmidt, R.; Brochhausen, C.; Schmitz, L.; Anspach, L.; Grünewald, B.; Kirkpatrick, C.J. Honeybees produce millimolar concentrations of non-neuronal acetylcholine for breeding: possible adverse effects of neonicotinoids. PLoS One 2016, 11, e0156886. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.H.; Nogueira-Neto, P.; Jaeger, C.P.; Ancona Lopez, A.A. Acetylcholine in the larval food, honey and stored pollen of a stingless bee, Melipona quadrifasciata. Bol. da Fac. Filos. Ciências e Let. Univ. São Paulo. Zool. 1965, 25, 105. [Google Scholar] [CrossRef]
- Valente, D.; Marques, L.A.C.; Mendes, E.G. The acetylcholine contents of honeys from different bees as determined in four-point-assays. Comp. Biochem. Physiol. Part C Comp. Pharmacol. 1981, 69, 161–164. [Google Scholar] [CrossRef]
- Shuel, R.W.; Dixon, S.E. Studies in the mode of action of royal jelly in honeybee development: II. Respiration of newly emerged larvae on various substrates. Can. J. Zool. 1959, 37, 803–813. [Google Scholar] [CrossRef]
- Jung-Hoffmann, I. Die Determination von Königin und Arbeiterin der Honigbiene. Z. Bienenforsch. 1966, 8, 296–322. [Google Scholar]
- Henschler, D.; von Rhein, W. Änderungen des Acetylcholingehaltes von Bienenfuttersäften in der Madenentwicklung. Naturwissenschaften 1960, 47, 326–327. [Google Scholar] [CrossRef]
- Colhoun, E.H.; Smith, M.V. Neurohormonal properties of royal jelly. Nature 1960, 188, 854–855. [Google Scholar] [CrossRef]
- Yang, E.-C.; Chang, H.-C.; Wu, W.-Y.; Chen, Y.-W. Impaired olfactory associative behavior of honeybee workers due to contamination of imidacloprid in the larval stage. PLoS One 2012, 7, e49472. [Google Scholar] [CrossRef]
- Tan, K.; Chen, W.; Dong, S.; Liu, X.; Wang, Y.; Nieh, J.C. A neonicotinoid impairs olfactory learning in Asian honey bees (Apis cerana) exposed as larvae or as adults. Sci. Rep. 2015, 5, 1–8. [Google Scholar] [CrossRef]
- Decourtye, A.; Devillers, J. Ecotoxicity of neonicotinoid insecticides to bees. Adv. Exp. Med. Biol. 2010, 683, 85–95. [Google Scholar] [PubMed]
- Wu, J.Y.; Anelli, C.M.; Sheppard, W.S. Sub-lethal effects of pesticide residues in brood comb on worker honey bee (Apis mellifera) development and longevity. PLoS One 2011, 6, e14720. [Google Scholar] [CrossRef] [PubMed]
- Abbott, V.A.; Nadeau, J.L.; Higo, H.A.; Winston, M.L. Lethal and sublethal effects of imidacloprid on Osmia lignaria and clothianidin on Megachile rotundata (Hymenoptera: Megachilidae). J. Econ. Entomol. 2008, 101, 784–796. [Google Scholar] [CrossRef]
- Böhme, F.; Bischoff, G.; Zebitz, C.P.W.; Rosenkranz, P.; Wallner, K. From field to food—Will pesticide-contaminated pollen diet lead to a contamination of royal jelly? Apidologie 2018, 49, 112–119. [Google Scholar]
- Wittmann, D. Tracer experiments on the passage of insecticides through nurse bees as a basis for determining intoxication routes in honeybee larvae. Apidologie 1982, 13, 328–330. [Google Scholar]
- Davis, A.R.; Shuel, R.W. Distribution of 14 C-labelled carbofuran and dimethoate in royal jelly, queen larvae and nurse honeybees. Apidologie 1988, 19, 37–50. [Google Scholar] [CrossRef]
- Rockstein, M. The relation of cholinesterase activity to change in cell number with age in the brain of the adult worker honeybee. J. Cell. Comp. Physiol. 1950, 35, 11–23. [Google Scholar] [CrossRef]
- Ament, S.A.; Wang, Y.; Robinson, G.E. Nutritional regulation of division of labor in honey bees: toward a systems biology perspective. Wiley Interdiscip. Rev. Syst. Biol. Med. 2010, 2, 566–576. [Google Scholar] [CrossRef]
