Towards Precision Nutrition: A Novel Concept Linking Phytochemicals, Immune Response and Honey Bee Health
Abstract
1. Introduction
2. Honey Bee Immunity
2.1. Social Immunity
2.1.1. Nest Hygiene
2.1.2. Antiseptic Compounds
2.1.3. Plant-Derived Compounds for Nutrition and Defense
2.1.4. Thermoregulation
2.1.5. Nest Defense
2.2. Individual Immunity: A Brief Summary
2.3. Interaction Between Social and Individual Immunity
3. Nutrition and Immunity
3.1. Generalities
3.2. Particularities: Phytochemicals as Dietary Treatments for Medication
4. Honey Bees Within the Context of Innate Immunity Research
4.1. Janus Kinases/signal Transducer and Activator of Transcription Proteins (JAK/STAT) and Nitric Oxide (NO) Signaling
4.2. Abscisic acid in Animals’ Immunity
4.3. JAK/STAT and Cold Stress
4.4. JAK/STAT and Wound-Healing
4.5. Wound-Healing, NO and ABA in Honey Bees
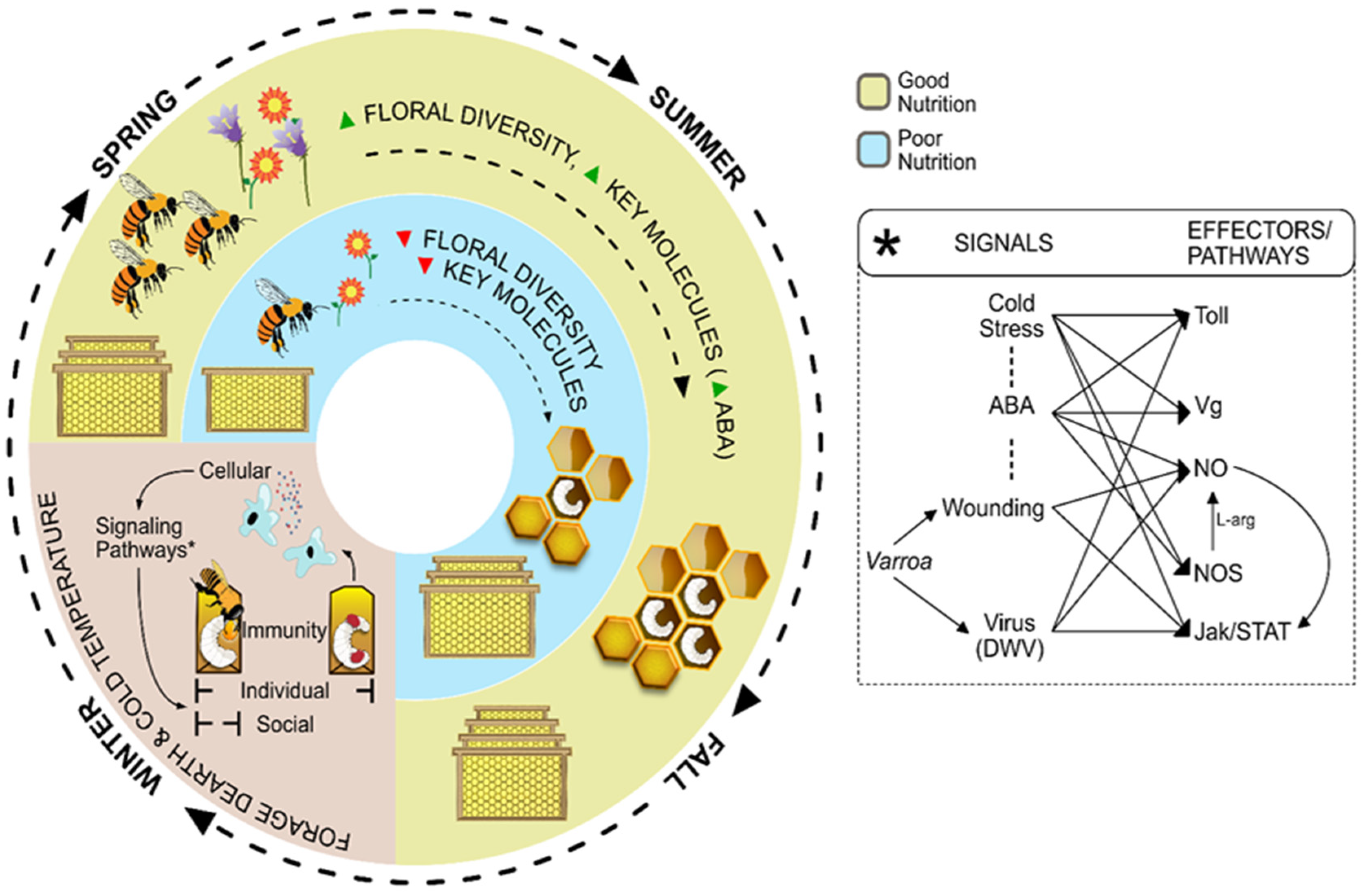
5. Varroa-DWV Within the Context of Innate Immune Responses
5.1. The Importance of the Hemocytes’ Role
5.2. Varroa-DWV and Immune Response: Pathways Shared by JAK/STAT, Toll, ABA and NO Signaling
5.3. Varroa-DWV and Environmental Factors: The Interaction Between the Immune System and Cold Temperatures
6. Key Molecules and Priming Effects
7. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Van Engelsdorp, D.A.; Meixner, M.D. A historical review of managed honey bee populations in Europe and the United States and the factors that may affect them. J. Invertebr. Pathol. 2010, 103, S80–S95. [Google Scholar] [CrossRef] [PubMed]
- Van Engelsdorp, D.; Caron, D.; Hayes, J.; Underwood, R.; Henson, M.; Rennich, K.; Spleen, A.; Andree, M.; Snyder, R.; Lee, K.; et al. A national survey of managed honey bee 2010–11 winter colony losses in the USA: Results from the Bee Informed Partnership. J. Apic. Res. 2012, 51, 115–124. [Google Scholar] [CrossRef]
- Maggi, M.; Antúnez, K.; Invernizzi, C.; Aldea, P.; Vargas, M.; Negri, P.; Brasesco, C.; de Jong, D.; Message, D.; Teixeira, E.W. Honeybee health in South America. Apidologie 2016, 47, 835–854. [Google Scholar] [CrossRef]
- Moritz, R.F.A.; Erler, S. Lost colonies found in a data mine: Global honey trade but not pests or pesticides as a major cause of regional honeybee colony declines. Agric. Ecosyst. Environ. 2016, 216, 44–50. [Google Scholar] [CrossRef]
- Van Engelsdorp, D.; Hayes, J., Jr.; Underwood, R.M.; Pettis, J.S. A survey of honey bee colony losses in the U.S., fall 2007 to spring 2008. PLoS ONE 2008, 3, e4071. [Google Scholar]
- Meixner, M.D.; Le Conte, Y. A current perspective on honey bee health. Apidologie 2016, 47, 273–275. [Google Scholar] [CrossRef]
- Grozinger, C.M.; Evans, J.D. From the lab to the landscape: Translational approaches to pollinator health. Curr. Opin. Insect Sci. 2015, 10, 7–9. [Google Scholar]
- Smart, M.; Pettis, J.; Rice, N.; Browning, Z.; Spivak, M. Linking measures of colony and individual honey bee health to survival among apiaries exposed to varying agricultural land use. PLoS ONE 2016, 11, e0152685. [Google Scholar] [CrossRef] [PubMed]
- De Grandi-Hoffman, G.; Chen, Y. Nutrition, immunity and viral infections in honey bees. Curr. Opin. Insect Sci. 2015, 10, 170–176. [Google Scholar] [CrossRef] [PubMed]
- McMenamin, A.J.; Brutscher, L.M.; Glenny, W.; Flenniken, M.L. Abiotic and biotic factors affecting the replication and pathogenicity of bee viruses. Curr. Opin. Insect Sci. 2016, 16, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Van Dooremalen, C.; Gerritsen, L.; Cornelissen, B.; van der Steen, J.J.M.; van Langevelde, F.; Blacquière, T. Winter survival of individual honey bees and honey bee colonies depends on level of Varroa destructor infestation. PLoS ONE 2012, 7, e36285. [Google Scholar] [CrossRef] [PubMed]
- Döke, M.A.; Frazier, M.; Grozinger, C.M. Overwintering honey bees: Biology and management. Curr. Opin. Insect Sci. 2015, 10, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Steinmann, N.; Corona, M.; Neumann, P.; Dainat, B. Overwintering is associated with reduced expression of immune genes and higher susceptibility to virus infection in honey bees. PLoS ONE 2015, 10, e0129956. [Google Scholar] [CrossRef] [PubMed]
- Mondet, F.; Kim, S.H.; de Miranda, J.R.; Beslay, D.; Le Conte, Y.; Mercer, A.R. Specific cues associated with honey bee social defence against Varroa destructor infested brood. Sci. Rep. 2016, 6, 25444. [Google Scholar] [CrossRef] [PubMed]
- Di Prisco, G.; Annoscia, D.; Margiotta, M.; Ferrara, R.; Varricchio, P.; Zanni, V.; Caprio, E.; Nazzi, F.; Pennacchio, F. A mutualistic symbiosis between a parasitic mite and a pathogenic virus undermines honey bee immunity and health. Proc. Natl. Acad. Sci. USA 2016, 113, 3203–3208. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xu, X.; Zhu, X.; Chen, L.; Zhou, S.; Huang, Z.Y.; Zhou, B. Low-temperature stress during capped brood stage increases pupal mortality, misorientation and adult mortality in honey bees. PLoS ONE 2016, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Cremer, S.; Armitage, S.A.O.; Schmid-Hempel, P. Social immunity. Curr. Biol. 2007, 17, R693–R702. [Google Scholar] [CrossRef] [PubMed]
- Simone-Finstrom, M. Social immunity and the superorganism: Behavioral defenses protecting honey bee colonies from pathogens and parasites. Bee World 2017, 94, 21–29. [Google Scholar] [CrossRef]
- Rothenbuhler, W.C. Behaviour genetics of nest cleaning in honey bees. I. Responses of four inbred lines to disease-killed brood. Anim. Behav. 1964, 12, 578–583. [Google Scholar] [CrossRef]
