Reproduction of Distinct Varroa destructor Genotypes on Honey Bee Worker Brood
Abstract
1. Introduction
2. Methods and Materials
2.1. Honey Bees and Varroa Mites
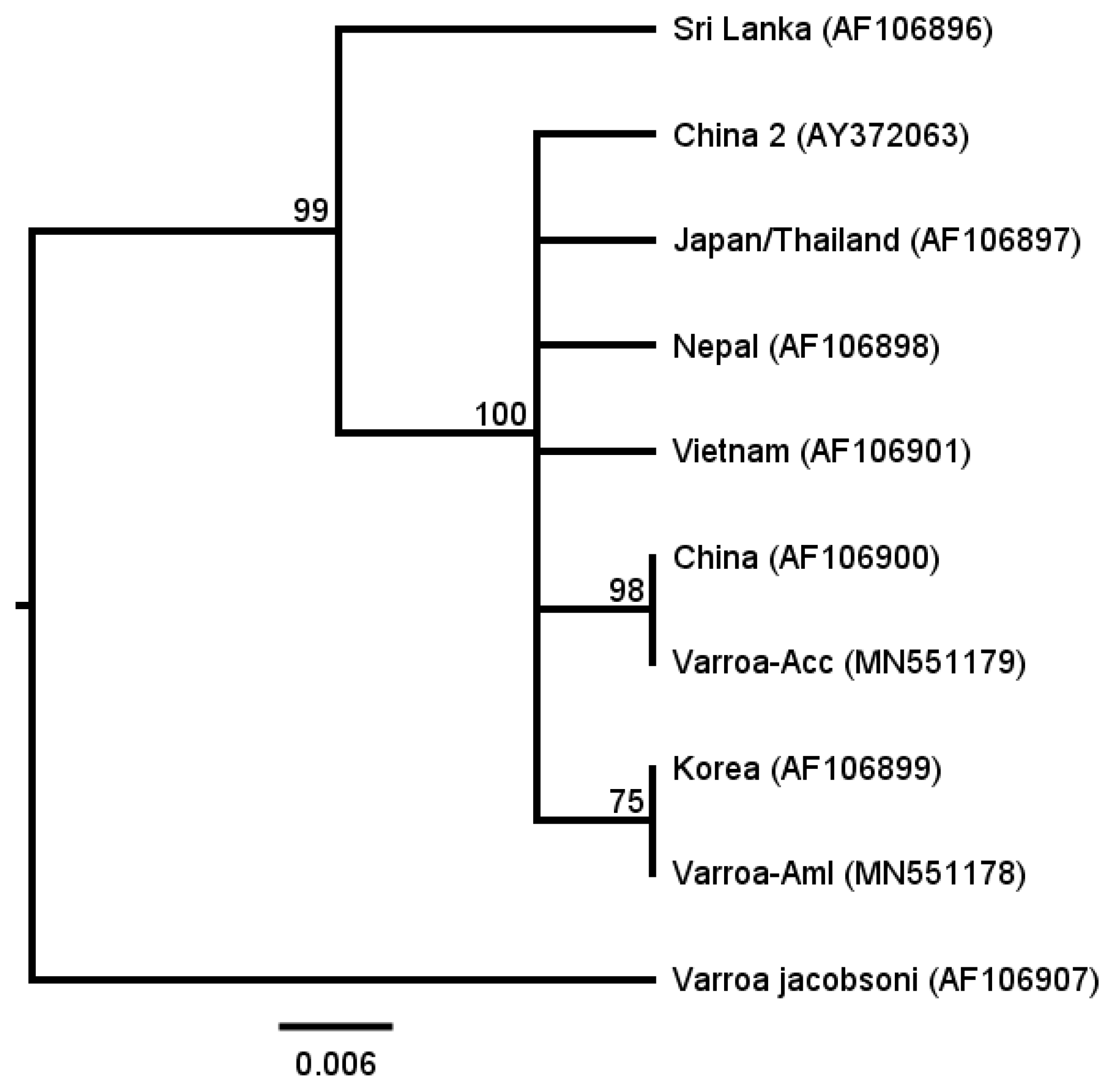
2.2. Genetic Identification of Varroa Mites
2.3. Preparation of Honey Bee Larvae
2.4. Collection and Inoculation of Varroa Mites
2.5. Experiment I. Reproduction of Varroa Mites Derived from A. mellifera Colonies
2.6. Experiment II. Reproduction of Varroa Mites Derived from A. cerana Colonies
2.7. Statistical Analyses
3. Results
3.1. Genetic Difference between Varroa Destructor from Aml and Acc Colonies
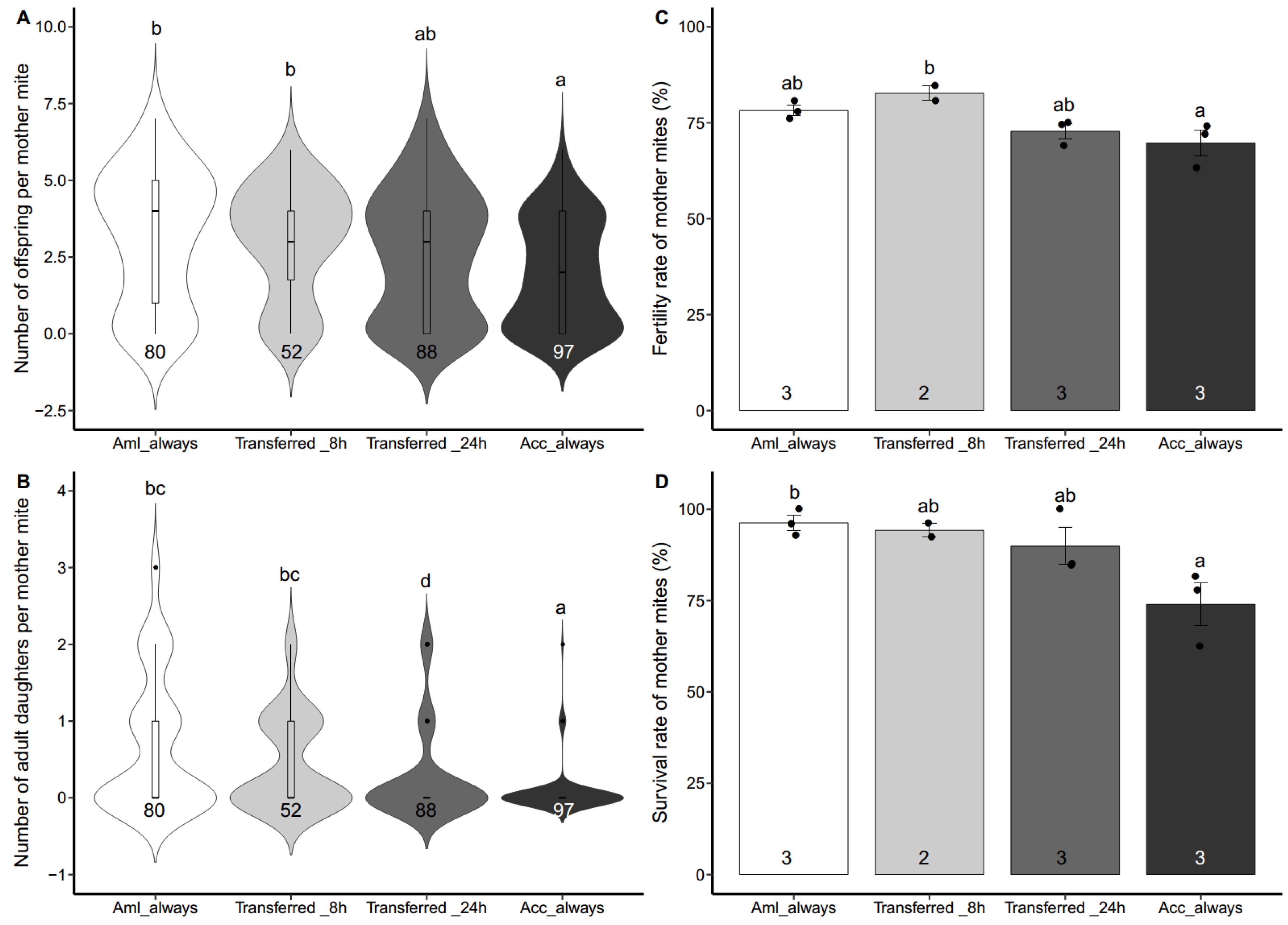
3.2. Reproduction of Aml-Derived Varroa Mites on both Acc and Aml Worker Brood
3.3. Reproduction of Acc-Derived Varroa Mites on Aml Worker Brood
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Klein, A.M.; Vaissière, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B 2007, 274, 303. [Google Scholar] [CrossRef] [PubMed]
- Potts, S.G.; Imperatriz-Fonseca, V.; Ngo, H.T.; Aizen, M.A.; Biesmeijer, J.C.; Breeze, T.D.; Dicks, L.V.; Garibaldi, L.A.; Hill, R.; Settele, J.; et al. Safeguarding pollinators and their values to human well-being. Nature 2016, 540, 220. [Google Scholar] [CrossRef] [PubMed]
- Neumann, P.; Carreck, N.L. Honey bee colony losses. J. Apic. Res. 2010, 49, 1–6. [Google Scholar] [CrossRef]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef] [PubMed]
- VanEngelsdorp, D.; Meixner, M.D. A historical review of managed honey bee populations in europe and the united states and the factors that may affect them. J. Invertebr. Pathol. 2010, 103, S80–S95. [Google Scholar] [CrossRef] [PubMed]
- Cox-Foster, D.L.; Conlan, S.; Holmes, E.C.; Palacios, G.; Evans, J.D.; Moran, N.A.; Quan, P.-L.; Briese, T.; Hornig, M.; Geiser, D.M.; et al. A metagenomic survey of microbes in honey bee colony collapse disorder. Science 2007, 318, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Ratnieks, F.L.W.; Carreck, N.L. Clarity on honey bee collapse? Science 2010, 327, 152–153. [Google Scholar] [CrossRef] [PubMed]
