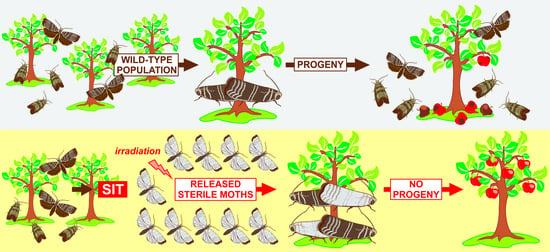
Advances and Challenges of Using the Sterile Insect Technique for the Management of Pest Lepidoptera
Abstract
1. Introduction
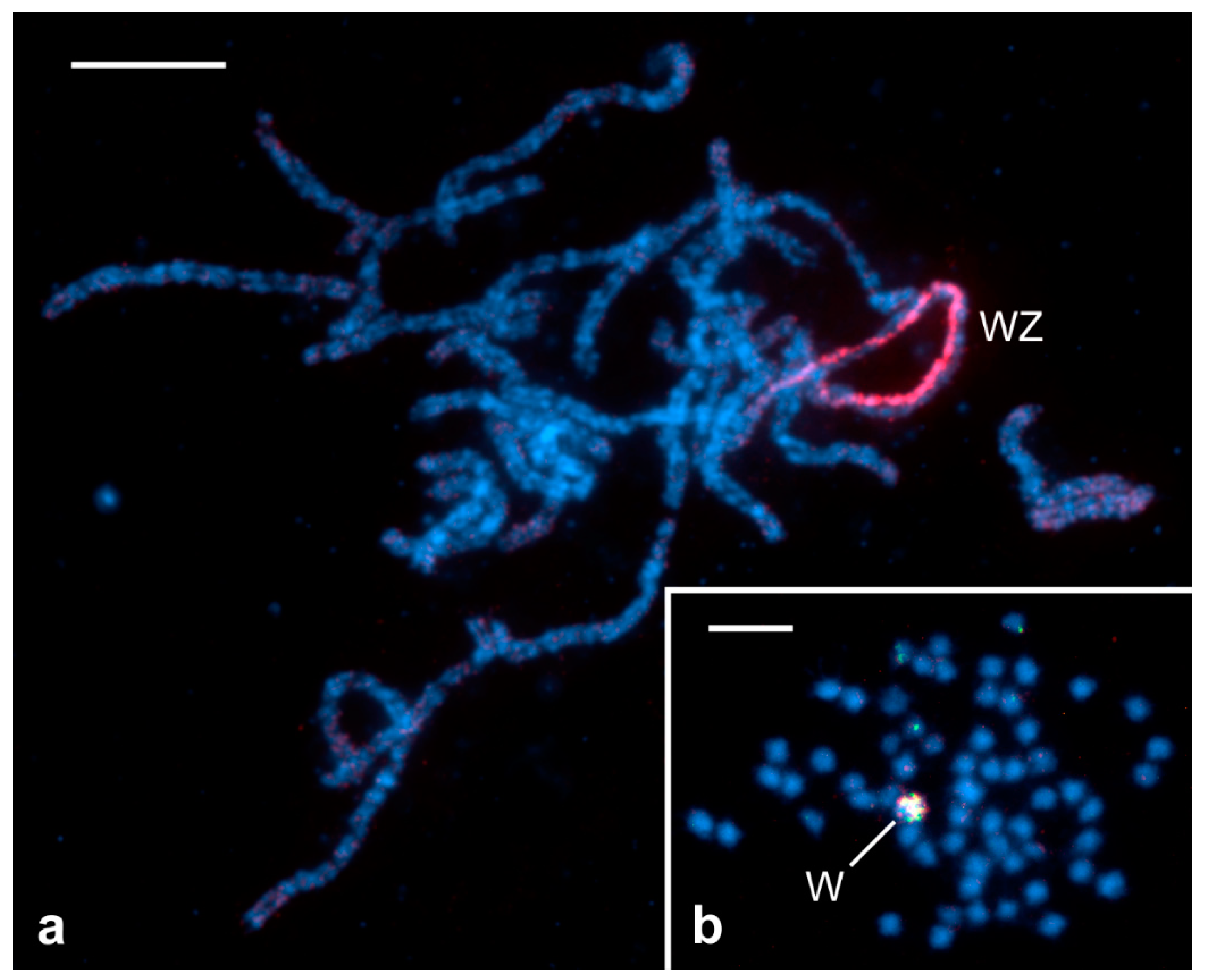
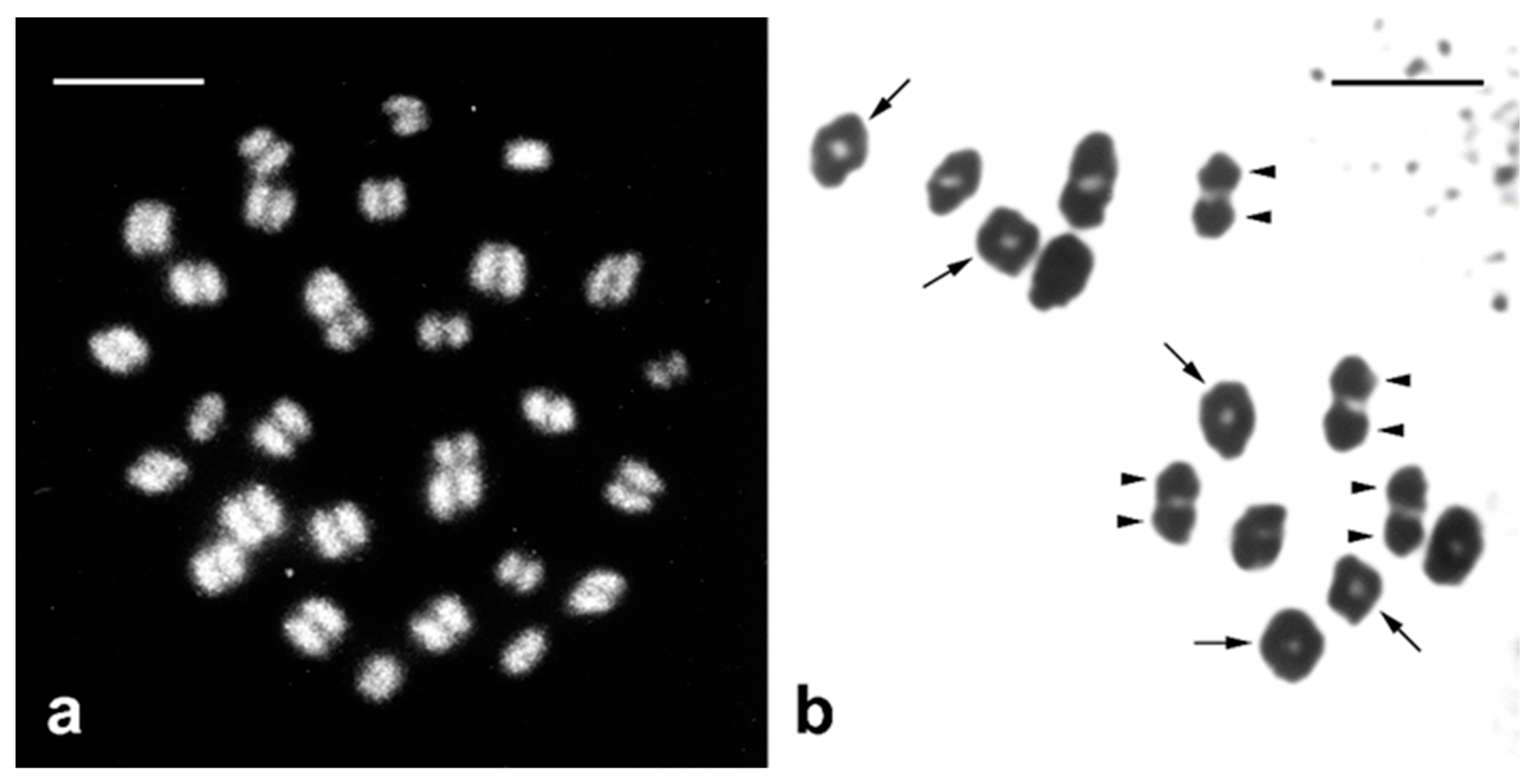
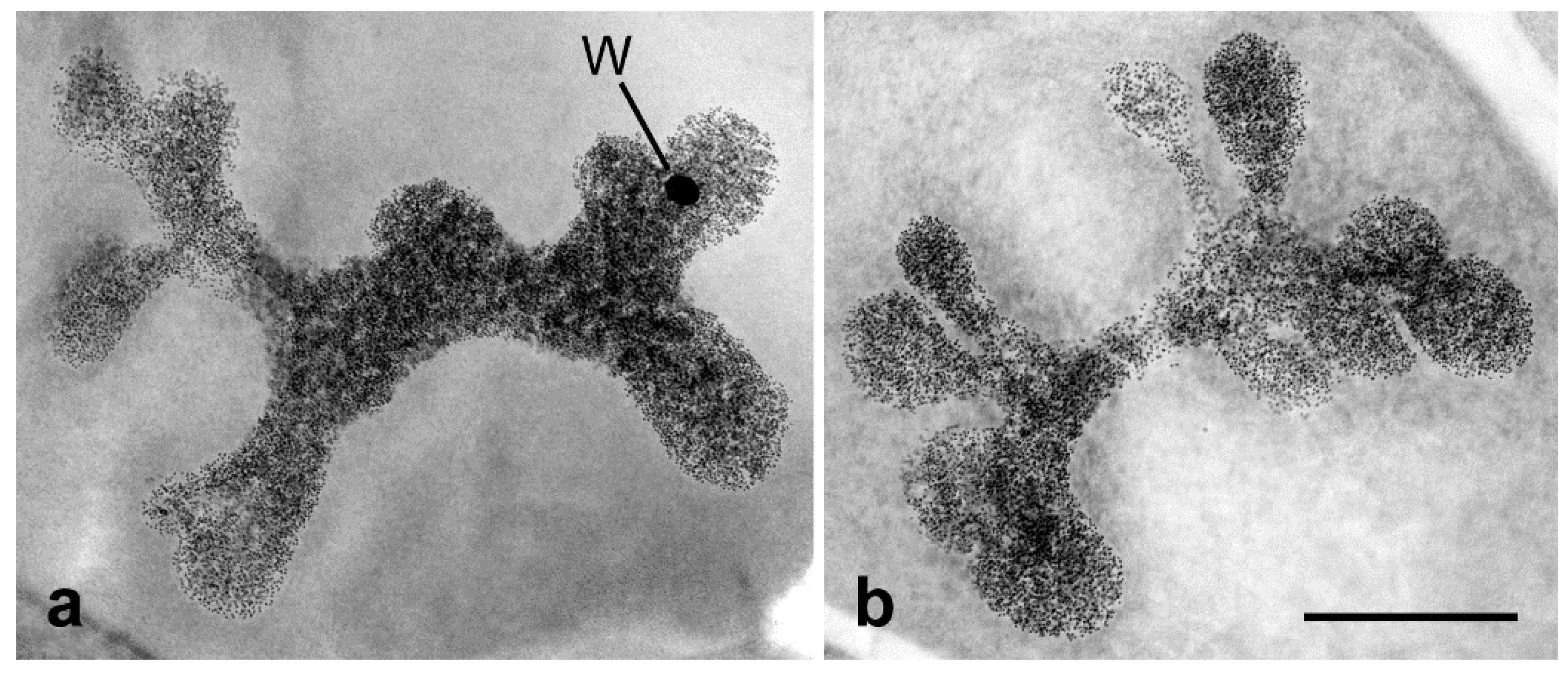
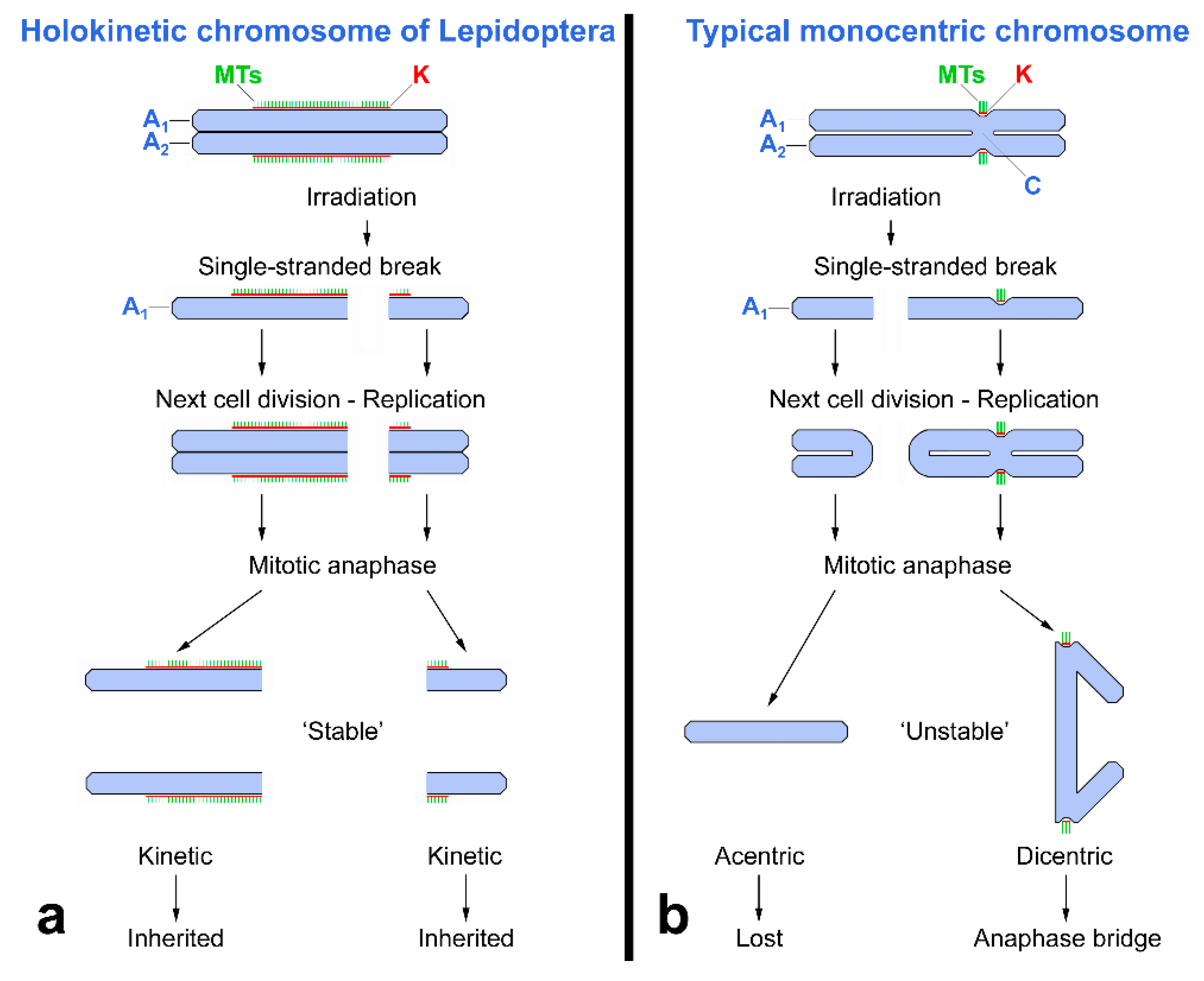
2. Specific Cytogenetic and Cytological Features of Lepidoptera Relevant to SIT
3. Resistance of Lepidoptera to Irradiation
4. Inherited Sterility in Lepidoptera
5. Successful Application of the SIT/IS Against Lepidopteran Pests
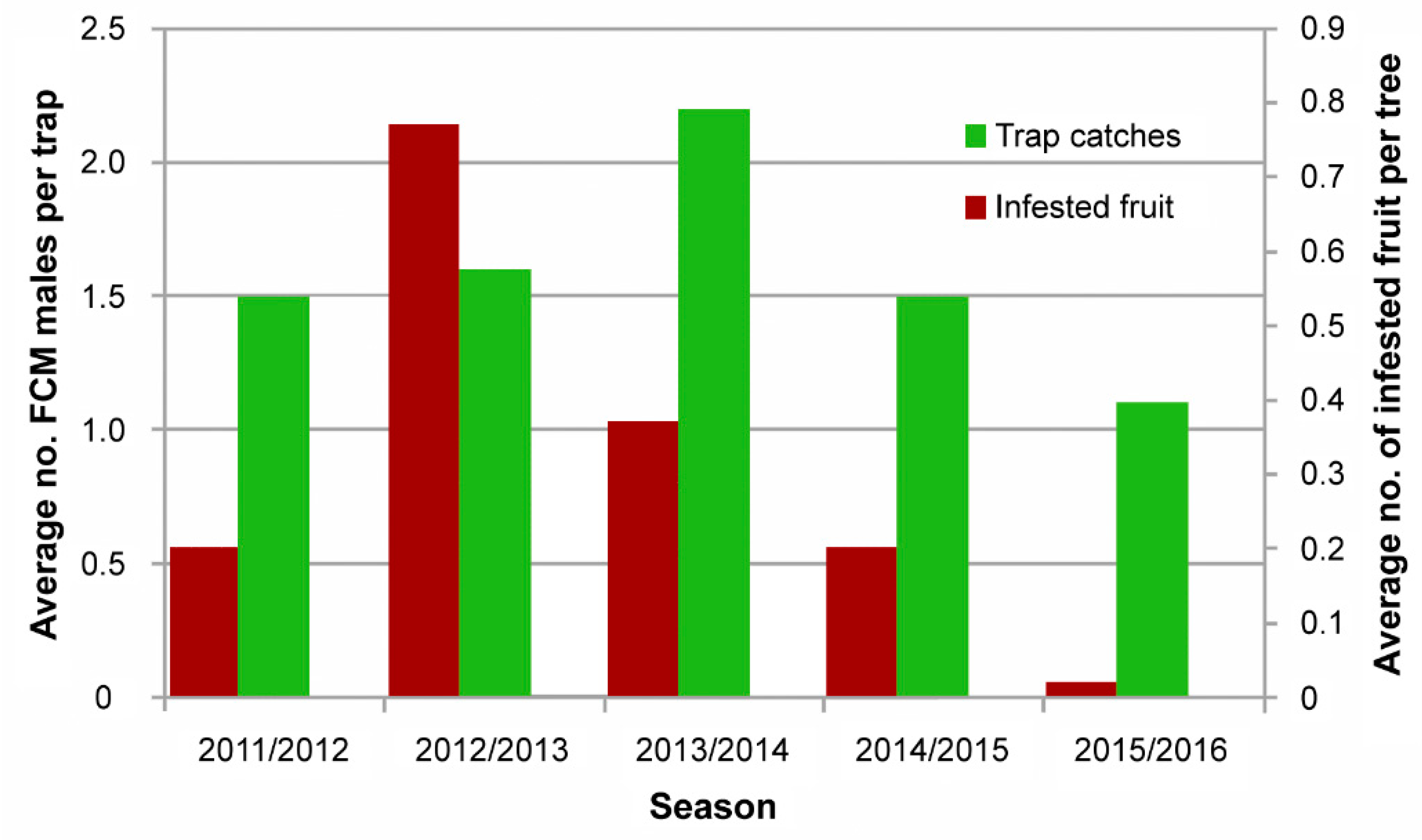
5.1. False Codling Moth Suppressed in Eastern and Western Cape, South Africa
5.2. Codling Moth Suppressed in the Okanagan Valley of British Columbia, Canada
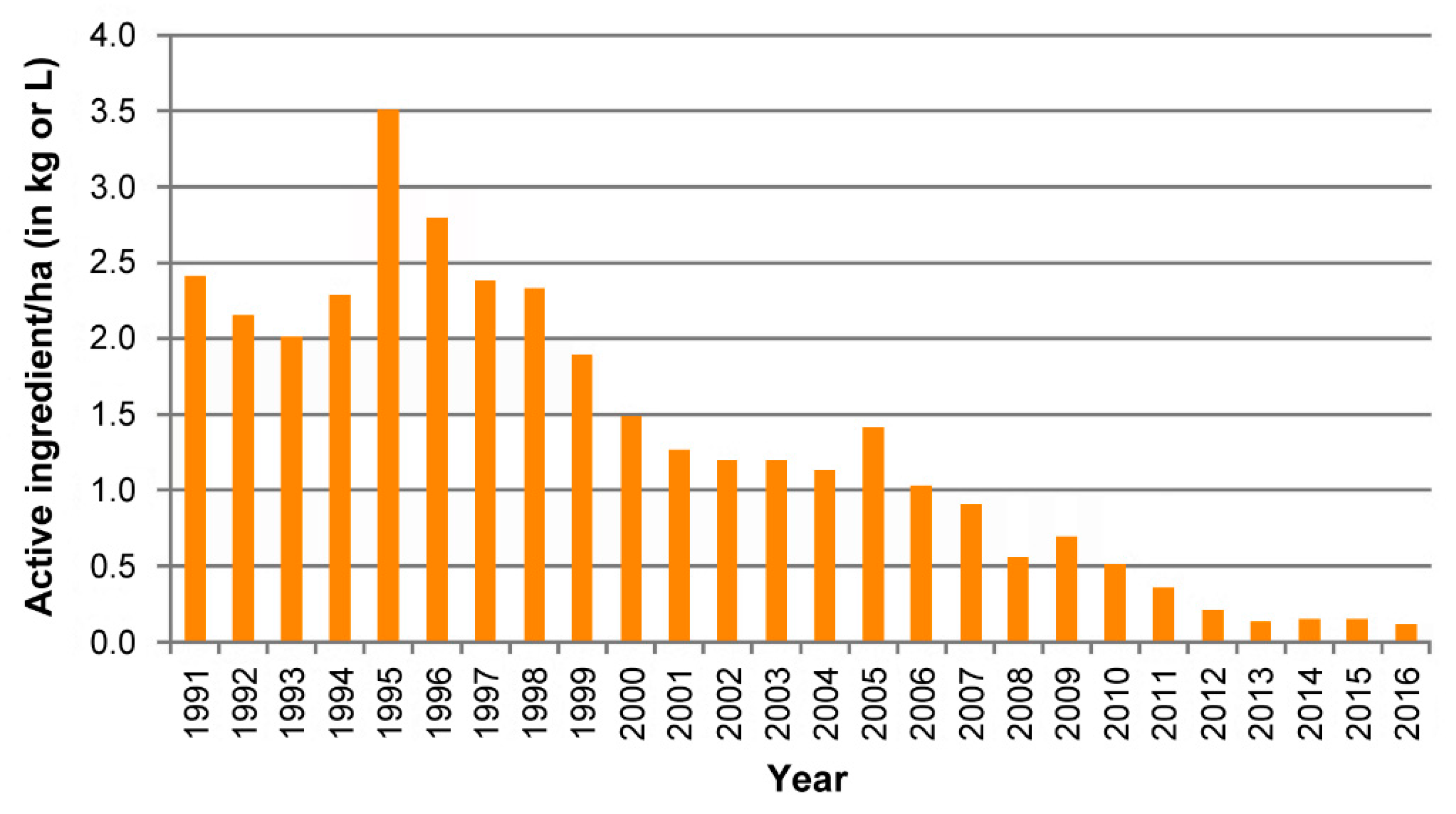
5.3. Pink Bollworm Eradication from Southern USA and Northern Mexico
5.4. Cactus Moth Eradicated from Two Islands in Mexico
5.5. Australian Painted Apple Moth Eradicated from New Zealand
6. Quality Control for Lepidoptera SIT or IS
6.1. Research on Factors and Variables that Affect the Quality of Lepidoptera Used in SIT/IS Programs
6.2. Research on Tools and Methods to Assess Sterile Moth Field Performance
7. Challenges
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Klassen, W. Area-wide integrated pest management and the sterile insect technique. In Sterile Insect Technique. Principles and Practice in Area-Wide Integrated Pest Management; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 39–68. [Google Scholar] [CrossRef]
- Knipling, E.F. The Basic Principles of Insect Suppression and Management; Agricultural Handbook 512; United States Department of Agriculture, Agricultural Research Service: Washington, DC, USA, 1979. [Google Scholar]
- Krafsur, E.S. Sterile insect technique for suppressing and eradicating insect populations: 55 years and counting. J. Agric. Entomol. 1998, 15, 303–317. [Google Scholar]
- Vreysen, M.J.B. Principles of area-wide integrated tsetse fly control using the sterile insect technique. Med. Trop. (Mars.) 2001, 61, 397–411. [Google Scholar] [PubMed]
- Vreysen, M.J.B.; Robinson, A.S. Ionising radiation and area-wide management of insect pests to promote sustainable agriculture. Agron. Sustain. Dev. 2011, 31, 233–250. [Google Scholar] [CrossRef]
- Lees, R.S.; Gilles, J.R.L.; Hendrichs, J.; Vreysen, M.J.B.; Bourtzis, K. Back to the future: The sterile insect technique against mosquito disease vectors. Curr. Opin. Insect Sci. 2015, 10, 156–162. [Google Scholar] [CrossRef] [PubMed]
- LaChance, L.E. The induction of dominant lethal mutations in insects by ionizing radiation and chemicals—As related to the sterile-male technique of insect control. In Genetics of Insect Vectors of Disease; Wright, J.W., Pal, R., Eds.; Elsevier: Amsterdam, The Netherlands, 1967; pp. 617–650. [Google Scholar]
- LaChance, L.E. Genetic Methods for the Control of Lepidopteran Species: Status and Potential; United States Department of Agriculture, Agricultural Research Service: Washington, DC, USA, 1985; Volume ARS-28, 44p. [Google Scholar] [CrossRef]
- Proverbs, M.D. Progress on the use of induced sexual sterility for the control of the codling moth Carpocapsa pomonella (L.) (Lepidoptera: Olethreutidae). Proc. Entomol. Soc. Ont. 1962, 92, 5–11. [Google Scholar]
- North, D.T. Inherited sterility in Lepidoptera. Annu. Rev. Entomol. 1975, 20, 167–182. [Google Scholar] [CrossRef]
- Carpenter, J.E.; Bloem, S.; Marec, F. Inherited sterility in insects. In Sterile Insect Technique. Principles and Practice in Area-Wide Integrated Pest Management; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 115–146. [Google Scholar] [CrossRef]
- Vreysen, M.J.B.; Carpenter, J.E.; Marec, F. Improvement of the sterile insect technique for codling moth Cydia pomonella (Linnaeus) (Lepidoptera, Tortricidae) to facilitate expansion of field application. J. Appl. Entomol. 2010, 134, 165–181. [Google Scholar] [CrossRef]
- Vreysen, M.J.B.; Klassen, W.; Carpenter, J.E. Overview of technological advances toward greater efficiency and efficacy in sterile insect-inherited sterility programs against moth pests. Fla. Entomol. 2016, 99, 1–12. [Google Scholar] [CrossRef]
- Fuková, I.; Traut, W.; Vítková, M.; Nguyen, P.; Kubíčková, S.; Marec, F. Probing the W chromosome of the codling moth, Cydia pomonella, with sequences from microdissected sex chromatin. Chromosoma 2007, 116, 135–145. [Google Scholar] [CrossRef]