- Spivak, M.; Reuter, G.S. Performance of hygienic honey bee colonies in a commercial apiary. Apidologie 1998, 29, 291–302. [Google Scholar] [CrossRef]
- Boecking, O.; Drescher, W. Response of Apis mellifera L. colonies infested with Varroa jacobsoni Oud. Apidologie 1991, 22, 237–241. [Google Scholar] [CrossRef][Green Version]
- Harbo, J.R.; Harris, J.W. Suppressed mite reproduction explained by the behaviour of adult bees. J. Apic. Res. 2005, 44, 21–23. [Google Scholar] [CrossRef]
- Panziera, D.; van Langevelde, F.; Blacquière, T. Varroa sensitive hygiene contributes to naturally selected varroa resistance in honey bees. J. Apic. Res. 2017, 56, 635–642. [Google Scholar] [CrossRef]
- Wilson-Rich, N.; Spivak, M.; Fefferman, N.H.; Starks, P.T. Genetic, individual, and group facilitation of disease resistance in insect societies. Ann. Rev. Entomol. 2009, 54, 405–423. [Google Scholar] [CrossRef] [PubMed]
- Boecking, O.; Spivak, M. Behavioral defenses of honey bees against Varroa jacobsoni Oud. Apidologie 1999, 30, 141–158. [Google Scholar] [CrossRef]
- Pritchard, D.J. Grooming by honey bees as a component of varroa resistant behaviour. J. Apic. Res. 2016, 55, 38–48. [Google Scholar] [CrossRef]
- Bucekova, M.; Valachova, I.; Kohutova, L.; Prochazka, E.; Klaudiny, J.; Majtan, J. Honeybee glucose oxidase—its expression in honeybee workers and comparative analyses of its content and H2O2-mediated antibacterial activity in natural honeys. Naturwissenschaften 2014, 101, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, S.; Imai, J.; Fujiwara, M.; Yaeshima, T.; Kawashima, T.; Kobayashi, K. A potent antibacterial protein in royal jelly purification and determination of the primary structure of royalisin. J. Biol. Chem. 1990, 265, 11333–11337. [Google Scholar] [PubMed]
- Blum, M.S.; Novak, A.F.; Taber, S. 10-Hydroxy-delta 2-decenoic acid, an antibiotic found in royal jelly. Science 1959, 130, 452–453. [Google Scholar] [CrossRef] [PubMed]
- Lihoreau, M.; Buhl, J.; Charleston, M.A.; Sword, G.A.; Raubenheimer, D.; Simpson, S.J. Nutritional ecology beyond the individual: A conceptual framework for integrating nutrition and social interactions. Ecol. Lett. 2015, 18, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.A.; Nicolson, S.W.; Shafir, S. Nutritional physiology and ecology of honey bees. Annu. Rev. Entomol. 2018, 63, 327–344. [Google Scholar] [CrossRef] [PubMed]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernández-López, J.; Pérez-Alvarez, J.A. Functional properties of honey, propolis, and royal jelly. J. Food Sci. 2008, 73, R117–R124. [Google Scholar] [CrossRef] [PubMed]
- Erler, S.; Moritz, R.F.A. Pharmacophagy and pharmacophory: Mechanisms of self-medication and disease prevention in the honeybee colony (Apis mellifera). Apidologie 2016, 47, 389–411. [Google Scholar] [CrossRef]
- Simone-Finstrom, M.D.; Spivak, M. Increased resin collection after parasite challenge: A case of self-medication in honey bees? PLoS ONE 2012, 7, e34601. [Google Scholar] [CrossRef] [PubMed]
- Borba, R.S.; Spivak, M. Propolis envelope in Apis mellifera colonies supports honey bees against the pathogen, Paenibacillus larvae. Sci. Rep. 2017, 7, 11429. [Google Scholar] [CrossRef] [PubMed]
- Gherman, B.I.; Denner, A.; Bobiş, O.; Dezmirean, D.S.; Mărghitaş, L.A.; Schlüns, H.; Moritz, R.F.A.; Erler, S. Pathogen-associated self-medication behavior in the honeybee Apis mellifera. Behav. Ecol. Sociobiol. 2014, 68, 1777–1784. [Google Scholar] [CrossRef]
- Münch, D.; Kreibich, C.D.; Amdam, G.V. Aging and its modulation in a long-lived worker caste of the honey bee. J. Exp. Biol. 2013, 216, 1638–1649. [Google Scholar] [CrossRef] [PubMed]
- Ricigliano, V.A.; Mott, B.M.; Maes, P.W.; Floyd, A.S.; Fitz, W.; Copeland, D.C.; Meikle, W.G.; Anderson, K.E. Honey bee colony performance and health are enhanced by apiary proximity to US Conservation Reserve Program (CRP) lands. Sci. Rep. 2019, 9, 4894. [Google Scholar] [CrossRef] [PubMed]
- Amdam, G.V. Social context, stress, and plasticity of aging. Aging Cell 2011, 10, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Amdam, G.V.; Simoes, Z.L.P.; Hagen, A.; Norberg, K.; Schroder, K.; Mikkelsen, O.; Kirkwoodd, T.B.; Omholt, S.W. Hormonal control of the yolk precursor vitellogenin regulates immune function and longevity in honeybees. Exp. Gerontol. 2004, 39, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Alaux, C.; Dantec, C.; Parrinello, H.; Le Conte, Y. Nutrigenomics in honey bees: Digital gene expression analysis of pollen’s nutritive effects on healthy and varroa-parasitized bees. BMC Genom. 2011, 12, 496. [Google Scholar] [CrossRef] [PubMed]
- Di Pasquale, G.; Salignon, M.; Le Conte, Y.; Belzunces, L.P.; Decourtye, A.; Kretzschmar, A.; Suchail, S.; Brunet, J.L.; Alaux, C. Influence of pollen nutrition on honey bee health: Do pollen quality and diversity matter? PLoS ONE 2013, 8, e72016-13. [Google Scholar] [CrossRef] [PubMed]
- Münch, D.; Amdam, G.V. The curious case of aging plasticity in honey bees. FEBS Lett. 2010, 584, 2496–2503. [Google Scholar] [CrossRef] [PubMed]
- Antúnez, K.; Mendoza, Y.; Santos, E.; Invernizzi, C. Differential expression of vitellogenin in honey bees (Apis mellifera) with different degrees of Nosema ceranae infection. J. Apic. Res. 2013, 52, 227–234. [Google Scholar] [CrossRef]
- Garrido, P.M.; Porrini, M.P.; Antúnez, K.; Branchiccela, B.; Martínez-Noël Zunino, P.; Salerno, G.; Eguaras, M.J.; Ieno, E. Sublethal effects of acaricides and Nosema ceranae infection on immune related gene expression in honeybees. Vet. Res. 2016, 47, 51. [Google Scholar] [CrossRef] [PubMed]
