Abstract
Fuel and water contents are inevitable in automotive engine oils. This study intends to investigate the impact of the addition of gasoline (3–20%) and water (1%) on the lubricating performance of synthetic base oil (PAO), with or without an anti-wear additive (ZDDP), for a steel-cast iron contact. Fuel-added PAO showed an increase in the load carrying capacity. Oil electrical conductivity and total acid number (TAN) measurements showed slightly increased conductivity and marginally increased acidity at a higher fuel concentration. In contrast, an increased wear rate, proportional to the fuel concentration, was observed in a prolonged test with constant-loading. Results suggested that the fuel addition is a double-edged sword: reducing the scuffing risk by providing stronger surface adsorption and increasing the sliding wear rate by bringing down the oil viscosity. The PAO-water blend formed an emulsion and resulted in a significantly increased load-carrying capacity, again likely due to the higher polarity and possibly acidity. For the ZDDP-containing PAO, the addition of 1% water and 3% fuel generated 24% and 52% higher wear. The phosphate polymerization level was reduced on the worn surfaces by the introduction of water but the thickness of ZDDP tribofilm was not significantly affected.
1. Introduction
Proper lubrication is essential for the performance and longevity of automotive engines; however, contaminants persistently exist in engine oils and result in changes of the oil viscosity and possible chemical changes of the additives. The two most common contaminants in engine oils are fuel and water. Unburned fuel is deposited on the cylinder wall, leaking past through the interface with the piston ring and into the crankcase. It has been reported that the fuel content can be as high as 9% in turbocharged gasoline direct injection (TGDI) engines, which consequently resulted in a 30% reduction in the oil viscosity [1]. The fuel concentration at the interface between the piston ring and cylinder wall near the combustion chamber could be much higher than that in the oil sump. By using a gas chromatography and a mass spectrometry, as much as 21.9% fuel was found in the engine oil at the top ring reversal zone after one-hour engine operation [2].
As to the impact of the fuel dilution on the engine oil lubricity, there is a lack of consensus in the literature. Ajayi et al. reported that the fuel content reached 6% in certain marine engine operating conditions with a start-and-stop cycle protocol; however, the fuel-diluted engine oil showed a minimal effect on friction and an inconclusive impact on wear, compared with fresh oil. In contrast, the load-carrying capacity was reported to be lowered by the fuel content with the hypothesis that the effectiveness of extreme pressure additives in the engine oil was reduced [3]. In another study, when 6% gasoline was added to an SAE 5W-50 engine oil, friction and wear were increased by 3–16% and 2–10%, respectively, under various loading conditions [4]. Song et al. reported that the addition of 15% diesel fuel significantly reduced the engine oil viscosity but did not cause any noticeable wear on a 2700 cc, 5 cylinder engine [5]. The impact of fuel content in a fully formulated engine oil was largely affected by the chemistry of oil additives. This study intended to separate the impacts of fuel on the base oil and an anti-wear additive.
Water vapor is produced during the combustion process. At a high engine temperature, water vapor exits through the exhaust; at a lower engine temperature water vapor condenses on the cylinder wall and mixes with the engine oil. The water content in the oil sump could be as high as 1%. The involvement of water in lubrication has long been studied. It was argued that water might influence friction and wear through modifying the lubricant/additive adsorption during boundary lubrication, disturbing the chemistry and structure of tribofilms and causing pitting on rolling elements [6]. Results are mixed for the effects of water on friction and wear, largely depending on testing conditions and contacting materials. Cai et al. showed that the addition of 1% water to a base oil increased the wear and friction of a steel–steel contact [7]. Fatima et al. reported the presence of water in a fully formulated automatic transmission fluid caused an increase in friction in a short period of time but a friction reduction over a longer term [8]. Interestingly, the wear of cast iron was reported to decrease with the increase of water content in polyalkylene glycol and polyolester oils [9]. The interaction between water and zinc dialkyldithiophosphate (ZDDP) and the impact on lubrication were reported in literature [10,11]. One study suggested that water might accelerate the decomposition of ZDDP through hydrolysis to supply more decomposed ZDDP products that are essential for the tribofilm buildup [12], while another study showed that water might inhibit the tribofilm growth by interfering with the ZDDP surface adsorption [13]. Costa et al. showed that ethanol reduced the thickness of ZDDP tribofilm significantly, while the combination of water and ethanol decreased the tribofilm adherence [14]. Parsaeian et al. applied controlled temperature and humidity on a steel-steel contact lubricated by a poly-alpha-olefin (PAO) base oil with 0.8% ZDDP. The increased humidity resulted in an increased wear that was attributed to the tribocorrosion from water [15]. The research of water in a base oil with ZDDP has usually been explored at an elevated temperature between 70 °C and 100 °C, there is a lack of attention on low temperatures when water condenses the most and forms an emulsion in engines.