- Dietemann, V.; Pflugfelder, J.; Anderson, D.; Charrière, J.-D.; Chejanovsky, N.; Dainat, B.; de Miranda, J.; Delaplane, K.; Dillier, F.-X.; Fuch, S.; et al. Varroa destructor: Research avenues towards sustainable control. J. Apic. Res. 2012, 51, 125–132. [Google Scholar] [CrossRef]
- Genersch, E. Honey bee pathology: Current threats to honey bees and beekeeping. Appl. Microbiol. Biotechnol. 2010, 87, 87–97. [Google Scholar] [CrossRef]
- Anderson, D.L.; Trueman, J.W.H. Varroa jacobsoni (acari: Varroidae) is more than one species. Exp. Appl. Acarol. 2000, 24, 165–189. [Google Scholar] [CrossRef]
- Oldroyd, B.P. Coevolution while you wait: Varroa jacobsoni, a new parasite of western honeybees. Trends Ecol. Evol. 1999, 14, 312–315. [Google Scholar] [CrossRef]
- Rosenkranz, P.; Aumeier, P.; Ziegelmann, B. Biology and control of Varroa destructor. J. Invertebr. Pathol. 2010, 103, S96–S119. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.-S.; Fang, Y.; Xu, S.; Ge, L. The resistance mechanism of the asian honey bee, Apis cerana fabr., to an ectoparasitic mite, Varroa jacobsoni oudemans. J. Invertebr. Pathol. 1987, 49, 54–60. [Google Scholar] [CrossRef]
- Rath, W. Co-adaptation of Apis cerana Fabr. and Varroa jacobsoni oud. Apidologie 1999, 30, 97–110. [Google Scholar] [CrossRef]
- Zhou, T.; Anderson, D.L.; Huang, Z.Y.; Huang, S.; Yao, J.; Ken, T.; Zhang, Q. Identification of Varroa mites (acari: Varroidae) infesting Apis cerana and Apis mellifera in China. Apidologie 2004, 35, 645–654. [Google Scholar] [CrossRef][Green Version]
- Navajas, M.; Anderson, D.L.; de Guzman, L.I.; Huang, Z.Y.; Clement, J.; Zhou, T.; Le Conte, Y. New Asian types of Varroa destructor: A potential new threat for world apiculture. Apidologie 2010, 41, 181–193. [Google Scholar] [CrossRef]
- Ramsey, S.D.; Ochoa, R.; Bauchan, G.; Gulbronson, C.; Mowery, J.D.; Cohen, A.; Lim, D.; Joklik, J.; Cicero, J.M.; Ellis, J.D.; et al. Varroa destructor feeds primarily on honey bee fat body tissue and not hemolymph. Proc. Natl. Acad. Sci. USA 2019, 116, 1792–1801. [Google Scholar] [CrossRef]
- De Jong, D. Varroa jacobsoni does reproduce in worker cells of Apis cerana in South Korea. Apidologie 1988, 19, 241–244. [Google Scholar] [CrossRef]