- Stabentheiner, A.; Kovac, H.; Brodschneider, R. Honeybee colony thermoregulation–regulatory mechanisms and contribution of individuals in dependence on age, location and thermal stress. PLoS ONE 2010, 5, e8967. [Google Scholar] [CrossRef] [PubMed]
- Starks, P.T.; Blackie, C.A.; Seeley, T.D. Fever in honeybee colonies. Naturwissenschaften 2000, 87, 229–231. [Google Scholar] [CrossRef] [PubMed]
- Gätschenberger, H.; Azzami, K.; Tautz, J.; Beier, H. Antibacterial immune competence of honey bees (A. mellifera) is adapted to different life stages and environmental risks. PLoS ONE 2013, 8, e66415. [Google Scholar] [CrossRef] [PubMed]
- Tautz, J.; Maier, S.; Groh, C.; Rössler, W.; Brockmann, A. Behavioral performance in adult honey bees is influenced by the temperature experienced during their pupal development. Proc. Natl. Acad. Sci. USA 2003, 100, 7343–7347. [Google Scholar] [CrossRef] [PubMed]
- Groh, C.; Tautz, J.; Rössler, W. Synaptic organization in the adult honey bee brain is influenced by brood-temperature control during pupal development. Proc. Natl. Acad. Sci. USA 2004, 101, 4268–4273. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, L.; Negri, P.; Sturla, L.; Guida, L.; Vigliarolo, T.; Maggi, M.; Eguaras, M.; Zocchi, E.; Lamattina, L. Abscisic acid enhances cold tolerance in honeybee larvae. Proc. Biol. Sci. 2017, 284, 20162140. [Google Scholar] [CrossRef] [PubMed]
- Alaux, C.; Robinson, G.E. Alarm pheromone induces immediate-early gene expression and slow behavioral response in honey bees. J. Chem. Ecol. 2007, 33, 1346–1350. [Google Scholar] [CrossRef] [PubMed]
- Nouvian, M.; Reinhard, J.; Giurfa, M. The defensive response of the honeybee Apis mellifera. J. Exp. Biol. 2016, 219, 3505–3517. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.; Medina, L.M. Africanized honeybees have unique tolerance to Varroa mites. Trends Parasitol. 2004, 20, 112–114. [Google Scholar] [CrossRef] [PubMed]
- Rittschof, C.C.; Coombs, C.B.; Frazier, M.; Grozinger, C.M.; Robinson, G.E. Early-life experience affects honey bee aggression and resilience to immune challenge. Sci. Rep. 2015, 5, 15572. [Google Scholar] [CrossRef] [PubMed]
- Arechavaleta-Velasco, M.; Guzman-Novoa, E. Relative effect of four characteristics that restrain the population growth of the mite Varroa destructor in honey bee (Apis mellifera) colonies. Apidologie 2001, 32, 157–174. [Google Scholar] [CrossRef]
- Rittschof, C.C.; Robinson, G.E. Manipulation of colony environment modulates honey bee aggression and brain gene expression. Genes Brain Behav. 2013, 12, 802–811. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.D.; Aronstein, K.; Chen, Y.P.; Hetru, C.; Imler, J.L.; Jiang, H.; Kanost, M.; Thompson, G.J.; Zou, Z.; Hultmark, D. Immune pathways and defence mechanisms in honey bees Apis mellifera. Insect Mol. Biol. 2006, 15, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Barribeau, S.M.; Sadd, B.M.; du Plessis, L.; Brown, M.J.; Buechel, S.D.; Cappelle, K.; Carolan, J.C.; Christiaens, O.; Colgan, T.J.; Erler, S.; et al. A depauperate immune repertoire precedes evolution of sociality in bees. Genome Biol. 2015, 16, 83–103. [Google Scholar] [CrossRef] [PubMed]
- Chan, Q.W.T.; Melathopoulos, A.P.; Pernal, S.F.; Foster, L.J. The innate and systemic response in honey bees to a bacterial pathogen, Paenibacillus larvae. BMC Genom. 2009, 10, 387. [Google Scholar] [CrossRef] [PubMed]
- Nappi, A.; Christensen, B. Melanogenesis and associated cytotoxic reactions: Applications to insect innate immunity. Insect Biochem. Mol. Biol. 2005, 35, 443–459. [Google Scholar] [CrossRef] [PubMed]
- González-Santoyo, I.; Córdoba-Aguilar, A. Phenoloxidase: A key component of the insect immune system. Entomol. Exp. Appl. 2012, 142, 1–16. [Google Scholar] [CrossRef]
- Wilson-Rich, N.; Dres, D.; Starks, P. The ontogeny of immunity: Development of innate immune strength in the honey bee (A. mellifera). J. Insect Physiol. 2008, 54, 1392–1399. [Google Scholar] [CrossRef] [PubMed]
- Schmid, M.R.; Brockmann, A.; Pirka, C.W.W.; Stanley, D.W.; Tautz, J. Adult honeybees (A. mellifera L.) abandon hemocytic, but not phenoloxidase-based immunity. J. Insect Physiol. 2008, 54, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Laughton, A.M.; Boots, M.; Siva-Jothy, M.T. The ontogeny of immunity in the honey bee, A. mellifera L. following an immune challenge. J. Insect Physiol. 2011, 57, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Korayem, A.M.; Khodairy, M.M.; Abdel-Aal, A.A.A.; El-Sonbaty, A.A. The protective strategy of antioxidant enzymes against hydrogen peroxide in honey bee, Apis mellifera during two different seasons. J. Biol. Earth Sci. 2012, 2, B93–B109. [Google Scholar]
- Weirich, G.F.; Coliins, A.M.; Williams, V.P. Antioxidant enzymes in the honey bee, Apis mellifera. Apidologie 2002, 33, 3–14. [Google Scholar] [CrossRef]
- Bogdan, C. NO synthase in innate and adaptive immunity: An update. Trends Immunol. 2015, 36, 161–178. [Google Scholar] [CrossRef] [PubMed]
- Rivero, A. Nitric oxide: An antiparasitic molecule of invertebrates. Trends Parasitol. 2006, 22, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.; Dow, J. Modulation of epithelial innate immunity by autocrine production of nitric oxide. Gen. Comp. Endocrinol. 2009, 162, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Hillyer, J.; Estévez-Lao, T. Nitric oxide is an essential component of the hemocyte-mediated mosquito immune response against bacteria. Dev. Comp. Immunol. 2010, 34, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Negri, P.; Maggi, M.; Correa-Aragunde, N.; Brasesco, C.; Eguaras, M.; Lamattina, L. NO participates at the first steps of A. mellifera cellular immune activation in response to non-self-recognition. Apidologie 2013, 44, 575–585. [Google Scholar] [CrossRef]