Here, we studied the impact of engine oil contaminants, fuel (3–20%) and water (1%), on the lubricating behavior of a PAO base oil both with and without a secondary ZDDP (0.8%) at room temperature, by comparing the wear and friction results. The load-carrying capacity of contaminated base oil has also been analyzed. The oil acidity and electrical conductivity measurements were performed to determine the anti-scuffing benefit from contaminants. Worn surface morphology and composition were studied by using microscopy and spectroscopy. The understandings between base oils and engine oil contaminants shed light on the fundamental mechanisms of their impact on engines.
2. Materials and Methods
A synthetic base oil, PAO 4 cSt (supplied by ExxonMobil Chemical, Houston, TX, USA) was used in this study. D.I. water at 1 wt % and regular grade unleaded gasoline with an octane level of 87 without ethanol at 3, 6, or 20 wt % were used as contaminants. A secondary ZDDP (supplied by Lubrizol, Wickliffe, OH, USA) was used as the anti-wear additive and the concentration was 0.8 wt % (800 ppm phosphorus). All the blends were sonicated for 15 min before tests. Both gasoline and ZDDP were fully dissolved in the base oil while an emulsion was formed when water was added. The viscosity of the blends was measured using a Petrolab Minivis II (Grabner Instruments, Vienna, Austria) viscometer at 23 °C. The total acid number (TAN) of the selected fluids was measured by using potentiometric titration following the ASTM standard D 664 (Method A). The electrical conductivity of selected fluids was measured at room temperature and 40 °C using a Precision Conductivity Meter (Model 1154, Emcee Electronics, Venice, FL, USA) following the procedure described in the ASTM standard D 4308. The post-tested lubricants were collected after the 1000-m wear tests.
In the tribological testing, a 10-mm diameter AISI E52100 hardened steel ball (Ra: 0.025–0.05 µm) was used to slide against a CL35 cast iron flat (2.54 cm by 2.54 cm, Ra: 0.08 µm) on a reciprocating tribometer (Plint TE77, Phoenix Tribology, Berkshire, UK). The steel was the reciprocating upper piece, while the cast iron flat was the stationary lower piece. The flats were polished using Buehler SiC abrasive papers in a sequence of P280, P800, and P1200 with water. The flats and balls were cleaned by using isopropyl alcohol before each test. The load-carrying tests were carried out starting at 20 N with a 50 N/min increasing rate until a 200 N normal load was reached. The wear tests were performed at 100 N for 1000 m. Each testing fluid was tested for 2–3 repeats. The sliding oscillation was 10 Hz with a 10-mm stroke. All tests were conducted in an ambient environment at room temperature (~23 °C), simulating the oil temperature at engine cold starts. The wear volumes were quantified using an optical interferometer (Wyko NT9100, Bruker, Tucson, AZ, USA). The worn surfaces were first examined using scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDX) (Hitachi S4800 field-emission, Hitachi, Tokyo, Japan). X-ray photoelectron spectroscopy (XPS) surface chemical analysis was performed using a Thermo Scientific K-Alpha XPS system (Thermo Fisher Scientific, Waltham, MA, USA) with the signal acquisition of 400 μm diameter spot on the wear track. The core-level spectra were obtained after 30-s argon-ion sputtering (2.0 keV) of the sample surface to remove any contamination or oil residual. The composition-depth profiling was achieved by using ion sputtering.