- Deseyn, J.; Billen, J. Age-dependent morphology and ultrastructure of the hypopharyngeal gland of Apis mellifera workers (Hymenoptera, Apidae). Apidologie 2005, 36, 49–57. [Google Scholar] [CrossRef]
- Crailsheim, K. Interadult feeding of jelly in honeybee (Apis mellifera L.) colonies. J. Comp. Physiol. B 1991, 161, 55–60. [Google Scholar] [CrossRef]
- Crailsheim, K. The flow of jelly within a honeybee colony. J. Comp. Physiol. B 1992, 162, 681–689. [Google Scholar] [CrossRef]
- Hatjina, F.; Papaefthimiou, C.; Charistos, L.; Dogaroglu, T.; Bouga, M.; Emmanouil, C.; Arnold, G. Sublethal doses of imidacloprid decreased size of hypopharyngeal glands and respiratory rhythm of honeybees in vivo. Apidologie 2013, 44, 467–480. [Google Scholar] [CrossRef]
- Heylen, K.; Gobin, B.; Arckens, L.; Huybrechts, R.; Billen, J. The effects of four crop protection products on the morphology and ultrastructure of the hypopharyngeal gland of the European honeybee, Apis mellifera. Apidologie 2011, 42, 103–116. [Google Scholar] [CrossRef]
- Škerl, M.I.S.; Gregorc, A. Heat shock proteins and cell death in situ localisation in hypopharyngeal glands of honeybee (Apis mellifera carnica) workers after imidacloprid or coumaphos treatment. Apidologie 2010, 41, 73–86. [Google Scholar] [CrossRef]
- De Smet, L.; Hatjina, F.; Ioannidis, P.; Hamamtzoglou, A.; Schoonvaere, K.; Francis, F.; Meeus, I.; Smagghe, G.; de Graaf, D.C. Stress indicator gene expression profiles, colony dynamics and tissue development of honey bees exposed to sub-lethal doses of imidacloprid in laboratory and field experiments. PLoS One 2017, 12, e0171529. [Google Scholar] [CrossRef]
- Menail, H.A.; Bouchema-Boutefnouchet, W.F.; Smagghe, G.; Ayad-Loucif, W. Thiamethoxam (neonicotinoid) and spinosad (bioinsecticide) affect hypopharyngeal glands and survival of Apis mellifera intermissa (Hymenoptera: Apidae). In Proceedings of the Recent Advances in Environmental Science from the Euro-Mediterranean and Surrounding Regions. EMCEI 2017. Advances in Science, Technology & Innovation (IEREK Interdisciplinary Series for Sustainable Development); Kallel, A., Ksibi, M., Ben Dhia, H., Khélifi, N., Eds.; Springer: Cham, Switzerland, 2018; pp. 347–349. [Google Scholar]
- Renzi, M.T.; Rodríguez-Gasol, N.; Medrzycki, P.; Porrini, C.; Martini, A.; Burgio, G.; Maini, S.; Sgolastra, F. Combined effect of pollen quality and thiamethoxam on hypopharyngeal gland development and protein content in Apis mellifera. Apidologie 2016, 47, 779–788. [Google Scholar] [CrossRef]
- Berenbaum, M.R.; Liao, L.-H. Honey bees and environmental stress: Toxicologic pathology of a superorganism. Toxicol. Pathol. 2019, 019262331987715. [Google Scholar] [CrossRef]
- Christen, V.; Kunz, P.Y.; Fent, K. Endocrine disruption and chronic effects of plant protection products in bees: Can we better protect our pollinators? Environ. Pollut. 2018, 243, 1588–1601. [Google Scholar] [CrossRef]
- Baines, D.; Wilton, E.; Pawluk, A.; De Gorter, M.; Chomistek, N. Neonicotinoids act like endocrine disrupting chemicals in newly-emerged bees and winter bees. Sci. Rep. 2017, 7, 1–18. [Google Scholar] [CrossRef]
- Cook, S.C. Compound and dose-dependent effects of two neonicotinoid pesticides on honey bee (Apis mellifera) metabolic physiology. Insects 2019, 10, 11–14. [Google Scholar] [CrossRef]
- Snodgrass, R.E. Anatomy of the Honey Bee; Cornell University Press: London, UK, 1956. [Google Scholar]