- Sahara, K.; Yoshido, A.; Shibata, F.; Fujikawa-Kojima, N.; Okabe, T.; Tanaka-Okuyama, M.; Yasukochi, Y. FISH identification of Helicoverpa armigera and Mamestra brassicae chromosomes by BAC and fosmid probes. Insect Biochem. Mol. Biol. 2013, 43, 644–653. [Google Scholar] [CrossRef]
- Traut, W.; Sahara, K.; Marec, F. Sex chromosomes and sex determination in Lepidoptera. Sex. Dev. 2007, 1, 332–346. [Google Scholar] [CrossRef] [PubMed]
- Marec, F.; Sahara, K.; Traut, W. Rise and fall of the W chromosome in Lepidoptera. In Molecular Biology and Genetics of the Lepidoptera; Goldsmith, M.R., Marec, F., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 49–63. [Google Scholar]
- Sahara, K.; Yoshido, A.; Traut, W. Sex chromosome evolution in moths and butterflies. Chromosome Res. 2012, 20, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Dalíková, M.; Zrzavá, M.; Hladová, I.; Nguyen, P.; Šonský, I.; Flegrová, M.; Kubíčková, S.; Voleníková, A.; Kawahara, A.Y.; Peters, R.S.; et al. New insights into the evolution of the W chromosome in Lepidoptera. J. Hered. 2017, 108, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Yoshido, A.; Šíchová, J.; Kubíčková, S.; Marec, F.; Sahara, K. Rapid turnover of the W chromosome in geographical populations of wild silkmoths, Samia cynthia ssp. Chromosome Res. 2013, 21, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Kost, S.; Heckel, D.G.; Yoshido, A.; Marec, F.; Groot, A.T. A Z-linked sterility locus causes sexual abstinence in hybrid females and facilitates speciation in Spodoptera frugiperda. Evolution 2016, 70, 1418–1427. [Google Scholar] [CrossRef]
- Yasukochi, Y.; Ohno, M.; Shibata, F.; Jouraku, A.; Nakano, R.; Ishikawa, Y.; Sahara, K. A FISH-based chromosome map for the European corn borer yields insights into ancient chromosomal fusions in the silkworm. Heredity 2016, 116, 75–83. [Google Scholar] [CrossRef]
- Nguyen, P.; Sýkorová, M.; Šíchová, J.; Kůta, V.; Dalíková, M.; Čapková Frydrychová, R.; Neven, L.G.; Sahara, K.; Marec, F. Neo-sex chromosomes and adaptive potential in tortricid pests. Proc. Nat. Acad. Sci. USA 2013, 110, 6931–6936. [Google Scholar] [CrossRef]
- Šíchová, J.; Nguyen, P.; Dalíková, M.; Marec, F. Chromosomal evolution in tortricid moths: Conserved karyotypes with diverged features. PLoS ONE 2013, 8, e64520. [Google Scholar] [CrossRef]
- Picq, S.; Lumley, L.; Šíchová, J.; Laroche, J.; Pouliot, E.; Brunet, B.; Levesque, R.C.; Sperling, F.A.H.; Marec, F.; Cusson, M. Insights into the structure of the spruce budworm (Choristoneura fumiferana) genome, as revealed by molecular cytogenetic analyses and a high-density linkage map. G3 (Bethesda) 2018, 8, 2539–2549. [Google Scholar] [CrossRef]
- Nokkala, S. Cytological characteristics of chromosome behaviour during female meiosis in Sphinx ligustri L. (Sphingidae, Lepidoptera). Hereditas 1987, 106, 169–179. [Google Scholar] [CrossRef]
- Marec, F. Synaptonemal complexes in insects. Int. J. Insect Morphol. Embryol. 1996, 25, 205–233. [Google Scholar] [CrossRef]
- Traut, W. A study of recombination, formation of chiasmata and synaptonemal complexes in female and male meiosis of Ephestia kuehniella (Lepidoptera). Genetica 1977, 47, 135–142. [Google Scholar] [CrossRef]
- Marec, F.; Traut, W. Synaptonemal complexes in female and male meiotic prophase of Ephestia kuehniella (Lepidoptera). Heredity 1993, 71, 394–404. [Google Scholar] [CrossRef][Green Version]
- Traut, W.; Marec, F. Sex chromatin in Lepidoptera. Q. Rev. Biol. 1996, 71, 239–256. [Google Scholar] [CrossRef] [PubMed]
- Buntrock, L.; Marec, F.; Krueger, S.; Traut, W. Organ growth without cell division: Somatic polyploidy in a moth, Ephestia kuehniella. Genome 2012, 55, 755–763. [Google Scholar] [CrossRef]
- Fuková, I.; Neven, L.G.; Bárcenas, N.M.; Gund, N.A.; Dalíková, M.; Marec, F. Rapid assessment of the sex of codling moth Cydia pomonella (Linnaeus) (Lepidoptera: Tortricidae) eggs and larvae. J. Appl. Entomol. 2009, 133, 249–261. [Google Scholar] [CrossRef]
- Traut, W.; Vogel, H.; Glöckner, G.; Hartmann, E.; Heckel, D.G. High-throughput sequencing of a single chromosome: A moth W chromosome. Chromosome Res. 2013, 21, 491–505. [Google Scholar] [CrossRef]
- Murakami, A.; Imai, H.T. Cytological evidence for holocentric chromosomes of the silkworms, Bombyx mori and B. mandarina, (Bombycidae, Lepidoptera). Chromosoma 1974, 47, 167–178. [Google Scholar] [CrossRef]
- Wolf, K.W. The structure of condensed chromosomes in mitosis and meiosis of insects. Int. J. Insect Morphol. Embryol. 1996, 25, 37–62. [Google Scholar] [CrossRef]