3. Results
3.1. Impact of Fuel and Water Content in PAO in Load-Carrying Tests
The load-carrying capacity of the additized PAO has been investigated by using variable loading tests. With the increase of the contact stress, the friction usually changes and serves as a good indicator of the lubricant performance. For PAO base oil, the system friction passed 0.15 at 75.6 ± 3.0 N (average ± SD), as shown in Figure 1. The addition of 3% fuel resulted in load carrying capacity of 77.7 ± 11.1 N for the COF passed 0.15. When the fuel concentration was increased to 20%, two out of three repeats tolerated a higher load at 85 N and 104 N for COF of 0.15, with a third test showed no signs of scuffing within the testing range. The discrepancies observed in the repeat tests for the PAO containing 3% and 20% fuel are due to the nature of scuffing. Scuffing is determined by many dynamic factors, for example, localized surface composition and roughness, transient asperity temperature and wear debris involvement. As a result, scuffing is not a definite event for a given material-lubricant system but a tendency (or probability): a better lubricated system would be less likely to scuff, not never; and a poorer lubricated system would be more likely to scuffing, not always. Three repeats from a statistical perspective are rather few (we wish we could run hundreds of repeats at each condition but it is not practical) and thus observing some discrepancies in scuffing behavior among the repeat tests is not surprising but well expected. Rather, what really matters is the trend that a higher fuel content increases the load carrying capacity. 1% of water in PAO led to a significantly increased loading tolerance such that the COF was lower than 0.09 throughout the testing range (20–200 N). Either fuel or water could increase the load carrying capacity of the base oil that water required a low concentration (1%) to be effective while fuel required a higher concentration (20% performed better than that at 3%). The results shown here on the base oil should be distinguished with the findings in the literature [3], where the contaminants reduced the load-carrying capacity of fully formulated engine oils by impairing the functions of additives.
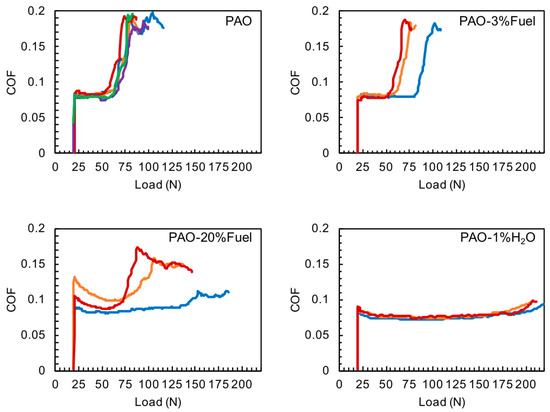
Figure 1.
Load-carrying tests of PAO, PAO-3%Fuel, PAO-20%Fuel and PAO-1%H2O. Colored lines represent individual repeats.
3.2. Impact of Fuel and Water Content in PAO in Constant-Load Wear Testing
The viscosity of fuel was much lower than that of PAO and our measured values at room temperature (23 °C) were 0.67 cSt for the fuel and 35.4 cSt for the PAO oil, respectively. As expected, adding fuel into PAO significantly reduced oil viscosity. The viscosity of the oil-fuel blends was measured to be 28.6, 23.2 and 9.5 cSt, respectively, for 3%, 6% and 20% fuel dilution. For a blend of two mutually miscible fluids, the viscosity of the blend supposedly is a function of the specific viscosities of each fluid and the blend ratio, as defined by the Refutas equation [16]. A simplified version for the oil-fuel blend is shown in Equation (1). The calculated viscosities for the oil-fuel blends are compared with the measured values in Figure 2a. The good agreement confirms the oil-fuel miscibility because the viscosity of an emulsion does not follow the Refutas equation.
where v is the kinematic viscosity in centistokes and Xoil and Xfuel represent the mass fractions of the oil and fuel in the blend, respectively.
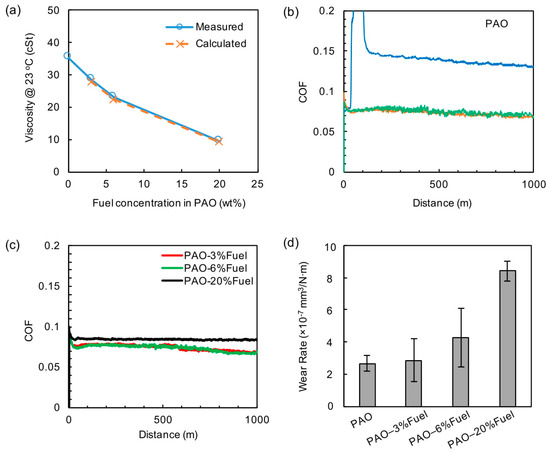
Figure 2.
Rheological and tribological properties of PAO-fuel blends. (a) Viscosities of PAO-fuel blends; (b) Friction behavior in the PAO base oil with colored lines represent individual repeats; (c) Friction behavior of PAO-fuel blends, the averaged profiles of repeats are shown; (d) Wear rates of PAO and PAO-fuel blends. Note: wear of a scuffed single test on PAO was not included.