- Yu, Y.-S.; Xue, S.; Wu, J.-C.; Wang, F.; Yang, G.-Q. Changes in levels of juvenile hormone and molting hormone in larvae and adult females of Chilo suppressalis (Lepidoptera: Pyralidae) after imidacloprid applications to rice. J. Econ. Entomol. 2007, 100, 1188–1193. [Google Scholar] [CrossRef]
- Kou, R. Cholinergic regulation of the corpora allata in adult male loreyi leafworm Mythimna loreyi. Arch. Insect Biochem. Physiol. 2002, 49, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Christen, V.; Mittner, F.; Fent, K. Molecular effects of neonicotinoids in honey bees (Apis mellifera). Environ. Sci. Technol. 2016, 50, 4071–4081. [Google Scholar] [CrossRef] [PubMed]
- Derecka, K.; Blythe, M.J.; Malla, S.; Genereux, D.P.; Guffanti, A.; Pavan, P.; Moles, A.; Snart, C.; Ryder, T.; Ortori, C.A.; et al. Transient exposure to low levels of insecticide affects metabolic networks of honeybee larvae. PLoS One 2013, 8, e68191. [Google Scholar] [CrossRef]
- Gregorc, A.; Evans, J.D.; Scharf, M.; Ellis, J.D. Gene expression in honey bee (Apis mellifera) larvae exposed to pesticides and Varroa mites (Varroa destructor). J. Insect Physiol. 2012, 58, 1042–1049. [Google Scholar] [CrossRef]
- Schmehl, D.R.; Teal, P.E.A.; Frazier, J.L.; Grozinger, C.M. Genomic analysis of the interaction between pesticide exposure and nutrition in honey bees (Apis mellifera). J. Insect Physiol. 2014, 71, 177–190. [Google Scholar] [CrossRef]
- Claudianos, C.; Ranson, H.; Johnson, R.M.; Biswas, S.; Schuler, M.A.; Berenbaum, M.R.; Feyereisen, R.; Oakeshott, J.G. A deficit of detoxification enzymes: pesticide sensitivity and environmental response in the honeybee. Insect Mol. Biol. 2006, 15, 615–636. [Google Scholar] [CrossRef]
- Mogren, C.L.; Lundgren, J.G. Neonicotinoid-contaminated pollinator strips adjacent to cropland reduce honey bee nutritional status. Sci. Rep. 2016, 6, 29608. [Google Scholar] [CrossRef]
- Wilde, J.; Frączek, R.J.; Siuda, M.; Bąk, B.; Hatjina, F.; Miszczak, A. The influence of sublethal doses of imidacloprid on protein content and proteolytic activity in honey bees (Apis mellifera L.). J. Apic. Res. 2016, 55, 212–220. [Google Scholar] [CrossRef]
- Rand, E.E.; Smit, S.; Beukes, M.; Apostolides, Z.; Pirk, C.W.W.; Nicolson, S.W. Detoxification mechanisms of honey bees (Apis mellifera) resulting in tolerance of dietary nicotine. Sci. Rep. 2015, 5, 11779. [Google Scholar] [CrossRef]
- Du Rand, E.E.; Human, H.; Smit, S.; Beukes, M.; Apostolides, Z.; Nicolson, S.W.; Pirk, C.W.W. Proteomic and metabolomic analysis reveals rapid and extensive nicotine detoxification ability in honey bee larvae. Insect Biochem. Mol. Biol. 2017, 82, 41–51. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grünewald, B.; Siefert, P. Acetylcholine and Its Receptors in Honeybees: Involvement in Development and Impairments by Neonicotinoids. Insects 2019, 10, 420. https://doi.org/10.3390/insects10120420
Grünewald B, Siefert P. Acetylcholine and Its Receptors in Honeybees: Involvement in Development and Impairments by Neonicotinoids. Insects. 2019; 10(12):420. https://doi.org/10.3390/insects10120420
Chicago/Turabian StyleGrünewald, Bernd, and Paul Siefert. 2019. "Acetylcholine and Its Receptors in Honeybees: Involvement in Development and Impairments by Neonicotinoids" Insects 10, no. 12: 420. https://doi.org/10.3390/insects10120420
APA StyleGrünewald, B., & Siefert, P. (2019). Acetylcholine and Its Receptors in Honeybees: Involvement in Development and Impairments by Neonicotinoids. Insects, 10(12), 420. https://doi.org/10.3390/insects10120420