- Melters, D.P.; Paliulis, L.V.; Korf, I.F.; Chan, S.W.L. Holocentric chromosomes: Convergent evolution, meiotic adaptations, and genomic analysis. Chromosome Res. 2012, 20, 579–593. [Google Scholar] [CrossRef]
- Robinson, R. Lepidoptera Genetics; Pergamon Press: Oxford, UK, 1971. [Google Scholar]
- Lukhtanov, V.A. Sex chromatin and sex chromosome systems in nonditrysian Lepidoptera (Insecta). J. Zool. Syst. Evol. Res. 2000, 38, 73–79. [Google Scholar] [CrossRef]
- Baxter, S.W.; Davey, J.W.; Johnston, J.S.; Shelton, A.M.; Heckel, D.G.; Jiggins, C.D.; Blaxter, M.L. Linkage mapping and comparative genomics using next-generation RAD sequencing of a non-model organism. PLoS ONE 2011, 6, e19315. [Google Scholar] [CrossRef] [PubMed]
- Van’t Hof, A.E.; Nguyen, P.; Dalíková, M.; Edmonds, N.; Marec, F.; Saccheri, I.J. Linkage map of the peppered moth, Biston betularia (Lepidoptera, Geometridae): A model of industrial melanism. Heredity 2013, 110, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Ahola, V.; Lehtonen, R.; Somervuo, P.; Salmela, L.; Koskinen, P.; Rastas, P.; Välimäki, N.; Paulin, L.; Kvist, J.; Wahlberg, N.; et al. The Glanville fritillary genome retains an ancient karyotype and reveals selective chromosomal fusions in Lepidoptera. Nat. Commun. 2014, 5, 4737. [Google Scholar] [CrossRef] [PubMed]
- Kandul, N.P.; Lukhtanov, V.A.; Pierce, N.E. Karyotypic diversity and speciation in Agrodiaetus butterflies. Evolution 2007, 61, 546–559. [Google Scholar] [CrossRef]
- Šíchová, J.; Ohno, M.; Dincă, V.; Watanabe, M.; Sahara, K.; Marec, F. Fissions, fusions, and translocations shaped the karyotype and multiple sex chromosome constitution in the northeast-Asian wood white butterfly, Leptidea amurensis. Biol. J. Linn. Soc. 2016, 118, 457–471. [Google Scholar] [CrossRef]
- Mediouni, J.; Fuková, I.; Frydrychová, R.; Dhouibi, M.H.; Marec, F. Karyotype, sex chromatin and sex chromosome differentiation in the carob moth, Ectomyelois ceratoniae (Lepidoptera: Pyralidae). Caryologia 2004, 57, 184–194. [Google Scholar] [CrossRef]
- Fuková, I.; Nguyen, P.; Marec, F. Codling moth cytogenetics: Karyotype, chromosomal location of rDNA, and molecular differentiation of sex chromosomes. Genome 2005, 2005 48, 1083–1092. [Google Scholar] [CrossRef]
- D’Alençon, E.; Sezutsu, H.; Legea, F.; Permal, E.; Bernard-Samain, S.; Gimenez, S.; Gagneur, C.; Cousserans, F.; Shimomura, M.; Brun-Barale, A.; et al. Extensive synteny conservation of holocentric chromosomes in Lepidoptera despite high rates of local genome rearrangements. Proc. Nat. Acad. Sci. USA 2010, 107, 7680–7685. [Google Scholar] [CrossRef]
- Friedländer, M.; Seth, R.K.; Reynolds, S.E. Eupyrene and apyrene sperm: Dichotomous spermatogenesis in Lepidoptera. Adv. Insect Physiol. 2005, 32, 206–308. [Google Scholar] [CrossRef]
- Cook, P.A.; Wedell, N. Ejaculate dynamics in butterflies: A strategy for maximizing fertilization success? Proc. R. Soc. Lond. B Biol. Sci. 1996, 263, 1047–1051. [Google Scholar] [CrossRef]
- Cook, P.A. Sperm numbers and female fertility in the moth Plodia interpunctella (Hubner) (Lepidoptera; Pyralidae). J. Insect Behav. 1999, 12, 767–779. [Google Scholar] [CrossRef]
- Cook, P.A.; Wedell, N. Non-fertile sperm delay female remating. Nature 1999, 397, 486. [Google Scholar] [CrossRef]
- Hiroki Sakai, H.; Oshima, H.; Yuri, K.; Gotoh, H.; Daimon, T.; Yaginuma, T.; Sahara, K.; Niimi, T. Dimorphic sperm formation by Sex-lethal. Proc. Nat. Acad. Sci. USA 2019, 116, 10412–10417. [Google Scholar] [CrossRef] [PubMed]
- LaChance, L.E. Dominant lethal mutations in insects with holokinetic chromosomes. 2. Irradiation of sperm of cabbage looper. Ann. Entomol. Soc. Am. 1974, 67, 35–39. [Google Scholar] [CrossRef]
- Berg, G.J.; LaChance, L.E. Dominant lethal mutations in insects with holokinetic chromosomes: Irradiation of pink bollworm sperm. Ann. Entomol. Soc. Am. 1976, 69, 971–976. [Google Scholar] [CrossRef]
- Marec, F.; Tothová, A.; Sahara, K.; Traut, W. Meiotic pairing of sex chromosome fragments and its relation to atypical transmission of a sex-linked marker in Ephestia kuehniella (Insecta: Lepidoptera). Heredity 2001, 87, 659–671. [Google Scholar] [CrossRef]
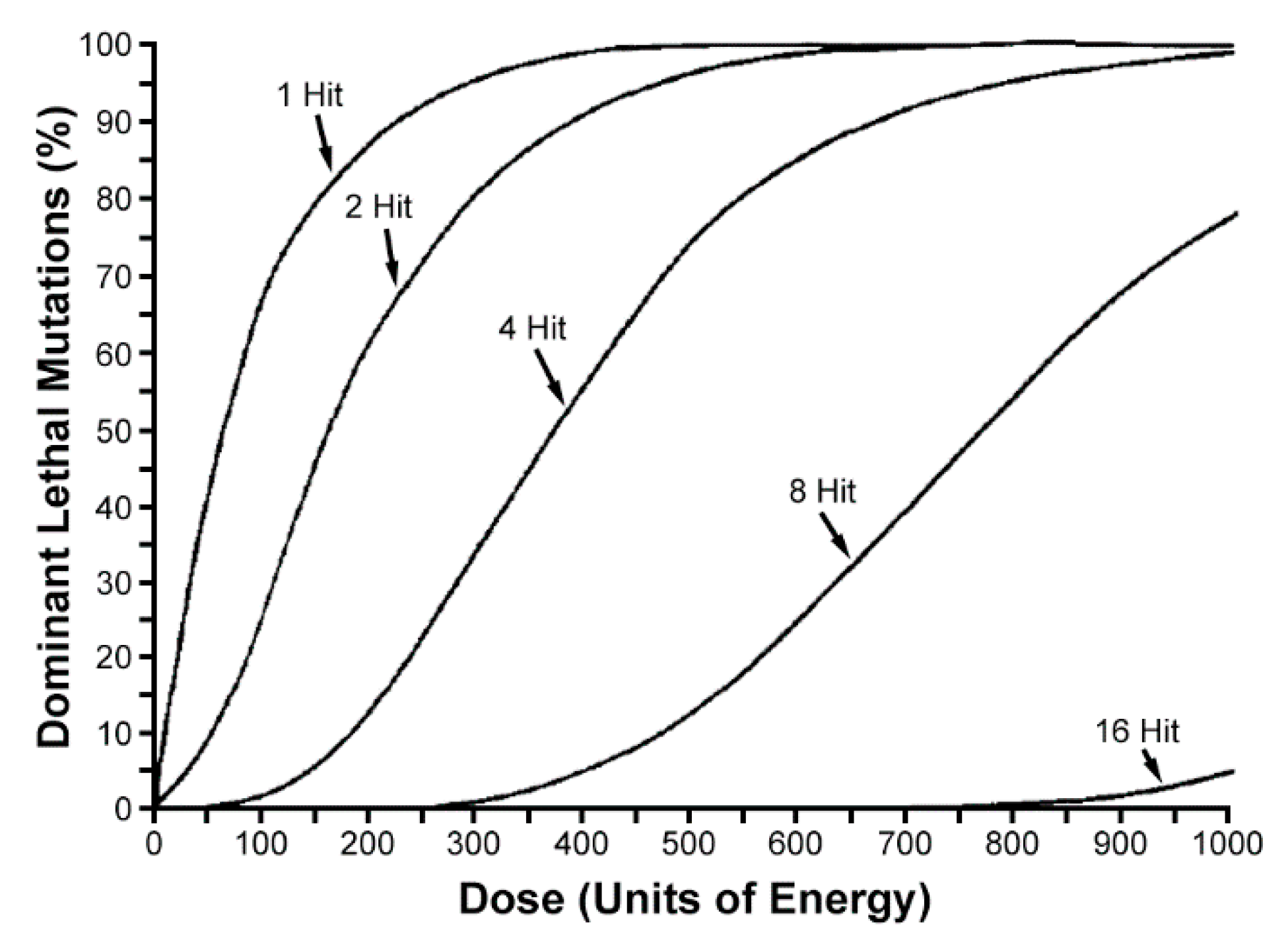
- LaChance, L.E.; Graham, C.K. Insect radiosensitivity: Dose curves and dose-fractionation studies of dominant lethal mutations in the mature sperm of 4 insect species. Mutat. Res. 1984, 127, 49–59. [Google Scholar] [CrossRef]