The lubricity of PAO base oil without and with fuel dilution was evaluated. Among the three repeating tests of the neat PAO base oil, one test showed the COF elevated to above 0.2 at the onset, indicating scuffing; while other two runs exhibited a relatively stable COF of 0.07–0.08 throughout the tests, as shown in Figure 2b. In contrast, no scuffing was observed in any run of the three oil-fuel blends, as shown in Figure 2c, which also supports the observation in the load-carrying tests. The COF was around 0.07–0.08 at all three fuel concentrations, similar to that for the neat PAO without scuffing. The wear rates of the cast iron flats are compared in Figure 2d (wear rates of the steel balls were two orders of magnitude lower and thus was neglected here). The wear rates of individual test were shown in Table S1. For the PAO base oil, the scuffed test had a wear rate >50× higher than the other two tests without scuffing and thus is not presented in the chart. The wear rate of PAO-3%Fuel seemed similar to that in the neat PAO without scuffing. With the increase of the fuel concentration from 3% to 6% and then 20%, the wear rate increased proportionally, correlating well to the viscosity reduction.
The addition of 1% water to the PAO base oil resulted in an emulsion (after 15 min sonication), which was relatively stable, as an observation over a period of 24 h was shown in Figure S1. Water in lubricants is known to cause corrosion and cavitation problems. The friction behavior of the PAO-water emulsion was rather random, as shown in Figure 3a. This may be due to the non-uniform distribution of the water mini-droplets in the oil. The water content seemed to have an insignificant impact on the wear rate compared with the non-scuffing cases of the neat PAO (Figure 3b). The cross-sectional profiles of the wear tracks on cast iron are shown in Figure S2. Signs of corrosion (e.g., pitting) were clearly observed outside the wear scar on the cast iron surface but not on the wear scar, where the tribocorrosion products were promptly removed by mechanical rubbing under high contact stress.
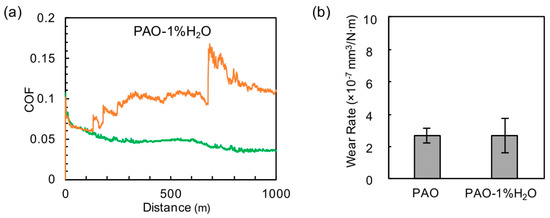
Figure 3.
(a) Friction profiles of PAO-water blend with colored lines represent individual repeats. (b) Wear rates of PAO and PAO-water blend.
3.3. Impact of Fuel and Water Content on the Anti-Wear Effectiveness of ZDDP
The impact of fuel and water contents on the performance of ZDDP was also studied. Figure 4 compares the friction behavior and wear rate of the ZDDP additized PAO without and with fuel (3%) or water (1%). The addition of ZDDP reduced the risk of scuffing failure for the PAO base oil, similarly to our previous experience [17,18]. The inclusion of fuel or water in the lubricant had little impact on the friction behavior but reduced the anti-wear effectiveness of ZDDP, as shown in Figure 4. The cross-sectional profiles of the wear tracks on cast iron are shown in Figure S3. Surface characterization revealed that the fuel and water content significantly affects the morphology and composition of the tribofilm produced by ZDDP, as described below.
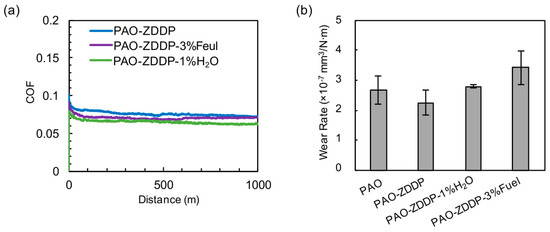
Figure 4.
The impact of fuel and water content on the (a) friction and (b) wear behavior of PAO-ZDDP. The averaged friction profiles of repeats are shown.
3.4. Characterization of Tested Lubricants and Worn Surfaces
We measured the electrical conductivity and TAN of the oil-fuel blends. The electrical conductivity of the neat PAO was below the instrument sensitivity (1 pS/m) at both room temperature (testing condition) and 40 °C (assumed peak temperature). While no change was observed after adding 3% fuel, the conductivity became measurable (~2 pS/m) at 40 °C when the fuel content was increased to 20%, due to the higher conductivity of pure fuel than that of pure PAO. The TANs of pre-tested lubricants were below the method sensitivity (0.1 mg KOH/g). After the wear tests, while the TAN of the used PAO stayed below 0.1 mg KOH/g, the TANs of the used PAO-fuel blends marginally increased to 0.11 and 0.13 mg KOH/g for 3% and 20% fuel concentration, respectively. Due to the methods’ sensitivity, the results were not as descriptive as hoped; however, both trends of the electrical conductivity and TAN can be correlated with the increased oil acidity by adding fuel. These tentative trends carry increased relevance in view of the increasing inclusion of polar oxygenated fuels derived from renewable sources. Recent studies identify the role of oxygenates in more complex fuel degradation or interaction chemistries beyond direct oxidation, including the accumulation of aqueous condensates into cool spots in fuel and lubricant systems [19]. Even the slightest lubricant trend toward increased polarity and acidity upon fuel mixing would be exacerbated in these new fuel systems.