- Negri, P.; Quintana, S.; Maggi, M.; Szawarski, N.; Lamattina, L.; Eguaras, M. Apis mellifera hemocytes generate increased amounts of nitric oxide in response to wounding/encapsulation. Apidologie 2015, 45, 610–617. [Google Scholar] [CrossRef]
- Negri, P.; Ramirez, L.; Quintana, S.; Szawarski, N.; Maggi, M.; Le Conte, Y.; Lamattina, L.; Eguaras, M. Dietary supplementation of honey bee larvae with arginine and abscisic acid enhances nitric oxide and granulocyte immune responses after trauma. Insects 2017, 8, 85. [Google Scholar] [CrossRef] [PubMed]
- Strand, M.R. The insect cellular immune response. Insect Sci. 2008, 15, 1–14. [Google Scholar] [CrossRef]
- Negri, P.; Maggi, M.; Ramirez, L.; Szawarski, N.; de Feudis, L.; Lamattina, L.; Eguaras, M. Cellular immunity in Apis mellifera: Studying hemocytes brings light about bees skills to confront threats. Apidologie 2015, 47, 379–388. [Google Scholar] [CrossRef]
- Burritt, N.L.; Foss, N.J.; Neeno-Eckwall, E.C.; Church, J.O.; Hilger, A.M.; Hildebrand, J.A.; Warshauer, D.M.; Perna, N.T.; Burritt, J.B. Sepsis and hemocyte loss in honey bees (Apis mellifera) infected with Serratia marcescens strain Sicaria. PLoS ONE 2016, 11, e0167752. [Google Scholar] [CrossRef] [PubMed]
- Walderdorff, L.; Laval-Gilly, P.; Bonnefoy, A.; Falla-Angel, J. Imidacloprid intensifies its impact on honeybee and bumblebee cellular immune response when challenged with LPS (lippopolysacharide) of Escherichia coli. J. Insect Physiol. 2018, 108, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Koleoglu, G.; Goodwin, P.H.; Reyes-Quintana, M.; Hamiduzzaman, M.M.; Guzman-Novoa, E. Varroa destructor parasitism reduces hemocyte concentrations and prophenol oxidase gene expression in bees from two populations. Parasitol. Res. 2018, 117, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Annoscia, D.; Brown, S.P.; Di Prisco, G.; de Paoli, E.; Del Fabbro, S.; Frizzera, D.; Zanni, V.; Galbraith, D.A.; Caprio, E.; Grozinger, C.M.; et al. Haemolymph removal by Varroa mite destabilizes the dynamical interaction between immune effectors and virus in bees, as predicted by Volterra’s model. Proc. Biol. Sci. B 2019, 286, 20190331. [Google Scholar] [CrossRef] [PubMed]
- Gábor, E.; Cinege, G.; Csordás, G.; Török, T.; Folkl-Medzihradszky, K.; Darula, Z.; Andó, I.; Kurucz, É. Hemolectin expression reveals functional heterogeneity in honey bee (Apis mellifera) hemocytes. Dev. Comp. Immunol. 2017, 76, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Richardson, R.T.; Ballinger, M.N.; Qian, F.; Christman, J.W.; Johnson, R.M. Morphological and functional characterization of honey bee, Apis mellifera, hemocyte cell communities. Apidologie 2018, 49, 397–410. [Google Scholar] [CrossRef]
- Page, P.; Lin, Z.; Buawangpong, N.; Zheng, H.; Hu, F.; Neumann, P.; Chantawannakul, P.; Dietemann, V. Social apoptosis in honey bee superorganisms. Sci. Rep. 2016, 6, 27210. [Google Scholar] [CrossRef] [PubMed]
- Richard, F.J.; Holt, H.L.; Grozinger, C.M. Effects of immunostimulation on social behavior, chemical communication and genome-wide gene expression in honey bee workers (Apis mellifera). BMC Genom. 2012, 13, 558. [Google Scholar] [CrossRef] [PubMed]
- Harpur, B.A.; Chernyshova, A.; Soltani, A.; Tsvetkov, N.; Mahjoorighasrodashti, M.; Xu, Z.; Zayed, A. No genetic tradeoffs between hygienic behaviour and individual innate immunity in the honey bee, Apis mellifera. PLoS ONE 2014, 9, e104214. [Google Scholar] [CrossRef] [PubMed]
- Genersch, E.; Ashiralieva, A.; Fries, I. Strain-and genotype-specific differences in virulence of Paenibacillus larvae subsp. larvae, a bacterial pathogen causing American foulbrood disease in honeybees. Appl. Environ. Microbiol. 2005, 71, 7551–7555. [Google Scholar] [CrossRef] [PubMed]
- Genersch, E.; Forsgren, E.; Pentikäinen, J.; Ashiralieva, A.; Rauch, S.; Kilwinski, J.; Fries, I. Reclassification of Paenibacillus larvae subsp. pulvifaciens and Paenibacillus larvae subsp. larvae as Paenibacillus larvae without subspecies differentiation. Int. J. Syst. Evol. Microbiol. 2006, 56, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Crailsheim, K.; Brodschneider, R.; Aupinel, P.; Behrens, D.; Genersch, E.; Vollmann, J.; Riessberger-Gallé, U. Standard methods for artificial rearing of Apis mellifera larvae. J. Apic. Res. 2013, 52, 1–15. [Google Scholar] [CrossRef]
- Sediva, M.; Klaudiny, J. The antimicrobial substances of royal jelly. Chem. Listy 2015, 109, 755–761. [Google Scholar]
- Buttstedt, A.; Moritz, R.F.A.; Erler, S. Origin and function of the major royal jelly proteins of the honeybee (Apis mellifera) as members of the yellow gene family. Biol. Rev. 2014, 89, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Buttstedt, A.; Moritz, R.F.A.; Erler, S. More than royal food-Major royal jelly protein genes in sexuals and workers of the honeybee Apis mellifera. Front. Zool. 2013, 10, 72. [Google Scholar] [CrossRef] [PubMed]
- Vezeteu, T.V.; Bobi¸s, O.; Moritz, R.F.A.; Buttstedt, A. Food to some, poison to others-honeybee royal jelly and its growth inhibiting effect on European Foulbrood bacteria. MicrobiologyOpen 2017, 6, e00397. [Google Scholar] [CrossRef] [PubMed]