- Boot, W.J.; Tan, N.Q.; Dien, P.C.; Huan, L.V.; Dung, N.V.; Long, L.T.; Beetsma, J. Reproductive success of Varroa jacobsoni in brood of its original host, Apis cerana, in comparison to that of its new host, A. mellifera (hymenoptera: Apidae). Bull. Entomol. Res. 1997, 87, 119–126. [Google Scholar] [CrossRef]
- Boot, W.J.; Calis, J.N.M.; Beetsma, J.; Hai, D.M.; Lan, N.K.; Toan, T.V.; Trung, L.Q.; Minh, N.H. Natural selection of Varroa jacobsoni explains the different reproductive strategies in colonies of Apis cerana and Apis mellifera. Exp. Appl. Acarol. 1999, 23, 133–144. [Google Scholar] [CrossRef]
- Koeniger, N.; Koeniger, G.; Delfinado-Baker, M. Observations on mites of the Asian honeybee species (Apis cerana, Apis dorsata, Apis florea). Apidologie 1983, 14, 197–204. [Google Scholar] [CrossRef]
- Koeniger, N.; Koeniger, G.; Wijayagunasekara, N.H. Boebachtungen uber die anpassung von Varroa jacobsoni an ihren naturlichen wirt Apis cerana in Sri Lanka. Apidologie 1981, 12, 37–40. [Google Scholar] [CrossRef]
- Rosenkranz, P.; Tewarson, N.C.; Rachinsky, A.; Strambi, A.; Strambi, C.; Engels, W. Juvenile hormone titer and reproduction of Varroa jacobsoni in capped brood stages of Apis cerana indica in comparison to Apis mellifera ligustica. Apidologie 1993, 24, 375–382. [Google Scholar] [CrossRef]
- Tewarson, N.C.; Singh, A.; Engels, W. Reproduction of Varroa jacobsoni in colonies of Apis cerana indica under natural and experimental conditions. Apidologie 1992, 23, 161–171. [Google Scholar] [CrossRef]
- Zhou, T. The Biological Characteristics and the Natural Distribution of Varroa Destructor in China. Ph.D. Thesis, China Agricultural University, Beijing, China, 2005. [Google Scholar]
- Anderson, D.L. Variation in the parasitic bee mite Varroa jacobsoni oud. Apidologie 2000, 31, 281–292. [Google Scholar] [CrossRef]
- Page, P.; Lin, Z.; Buawangpong, N.; Zheng, H.; Hu, F.; Neumann, P.; Chantawannakul, P.; Dietemann, V. Social apoptosis in honey bee superorganisms. Sci. Rep. 2016, 6, 27210. [Google Scholar] [CrossRef]
- Le Conte, Y.; Huang, Z.Y.; Roux, M.; Zeng, Z.J.; Christidès, J.P.; Bagnères, A.G. Varroa destructor changes its cuticular hydrocarbons to mimic new hosts. Biol. Lett. 2015, 11, 20150233. [Google Scholar] [CrossRef]