Surface characterization using SEM, EDX and XPS was carried out on the wear tracks of cast iron flats to further understand the impact of the fuel and water content in the oil. SEM images and the EDS spectra of selected wear scars are shown in Figure 5. It seems that the worn surface became smoother when the fuel concentration increased from 3% to 6% and then to 20%, which might be a result of chemical-mechanical polishing at the contact interface promoted by the increasing oil acidity. The ZDDP, as expected, produced a relatively rough surface with a tribofilm containing its signature elements, Zn, P and S, as detected by using EDX. The O peak was significantly reduced, likely because of the anti-oxidant functionality of ZDDP that reduced iron oxide formation the contact surfaces. The addition of fuel and water evidently changed the morphology of the ZDDP-based tribofilm with much smaller pad sizes.
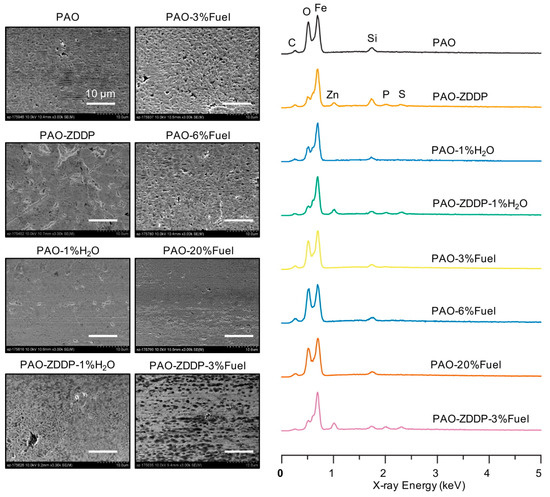
Figure 5.
Scanning electron microscopy (SEM) surface images and energy dispersive X-ray spectroscopy (EDX) spectra of the cast iron worn surfaces. Scale bars represent 10 μm.
Figure 6 shows the XPS core-level spectra of C 1s, O 1s and Fe 2P3/2 on the cast iron worn surfaces lubricated by PAO-1%H2O, PAO-ZDDP and PAO-ZDDP-1%H2O, obtained after 30 s iron sputtering. There were three C 1s compounds identified based on the binding energy: a carbon group at 285 eV, an alcohol group at 286.4 eV and a carboxylate group at 288.5 eV. For PAO-1%H2O, a stronger peak of carboxylate group was recognized, compared to that of PAO-ZDDP and PAO-ZDDP-1%H2O. The O 1s peak was resolved into two separate peaks: metal oxides and others. Higher iron oxides content has been detected on PAO-1%H2O lubricated surface, due to the lack of anti-wear additive. Between 531 eV and 532 eV, there is an energy overlapping of O 1s in hydroxides, phosphates and carbonates. On the PAO-1%H2O lubricated surface, there were both hydroxides and carbonates, while phosphates replaced them in ZDDP-involved lubrication. For Fe 2P3/2, there was mainly Fe (III) at 710.9 eV and no major difference was noticed in the corresponding iron binding energy between worn surfaces. The P 2p peak was at 133.7 eV and 133.3 eV for PAO-ZDDP and PAO-ZDDP-1%H2O, respectively. The lowered binding energy of P for PAO-ZDDP-1%H2O stands for a lower degree of polymerization of phosphate, which agrees with the results in the literature [13,20,21]. The main peak of S 2p was positioned at 162.5 eV, indicating sulfide and the major peaks of Zn 2p around 1022.5 suggest Zn (II) with the preference of ZnO and/or ZnS.
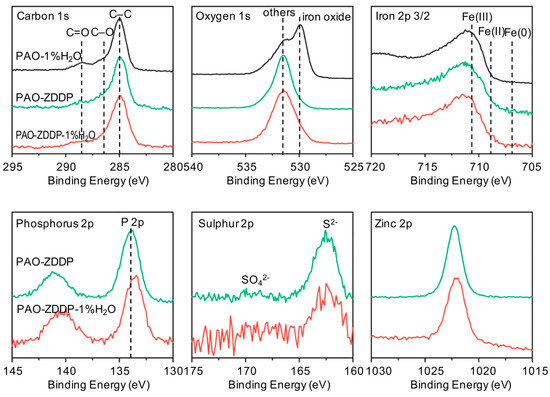
Figure 6.