- Buttstedt, A.; Mure¸san, C.I.; Lilie, H.; Hause, G.; Ihling, C.H.; Schulze, S.H.; Pietzsch, M.; Moritz, R.F.A. How honeybees defy gravity with royal jelly to raise queens. Curr. Biol. 2018, 28, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Goulson, D. Effects of introduced bees on native ecosystems. Annu. Rev. Ecol. Syst. 2003, 34, 1–26. [Google Scholar] [CrossRef]
- Vaudo, A.D.; Tooker, J.F.; Grozinger, C.M.; Patch, H.M. Bee nutrition and floral resource restoration. Curr. Opin. Insect Sci. 2015, 10, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Alaux, C.; Ducloz, F.; Crauser, D.; Le Conte, Y. Diet effects on honeybee immunocompetence. Biol. Lett. 2010, 6, 562–565. [Google Scholar] [CrossRef] [PubMed]
- Branchiccela, B.; Castelli, L.; Corona, M.; Díaz-Cetti, S.; Invernizzi, C.; Martínez de la Escalera, G.; Mendoza, Y.; Santos, E.; Silva, C.; Zunino, P.; et al. Impact of nutritional stress on the honeybee colony health. Sci. Rep. 2019, 9, 10156. [Google Scholar] [CrossRef] [PubMed]
- Di Pasquale, G.; Alaux, C.; Le Conte, Y.; Odoux, J.-F.; Pioz, M.; Vaissière, B.E.; Belzunces, L.P.; Decourtye, A. Variations in the availability of pollen resources affect honey bee health. PLoS ONE 2016, 11, e0162818. [Google Scholar] [CrossRef] [PubMed]
- Kriesell, L.; Hilpert, A.; Leonhardt, S.D. Different but the same: Bumblebee species collect pollen of different plant sources but similar amino acid profiles. Apidologie 2017, 48, 102–116. [Google Scholar] [CrossRef]
- Mao, W.; Schuler, M.A.; Berenbaum, M.R. Honey constituents up-regulate detoxification and immunity genes in the western honey bee Apis mellifera. Proc. Natl. Acad. Sci. USA 2013, 110, 8842–8846. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, M.M.; Robinson, G.E. Diet-dependent gene expression in honey bees: Honey vs. sucrose or high fructose corn syrup. Sci. Rep. 2014, 4, 5726. [Google Scholar] [CrossRef] [PubMed]
- Schmehl, D.R.; Teal, P.E.; Frazier, J.L.; Grozinger, C.M. Genomic analysis of the interaction between pesticide exposure and nutrition in honey bees (Apis mellifera). J. Insect Physiol. 2014, 71, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Simone, M.; Evans, J.; Spivak, M. Resin collection and social immunity in honey bees. Evolution 2009, 63, 3016–3022. [Google Scholar] [CrossRef] [PubMed]
- Niu, G.; Johnson, R.; Berenbaum, M. Toxicity of mycotoxins to honeybees and its amelioration by propolis. Apidologie 2011, 42, 79–87. [Google Scholar] [CrossRef]
- Erler, S.; Denner, A.; Bobis, O.; Forsgren, E.; Moritz, R.F.A. Diversity of honey stores and their impact on pathogenic bacteria of the honeybee, Apis mellifera. Ecol. Evol. 2014, 4, 3960–3967. [Google Scholar] [CrossRef] [PubMed]
- Negri, P.; Maggi, M.D.; Ramirez, L.; de Feudis, L.; Szawarski, N.; Quintana, S.; Eguaras, M.; Lamattina, L. Abscisic acid enhances the immune response in Apis mellifera and contributes to the colony fitness. Apidologie 2015, 46, 542–557. [Google Scholar] [CrossRef]
- Richardson, L.L.; Adler, L.S.; Leonard, A.S.; Andicoechea, J.; Regan, K.H.; Anthony, W.E.; Manson, J.S.; Irwin, R.E. Secondary metabolites in floral nectar reduce parasite infections in bumblebees. Proc. R. Soc. B 2015, 282, 2014–2471. [Google Scholar] [CrossRef] [PubMed]
- Negri, P.; Ramirez, L.; Quintana, S.; Szawarski, N.; Maggi, M.; Eguaras, M.; Lamattina, L. Immune-Related Gene Expression of Apis mellifera Larvae In Response to Cold Stress and Abscisic Acid (ABA) Dietary Supplementation. J. Apic. Res. 2019. accepted for publication. [Google Scholar]
- Wong, M.J.; Liao, L.-H.; Berenbaum, M.R. Biphasic concentration-dependent interaction between imidacloprid and dietary phytochemicals in honey bees (Apis mellifera). PLoS ONE 2018, 13, e0206625. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.-H.; Wu, W.Y.; Berenbaum, M. Impacts of dietary phytochemicals in the presence and absence of pesticides on longevity of honey bees (Apis mellifera). Insects 2017, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Palmer-Young, E.C.; Tozkar, C.Ö.; Schwarz, R.S.; Chen, Y.; Irwin, R.E.; Adler, L.S.; Evans, J.D. Nectar and pollen phytochemicals stimulate honey bee (Hymenoptera: Apidae) immunity to viral infection. J. Econ. Entomol. 2017, 110, 1959–1972. [Google Scholar] [CrossRef] [PubMed]
- Strachecka, A.; Krauze, M.; Olszewski, K.; Borsuk, G.; Paleolog, J.; Merska, M.; Chobotow, J.; Bajda, M.; Grzywnowicz, K. Unexpectedly strong effect of caffeine on the vitality of western honeybees (Apis mellifera). Biochem. Mosc. 2014, 79, 1192–1201. [Google Scholar] [CrossRef] [PubMed]
- Bernklau, E.; Bjostad, L.; Hogeboom, A.; Carlisle, A.; Arathi, H.S. Dietary phytochemicals, honey bee longevity and pathogen tolerance. Insects 2019, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Baracchi, D.; Brown, M.J.; Chittka, L. Behavioural evidence for self-medication in bumblebees? F1000Research 2015, 4, 73. [Google Scholar] [CrossRef] [PubMed]
- Thorburn, L.P.; Adler, L.S.; Irwin, R.E.; Palmer-Young, E.C. Variable effects of nicotine, anabasine, and their interactions on parasitized bumble bees. F1000Research 2015, 4, 880. [Google Scholar] [CrossRef] [PubMed]