- Lin, Z.; Qin, Y.; Page, P.; Wang, S.; Li, L.; Wen, Z.; Hu, F.; Neumann, P.; Zheng, H.; Dietemann, V. Reproduction of parasitic mites Varroa destructor in original and new honeybee hosts. Ecol. Evol. 2018, 8, 2135–2145. [Google Scholar] [CrossRef]
- Sasaki, M. The reason why Apis cerana japonica is resistant to Varroa mite. Honeybee Sci. 1989, 10, 28–36. [Google Scholar]
- Anderson, D.L.; Fuchs, S. Two genetically distinct populations of Varroa jacobsoni with contrasting reproductive abilities on Apis mellifera. J. Apic. Res. 1998, 37, 69–78. [Google Scholar] [CrossRef]
- Nazzi, F.; Milani, N. A technique for reproduction of Varroa jacobsoni Oud under laboratory conditions. Apidologie 1994, 25, 579–584. [Google Scholar] [CrossRef]
- Di Prisco, G.; Annoscia, D.; Margiotta, M.; Ferrara, R.; Varricchio, P.; Zanni, V.; Caprio, E.; Nazzi, F.; Pennacchio, F. A mutualistic symbiosis between a parasitic mite and a pathogenic virus undermines honey bee immunity and health. Proc. Natl. Acad. Sci. USA 2016, 113, 3203–3208. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 24 May 2019).
- Beaurepaire, A.L.; Truong, T.A.; Fajardo, A.C.; Dinh, T.Q.; Cervancia, C.; Moritz, R.F.A. Host specificity in the honeybee parasitic mite, Varroa spp. in Apis mellifera and Apis cerana. PLoS ONE 2015, 10, e0135103. [Google Scholar] [CrossRef] [PubMed]
- Peck, D.T.; Seeley, T.D. Mite bombs or robber lures? The roles of drifting and robbing in Varroa destructor transmission from collapsing honey bee colonies to their neighbors. PLoS ONE 2019, 14, e0218392. [Google Scholar] [CrossRef] [PubMed]
- Dietemann, V.; Beaurepaire, A.; Page, P.; Yañez, O.; Buawangpong, N.; Chantawannakul, P.; Neumann, P. Population genetics of ectoparasitic mites Varroa spp. in Eastern and Western honey bees. Parasitology 2019, 146, 1429–1439. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Han, R. A saliva protein of Varroa mites contributes to the toxicity toward Apis cerana and the DWV elevation in A. mellifera. Sci. Rep. 2018, 8, 3387. [Google Scholar] [CrossRef]