XPS core-level spectra of C 1s, O 1s, Fe 2P3/2, P 2p, S 2p and Zn 2p on the cast iron worn surfaces lubricated by PAO-1%H2O, PAO-ZDDP and PAO-ZDDP-1%H2O. (Note: some signal strength has been normalized).
The composition-depth profiles of the worn surfaces lubricated by PAO-1%H2O, PAO-ZDDP and PAO-ZDDP-1%H2O are shown in Figure 7. The atomic composition was plotted against the ion sputtering time. The sputtering rate was calibrated to be roughly 10 nm/min. For PAO-1%H2O lubricated surface, the O content was above 60%, indicating high oxide content in the surface film and the O:Fe ratio surpassed 3. As shown in Figure 6, the iron mainly existed in the Fe (III) form. Theoretically, O:Fe ratio in iron hydroxide (Fe(OH)3) and iron carbonate (Fe2(CO3)3) ranges from 3:1 to 4.5:1 that is much higher than that of iron oxide (1.5:1 in Fe2O3). Moreover, the iron hydroxides are capable of enclosing water molecules in their structure and transforming to hydrates, FeO(OH)·xH2O, that also contains a high O content. The carbon in PAO-ZDDP lubrication reached as high as 30%, which was greater than those in PAO-1%H2O and PAO-ZDDP-1%H2O. Carbon constituents in the tribofilm are a critical part in the phosphate polymerization and the presence of water hindered the polymerization process. For PAO-ZDDP-1%H2O, phosphorus content was only slightly reduced, compared with PAO-ZDDP. The thickness of ZDDP tribofilm was not significantly altered by the arrival of water.
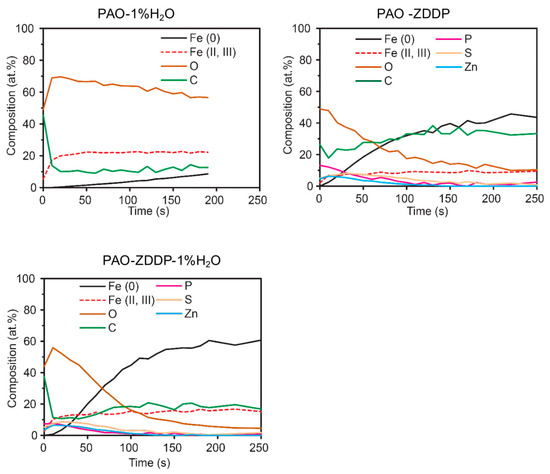
Figure 7.
XPS composition-depth profiles of the cast iron worn surfaces lubricated by PAO-1%H2O, PAO-ZDDP, and PAO-ZDDP-1%H2O.
4. Discussion
It is interesting to observe the phenomenon that the fuel and water help mitigate scuffing to some extents, as shown in the load-carrying tests. We hypothesize that the addition of fuel and water increases the polarity of the lubricant and therefore improves the load carrying performance. PAO has a complex branched structure and is produced by the polymerization of alpha olefins where olefins were saturated with hydrogen. Due to the lack of polar functional groups, the interaction (physical and chemical adsorptions) between the PAO oil molecules and the metal surface is rather weak. Gasoline is a mixture of various hydrocarbons (linear/branched/cyclic hydrocarbons; aromatics, olefins, etc.) plus some fuel additives, whose compositions may vary significantly depending on the source of crude oils, the refinery process, as well as the formulations. Typically, the gasoline contains approximately 20~40 vol % unsaturated hydrocarbons (aromatics and olefins) which are more polar than saturated hydrocarbons. Fuel additives, such as anti-knock agents, anti-oxidants, metal deactivators, anti-rust agents, anti-icing agents, detergents and so forth, are introduced to improve the combustion performance and thermochemical stability of the gasoline. Although fuel additives treat levels are generally low (0.035~0.35 wt %), most of these additives are polar such as alcohols, carboxylic acids, amines and so forth, which have strong tendency to adsorb onto the metal surface. The oil conductivity is supposed to be higher for a more polarized lubricant. Water is also more polar than PAO, hence, water droplets absorb onto metal surface more effectively. Due to the short test duration in the load-carrying the tests, the load carrying capacity is dominated by the strength of adsorption film rather than the viscosity. Moreover, acidic or even di-ketone compounds could promote oxidation or oxidative complexation of the surface areas in contact and the surface oxides reduce metal-metal adhesion and thus prevent scuffing.