- Szawarski, N.; Saez, A.; Domínguez, E.; Dickson, R.; Dematteis, A.; Eciolaza, C.; Justel, M.; Aliano, A.; Solar, P.; Bergara, I.; et al. Effect of abscisic acid (ABA) combined with two different beekeeping nutritional strategies to confront overwintering: Studies on honey bees’ population dynamics and nosemosis. Insects 2019, 10, 329. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, E.; Moliné, M.P.; Churio, M.S.; Arce, V.B.; Mártire, D.O.; Mendiara, S.N.; Álvarez, B.S.; Gende, L.B.; Damiani, N. Bioactivity of gallic acid–conjugated silica nanoparticles against Paenibacillus larvae and their host, Apis mellifera honeybee. Apidologie 2019, 50, 616–631. [Google Scholar] [CrossRef]
- Lipp, J. Nachweis und herkunft von abscisinsäure und prolin in honig. Apidologie 1990, 21, 249–259. [Google Scholar] [CrossRef]
- Tuberoso, C.I.; Bifulco, E.; Caboni, P.; Cottiglia, F.; Cabras, P.; Floris, I. Floral markers of strawberry tree (Arbutus unedo L.) honey. J. Agric. Food Chem. 2010, 58, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, J.A. The immune response of Drosophila. Nature 2003, 426, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Beutler, B. Innate immunity: An overview. Biochem. Mol. Immunol. 2004, 40, 845–859. [Google Scholar] [CrossRef] [PubMed]
- Kingsolver Megan, B.; Huang, Z.; Hardy, R.W. Insect antiviral innate immunity: Pathways, effectors, and connections. J. Mol. Biol. 2013, 425, 4921–4936. [Google Scholar] [CrossRef] [PubMed]
- Gupta, L.; Molina-Cruz, A.; Kumar, S.; Rodrigues, J.; Dixit, R.; Zamora, R.E.; Barillas-Mury, C. The STAT pathway mediates late phase immunity against Plasmodium in the mosquito Anopheles gambiae. Cell Host Microbe 2009, 5, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Agaisse, H.; Perrimon, N. The roles of JAK/STAT signaling in Drosophila immune responses. Immunol. Rev. 2004, 198, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Nappi, A.J.; Vass, E.; Frey, F.; Carton, Y. Nitric oxide involvement in Drosophila immunity. Nitric Oxide 2000, 4, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Reiss, C.S.; Komatsu, T. Does no play a critical role in viral infections? J. Virol. 1998, 72, 4547–4551. [Google Scholar] [PubMed]
- Chen, C.W.; Chao, Y.; Chang, Y.H.; Hsu, M.J.; Lin, W.W. Inhibition of cytokine-induced JAK-STAT signalling pathways by an endonuclease inhibitor aurintricarboxylic acid. Br. J. Pharmacol. 2002, 137, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Dostert, C.; Jouanguy, E.; Irving, P.; Troxler, L.; Galiana-Arnoux, D.; Hetru, C.; Hoffmann, J.A.; Imler, J.L. The Jak-STAT signaling pathway is required but not sufficient for the antiviral response of Drosophila. Nat. Immunol. 2005, 6, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Barillas-Mury, C.; Han, Y.S.; Seeley, D.; Kafatos, F.C. Anopheles gambiae Ag-STAT, a new insect member of the STAT family, is activated in response to bacterial infection. EMBO J. 1999, 18, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Glennon, E.K.K.; Adams, L.G.; Hicks, D.R.; Dehesh, K.; Luckhart, S. Supplementation with abscisic acid reduces malaria disease severity and parasite transmission. Am. J. Trop. Med. Hyg. 2016, 94, 1266–1275. [Google Scholar] [CrossRef] [PubMed]
- Nambara, E.; Marion-Poll, A. Abscisic acid biosynthesis and catabolism. Annu. Rev. Plant. Biol. 2005, 56, 165–185. [Google Scholar] [CrossRef] [PubMed]
- Adie, B.A.T.; Pérez-Pérez, J.; Pérez-Pérez, M.M.; Godoy, M.; Sánchez-Serrano, J.; Schmelz, E.A.; Solanoa, R. ABA is an essential signal for plant resistanceto pathogens affecting JA biosynthesis and the activation of defences in Arabidopsis. Plant. Cell 2007, 19, 1665–1681. [Google Scholar] [CrossRef] [PubMed]
- Frascaroli, E.; Tuberosa, R. Effect of abscisic acid on pollen germination and tube growth of maize genotypes 1. Plant. Breed. 1993, 110, 250–254. [Google Scholar] [CrossRef]
- Ferreres, F.; Andrade, P.; Tomás-Barberán, F. Natural occurrence of abscisic acid in heather honey and floral nectar. J. Agric. Food Chem. 1996, 44, 2053–2056. [Google Scholar] [CrossRef]
- Saxena, S.; Tripathi, J.; Chatterjee, S.; Gautam, S. Natural predominance of abscisic acid in Pongammia pinnata (“Karanj”) honey contributed to its strong antimutagenicity. J. Agric. Food Chem. 2017, 65, 4624–4633. [Google Scholar] [CrossRef] [PubMed]
- Bruzzone, S.; Moreschi, I.; Usai, C.; Guida, L.; Damonte, G.; Salis, A.; Scarfì, S.; Millo, E.; de Flora, A.; Zocchi, E. Abscisic acid is an endogenous cytokine in human granulocytes with cyclic ADP-ribose as second messenger. Proc. Natl. Acad. Sci. USA 2007, 104, 5759–5764. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Marshall, K.E.; Westwood, J.T.; Clark, M.S.; Sinclair, B.J. Divergent transcriptomic responses to repeated and single cold exposures in D. melanogaster. J. Exp. Biol. 2011, 214, 4021–4029. [Google Scholar] [CrossRef] [PubMed]
- Krams, I.; Daukste, J.; Kivleniece, I.; Krama, T.; Rantala, M.J. Overwinter survival depends on immune defense and body length in male Aquarius najas water striders. Entomol. Exp. Appl. 2011, 140, 45–51. [Google Scholar] [CrossRef]
- Vermeulen, C.J.; Sørensen, P.; Gagalova, K.K.; Loeschcke, V. Transcriptomic analysis of inbreeding depression in cold-sensitive D. melanogaster shows upregulation of the immune response. J. Evol. Biol. 2013, 26, 1890–1902. [Google Scholar] [CrossRef] [PubMed]
- Salehipourshirazi, G. Cold-Activation of the D. Melanogaster Immune System. Master’s Thesis, University of Western Ontario London, Ontario, ON, Canada, 2013. [Google Scholar]