- Steiner, J.; Dittmann, F.; Rosenkranz, P.; Engels, W. The first gonocycle of the parasitic mite (Varroa juobsoni) in relation to preimaginal development of its host, the honey bee (Apis mellifera carnicar). Invertebr. Reprod. Dev. 1994, 25, 175–183. [Google Scholar] [CrossRef]
- Nazzi, F.; Le Conte, Y. Ecology of Varroa destructor, the major ectoparasite of the western honey bee, Apis mellifera. Annu. Rev. Entomol. 2016, 61, 417–432. [Google Scholar] [CrossRef]
- Frey, E.; Odemer, R.; Blum, T.; Rosenkranz, P. Activation and interruption of the reproduction of Varroa destructor is triggered by host signals (Apis mellifera). J. Invertebr. Pathol. 2013, 113, 56–62. [Google Scholar] [CrossRef]
- Garrido, C.; Rosenkranz, P. Volatiles of the honey bee larva initiate oogenesisin the parasitic mite Varroa destructor. Chemoecology 2004, 14, 193–197. [Google Scholar]
- Trouiller, J.; Milani, N. Stimulation of Varroa jacobsoni oud. oviposition with semiochemicals from honeybee brood. Apidologie 1999, 30, 3–12. [Google Scholar] [CrossRef]
- Milani, N.; Vedova, G.D.; Nazzi, F. (Z)-8-heptadecene reduces the reproduction of Varroa destructor in brood cells. Apidologie 2004, 35, 265–273. [Google Scholar] [CrossRef][Green Version]
- Nazzi, F.; Milani, N.; Vedova, G.D. (Z)-8-heptadecene from infested cells reduces the reproduction of Varroa destructor under laboratory conditions. J. Chem. Ecol. 2002, 28, 2181–2190. [Google Scholar] [CrossRef] [PubMed]
- Milani, N.; Chiesa, F. Some factors affecting the reproduction of Varroa jacobsoni Oud. under laboratory conditions. Apicoltura 1990, 6, 33–42. [Google Scholar]
- De Ruijter, A. Reproduction of Varroa jacobsoni during successive brood cycles of the honeybee. Apidologie 1987, 18, 321–326. [Google Scholar] [CrossRef]
- Huang, Z. Varroa mite reproductive biology. Am. Bee J. 2012, 152, 981. [Google Scholar]
- Posada-Florez, F.; Sonenshine, D.E.; Egekwu, N.I.; Rice, C.; Lupitskyy, R.; Cook, S.C. Insights into the metabolism and behaviour of Varroa destructor mites from analysis of their waste excretions. Parasitology 2018, 146, 527–532. [Google Scholar] [CrossRef]