- Marec, F.; Kollárová, I.; Pavelka, J. Radiation-induced inherited sterility combined with a genetic sexing system in Ephestia kuehniella (Lepidoptera: Pyralidae). Ann. Entomol. Soc. Am. 1999, 92, 250–259. [Google Scholar] [CrossRef]
- Bakri, A.; Metha, K.; Lance, D.R. Sterilizing insects with ionizing radiation. In Sterile Insect Technique. Principles and Practice in Area-Wide Integrated Pest Management; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 233–268. [Google Scholar] [CrossRef]
- Cladera, J.L.; Vilardi, J.C.; Juri, M.; Paulin, L.E.; Giardini, M.C.; Gómez Cendra, P.V.; Segura, D.F.; Lanzavecchia, S.B. Genetics and biology of Anastrepha fraterculus: Research supporting the use of the sterile insect technique (SIT) to control this pest in Argentina. BMC Genet. 2014, 15, S12. [Google Scholar] [CrossRef]
- Koval, T.M. Moths: Myths and mysteries of stress resistance. Bioessays 1996, 18, 149–156. [Google Scholar] [CrossRef]
- Chandna, S.; Dwarakanath, B.S.; Seth, R.K.; Khaitan, D.; Adhikari, J.S.; Jain, V. Radiation responses of Sf9, a highly radioresistant lepidopteran insect cell line. Int. J. Radiat. Biol. 2004, 80, 301–315. [Google Scholar] [CrossRef] [PubMed]
- Suman, S.; Khan, Z.; Zarin, M.; Chandna, S.; Seth, R.K. Radioresistant Sf9 insect cells display efficient antioxidant defence against high dose of γ-radiation. Int. J. Radiat. Biol. 2015, 91, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Kumar, A.; Chandna, S. Constitutive hyperactivity of histone deacetylases enhances radioresistance in Lepidopteran Sf9 insect cells. Biochim. Biophys. Acta 2016, 1860, 1237–1246. [Google Scholar] [CrossRef] [PubMed]
- Tothová, A.; Marec, F. Chromosomal principle of radiation-induced F1 sterility in Ephestia kuehniella (Lepidoptera: Pyralidae). Genome 2001, 44, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Anisimov, A.I.; Lazurkina, N.V.; Shvedov, A.N. Influence of radiation-induced genetic damage on the suppressive effect of inherited sterility in the codling moth (Lepidoptera: Tortricidae). Ann. Entomol. Soc. Am. 1989, 82, 769–777. [Google Scholar] [CrossRef]
- Bloem, S.; Carpenter, J.E.; Hofmeyr, J.H. Radiation biology and inherited sterility in false codling moth (Lepidoptera: Tortricidae). J. Econ. Entomol. 2003, 96, 1724–1731. [Google Scholar] [CrossRef]
- Astaurov, B.I.; Frolova, S.L. Artificial mutations in the silkworm (Bombyx mori L.). V. Sterility and spermatogenic anomalies in the progeny of irradiated moths concerning some questions of general biological and mutagenic action of X-rays. Biologicheskii Zhurnal 1935, 4, 861–894. [Google Scholar]
- Ostriakova-Varshaver, V.P. The bee moth, Galleria mellonella, as a new object for genetic investigations. II. Cytogenetic analysis of sterility initiated by X-rays in males. Biologicheskii Zhurnal 1937, 6, 816–836. [Google Scholar]
- Knipling, E.F. Suppression of pest Lepidoptera by releasing partially sterile males: A theoretical appraisal. BioScience 1970, 20, 456–470. [Google Scholar] [CrossRef]
- Carabajal Paladino, L.Z.; Ferrari, M.E.; Lauría, J.P.; Cagnotti, C.L.; Šíchová, J.; López, S.N. The effect of X-rays on cytological traits of Tuta absoluta (Lepidoptera: Gelechiidae). Fla. Entomol. 2016, 99, 43–53. [Google Scholar] [CrossRef]
- Lukhtanov, V.A.; Dincă, V.; Friberg, M.; Šíchová, J.; Olofsson, M.; Vila, R.; Marec, F.; Wiklund, C. Versatility of multivalent orientation, inverted meiosis, and rescued fitness in holocentric chromosomal hybrids. Proc. Nat. Acad. Sci. USA 2018, 115, E9610–E9619. [Google Scholar] [CrossRef] [PubMed]
- Koudelová, J.; Cook, P.A. Effect of gamma radiation and sex-linked recessive lethal mutations on sperm transfer in Ephestia kuehniella (Lepidoptera: Pyralidae). Fla. Entomol. 2001, 84, 172–182. [Google Scholar] [CrossRef]
- Hendrichs, J.; Vreysen, M.J.B.; Enkerlin, W.R.; Cayol, J.P. Strategic options in using sterile insects for area-wide integrated pest management. In Sterile Insect Technique. Principles and Practice in Area-Wide Integrated Pest Management; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 563–600. [Google Scholar] [CrossRef]
- Hofmeyr, J.H.; Carpenter, J.E.; Bloem, S.; Slabbert, J.P.; Hofmeyr, M.; Groenewald, S.S. Development of the sterile insect technique to suppress false codling moth Thaumatotibia leucotreta (Lepidoptera: Tortricidae) in citrus fruit: Research to implementation (Part 1). Afr. Entomol. 2015, 23, 180–186. [Google Scholar] [CrossRef]