5. Conclusions
In summary, we investigated the impact of fuel and water content on the tribological performance of a PAO base oil and a secondary ZDDP. The key observations including the following:
- Fuel was added to a PAO base oil at 3%, 6% and 20% and the resulting viscosity reduction of PAO was 19%, 35% and 73%, respectively.
- The fuel content increased the load-carrying capacity of PAO, probably due to the increased the polarity and acidity in the lubricant.
- Adding 3%, 6% and 20% fuel into the PAO base oil resulted in a wear increase of the cast iron flat by 7%, 59% and 212%, contributed by the reduced lubricant viscosity.
- Adding 1% water into PAO led to a load-carrying capacity higher than 200 N with the COF below 0.1 but had an insignificant impact on the wear rate under constant-load sliding. 0.8% ZDDP had marginal protection of the cast iron surfaces at room temperature with 16% wear decrease compared with PAO. The addition of 1% water and 3% fuel gave rise to a 24% and a 52% wear increase, compared with PAO-ZDDP.
- A higher fuel concentration resulted in a smoother wear scar, suggesting chemical-mechanical polishing process as a result of the acidic compounds and reduced viscosity. The phosphate polymerization level of ZDDP tribofilm was reduced by the addition of H2O but the thickness of ZDDP tribofilm was not significantly altered.
The results of fuel and water in the base oil reveal their direct impacts on engines performances.
Supplementary Materials
Supplementary materials can be found at http://www.mdpi.com/2075-4442/6/3/79/s1. Table S1. Comparison of wear rates of cast iron flats (10−7 mm3/(N·m)). Figure S1. Emulsion of 1% water in PAO after 15 min sonication (0 h), and after 1 h, 2 h and 24 h. Figure S2. Wear track morphology and cross-sectional 2D profiles of PAO, PAO-3%Fuel, and PAO-1%H2O. Figure S3. Wear track morphology and cross-sectional 2D profiles of PAO-ZDDP, PAO-ZDDP-1%H2O, and PAO-ZDDP-3%Fuel.
Author Contributions
Conceptualization, Y.Z. and J.Q.; Load-Carrying Test, B.C.S.; TAN Measurement, R.M.C.; Electrical Conductivity Measurement, S.L.; Writing—Original Draft Preparation, Y.Z. and J.Q.; Writing—Review & Editing, Y.Z., W.L. and J.Q.; Funding Acquisition, J.Q.
Funding
The research was sponsored by the Vehicle Technologies Office, Office of Energy Efficiency and Renewable Energy, U.S. Department of Energy (DOE).
Acknowledgments
The authors thank Ewa Bardasz from Lubrizol and Andrew G. Bro and Don Mattran from ExxonMobil for providing the ZDDP and the PAO base oil, respectively.
Conflicts of Interest
The authors declare no conflict of interest.
Notice
This manuscript has been authored by UT-Battelle, LLC, under Contract No. DE-AC05-00OR22725 with the U.S. Department of Energy. The United States Government retains and the publisher, by accepting the article for publication, acknowledges that the United States Government retains a non-exclusive, paid-up, irrevocable, world-wide license to publish or reproduce the published form of this manuscript, or allow others to do so, for United States Government purposes.