- Ekengren, S.; Hultmark, D. A family of Turandot-related genes in the humoral stress response of Drosophila. Biochem. Biophys. Res. Commun. 2001, 284, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- Richards, E.; Jones, B.; Bowman, A. Salivary secretions from the honey bee mite, V. destructor: Effects on insect hemocytes and preliminary biochemical characterization. Parasitology 2011, 138, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.L.; Cox-Foster, D.L. Impact of an ectoparasite on the immunity and pathology of an invertebrate: Evidence for host immunosuppression and viral amplification. Proc. Natl. Acad. Sci. USA 2005, 102, 7470–7475. [Google Scholar] [CrossRef] [PubMed]
- Zaobidna, E.A.; Żółtowska, K.; Łopieńska-Biernat, E. Expression of the prophenoloxidase gene and phenoloxidase activity, during the development of A. mellifera brood infected with Varroa destructor. J. Apic. Sci. 2015, 59, 85–93. [Google Scholar] [CrossRef]
- Martin, S.J.; Highfield, A.C.; Brettell, L.; Villalobos, E.M.; Budge, G.E.; Powell, M.; Nikaido, S.; Schroeder, D.C. Global honey bee viral landscape altered by a parasitic mite. Science 2012, 336, 1304. [Google Scholar] [CrossRef] [PubMed]
- Rosenkranz, P.; Aumeier, P.; Ziegelmann, B. Biology and control of Varroa destructor. J. Invertebr. Pathol. 2010, 103, 96–119. [Google Scholar] [CrossRef] [PubMed]
- Nazzi, F.; Brown, S.P.; Annoscia, D.; Del Piccolo, F.; Di Prisco, G.; Varricchio, P.; Vedova, G.D.; Cattonaro, F.; Caprio, E.; Pennacchio, F. Synergistic parasite-pathogen interactions mediated by host immunity can drive the collapse of honeybee colonies. PLoS Pathog. 2012, 8, e1002735. [Google Scholar] [CrossRef] [PubMed]
- Kanbar, G.; Engels, W. Ultrastructure and bacterial infection of wounds in honey bee (A. mellifera) pupae punctured by Varroa mites. Parasitol. Res. 2003, 90, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Kanbar, G.; Engels, W. Communal use of integumental wounds in honey bee (A. mellifera) pupae multiply infested by the ectoparasitic mite Varroa destructor. Genet. Mol. Res. 2005, 4, 465–472. [Google Scholar] [PubMed]
- Ramsey, S.D.; Ochoa, R.; Bauchan, G.; Gulbronson, C.; Mowery, J.D.; Cohen, A.; Lim, D.; Joklik, J.; Cicero, J.M.; Ellis, J.M.; et al. Varroa destructor feeds primarily on honey bee fat body tissue and not hemolymph. Proc. Natl. Acad. Sci. USA 2019, 116, 1792–1801. [Google Scholar] [CrossRef] [PubMed]
- Eleftherianos, I.; Revenis, C. Role and importance of phenoloxidase in insect hemostasis. J. Innate Immun. 2011, 3, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Yang Wang, Y.; Deng, J.; Jiang, H. The structure of a prophenoloxidase (PPO) from Anopheles gambiae provides new insights into the mechanism of PPO activation. BMC Biol. 2016, 14, 2. [Google Scholar] [CrossRef] [PubMed]
- Ling, E.; Yu, X.Q. Prophenoloxidase binds to the surface of hemocytes and is involved in hemocyte melanization in Manduca sexta. Insect Biochem. Mol. Biol. 2005, 35, 1356–1366. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Heerman, M.; Peng, W.; Evans, J.D.; Rose, R.; DeGrandi-Hoffman, G.; Simone-Finstrom, M.; Li, J.; Li, Z.; Cook, S.C.; et al. The dynamics of Deformed wing virus concentration and host defensive gene expression after varroa mite parasitism in honey bees, Apis Mellifera. Insects 2019, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Quintana, S.; Brasesco, C.; Negri, P.; Marín, M.; Pagnuco, I.; Szawarski, N.; Reynaldi, F.; Larsen, A.; Eguaras, M.; Maggi, M. Up-regulated pathways in response to Deformed Wing Virus infection in Apis mellifera (Hymenoptera: Apidae). Rev. Soc. Entomol. Argent. 2019, 78, 1–11. [Google Scholar] [CrossRef]
- Gregorc, A.; Evans, J.D.; Scharf, M.; Ellis, J.D. Gene expression in honey bee (Apis mellifera) larvae exposed to pesticides and Varroa mites (Varroa destructor). J. Insect Physiol. 2012, 58, 1042–1049. [Google Scholar] [CrossRef] [PubMed]
- Ryabov, E.V.; Fannon, J.M.; Moore, J.D.; Wood, G.R.; Evans, D.J. The Iflaviruses Sacbrood virus and Deformed wing virus evoke different transcriptional responses in the honeybee which may facilitate their horizontal or vertical transmission. PeerJ 2016, 4, e1591. [Google Scholar] [CrossRef] [PubMed]
- Mullin, C.A.; Frazier, M.; Frazier, J.L.; Ashcraft, S.; Simonds, R.; van Engelsdorp, D.; Pettis, J.S. High levels of miticides and agrochemicals in North American apiaries: Implications for honey bee health. PLoS ONE 2010, 5, e9754. [Google Scholar] [CrossRef] [PubMed]
- Medici, S.K.; Blando, M.; Sarlo, E.; Maggi, M.; Espinosa, J.P.; Ruffinengo, S.; Bianchi, B.; Eguaras, M.; Recavarren, M. Pesticide residues used for pest control in honeybee colonies located in agroindustrial areas of Argentina. Int. J. Pest. Manag. 2019. [Google Scholar] [CrossRef]
- Valanne, S.; Wang, J.H.; Rämet, M. The Drosophila toll signaling pathway. J. Immunol. 2011, 186, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Di Prisco, G.; Cavaliere, V.; Annoscia, D.; Varricchio, P.; Caprio, E.; Nazzi, F.; Gargiulo, G.; Pennacchio, F. Neonicotinoid clothianidin adversely affects insect immunity and promotes replication of a viral pathogen in honey bees. Proc. Natl. Acad. Sci. USA 2013, 110, 18466–18471. [Google Scholar] [CrossRef] [PubMed]
- Arefin, B.; Kucerova, L.; Krautz, R.; Kranenburg, H.; Parvin, F.; Theopold, U. Apoptosis in hemocytes induces a shift in effector mechanisms in the Drosophila Immune System and leads to a Pro-Inflammatory State. PLoS ONE 2015, 10, e0136593. [Google Scholar] [CrossRef] [PubMed]