- Hofmeyr, J.H.; Hofmeyr, M.; Carpenter, J.E.; Bloem, S.; Slabbert, J.P. Sterile insect releases for control of Thaumatotibia leucotreta (Lepidoptera: Tortricidae): An assessment on semi-commercial scale. Afr. Entomol. 2016, 24, 80–89. [Google Scholar] [CrossRef]
- Boersma, N. (XSIT, Citrusdal, South Africa). Personal communication, 2018.
- Bloem, S.; McCluskey, A.; Fugger, R.; Arthur, S.; Wood, S.; Carpenter, J. Suppression of the codling moth Cydia pomonella in British Columbia, Canada using an area-wide integrated approach with an SIT component. In Area-Wide Control of Insect Pests. From Research to Field Implementation; Vreysen, M.J.B., Robinson, A.S., Hendrichs, J., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 591–602. [Google Scholar] [CrossRef]
- Proverbs, M.D.; Newton, J.R. Influence of gamma radiation on the development and fertility of the codling moth, Carpocapsa pomonella (L.) (Lepidoptera: Olethreutidae). Can. J. Zool. 1962, 40, 401–420. [Google Scholar] [CrossRef]
- Proverbs, M.D.; Newton, J.R. Some effects of gamma radiation on the reproductive potential of the codling moth, Carpocapsa pomonella (L.) (Lepidoptera: Olethreutidae). Can. Entomol. 1962, 94, 1162–1170. [Google Scholar] [CrossRef]
- Proverbs, M.D.; Newton, J.R.; Campbell, C.J. Codling moth: A pilot program of control by sterile insect release in British Columbia. Can. Entomol. 1982, 114, 363–376. [Google Scholar] [CrossRef]
- Bloem, S.; Bloem, K.A.; Knight, A.L. Assessing the quality of mass-reared codling moths (Lepidoptera: Tortricidae) by using field release-recapture tests. J. Econ. Entomol. 1998, 91, 1122–1130. [Google Scholar] [CrossRef]
- Bloem, S.; Carpenter, J.E.; Bloem, K.A.; Tomlin, L.; Taggart, S. Effect of rearing strategy and gamma radiation on field competitiveness of mass-reared codling moths (Lepidoptera: Tortricidae). J. Econ. Entomol. 2004, 97, 1891–1898. [Google Scholar] [CrossRef][Green Version]
- Judd, G.J.R.; Thistlewood, H.M.A.; Gardiner, M.G.T.; Lannard, B.L. Is lack of mating competitiveness in spring linked to mating asynchrony between wild and mass-reared codling moths from an operational sterile insect programme? Entomol. Exp. Appl. 2006, 120, 113–124. [Google Scholar] [CrossRef]
- Tyson, R.; Thistlewood, H.; Judd, G.J.R. Modelling dispersal of sterile male codling moths, Cydia pomonella, across orchard boundaries. Ecol. Model. 2007, 205, 1–12. [Google Scholar] [CrossRef]
- Judd, G.J.R.; Arthur, S.; Deglow, K.; Gardiner, M.G.T. Operational mark-release-recapture field tests comparing competitiveness of wild and differentially mass-reared codling moths from the Okanagan Kootenay sterile insect program. Can. Entomol. 2011, 143, 300–316. [Google Scholar] [CrossRef]
- Thistlewood, H.M.A.; Judd, G.J.R. Twenty-five years of research experience with the sterile insect technique and area-wide management of codling moth, Cydia pomonella (L.), in Canada. Insects 2019, 10, 292. [Google Scholar] [CrossRef] [PubMed]
- Philip, H. (Okanagan Kootenay Sterile Insect Release Program, Kelowna, BC, Canada). Personal communication, 2018.
- A Benefit-Cost Analysis of the Okanagan Kootenay Sterile Insect Release Program. Available online: http://www.oksirconnect.com/uploads/5/3/7/6/53763585/oksir_b-c_analysis_report_-_lee_cartier_okanagan_college_school_of_business.pdf (accessed on 13 April 2019).
- Nelson, C. (Okanagan Kootenay Sterile Insect Release Program, Kelowna, BC, Canada). Personal communication, 2018.
- Staten, R.T.; Rosander, R.W.; Keaveny, D.F. Genetic control of cotton insects. The pink bollworm as a working programme. In Management of Insect Pests: Nuclear and Related Molecular and Genetic Techniques; Howard-Kitto, P., Kelleher, R.F., Ramesh, G.V., Eds.; International Atomic Energy Agency (IAEA): Vienna, Austria, 1993; pp. 269–283. [Google Scholar]
- Henneberry, T.J. Integrated systems for control of the pink bollworm Pectinophora gossypiella in cotton. In Area-Wide Control of Insect Pests. From Research to Field Implementation; Vreysen, M.J.B., Robinson, A.S., Hendrichs, J., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 567–580. [Google Scholar] [CrossRef]
- USDA Announces Pink Bollworm Eradication Significantly Saving Cotton Farmers in Yearly Control Costs. Available online: https://www.usda.gov/media/press-releases/2018/10/19/usda-announces-pink-bollworm-eradication-significantly-saving (accessed on 13 April 2019).
- Davis, E. (USDA-APHIS, Phoenix, AZ, USA). Personal communication, 2018.