References
- Hu, T.; Teng, H.; Luo, X.; Chen, B. Impact of fuel injection on dilution of engine crankcase oil for turbocharged gasoline direct-injection engines. SAE Int. J. Engines 2015, 8, 1107–1116. [Google Scholar] [CrossRef]
- Splitter, D.; Burrows, B.; Lewis, S. Direct Measurement and Chemical Speciation of Top Ring Zone Liquid during Engine Operation. SAE Tech. Pap. 2015. [Google Scholar] [CrossRef]
- Ajayi, O.O.; Lorenzo-Martin, C.; Fenske, G.; Corlett, J.; Murphy, C.; Przesmitzki, S. Bioderived fuel blend dilution of marine engine oil and impact on friction and wear behavior. J. Tribol. 2016, 138, 021603. [Google Scholar] [CrossRef]
- Khuong, L.S.; Masjuki, H.H.; Zulkifli, N.W.M.; Mohamad, E.N.; Kalam, M.A.; Alabdulkarem, A.; Arslan, A.; Mosarof, M.H.; Syahir, A.Z.; Jamshaid, M. Effect of gasoline-bioethanol blends on the properties and lubrication characteristics of commercial engine oil. RSC Adv. 2017, 7, 15005–15019. [Google Scholar] [CrossRef]
- Song, B.H.; Choi, Y.H. Investigation of variations of lubricating oil diluted by post-injected fuel for the regeneration of CDPF and its effects on engine wear. J. Mech. Sci. Technol. 2008, 22, 2526–2533. [Google Scholar] [CrossRef]
- Lancaster, J.K. A review of the influence of environmental humidity and water on friction, lubrication and wear. Tribol. Int. 1990, 23, 371–389. [Google Scholar] [CrossRef]
- Cai, Z.B.; Zhou, Y.; Qu, J. Effect of oil temperature on tribological behavior of a lubricated steel-steel contact. Wear 2015, 332, 1158–1163. [Google Scholar] [CrossRef]
- Fatima, N.; Holmgren, A.; Marklund, P.; Minami, I.; Larsson, R. Degradation mechanism of automatic transmission fluid by water as a contaminant. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2015, 229, 74–85. [Google Scholar] [CrossRef]
- Sheiretov, T.; Van Glabbeek, W.; Cusano, C. The effect of dissolved water on the tribological properties of polyalkylene glycol and polyolester lubricants. Lubr. Eng. 1996, 52, 463–480. [Google Scholar]
- Spikes, H. The history and mechanisms of ZDDP. Tribol. Lett. 2004, 17, 469–489. [Google Scholar] [CrossRef]
- Parsaeian, P.; Ghanbarzadeh, A.; Wilson, M.; Van Eijk, M.C.P.; Nedelcu, I.; Dowson, D.; Neville, A.; Morina, A. An experimental and analytical study of the effect of water and its tribochemistry on the tribocorrosive wear of boundary lubricated systems with ZDDP-containing oil. Wear 2016, 358, 23–31. [Google Scholar] [CrossRef]
- Rounds, F.G. Some factors affecting the decomposition of three commercial zinc organodithiophosphates. ASLE Trans. 1975, 18, 79–89. [Google Scholar] [CrossRef]
- Nedelcu, I.; Piras, E.; Rossi, A.; Pasaribu, H.R. XPS analysis on the influence of water on the evolution of zinc dialkyldithiophosphate–derived reaction layer in lubricated rolling contacts. Surf. Interface Anal. 2012, 44, 1219–1224. [Google Scholar] [CrossRef]
- Costa, H.L.; Spikes, H.A. Impact of ethanol on the formation of antiwear tribofilms from engine lubricants. Tribol. Int. 2016, 93, 364–376. [Google Scholar] [CrossRef]
- Parsaeian, P.; Van Eijk, M.C.P.; Nedelcu, I.; Neville, A.; Morina, A. Study of the interfacial mechanism of ZDDP tribofilm in humid environment and its effect on tribochemical wear; Part I: Experimental. Tribol. Int. 2017, 107, 135–143. [Google Scholar] [CrossRef]
- Maples, R.E. Petroleum Refinery Process Economics; PennWell Corporation: Tulsa, OK, USA, 2000. [Google Scholar]
- Wright, R.A.E.; Wang, K.; Qu, J.; Zhao, B. Oil-Soluble Polymer Brush Grafted Nanoparticles as Effective Lubricant Additives for Friction and Wear Reduction. Angew. Chem. Int. Ed. 2016, 55, 8656–8660. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Luo, H.; Chi, M.; Ma, C.; Blau, P.J.; Dai, S.; Viola, M.B. Comparison of an oil-miscible ionic liquid and ZDDP as a lubricant anti-wear additive. Tribol. Int. 2014, 71, 88–97. [Google Scholar] [CrossRef]
- Jenkins, R.W.; Moore, C.M.; Semelsberger, T.A.; Chuck, C.J.; Gordon, J.C.; Sutton, A.D. The Effect of Functional Groups in Bio-Derived Fuel Candidates. ChemSusChem 2016, 9, 922–931. [Google Scholar] [CrossRef] [PubMed]
- Soltanahmadi, S.; Morina, A.; van Eijk, M.C.P.; Nedelcu, I.; Neville, A. Tribochemical study of micropitting in tribocorrosive lubricated contacts: The influence of water and relative humidity. Tribol. Int. 2017, 107, 184–198. [Google Scholar] [CrossRef]
- Cen, H.; Morina, A.; Neville, A.; Pasaribu, R.; Nedelcu, I. Effect of water on ZDDP anti-wear performance and related tribochemistry in lubricated steel/steel pure sliding contacts. Tribol. Int. 2012, 56, 47–57. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).