- Donzé, G.; Guerin, P.M. Behavioral attributes and parental care of Varroa mites parasitizing honeybee brood. Behav. Ecol. Sociobiol. 1994, 34, 305–319. [Google Scholar] [CrossRef]
- Cornman, R.S.; Schatz, M.C.; Johnston, J.S.; Chen, Y.-P.; Pettis, J.; Hunt, G.; Bourgeois, L.; Elsik, C.; Anderson, D.; Grozinger, C.M.; et al. Genomic survey of the ectoparasitic mite Varroa destructor, a major pest of the honey bee Apis mellifera. BMC Genom. 2010, 11, 1–15. [Google Scholar] [CrossRef]
- Techer, M.A.; Rane, R.V.; Grau, M.L.; Roberts, J.M.K.; Sullivan, S.T.; Liachko, I.; Childers, A.K.; Evans, J.D.; Mikheyev, A.S. Divergent evolutionary trajectories following speciation in two ectoparasitic honey bee mites. Commun. Biol. 2019, 2, 357. [Google Scholar] [CrossRef]
| First Infestation | Second Infestation | |||||
|---|---|---|---|---|---|---|
| Varroa-Acc a (n = 13) | Varroa-Aml b (n = 19) | Significance d | Varroa-Acc (n = 11) | Varroa-Aml (n = 17) | Significance d | |
| Number of offspring per mother mite | 0 | 2.47 ± 0.52 c | p < 0.001 | 0 | 1.65 ± 0.44 c | p = 0.005 |
| Number of adult daughters per mother mite | 0 | 0.63 ± 0.24 c | p = 0.030 | 0 | 0.29 ± 0.11 c | p = 0.056 |
| Fertility rate of mother mites (%) | 0 | 63.16 | p = 0.001 | 0 | 52.94 | p = 0.012 |
| Survival rate of mother mites (%) | 100 | 89.47 | p = 0.640 | 100 | 100 | p = 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Wang, C.; Huang, Z.Y.; Chen, Y.; Han, R. Reproduction of Distinct Varroa destructor Genotypes on Honey Bee Worker Brood. Insects 2019, 10, 372. https://doi.org/10.3390/insects10110372
Li W, Wang C, Huang ZY, Chen Y, Han R. Reproduction of Distinct Varroa destructor Genotypes on Honey Bee Worker Brood. Insects. 2019; 10(11):372. https://doi.org/10.3390/insects10110372
Chicago/Turabian StyleLi, Wenfeng, Cheng Wang, Zachary Y. Huang, Yanping Chen, and Richou Han. 2019. "Reproduction of Distinct Varroa destructor Genotypes on Honey Bee Worker Brood" Insects 10, no. 11: 372. https://doi.org/10.3390/insects10110372
APA StyleLi, W., Wang, C., Huang, Z. Y., Chen, Y., & Han, R. (2019). Reproduction of Distinct Varroa destructor Genotypes on Honey Bee Worker Brood. Insects, 10(11), 372. https://doi.org/10.3390/insects10110372