- Zimmermann, H.G.; Perez-Sandi, M.; Bello-Rivera, A. The Status of Cactoblastis cactorum (Lepidoptera: Pyralidae) in the Caribbean and the Likelihood of Its Spread to Mexico; Unpublished Report to International Atomic Energy Agency (Project TC MEX/5/029): Vienna, Austria, 2005; 60p. [Google Scholar]
- Zimmermann, H.G.; Bloem, S.; Klein, H. Biology, History, Threat, Surveillance and Control of the Cactus Moth, Cactoblastis cactorum; IAEA/FAO-BSC/CM: Vienna, Austria, 2004; 40p. [Google Scholar]
- Soberon, J.; Golubov, J.; Sarukhán, J. The importance of Opuntia in Mexico and routes of invasion and impact of Cactoblastis cactorum (Lepidoptera: Pyralidae). Fla. Entomol. 2001, 84, 486–492. [Google Scholar] [CrossRef]
- Hernández, J.; Sánchez, H.; Bello, A.; González, G. Preventive programme against the cactus moth Cactoblasis cactorum in Mexico. In Area-wide Control of Insect Pests. From Research to Field Implementation; Vreysen, M.J.B., Robinson, A.S., Hendrichs, J., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 345–350. [Google Scholar] [CrossRef]
- Carpenter, J.E. (retired, Tifton, GA, USA; former USDA/ARS). Personal communication, 2019.
- Bello-Rivera, A.; Pereira, R.; Enkerlin-Hoeflich, W.; Bloem, S.; Bloem, K.; Hights, S.; Carpenter, J.E.; Trujillo-Arriaga, F.J.; Sanchez-Anguiano, H.M.; Zetina-Rodriguez, R. Successful area-wide programme in Mexico that eradicated outbreaks of the cactus moth. In Area-Wide Integrated Pest Management: Development and Field Application; Hendrichs, J., Pereira, R., Vreysen, M.J.B., Eds.; CRC Press: Boca Raton, FL, USA, 2019; in press. [Google Scholar]
- Suckling, D.M.; Hackett, J.; Daly, J. Sterilisation of painted apple moth Teia anartoides (Lepidoptera: Lymantriidae) by irradiation. N. Z. Plant Prot. 2002, 55, 7–11. [Google Scholar]
- Suckling, D.M.; Charles, J.; Allan, D.; Chhagan, A.; Barrington, A.; Burnip, G.M.; El-Sayed, A.M. Performance of irradiated Teia anartoides (Lepidoptera: Lymantriidae) in urban Auckland, New Zealand. J. Econ. Entomol. 2005, 98, 1531–1538. [Google Scholar] [CrossRef]
- Stephens, A.E.A.; Suckling, D.M.; Burnip, G.M.; Richmond, J.; Flynn, A. Field records of painted apple moth (Teia anartoides Walker: Lepidoptera: Lymantriidae) on plants and inanimate objects in Auckland, New Zealand. Aust. J. Entomol. 2007, 46, 152–159. [Google Scholar] [CrossRef]
- Self, M. Biosecurity: The implications for international forestry trade. In Proceedings of the Australian and New Zealand Institutes of Forestry Conference, Queenstown, New Zealand, 27 April–1 May 2003; Mason, E.G., Perley, C.J., Eds.; New Zealand Institute of Forestry: Queenstown, New Zealand, 2003; pp. 59–63. [Google Scholar]
- El-Sayed, A.M.; Gibb, A.R.; Suckling, D.M.; Bunn, B.; Fiedler, S.; Comeskey, D.; Manning, L.A.; Foster, S.P.; Morris, B.D.; Ando, T.; et al. Identification of sex pheromone components of the painted apple moth: A tussock moth with a thermally labile pheromone component. J. Chem. Ecol. 2005, 31, 621–646. [Google Scholar] [CrossRef]
- Suckling, D.M.; Barrington, A.M.; Chhagan, A.; Stephens, A.E.A.; Burnip, G.M.; Charles, J.G.; Wee, S.L. Eradication of the Australian painted apple moth Teia anartoides in New Zealand: Trapping, inherited sterility, and male competitiveness. In Area-Wide Control of Insect Pests. From Research to Field Implementation; Vreysen, M.J.B., Robinson, A.S., Hendrichs, J., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 603–615. [Google Scholar] [CrossRef]
- Charles, J.G.; Allan, D.J.; Chhagan, A.; Jamieson, L.E. Effectiveness of Foray 48B over time after application against the painted apple moth. N. Z. Plant Prot. 2005, 58, 17–23. [Google Scholar] [CrossRef]
- Richardson, B.; Kay, M.K.; Kimberley, M.O.; Charles, J.G.; Gresham, B.A. Evaluating the benefits of dose-response bioassays during aerial pest eradication operations. N. Z. Plant Prot. 2005, 58, 12–16. [Google Scholar]
- Suckling, D.M. Applying the sterile insect technique for biosecurity: Benefits and constraints. N. Z. Plant Prot. 2003, 56, 21–26. [Google Scholar] [CrossRef]
- Simmons, G.S.; Suckling, D.M.; Carpenter, J.E.; Addison, M.F.; Dyck, V.A.; Vreysen, M.J.B. Improved quality management to enhance the efficacy of the sterile insect technique for lepidopteran pests. J. Appl. Entomol. 2010, 134, 261–273. [Google Scholar] [CrossRef]
- Kleynhans, E.; Conlong, D.E.; Terblanche, J.S. Host plant related variation in thermal tolerance of Eldana saccharina. Entomol. Exp. Appl. 2014, 150, 113–122. [Google Scholar] [CrossRef]
- Mudavanhu, P.; Conlong, D.E.; Addison, P. Impact of mass rearing and gamma radiation on thermal tolerance of Eldana saccharina Walker (Lepidoptera: Pyralidae). Proc. S. Afr. Sugar Technol. Assoc. 2012, 85, 139–143. [Google Scholar]
- Stotter, R.L.; Terblanche, J.S. Low-temperature tolerance of false codling moth Thaumatotibia leucotreta (Meyrick) (Lepidoptera: Tortricidae) in South Africa. J. Therm. Biol. 2009, 34, 320–325. [Google Scholar] [CrossRef]
- Chidawanyika, F.; Terblanche, J.S. Costs and benefits of thermal acclimation for codling moth, Cydia pomonella (Lepidoptera: Tortricidae): Implications for pest control and the sterile insect release programme. Evol. Appl. 2011, 4, 534–544. [Google Scholar] [CrossRef]
- Blomefield, T.L.; Bloem, S.; Carpenter, J.E. Effect of radiation on fecundity and fertility of a South African codling moth (Lepidoptera: Tortricidae) strain. J. Appl. Entomol. 2010, 134, 216–220. [Google Scholar] [CrossRef]
- Saour, G. Sterile insect technique and F1 sterility in the European grapevine moth, Lobesia botrana. J. Insect Sci. 2014, 14, 8. [Google Scholar] [CrossRef][Green Version]
- Walton, A.; Conlong, D.E. Radiation biology of Eldana saccharina (Lepidoptera: Pyralidae). Fla. Entomol. 2016, 99, 36–42. [Google Scholar] [CrossRef]
- Cagnotti, C.L.; Viscarret, M.M.; Riquelme, M.B.; Botto, E.N.; Carabajal, L.Z.; Segura, D.F.; López, S.N. Effects of X rays on Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) for use in inherited sterility programmes. J. Pest Sci. 2012, 85, 413–421. [Google Scholar] [CrossRef]
- Cagnotti, C.L.; Andorno, A.V.; Hernández, C.M.; Carabajal Paladino, L.; Botto, E.N.; López, S.N. Inherited sterility in Tuta absoluta (Lepidoptera: Gelechiidae): Pest population suppression and potential for combined use with a generalist predator. Fla. Entomol. 2016, 99, 87–94. [Google Scholar] [CrossRef]
- Fu, H.; Zhu, F.W.; Deng, Y.Y.; Weng, Q.F.; Hu, M.Y.; Zhang, T.Z. Development, reproduction and sexual competitiveness of irradiated Conopomorpha sinensis (Lepidoptera: Gracillaridae) pupae and adults. Fla. Entomol. 2016, 99, 66–72. [Google Scholar] [CrossRef]