- Kraaijeveld, A.R.; Elrayes, N.P.; Schuppe, H.; Newland, P.L. L-arginine enhances immunity to parasitoids in Drosophila melanogaster and increases NO production in lamellocytes. Dev. Comp. Immunol. 2011, 35, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Sanzhaeva, U.; Vorontsova, Y.; Glazachev, Y.; Slepneva, I. Dual effect of nitric oxide on phenoloxidase-mediated melanisation. J. Enzym. Inhib. Med. Chem. 2015, 31, 1063–1068. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Semenova, A.D.; Glazachev, Y.I.; Slepneva, I.A.; Glupov, V.V. Quantitative determination of nitric oxide production in haemocytes: Nitrite reduction activity as a potential pathway of NO formation in haemolymph of Galleria mellonella larvae. Nitric Oxide 2014, 37, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Schmid-Hempel, P. Evolutionary ecology of insect immune defenses. Annu. Rev. Entomol. 2005, 50, 529–551. [Google Scholar] [CrossRef] [PubMed]
- Moret, Y.; Schmid-Hempel, P. Survival for immunity: The price of immune system activation for bumblebee workers. Science 2000, 290, 1166–1168. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; James, R.R. Temperature stress affects the expression of immune response genes in the alfalfa leafcutting bee, Megachile rotundata. Insect Mol. Biol. 2012, 21, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, B.J.; Ferguson, L.V.; Salehipourshirazi, G.; MacMillan, H.A. Cross-tolerance and cross-talk in the cold: Relating low temperatures to desiccation and immune stress in insects. Integr. Comp. Biol. 2013, 53, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Miyado, K.; Takezawa, Y.; Ohnami, N.; Sato, M.; Ono, C.; Harada, Y.; Yoshida, K.; Kawano, N.; Kanai, S.; et al. Innate immune system still works at diapause, a physiological state of dormancy in insects. Biochem. Biophys. Res. Commun. 2011, 410, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Linderman, J.A.; Chambers, M.C.; Gupta, A.S.; Schneider, D.S. Infection-related declines in chill coma recovery and negative geotaxis in Drosophila melanogaster. PLoS ONE 2012, 7, e41907. [Google Scholar] [CrossRef] [PubMed]
- Sadd, B.M.; Kleinlogel, Y.; Schmid-Hempel, R.; Schmid-Hempel, P. Trans-generational immune priming in a social insect. Biol. Lett. 2005, 1, 386–388. [Google Scholar] [CrossRef] [PubMed]
- Tidbury, H.J.; Pedersen, A.B.; Boots, M. Within and transgenerational immune priming in an insect to a DNA virus. Proc. Biol. Sci. 2011, 278, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Le Bourg, É.; Massou, I.; Gobert, V. Cold stress increases resistance to fungal infection throughout life in Drosophila melanogaster. Biogerontology 2009, 10, 613. [Google Scholar] [CrossRef] [PubMed]
- Richard, F.J.; Aubert, A.; Grozinger, C.M. Modulation of social interactions by immune stimulation in honey bee, Apis mellifera, workers. BMC Biol. 2008, 6, 50. [Google Scholar] [CrossRef] [PubMed]
- Youngsteadt, E.; Appler, R.H.; López-Uribe, M.M.; Tarpy, D.R.; Frank, S.D. Urbanization increases pathogen pressure on feral and managed honey bees. PLoS ONE 2015, 10, e0142031. [Google Scholar] [CrossRef] [PubMed]
- López-Uribe, M.M.; Appler, R.H.; Youngsteadt, E.; Dunn, R.R.; Frank, S.D.; Tarpy, D.R. Higher immunocompetence is associated with higher genetic diversity in feral honey bee colonies (Apis mellifera). Conserv. Genet. 2017, 18, 659–666. [Google Scholar] [CrossRef]
| Evidences | Phytochemical | ABA | CouA | Quer | Nic | Ana | Caff |
|---|---|---|---|---|---|---|---|
| Level of organization | Molecular | [52,107,109] | [101] | [110,111] | [112] | [112] | [113,114] |
| Cellular | [75,107] | ||||||
| Individual (organism) | [52,107] | [110,111,114] | [110,111] | [112,115,116] | [112,116] | [113,114] | |
| Colony (superorganism) | [107,117] | ||||||
| Experimental system | In-vitro rearing | [52,75,109] | [112] | ||||
| Laboratory | [111] | [101,110,111,114] | [110,111] | [112,115,116] | [112,116] | [113,114] | |
| Field | [107,117] | ||||||
| Ontogeny | Larva | [52,75,107,109,117] | |||||
| Pupa | [52,117] | ||||||
| Adult | [52,107,117] | [101,110,111,114] | [110,111] | [112,115,116] | [112,116] | [113,114] | |
| Related with | Immune response | [75,107,109] | [112] | ||||
| Parasite/Pathogen | [107,117] | [114] | [112,115,116] | [112,116] | [113,114] | ||
| Pesticides | [107] | [101,110,111] | [110,111] | ||||
| Abiotic stressors (not pesticides) | [52,109] | ||||||
| Total references | 5 | 4 | 2 | 3 | 2 | 2 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Negri, P.; Villalobos, E.; Szawarski, N.; Damiani, N.; Gende, L.; Garrido, M.; Maggi, M.; Quintana, S.; Lamattina, L.; Eguaras, M. Towards Precision Nutrition: A Novel Concept Linking Phytochemicals, Immune Response and Honey Bee Health. Insects 2019, 10, 401. https://doi.org/10.3390/insects10110401
Negri P, Villalobos E, Szawarski N, Damiani N, Gende L, Garrido M, Maggi M, Quintana S, Lamattina L, Eguaras M. Towards Precision Nutrition: A Novel Concept Linking Phytochemicals, Immune Response and Honey Bee Health. Insects. 2019; 10(11):401. https://doi.org/10.3390/insects10110401
Chicago/Turabian StyleNegri, Pedro, Ethel Villalobos, Nicolás Szawarski, Natalia Damiani, Liesel Gende, Melisa Garrido, Matías Maggi, Silvina Quintana, Lorenzo Lamattina, and Martin Eguaras. 2019. "Towards Precision Nutrition: A Novel Concept Linking Phytochemicals, Immune Response and Honey Bee Health" Insects 10, no. 11: 401. https://doi.org/10.3390/insects10110401
APA StyleNegri, P., Villalobos, E., Szawarski, N., Damiani, N., Gende, L., Garrido, M., Maggi, M., Quintana, S., Lamattina, L., & Eguaras, M. (2019). Towards Precision Nutrition: A Novel Concept Linking Phytochemicals, Immune Response and Honey Bee Health. Insects, 10(11), 401. https://doi.org/10.3390/insects10110401