- Jang, E.B.; McInnis, D.O.; Kurashima, R.; Woods, B.; Suckling, D.M. Irradiation of adult Epiphyas postvittana (Lepidoptera: Tortricidae): Egg sterility in parental and F1 generations. J. Econ. Entomol. 2012, 105, 54–61. [Google Scholar] [CrossRef]
- Chakroun, S.; Rempoulakis, C.; Lebdi-Grissa, K.; Vreysen, M.J.B. Gamma irradiation of the carob or date moth Ectomyelois ceratoniae: Dose-response effects on egg hatch, fecundity and survival. Entomol. Exp. Appl. 2017, 164, 257–268. [Google Scholar] [CrossRef]
- Carpenter, J.E.; Bloem, S.; Taggart, S. Effect of rearing strategy and gamma radiation on the fecundity and fertility of mass-reared codling moth. J. Appl. Entomol. 2010, 134, 221–226. [Google Scholar] [CrossRef]
- López-Martínez, G.; Carpenter, J.E.; Hight, S.D.; Hahn, D.A. Low-oxygen atmospheric treatment improves the performance of irradiation-sterilized male cactus moths used in SIT. J. Econ. Entomol. 2014, 107, 185–197. [Google Scholar] [CrossRef]
- López-Martínez, G.; Carpenter, J.E.; Hight, S.D.; Hahn, D.A. Anoxia-conditioning hormesis alters the relationship between irradiation doses for survival and sterility in the cactus moth, Cactoblastis cactorum (Lepidoptera: Pyralidae). Fla. Entomol. 2016, 99, 95–105. [Google Scholar] [CrossRef]
- Vreysen, M.J.B. Monitoring sterile and wild insects in area-wide integrated pest management programmes. In Sterile Insect Technique. Principles and Practice in Area-Wide Integrated Pest Management; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 325–361. [Google Scholar] [CrossRef]
- Cayol, J.P.; Vilardi, J.; Rial, E.; Vera, M.T. New indices and method to measure the sexual compatibility and mating performance of Ceratitis capitata (Diptera: Tephritidae) laboratory-reared strains under field cage conditions. J. Econ. Entomol. 1999, 99, 140–145. [Google Scholar] [CrossRef]
- Vera, M.T.; Cáceres, C.; Wornoayporn, V.; Islam, A.; Robinson, A.S.; de la Vega, M.H.; Hendrichs, J.; Cayol, J.P. Mating incompatibility among populations of the South American fruit fly, Anastrepha fraterculus (Diptera: Tephritidae). Ann. Entomol. Soc. Am. 2006, 99, 387–397. [Google Scholar] [CrossRef]
- Taret, G.; Sevilla, M.; Wornoayporn, V.; Islam, A.; Ahmad, S.; Caceres, C.; Robinson, A.S.; Vreysen, M.J.B. Mating compatibility among populations of codling moth Cydia pomonella (Linnaeus) (Lepidoptera: Tortricidae) from different geographic origins. J. Appl. Entomol. 2010, 134, 207–215. [Google Scholar] [CrossRef]
- Mudavanhu, P.; Addison, P.; Carpenter, J.E.; Conlong, D.E. Mating compatibility and competitiveness between wild and laboratory strains of Eldana saccharina (Lepidoptera: Pyralidae) after radiation treatment. Fla. Entomol. 2016, 99, 54–65. [Google Scholar] [CrossRef]
- Stringer, L.D.; Sullivan, N.J.; Sullivan, T.E.S.; Mitchell, V.J.; Manning, L.-A.M.; Mas, F.; Hood-Nowotny, R.C.; Suckling, D.M. Attractiveness and competitiveness of irradiated light brown apple moths. Entomol. Exp. Appl. 2013, 148, 203–212. [Google Scholar] [CrossRef]
- Suckling, D.M.; Stringer, L.D.; Mitchell, V.J.; Sullivan, T.E.; Simmons, G.S.; Barrington, A.M.; El-Sayed, A.M. Comparative fitness of irradiated light brown apple moths (Lepidoptera: Tortricidae) in a wind tunnel, hedgerow and vineyard. J. Econ. Entomol. 2011, 104, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Saour, G. Flight ability and dispersal of the European grapevine moth gamma-irradiated males (Lepidoptera: Tortricidae). Fla. Entomol. 2016, 99, 73–78. [Google Scholar] [CrossRef][Green Version]
- Zhang, K.; Fu, H.; Zhu, S.; Li, Z.; Weng, Q.F.; Hu, M.Y. Influence of irradiation on flight ability and dispersal of Conopomorpha sinensis (Lepidoptera: Gracillariidae). Fla. Entomol. 2016, 99, 79–86. [Google Scholar] [CrossRef]
- Brown, R.L.; Stanbury, M.; El-Sayed, A.M.; Laban, J.; Butler, R.; Suckling, D.M. Locomotion activity meter for quality assessment of mass-reared sterile male moths. Fla. Entomol. 2016, 99, 131–137. [Google Scholar] [CrossRef]
- Carpenter, J.E.; Blomefield, T.; Vreysen, M.J.B. A flight cylinder bioassay as a simple, effective quality control test for Cydia pomonella. J. Appl. Entomol. 2012, 136, 711–720. [Google Scholar] [CrossRef]
- Carpenter, J.E.; Blomefield, T.; Hight, S.D. Comparison of laboratory and field bioassays to examine lab-reared Cydia pomonella (Lepidoptera: Tortricidae) quality and field performance. J. Appl. Entomol. 2013, 137, 631–640. [Google Scholar] [CrossRef]
- Woods, B.; McInnis, D.; Steiner, E.; Soopaya, A.; Lindsey, J.; Lacey, I.; Virdi, A.; Fogliani, R. Developing field cage tests to measure mating competitiveness of sterile light brown apple moth (Lepidoptera: Torticidae) in Western Australia. Fla. Entomol. 2016, 99, 138–145. [Google Scholar] [CrossRef]
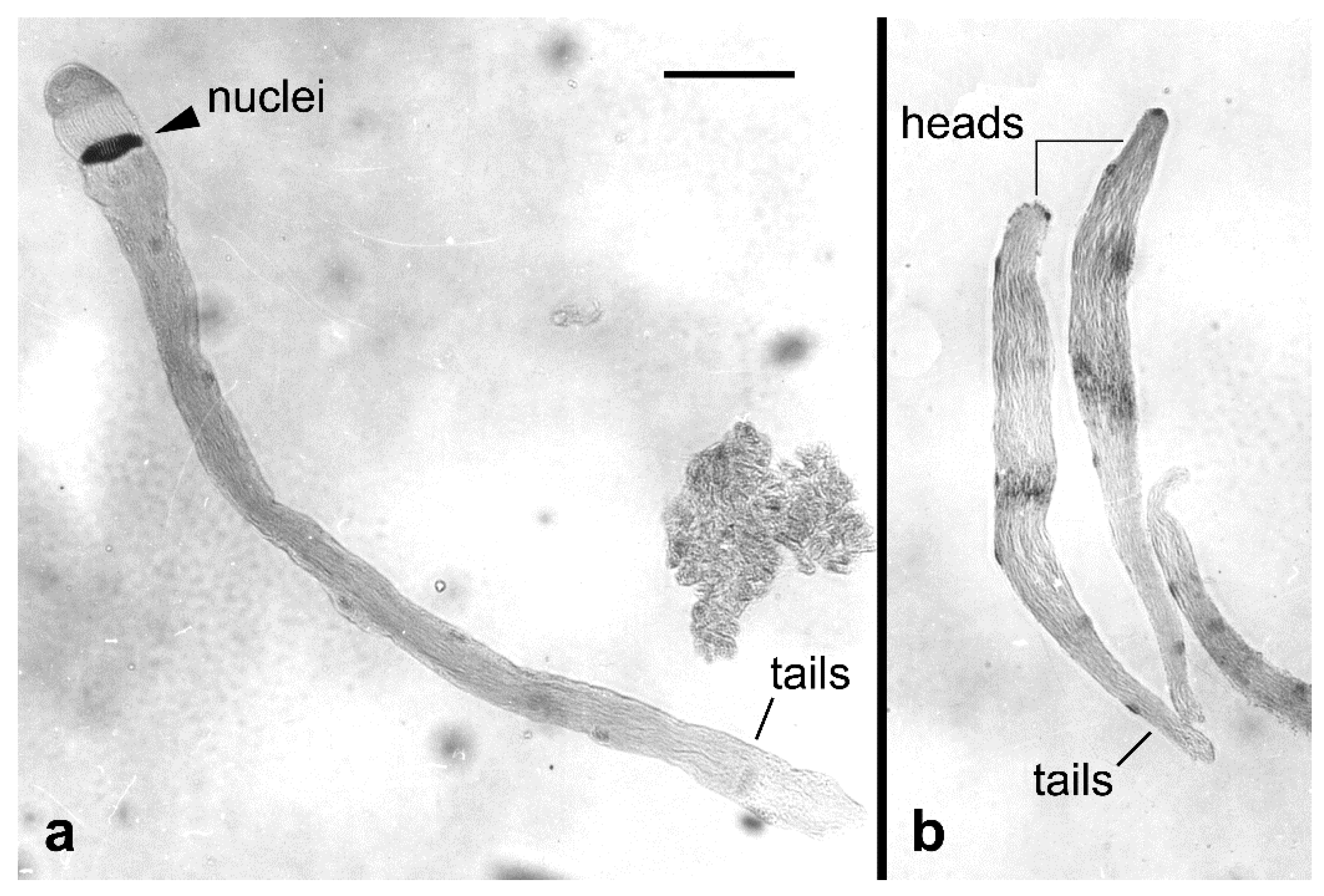
- Carpenter, J.E.; Marti, O.G.; Wee, S.L.; Suckling, D.M. Cytological attributes of sperm bundles unique to F1 progeny of irradiated male Lepidoptera: Relevance to sterile insect technique programs. Fla. Entomol. 2009, 92, 80–86. [Google Scholar] [CrossRef]
- Wee, S.L.; Suckling, D.M.; Barrington, A.M. Feasibility study on cytological sperm bundle assessment of F1 progeny of irradiated male painted apple moth (Teia anartoides Walker; Lepidoptera: Lymantriidae) for the sterile insect technique. Aust. J. Entomol. 2011, 50, 269–275. [Google Scholar] [CrossRef]
- Light, D.M.; Knight, A.L.; Henrick, C.A.; Rajapaska, D.; Lindgren, B.; Dickens, J.C.; Reynolds, K.M.; Buttery, R.G.; Merrill, G.; Roitman, J.; et al. A pear derived kairomone with pheromonal potency that attracts male and female codling moth, Cydia pomonella (L.). Naturwissenschaften 2001, 88, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Wee, S.L. Effects of conspecific herbivory and mating status on host searching and oviposition behavior of Plutella xylostella (Lepidoptera: Plutellidae) in relation to its host, Brassica oleracea (Brassicales: Brassicaceae). Fla. Entomol. 2016, 99, 159–165. [Google Scholar] [CrossRef]
- Simmons, G.S.; McKemey, A.; Morrison, N.; O’Connell, S.; Tabashnik, B.; Claus, J.; Fu, G.; Tang, G.; Sledge, M.; Walker, A.; et al. Field performance of a genetically engineered strain of pink bollworm. PLoS ONE 2011, 6, e24110. [Google Scholar] [CrossRef] [PubMed]
- Hood-Nowotny, R.; Mayr, L.; Islam, A.; Robinson, A.S.; Caceres, C. Routine isotope marking for the Mediterranean fruit fly (Diptera: Tephritidae). J. Econ. Entomol. 2009, 102, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Hood-Nowotny, R.; Watzka, M.; Mayr, L.; Mekonnen, S.; Kapitano, B.; Parker, A. Intrinsic and synthetic stable isotope marking of tsetse flies. J. Insect Sci. 2011, 11, 79. [Google Scholar] [CrossRef]
- Hood-Nowotny, R.; Harari, A.; Seth, R.; Wee, S.-L.; Conlong, D.E.; Suckling, D.M.; Lebdi-Grissa, K.; Simmons, G.; Carpenter, J.E. Stable isotope markers differentiate between mass-reared and wild Lepidoptera in sterile insect technique programs. Fla. Entomol. 2016, 99, 166–176. [Google Scholar] [CrossRef][Green Version]
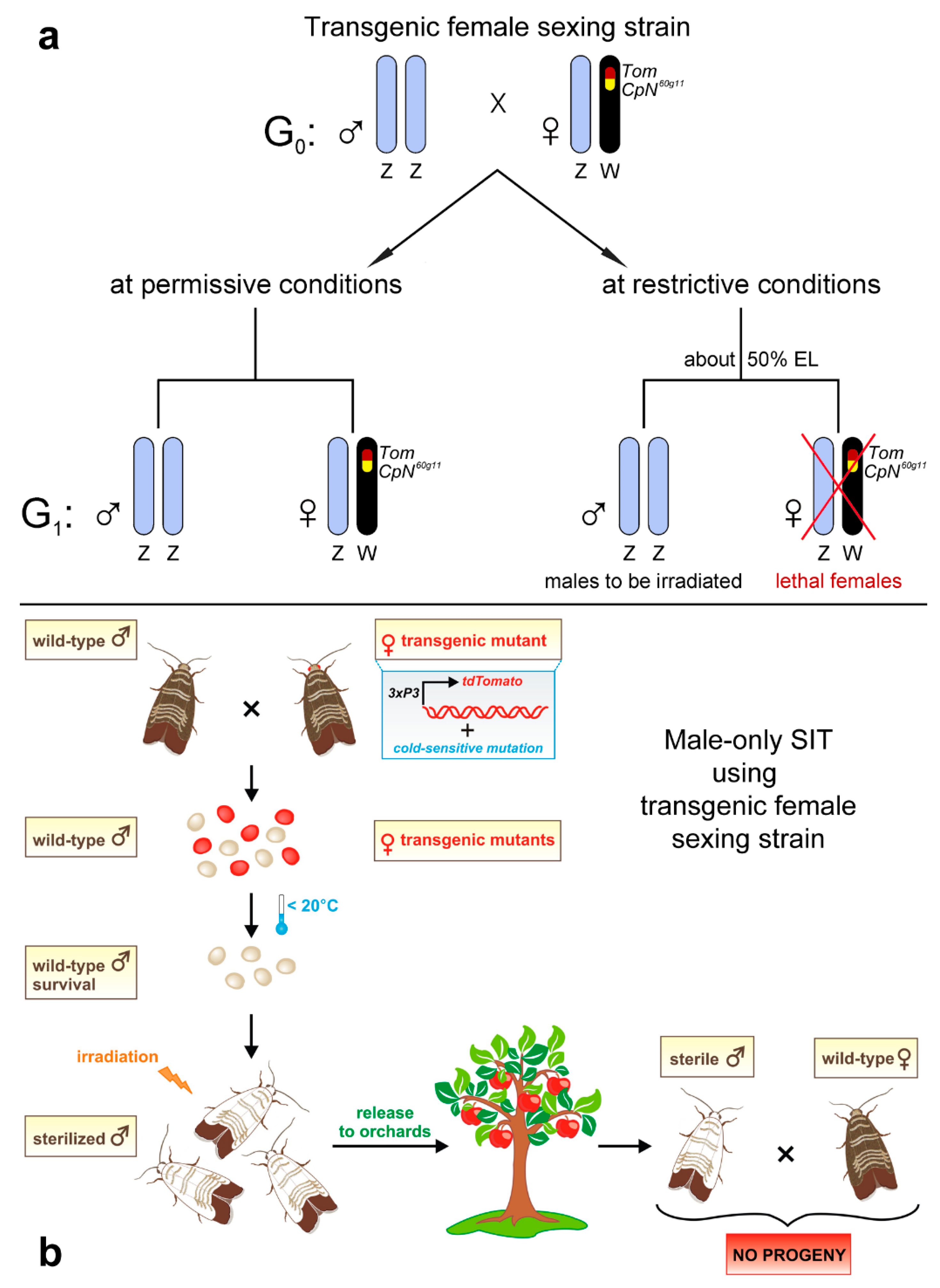
- Marec, F.; Neven, L.G.; Robinson, A.S.; Vreysen, M.; Goldsmith, M.R.; Nagaraju, J.; Franz, G. Development of genetic sexing strains in Lepidoptera: From traditional to transgenic approaches. J. Econ. Entomol. 2005, 98, 248–259. [Google Scholar] [CrossRef]
- Rendón, P.; McInnes, D.; Lance, D.; Stewart, J. Medfly (Diptera: Tephritidae) genetic sexing: Large scale field comparison of males-only and bisexual sterile fly releases in Guatemala. J. Econ. Entomol. 2004, 97, 1547–1553. [Google Scholar] [CrossRef]
- Hight, S.D.; Carpenter, J.E.; Bloem, S.; Bloem, K.A. Developing a sterile insect release program for Cactoblastis cactorum (Berg) (Lepidoptera: Pyralidae): Effective overflooding ratios and release-recapture field studies. Environ. Entomol. 2005, 34, 850–856. [Google Scholar] [CrossRef]
- Strunnikov, V.A. Sex control in the silkworms. Nature 1975, 255, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Ohnuma, A. Establishment of the practical male-rearing technology by a balanced sex-linked lethal (in Japanese). J. Sericult. Sci. Jpn. 2005, 74, 81–87. [Google Scholar] [CrossRef]
- Marec, F. Genetic control of pest Lepidoptera: Construction of a balanced lethal strain in Ephestia kuehniella. Entomol. Exp. Appl. 1991, 61, 271–283. [Google Scholar] [CrossRef]
- Peloquin, J.J.; Thibault, S.T.; Staten, R.; Miller, T.A. Germ-line transformation of pink bollworm (Lepidoptera: Gelechiidae) mediated by the piggyBac transposable element. Insect Mol. Biol. 2000, 9, 323–333. [Google Scholar] [CrossRef]
- Tamura, T.; Thibert, C.; Royer, C.; Kanda, T.; Abraham, E.; Kamba, M.; Komoto, N.; Thomas, J.L.; Mauchamp, B.; Chavancy, G.; et al. Germline transformation of the silkworm Bombyx mori L. using a piggyBac transposon derived vector. Nat. Biotechnol. 2000, 18, 81–84. [Google Scholar] [CrossRef]
- Marec, F.; Neven, L.G.; Fuková, I. Developing transgenic sexing strains for the release of non-transgenic sterile male codling moths Cydia pomonella. In Area-Wide Control of Insect Pests. From Research to Field Implementation; Vreysen, M.J.B., Robinson, A.S., Hendrichs, J., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 103–111. [Google Scholar] [CrossRef]
- Ma, S.; Wang, X.; Fei, J.; Liu, Y.; Duan, J.; Wang, F.; Xu, H.; Zhao, P.; Xia, Q. Genetic marking of sex using a W chromosome-linked transgene. Insect Biochem. Mol. Biol. 2013, 43, 1079–1086. [Google Scholar] [CrossRef]
- Tan, A.; Fu, G.; Jin, L.; Guo, Q.; Li, Z.; Niu, B.; Meng, Z.; Morrison, N.I.; Alphey, L.; Huang, Y. Transgene-based, female-specific lethality system for genetic sexing of the silkworm, Bombyx mori. Proc. Nat. Acad. Sci. USA 2013, 110, 6766–6770. [Google Scholar] [CrossRef]
- Jin, L.; Walker, A.S.; Fu, G.; Harvey-Samuel, T.; Dafa’alla, T.; Miles, A.; Marubbi, T.; Granville, D.; Humphrey-Jones, N.; O’Connell, S.; et al. Engineered female-specific lethality for control of pest Lepidoptera. ACS Synth. Biol. 2013, 2, 160–166. [Google Scholar] [CrossRef]
- Kiuchi, T.; Sugano, Y.; Shimada, T.; Katsuma, S. Two CCCH-type zinc finger domains in the Masc protein are dispensable for masculinization and dosage compensation in Bombyx mori. Insect Biochem. Mol. Biol. 2019, 104, 30–38. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Liu, Z.; Xu, J.; Li, X.; Bi, H.; Andongma, A.A.; Niu, C.; Huang, Y. Mutation of doublesex induces sex-specific sterility of the diamondback moth Plutella xylostella. Insect Biochem. Mol. Biol. 2019, 112, 103180. [Google Scholar] [CrossRef] [PubMed]
- Ohbayashi, F.; Suzuki, M.G.; Mita, K.; Okano, K.; Shimada, T. A homologue of the Drosophila doublesex gene is transcribed into sex-specific mRNA isoforms in the silkworm, Bombyx mori. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2001, 128, 145–158. [Google Scholar] [CrossRef]
- Suzuki, M.G. Sex determination: Insights from the silkworm. J. Genet. 2010, 89, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Kunte, K.; Zhang, W.; Tenger-Trolander, A.; Palmer, D.H.; Martin, A.; Reed, R.D.; Mullen, S.P.; Kronforst, M.R. doublesex is a mimicry supergene. Nature 2014, 507, 229–232. [Google Scholar] [CrossRef]
- Nagaraju, J.; Gopinath, G.; Sharma, V.; Shukla, J.N. Lepidopteran sex determination: A cascade of surprises. Sex. Dev. 2014, 8, 104–112. [Google Scholar] [CrossRef]
- Wang, X.Y.; Zheng, Z.Z.; Song, H.S.; Xu, Y.Z. Conserved RNA cis-elements regulate alternative splicing of Lepidopteran doublesex. Insect Biochem. Mol. Biol. 2014, 44, 1–11. [Google Scholar] [CrossRef]
- Fujii, T.; Shimada, T. Sex determination in the silkworm, Bombyx mori: A female determinant on the W chromosome and the sex-determining gene cascade. Semin. Cell Dev. Biol. 2007, 18, 379–388. [Google Scholar] [CrossRef]
- Kiuchi, T.; Koga, H.; Kawamoto, M.; Shoji, K.; Sakai, H.; Arai, Y.; Ishihara, G.; Kawaoka, S.; Sugano, S.; Shimada, T.; et al. A single female-specific piRNA is the primary determiner of sex in the silkworm. Nature 2014, 509, 633–636. [Google Scholar] [CrossRef]
- Yoshido, A.; Marec, F.; Sahara, K. The fate of W chromosomes in hybrids between wild silkmoths, Samia cynthia ssp.: No role in sex determination and reproduction. Heredity 2016, 116, 424–433. [Google Scholar] [CrossRef]
- Gempe, T.; Beye, M. Function and evolution of sex determination mechanisms, genes and pathways in insects. Bioessays 2011, 33, 52–60. [Google Scholar] [CrossRef]
- Kean, J.M.; Suckling, D.M.; Sullivan, N.J.; Tobin, P.C.; Stringer, L.D.; Lee, D.C.; Smith, G.R.; Flores Vargas, R.; Fletcher, J.; Macbeth, F.; et al. Global Eradication and Response Database. 2016. Available online: http://b3.net.nz/gerda/index.php (accessed on 22 April 2019).
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marec, F.; Vreysen, M.J.B. Advances and Challenges of Using the Sterile Insect Technique for the Management of Pest Lepidoptera. Insects 2019, 10, 371. https://doi.org/10.3390/insects10110371
Marec F, Vreysen MJB. Advances and Challenges of Using the Sterile Insect Technique for the Management of Pest Lepidoptera. Insects. 2019; 10(11):371. https://doi.org/10.3390/insects10110371
Chicago/Turabian StyleMarec, František, and Marc J. B. Vreysen. 2019. "Advances and Challenges of Using the Sterile Insect Technique for the Management of Pest Lepidoptera" Insects 10, no. 11: 371. https://doi.org/10.3390/insects10110371
APA StyleMarec, F., & Vreysen, M. J. B. (2019). Advances and Challenges of Using the Sterile Insect Technique for the Management of Pest Lepidoptera. Insects, 10(11), 371. https://doi.org/10.3390/insects10110371